Abstract
Essential oils (EO) are secondary metabolites usually made up of terpenoids and phenylpropanoids and have antimicrobial properties. However, the feeding effects of EO-Cobalt (EOC) on the performance of goats are largely unknown. Herein we investigated and reported the effects of dietary EOC (0, 52, and 91 mg daily) on fiber producing cashmere goats. We determined the resulting phenotypes including live growth, carcass weight, meat quality, and cashmere fiber traits. We show that dietary supplement of EOC significantly promoted average daily gain (P < 0.05), and significantly improved carcass weight, and meat and hair fiber quality (P < 0.05). We further conducted RNA-seq using skin and liver tissues from each group to assess the molecular mechanism conferring these phenotypic changes. A total of 191 differentially expressed genes were found in the skin tissues (0 vs 91 mg), while 1,127 DEGs were found in livers. Analyses of liver samples for differential gene action and functional prediction found that EOC stimulated physiological changes in the body’s immune system at both blood and cell levels. Our results demonstrated the potential of using EO-based feed ingredient to improve animal growth performance, meat quality and fiber quality, and further illustrated the molecular basis that contribute to phenotypes at physiological levels.
Introduction
Essential oils (EO) are volatile aromatic compounds produced by plants (herbs and spices) as complex mixtures of secondary metabolites, EO have a large number of beneficial properties on health through their well-known antioxidant and free radical scavenging activities1. An active component in essential oils (EO) has been reported to possess high antifungal, antioxidant and antimicrobial activity2. Antimicrobial activity contributes to promoting a dynamic balance microflora population in the rumen and the lower gut which contributes directly to gut barrier integrity, reduced inflammation, improved competitive exclusion of pathogenic bacteria resulting in a stronger immune system. Essential oils appeared to be very promising compounds as they selectively reduced methane production and protein breakdown in both in vitro and in vivo studies3, while the use of EO as feed additives was accompanied with decreased feed degradability and lowered volatile fatty acid4. Previous studies have found that dietary incorporation of oregano essential oil (OEO) exerted strong antioxidant effects retarding lipid oxidation in meat during refrigerated and long-frozen storage so frozen stored meats have extended shelf life5. In addition, the study of dietary effects of OEO on pig performance, oxidative status and pork quality traits revealed that the meat, received higher organoleptic scores for color, taste, juiciness, and overall acceptance in both the blind and the labelled consumer tests6.
The antimicrobial action of essential oils in feed systems is well documented and there is a growing interest in studies of natural additives as potential antioxidants and as substitutes for antibiotics when illegality for antibiotic use is totally enforced worldwide7. Many sources of antioxidants of plant origin have been studied in recent years. Among these, the antioxidant properties of many aromatic plants and spices have shown to be effective in retarding the process of lipid peroxidation in oils and fatty foods8,9. Previous studies have found that EOC is beneficial in alleviating transportation stress and improving antioxidant activity10,11. In addition, Cobalt is the activator of various enzymes in the body, mainly involved in the synthesis of vitamin B12 in the form of Co3+. A small amount of cobalt can enhance the reproductive capacity of ruminants, Lack of cobalt often lead to anemia and dysplasia, livestock will appear low birth rate, reduced milk, young chicks born weak, low rate of weaning survival12,13.
Mammalian hair is produced by hair follicle (HF) cell proliferation and differentiation, the formation of HF is associated with epidermal and mesenchymal cells14,15. HF are unique to mammals, with a high degree of self-renewal ability, the only lifelong growth of organs. The studies of HF related to the mechanism at the transcriptional level of shows that many factors and their receptors plays an extremely important role in the regulation HF as an important link in the regulation of genes and environment in vivo16. RNA-seq was sequenced using high-throughput sequencing techniques for reverse transcription of all RNA’s in tissues or cells17, and useful for studying animal production and health and have become important components of systems genomic or systems biology methods18. The objective of the present study was to identify potential regulatory genes in supplementation with EOC for cashmere goats by characterizing the skin and liver transcriptome based on RNA-seq technologies. And this study reports important finding regarding potential regulatory genes and effects of EOC, including hair fiber and meat quality in farm animals.
Material and Methods
Ethical approval
All animal handing protocols in this study were approved by the Gansu Agricultural University Animal Care and Use Committee guidelines (approved ID: 2012-2-159), in compliance with the Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of P. R. China, 1988).
