Abstract
Ultrasonography has been rarely used to measure musculoskeletal and joint activity in systemic lupus erythematosus (SLE). The aim of this review is to discuss the utility and reliability of this non-invasive diagnostic tool for the assessment of joint disease in SLE patients. In the last decade, several reports have highlighted the role of ultrasonography for a better evaluation of SLE-related musculoskeletal symptoms. The symptoms have also been associated with worse outcomes in SLE; therefore, it is essential to seek useful and accessible techniques for better understanding of such patients who are insufficiently assessed by standard physical examination.
Keywords: Systemic lupus erythematosus, ultrasound, musculoskeletal
Musculoskeletal involvement in systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect any organ or system (1). The early diagnosis and control of symptoms through disease outcome is important for rheumatologists not only to initiate adequate treatments but also to avoid unneeded medications (2). Musculoskeletal (MS) involvement is the most common group of symptoms in patients with SLE (70%–95% over the disease course and up to 60% during disease flares), or even as a first presenting symptom in up to 50% of cases (3–5). Therefore, the clinical picture varies from transient or migratory arthralgia, non-deforming and nonerosive arthritis, and tenosynovitis to Jaccoud’s arthropathy, which shows deformities with none or few bone erosions, depending on the imaging technique used (3, 4). According to a variety of descriptive cohorts, other uncommon MS manifestations in SLE patients are myositis, fibromyalgia, fragility fractures, and osteonecrosis (5–12). Interestingly, it is very important to appropriately assess joint disease to help physicians establish early SLE diagnosis and to give an interesting window of opportunity to treat SLE. Thereafter, the increasing implementation of T2T treatment strategies on patients may allow a better control and clinical monitoring along with decreasing further complications, thereby leading to a better prognosis. Ozbek et al. (13) demonstrated that despite MS symptoms being the most common initial symptom, they were barely diagnosed as SLE only in 27% of cases within the first 3 months of the disease (9). The delayed diagnosis may have a further impact on accurate diagnosis, morbidity, and mortality, and the authors attributed the low intensity of MS symptoms (“arthralgia”) to this situation.
Classically, MS involvement in SLE has been considered a mild complaint for many years, thereby disparaging its potential role on disability or joint destruction. However, in the last decade, several studies have raised concerns in the outcomes for patients with MS involvement in terms of impaired health-related quality of life (HRQoL), disability, hand functioning, and work productivity (14–18). Even in the absence of clinical synovitis, MS involvement causes important disability, loss of function, and a socioeconomic impact (19, 20). Therefore, MS SLE disease activity has been highlighted as a major determinant of HRQoL impairment (21). However, arthritis manifestation per se has been associated with the presence of specific subsets of patients when clusters of clinical manifestations were evaluated (22). Moreover, studying specific subsets of SLE patients adds new data on the SLE outcome (23–25). SLE patients with RNP, Sm, and APL antibodies showed a higher frequency of arthritis (and serositis) than patients with other antibody profiles (DNAds, Ro, and La). Besides, in a study that assessed clusters and mortality and flares per year, a cluster with arthritis as the main involvement (alongside higher use of glucocorticoids and immunosuppressant than of other clusters) was found to be associated with worse outcomes (26). It is noteworthy that MS damage domains appear as one of the most chiefly affected domains when assessing SLE clusters by damage with an important impact on the overall SLE mortality compared to other damage clusters (27).
Based on the abovementioned observations, it seems crucial to confirm joint disease as arthritis. Meanwhile, the unspecific “musculoskeletal” involvement has been associated with higher rates of mortality, intense treatment strategies, and specific clinical subsets (28).
SLE disease activity-validated tools are consistent with the presence of MS activity, although these are based on the clinical identification of joint swelling (29). However, many patients do not show consistent signs of joint inflammation upon physical examination, although they show many other symptoms that rheumatologists may attribute to SLE MS involvement. Considering these ideas, a need for a more sensitive and specific tool to assess joints and tendons involvement and damage in SLE certainly seems the goal. Physicians and patients need a more reliable, accessible, and specific MS assessment diagnostic tool, such as Musculoskeletal Ultrasound (MSKUS), in SLE. MSKUS gains more potential when the possible confounding factors, such as fibromyalgia, fragility fractures, and Jaccoud’s arthropathy, may confound patient’s HRQoL perception, thereby leading to better strategies than those only taken from patients’ opinion obtained from the clinical interview (20, 30). Notably, an increasing number of projects nowadays focus on the latter, and the role of ultrasonography (US) is likely to become a priority in the clinical setting.
Role of ultrasound in systemic lupus erythematosus musculoskeletal manifestations and joint disease
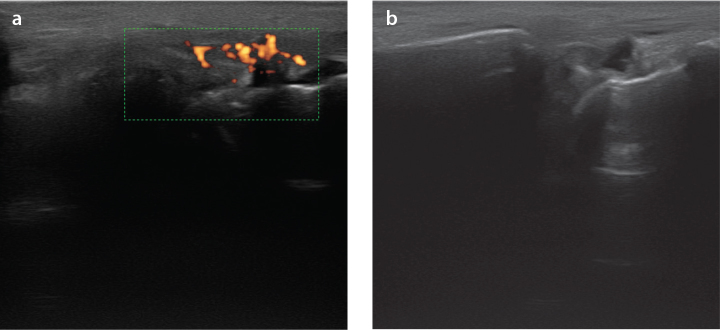
It is widely accepted that MSKUS increases the detection of a higher number of inflamed joints and tendons than the clinical exam findings in inflammatory arthropathies (31). Furthermore, Ogura et al. (32) have recently demonstrated that, similar to what occurs in rheumatoid arthritis, the predominance of tendon involvement (tenosynovitis and peri-extensor tendon inflammation) over synovitis when assessing treatment-naïve SLE patients by MSKUS is crucial. Interestingly, similar findings have been described in SLE-active patients, wherein Torrente-Segarra et al. (33) found a higher frequency of MSKUS abnormalities in patients complaining of arthralgia without confirmed arthritis. Moreover, Dreyer et al. (34) confirmed these findings in a more robust effort with more accurate evaluations, assessing all hand joints and tendons through US and clinical assessments. Based on these two studies, compared to MSKUS in the daily practice, a clinical joint examination seems to underestimate synovitis in SLE patients (27). Moreover, erosions have been shown in SLE patients with arthritis without rhupus or Jaccoud arthropathy patterns while receiving treatment for organic involvement of its SLE (Figure 1).
Figure 1. a, b.
Ultrasonography of a 55 yo woman with SLE presenting radio-carpal synovitis (synovial hypertrophy and fluid) (G1) (a); radio-carpal joint with power doppler signal (G3) in same SLE patient (b)
Interestingly, a recent review by Zayat et al. (29) focused on the role of MSKUS in assessing MS symptoms in SLE (27). Considering the application of quality measurement tools, the authors reinforce the potential use of the US in the evaluation of SLE disease activity in nine selected articles on this issue. However, they also raised the need to improve this knowledge since the nature of these articles presented heterogeneity in patient inclusion criteria, risk of bias, collected data, activity measures, outcome, US abnormalities definitions, assessed areas/joints, comparison with healthy controls, and previous treatments). Some studies have suggested an association between US abnormalities and SLE disease activity measures; nevertheless, one study found the opposite (35–37). The majority of those studies found the presence of US abnormalities in patients with MS symptoms; however, asymptomatic patients also showed US abnormalities (mostly in tendons) in four studies (24, 25, 30, 37). Erosions were also observed in several patients (2–41%), mainly in Jaccoud’s arthropathy and rhupus patients. Only three studies clearly separated rhupus from non-rhupus patients, and the other studies with included rhupus patients led to a bias in data collection. The latter could explain the wide range found in terms of erosion detection. Interestingly, the presence of synovitis by MSKUS in symptomatic patients without swelling clinically observed upon physical examination was present in up to 82% of cases, which seemed important for assessment. The given scenario has a deep impact on the treatment strategy. Grade 1 gray scale without Power Doppler signal (PD) was also reported, and we currently know that this observation may also be found in other rheumatologic conditions, such osteoarthritis and hypermobility syndrome undergoing stretching maneuvers over the joints.