Experimental design and animal management
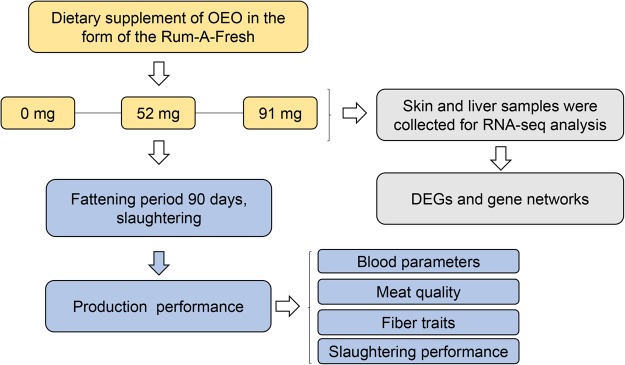
A total of 45 castrated male cashmere goats were randomly divided into three groups. The cashmere goats in each group were animals of similar live weight (average weight 33.73 ± 2.11 kg) at fasting. There was no significant difference in body weight between three groups (P > 0.05). the EO and Co are added as product of Rum-A-fresh (RAF), the organic cobalt level is 0.75% in the RAF product. the essential oil amount is 1.3% of the RAF product. For each group, 0, 52, and/or 91 mg per head/day of EOC were added in daily diet in the form of an essential oil, lactic acid, and cobalt carbonate with clinoptilolite used as an ingredient dispersant. The fattening period lasted 90 days and was divided into three stages. Each fattening period was 30 days. Each test group of goats was evaluated for 90 days. There was a 15-day pre-feeding adjustment period at the beginning of fattening period. Uniform live body weights were obtained following a 12-hour fast. This was done to eliminate variation in body weight that might be related to weight changes associated with potential drinking or eating immediately before weighing. During the trial period, the goats were allowed to freely drink water, and feed twice every day. Table S9 shows the chemical composition of the commercial diet for each group. Three goats from each group were randomly selected for slaughtering at day 90. All goats were slaughtered in an accredited abattoir using carbon dioxide to stun the animal. The specific experimental design is shown in Fig. 1.
Figure 1.
Study design of the present study.
Analytical Methods
Analysis of growth and slaughter performance
The weight of goats was determined before morning feeding at day 0 and day 90, to calculate the average daily gain (ADG). Animals were then processed following the standard industrial techniques. For each animal, the weight of pelt, full gastrointestinal liver, spleen, left kidney tract, heart, and lungs were collected. Hot carcass weight was then recorded in order to evaluate hot dressing percentage as a ratio of hanging carcass weight and live weight.
Analysis of meat quality
The meat quality was determined from the sample removed from the Longissimus dorsi (LD) muscle after carcass dissection, with no external fat or connective tissue. The measurement of pH, lightness (L*), redness (a*), yellowness (b*) were measured. Water loss rate, drip loss, cooking losses, and tenderness were conducted according to a previous study19. The first pH reading (pH1) was taken in situ on the LD muscle of the right side between the 4th and the 5th lumbar vertebra using penetrating electrode and a portable pH meter. Drip losses and other cooking losses were performed on LD muscle as described by Honikel20. The detailed method steps refer to a previous report19. The LD muscles were extracted according to the method of Folch21.The one-step extractive methylation procedure for fatty acids gain was performed in a gas chromatograph (Trace DSQGC/MS) with a capillary column (HP-5MS) using margaric acid (C17:0) as an internal standard. The oven temperature was programmed to provide three consecutive ramps, the first had an initial temperature of 120 °C maintained for 1 min then increased by 10 °C/min until it reached 175 °C, where it was maintained for 10 min; the second increased by 5 °C/min until it reached 210 °C, where it was maintained for 5 min and the third ramp increased by 5 °C/min to 230 °C, where it was maintained for 5 min. The carrier gas was helium at a flow rate of 2 mL/min. An automatic split less injector with a 1/50 split and a temperature of 250 °C was used. The injection volume was 1 μL. A flame ionization detector (FID) was used with an air flow of 450 mL/min, hydrogen flow of 40 mL/min and a detector temperature of 280 °C. Fatty acids were expressed in gravimetric concentrations (mg/g of freeze dried sample)22,23.
Analysis of serum physiological and biochemical indexes
Jugular vein blood was collected from the goats 1 hour before slaughtering, the blood was preserved in the vacuum tube containing coagulant, blood was kept at 4 °C for 3 h, and then centrifuged (4000 r/min for 10 min at 4 °C) to extract the serum. The serum was used to determine blood physiological parameters.