Our group demonstrated that arthralgia was related to the overall SLE activity and the existence of US and clinical abnormalities, such as tenosynovitis and synovitis (based on OMERACT) (33). Recently, Piga et al. (21) suggested the power of US by PD signal in predicting MS flares within the first 24 months. They also showed that erosions do not lead to worse HRQoL per se. In contrast, active MS SLE activity and Jaccoud’s arthropathy are associated with impaired HRQoL. Another recent article by Corzo et al. (28) shows approximately 6-year patient outcome measurement after US baseline assessment in patients with arthralgia and US abnormalities. Those who presented US abnormalities at baseline showed higher need of methotrexate and hydroxychloroquine use to control MS SLE activity than those who did not show any US abnormalities or MS symptoms at baseline (26). This study is the first prospective study to assess the long-term outcome of SLE patients who showed US joint abnormalities at some point of disease progression. Patients who initially showed US abnormalities (synovitis and tenosynovitis) and were followed and treated in clinical practice settings by standard of care based on Spanish Society of Rheumatology guidelines mainly experienced MS symptoms throughout the study. The main reason to start anew the aforementioned disease-modifying drugs was the persistence of MS symptoms, in which the presence of US abnormalities was very common (more than 70% of cases, mostly tenosynovitis in 39% of cases) compared to those who did not show MS involvement or US abnormalities. Unfortunately, possibly because of the small sample of remaining patients at the end of follow-up, no association was found between the increasing need of treatment and a specific US finding.
Conclusion
Our clinical research pathway on MSKUS and SLE clinical assessment reinforces the use of MSKUS together with functional and quality of life measurement tools to better depict and understand the symptoms. Considering the previous experience with rheumatoid arthritis and psoriatic arthritis, US in SLE is likely to become an essential diagnostic tool in the diagnosis, monitoring, and treatment strategies of patients as soon as they present the flare. We essentially do not have enough experience or information, but as it was initially wiped out for rheumatoid arthritis and posteriorly widely accepted, we encourage SLE experts to use US at the onset of SLE to set up a baseline mapping of joint activity on the basis of US. The agreement on the future value of the MSKUS in SLE for MS involvement disease progression, prognosis, and outcome seems to ease management of SLE patients in a clinical setting. Further studies are needed to globally clarify and validate US as a technique of sensitive, specific, and predictive value to confirm joint inflammatory arthritis in SLE.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.T.S.; T.C.S.M., H.C.; Design - V.T.S.; T.C.S.M., H.C.; Supervision - V.T.S.; T.C.S.M., H.C.; Analysis and/or Interpretation - V.T.S.; T.C.S.M., H.C.; Literature Search - V.T.S.; T.C.S.M., H.C.; Writing - V.T.S.; T.C.S.M., H.C.; Critical Reviews - V.T.S.; T.C.S.M., H.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lahita RG. The immunoendocrinology of systemic lupus erythematosus. Clin Immunol. 2016;172:98–100. doi: 10.1016/j.clim.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Larosa M, Iaccarino L, Gatto M, Punzi L, Doria A. Advances in the diagnosis and classification of systemic lupus erythematosus. Expert Rev Clin Immunol. 2016;12:1309–20. doi: 10.1080/1744666X.2016.1206470. [DOI] [PubMed] [Google Scholar]
- 3.Ball EM, Bell AL. Lupus arthritis-do we have a clinically useful classification? Rheumatology (Oxford) 2012;51:771–9. doi: 10.1093/rheumatology/kes139. [DOI] [PubMed] [Google Scholar]
- 4.Santiago MB, Galvao V. Jaccoud arthropathy in systemic lupus erythematosus: analysis of clinical characteristics and review of the literature. Medicine (Baltimore) 2008;87:37–44. doi: 10.1097/MD.0b013e3181632d18. [DOI] [PubMed] [Google Scholar]
- 5.Zoma A. Musculoskeletal involvement in systemic lupus erythematosus. Lupus. 2004;13:851–3. doi: 10.1191/0961203303lu2021oa. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Wu H, Huang X, Xu D, Zheng W, Zhao Y, et al. Clinical analysis of 56 patients with rhupus syndrome: manifestations and comparisons with systemic lupus erythematosus: a retrospective case-control study. Medicine (Baltimore) 2014;93:e49. doi: 10.1097/MD.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zayat AS, Md Yusof MY, Wakefield RJ, Conaghan PG, Emery P, Vital EM. The role of ultrasound in assessing musculoskeletal symptoms of systemic lupus erythematosus: a systematic literature review. Rheumatology (Oxford) 2016;55:485–94. doi: 10.1093/rheumatology/kev343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy G, Isenberg DA. Connective tissue diseases: SLE-associated arthrop- athy: truly a benign entity? Nat Rev Rheumatol. 2012;8:695–6. doi: 10.1038/nrrheum.2012.200. [DOI] [PubMed] [Google Scholar]
- 9.Delle Sedie A, Riente L, Scire CA, Iagnocco A, Filippucci E, Meenagh G, et al. Ultrasound imaging for the rheumatologist. XXIV. Sonographic evaluation of wrist and hand joint and tendon involvement in systemic lupus erythematosus. Clin Exp Rheumatol. 2009;27:897–901. [PubMed] [Google Scholar]
- 10.Gabba A, Piga M, Vacca A, Porru G, Garau P, Cauli A, et al. Joint and tendon involvement in systemic lupus erythematosus: an ultrasound study of hands and wrists in 108 patients. Rheumatology (Oxford) 2012;51:2278–85. doi: 10.1093/rheumatology/kes226. [DOI] [PubMed] [Google Scholar]
- 11.Iagnocco A, Ceccarelli F, Rizzo C, Truglia S, Massaro L, Spinelli FR, et al. Ultrasound evaluation of hand, wrist and foot joint synovitis in systemic lupus erythematosus. Rheumatology (Oxford) 2014;53:465–72. doi: 10.1093/rheumatology/ket376. [DOI] [PubMed] [Google Scholar]
- 12.Mosca M, Tani C, Carli L, Vagnani S, Possemato N, Delle Sedie A, et al. The role of imaging in the evaluation of joint involvement in 102 consecutive patients with systemic lupus erythematosus. Autoimmun Rev. 2015;14:10–5. doi: 10.1016/j.autrev.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Ozbek S, Sert M, Paydas S, Soy M. Delay in the diagnosis of SLE: the importance of arthritis/arthralgia as the initial symptom. Acta Med Okayama. 2003;57:187–90. doi: 10.18926/AMO/32807. [DOI] [PubMed] [Google Scholar]
- 14.Drenkard C, Bao G, Dennis G, Kan HJ, Jhingran PM, Molta CT, et al. Burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res. 2014;66:878–87. doi: 10.1002/acr.22245. [DOI] [PubMed] [Google Scholar]
- 15.Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Relationship between flare and health-related quality of life in patients with systemic lupus erythematosus. J Rheumatol. 2010;37:568–73. doi: 10.3899/jrheum.090876. [DOI] [PubMed] [Google Scholar]
- 16.Malcus Johnsson P, Sandqvist G, Bengtsson A, Nived O. Hand function and performance of daily activities in systemic lupus erythematosus. Arthritis Rheum. 2008;59:1432–8. doi: 10.1002/art.24108. [DOI] [PubMed] [Google Scholar]
- 17.Doria A, Rinaldi S, Ermani M, Salaffi F, Iaccarino L, Ghirardello A, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. II. Role of clinical, immunological and psychological determinants. Rheumatology (Oxford) 2004;43:1580–6. doi: 10.1093/rheumatology/keh392. [DOI] [PubMed] [Google Scholar]
- 18.Grossman JM. Lupus arthritis. Best Pract Res Clin Rheumatol. 2009;23:495–506. doi: 10.1016/j.berh.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Drenkard C, Bao G, Dennis G, Kan HJ, Jhingran PM, Molta CT, et al. The burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res. 2014;66:878–87. doi: 10.1002/acr.22245. [DOI] [PubMed] [Google Scholar]
- 20.Eilertsen GO, Nikolaisen C, Becker-Merok A, Nossent JC. Interleukin-6 promotes arthritis and joint deformation in patients with systemic lupus erythematosus. Lupus. 2011;20:607–13. doi: 10.1177/0961203310392432. [DOI] [PubMed] [Google Scholar]
- 21.Piga M, Congia M, Gabba A, Figus F, Floris A, Mathieu A, Cauli A. Musculoskeletal manifestations as determinants of quality of life impairment in patients with systemic lupus erythematosus. Lupus. 2018;27:190–8. doi: 10.1177/0961203317716319. [DOI] [PubMed] [Google Scholar]