Skin staining
Skin tissues were used for histological sectioning, the procedures of histological sectioning according to the method of Carter and Clarke24. skin samples were extracted from the different goats and placed in tubes containing 4% paraformaldehyde solution (made with 0.1 M sodium phosphate buffer, pH = 7.4). After the following steps are embedding, cutting into slices, baking slides, H&E straining, mounting25.
Total RNA isolation, library construction and sequencing
Total RNA was isolated from skin and liver samples using Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. The quality and concentration of the total RNA were determined using an Agilent 2100 Bioanalyzer (Agilent). RNA samples were stored at -80 °C for later library construction and sequencing. Nine RNA libraries for each skin sample were constructed. Oligo (dTs) were used to isolate poly (A) mRNA. The mRNA was fragmented, and reverse transcribed using random primers. Second-strand cDNAs were synthesized using RNase H and DNA polymerase I. The double-strand cDNAs were then purified using the QiaQuick PCR extraction kit. The required fragments were purified via agarose gel electrophoresis and were enriched through PCR amplification. Finally, the amplified fragments were sequenced using Illumina HiSeq™ 2000 (Novogene, Beijing, China) according to the manufacturer’s specifications.
Mapping reads to the reference genome
The original sequencing-received image data were transferred into sequence data via base calling, which is defined as raw data or raw reads stored in the appropriate format. Raw reads of all nine samples were pre-processed through the removal of containing adaptors-read with more than 17% unknown nucleotides (the minimum read length is 400 bp). The clean reads of each stage were aligned to the goat genome assembly (ARS1). At the same time, STAR add parameters of out Filter Intron Motifs Remove Non-canonical, In-the- post-sequences, so the resulting bam file supports the use of Cuffdiff26.
Expression annotation
For gene expression analysis, the number of unique-match reads was calculated and normalized to FPKM (Fragment Per Kilo base of exon model per Million mapped reads). Expression levels of each gene between two groups were compared to give an expression difference using Cuffdiff as outlined by Trapnell27. The P value corresponded to differential gene expression at statistically significant levels (P < 0.05)28. False Discovery Rate (FDR) was used to determine the P value threshold. DEGs were defined as FDR ≤ 0.05 and absolute value of |log (fold change)| >1. Functional classification of the DEGs was performed using WEGO software. The KEGG (Kyoto encyclopedias of genes and genomes) pathway annotation was carried out using DAVID 6.729.
Statistical analysis
The analyses associated with RNA-seq were analyzed using R. We performed t-test using a one-way ANOVA with Dunnett’s post-hoc comparison procedure of the statistical analyses software SPSS version 22.0 to separate statistical means and to establish the probabilities of larger ‘P’ values.
Results
Finishing performance and carcass characteristics
The growth performance results from the goats during the finishing phases are shown in Table 1. The initial body weights by treatment were not significantly different (P > 0.05). The final body weight and ADG was affected by the feed levels; consequently, means of two treatments of 0 mg group and 91 mg group were significant different at the 5% level of probability (P < 0.05). The feed conversion rate (FCR) were significant increase in EOC addition group (P < 0.05). The meat marbling score of the 91 mg group was significantly higher also at the 5% level of probability than that of the control group (P < 0.05). Carcass weight, dressing percentage, net meat percentage, meat-bone ratio, and perineal fat are significantly different when contrasting the 91 mg group mean with the control group mean (P < 0.05).
Table 1.
Effects of the EOC on growth and slaughter performance of goats.
| Item | Group | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 mg | 52 mg | 91 mg | |||
| Initial body weight (kg) | 33.38 | 33.41 | 34.38 | 0.49 | 0.95 |
| Final body weight (kg) | 42.05a | 48.91b | 49.66b | 1.23 | 0.50 |
| Average daily gain | 0.09a | 0.17b | 0.17b | 0.01 | 0.51 |
| Feed conversion efficiency (%) | 11.14a | 31.69b | 19.77b | 3.40 | 0.03 |
| Marbling score | 2.53a | 4.33b | 4.00b | 0.19 | 0.00 |
| Longissimus Doris (g) | 556.3 | 584.57 | 625.01 | 15.38 | 0.19 |
| Carcass weight (kg) | 20.23a | 24.19b | 23.9b | 0.59 | 0.00 |
| Dressing percentage (%) | 49.00a | 51.27ab | 51.56b | 0.50 | 0.07 |
| Net meat percentage (%) | 33.57a | 34.07ab | 35.98b | 0.45 | 0.07 |
| Meat-bone radio (%) | 34.55a | 30.38a | 28.66b | 0.89 | 0.01 |
| Perirenal fat (kg) | 0.92a | 1.40b | 1.52b | 0.09 | 0.01 |
| Left half carcass muscle area (cm2) | 19.38 | 19.88 | 22.75 | 0.87 | 0.25 |
SEM = standard error measurement. a,bMeans with different superscripts differ significantly (P < 0.05).