- 22.Li PH, Wong WH, Lee TL, Lau CS, Chan TM, Leung AM. Relationship between autoantibody clustering and clinical subsets in SLE: cluster and association analyses in Hong Kong Chinese. Rheumatology (Oxford) 2013;52:337–45. doi: 10.1093/rheumatology/kes261. [DOI] [PubMed] [Google Scholar]
- 23.Grönwall C, Hardt U, Gustafsson JT, Elvin K, Jensen-Urstad K, Kvarnström M, et al. Depressed serum IgM levels in SLE are restricted to defined subgroups. Clin Immunol. 2017;183:304–15. doi: 10.1016/j.clim.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Grönwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–8. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M, Sing S, Golabkesh Z, Fiskesund R, Gustafsson T, Jogestrand T, et al. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Clin Immunol. 2016;166–167:27–37. doi: 10.1016/j.clim.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 26.De Marchi G, Quartuccio L, Zuliani F, Bond M, De Vita S. The relevance of cluster analyses to stratify systemic lupus erythematosus: increased mortality with heavier treatment. Clin Exp Rheumatol. 2017;35:873–4. [PubMed] [Google Scholar]
- 27.Pego-Reigosa JM, Lois-Iglesias A, Rúa-Figueroa Í, Galindo M, Calvo-Alén J, de Uña-Álvarez J, et al. Relationship between damage clustering and mortality in systemic lupus erythematosus in early and late stages of the disease: cluster analyses in a large cohort from the Spanish Society of Rheumatology Lupus Registry. Rheumatology (Oxford) 2016;55:1243–50. doi: 10.1093/rheumatology/kew049. [DOI] [PubMed] [Google Scholar]
- 28.Corzo P, Salman-Monte TC, Torrente-Segarra V, Polino L, Mojal S, Carbonell-Abelló J. Joint ultrasound baseline abnormalities predict a specific long-term clinical outcome in systemic lupus erythematosus patients. Lupus. 2017;26:729–33. doi: 10.1177/0961203316676376. [DOI] [PubMed] [Google Scholar]
- 29.Zayat AS, Md Yusof MY, Wakefield RJ, Conaghan PG, Emery P, Vital EM. The role of ultrasound in assessing musculoskeletal symptoms of systemic lupus erythematosus: a systematic literature review. Rheumatology (Oxford) 2016;55:485–94. doi: 10.1093/rheumatology/kev343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrente-Segarra V, Carbonell-Abelló J, Castro-Oreiro S, Manresa Domínguez JM. Association between fibromyalgia and psychiatric disorders in systemic lupus erythematosus. Clin Exp Rheumatol. 2010;28:22–6. [PubMed] [Google Scholar]
- 31.Dale J, Purves D, McConnachie A, McInnes I, Porter D. Tightening up? Impact of musculoskeletal ultrasound disease activity assessment on early rheumatoid arthritis patients treated using a treat to target strategy. Arthritis Care Res. 2014;66:19–26. doi: 10.1002/acr.22218. [DOI] [PubMed] [Google Scholar]
- 32.Ogura T, Hirata A, Hayashi N, Takenaka S, Ito H, Mizushina K, et al. Comparison of ultrasonographic joint and tendon findings in hands between early, treatment-naïve patients with systemic lupus erythematosus and rheumatoid arthritis. Lupus. 2017;26:707–14. doi: 10.1177/0961203316676375. [DOI] [PubMed] [Google Scholar]
- 33.Torrente-Segarra V, Lisbona MP, Rotés-Sala D, Muñoz-Ortego J, Padró-Blanch I, Maymó-Guarch J, et al. Hand and wrist arthralgia in systemic lupus erythematosus is associated to ultrasonographic abnormalities. Joint Bone Spine. 2013;80:402–6. doi: 10.1016/j.jbspin.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Dreyer L, Jacobsen S, Juul L, Terslev L. Ultrasonographic abnormalities and inter-reader reliability in Danish patients with systemic lupus erythematosus - a comparison with clinical examination of wrist and metacarpophalangeal joints. Lupus. 2015;24:712–9. doi: 10.1177/0961203314561666. [DOI] [PubMed] [Google Scholar]
- 35.Iagnocco A, Ossandon A, Coari G, Conti F, Priori R, Alessandri C, et al. Wrist joint involvement in systemic lupus erythematosus. An ultrasonographic study. Clin Exp Rheumatol. 2004;22:621–4. [PubMed] [Google Scholar]
- 36.Ball E, Gibson D, Bell A, Rooney M. Plasma IL-6 levels correlate with clinical and ultrasound measures of arthritis in patients with systemic lupus erythematosus. Lupus. 2014;23:46–56. doi: 10.1177/0961203313512882. [DOI] [PubMed] [Google Scholar]
- 37.Ossandon A, Iagnocco A, Alessandri C, Priori R, Conti F, Valesini G. Ultrasonographic depiction of knee joint alterations in systemic lupus erythematosus. Clin Exp Rheumatol. 2009;27:329–32. [PubMed] [Google Scholar]