Meat quality
The muscle quality traits are shown in Table 2. The pH1 and pH24 were not affected by the feed levels, the cooked meat percentage was significantly affected by the EOC (P < 0.05), the cooking loss. water-holding capacity and drip loss are not significantly different when comparisons of the 91 mg group and the control group are considered (P > 0.05). The fatty acid composition of LD muscle is shown in Table 3, Saturated hypercholesterolemia FA (14:0) was significantly affected by EOC (P < 0.05). The contents of polyunsaturated fatty acid (PUFA), monounsaturated fatty acid (MUFA), saturated fatty acid (UFA) and saturated fatty acid (SFA) were not significantly influenced by the EOC (P > 0.05).
Table 2.
Effects of the EOC on quality traits of goat’s LM.
| Item | Group | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 mg | 52 mg | 91 mg | |||
| pH1 | 6.62 | 6.61 | 6.54 | 0.07 | 0.90 |
| pH24 | 6.25 | 6.23 | 6.36 | 0.11 | 0.91 |
| L* | 49.94 | 49.71 | 50.16 | 0.18 | 0.67 |
| a* | 8.46 | 4.71 | 5.51 | 0.81 | 0.13 |
| b* | 12.99 | 12.51 | 12.82 | 0.16 | 0.57 |
| Cooking loss (%) | 14.69 | 15.84 | 18.09 | 1.36 | 0.65 |
| Water-holding capacity (%) | 5.56 | 8.24 | 4.79 | 1.08 | 0.45 |
| Cooked meat percentage (%) | 25.71b | 31.38b | 40.72a | 2.53 | 0.02 |
| Drip loss (%) | 5.52 | 4.55 | 6.93 | 0.75 | 0.50 |
SEM = standard error measurement. a,bMeans with different superscripts differ significantly (P < 0.05).
Table 3.
Effects of the EOC on total fatty acid concentration (mg/g muscle dry matter) and composition (g/100 g total FA) composition of goat’s LM.
| Item | Group | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 mg | 52 mg | 91 mg | |||
| C14:0 | 1.34a | 1.61a | 0.32b | 0.05 | 0.04 |
| C16:0 | 20.02 | 21.94 | 21.21 | 0.36 | 0.09 |
| C18:1 9t | 2.15 | 1.79 | 1.67 | 0.11 | 0.19 |
| C18:1 9c | 51.01 | 50.74 | 51.78 | 0.49 | 0.70 |
| C18:1 10c | 1.99 | 2.08 | 2.04 | 0.05 | 0.84 |
| C18:1 11c | 0.27 | 0.34 | 0.25 | 0.03 | 0.47 |
| C18:1 12c | 0.14 | 0.19 | 0.14 | 0.01 | 0.45 |
| C22:1n9 | 0.47 | 0.32 | 0.55 | 0.04 | 0.17 |
| Total acid in muscle (mg/g) | 121.70b | 216.91a | 107.67b | 20.23 | 0.04 |
| Polyunsaturated fatty acid (PUFA) | 3.09 | 2.93 | 3.44 | 0.16 | 0.44 |
| Monounsaturated fatty acid (MUFA) | 58.29 | 57.62 | 58.52 | 0.52 | 0.78 |
| Unsaturated fatty acid (UFA) | 61.38 | 60.56 | 61.96 | 0.61 | 0.67 |
| Saturated fatty acid (SFA) | 38.61 | 39.44 | 38.03 | 0.61 | 0.67 |
SEM = standard error measurement. a,bMeans with different superscripts differ significantly (P < 0.05).
Serum physiological and biochemical analyses
The contents of calcium, magnesium, creatinine, carbon dioxide-combining power, bilirubin acid, total protein and albumin in the 91 mg group were significantly higher than the control group at the 5% level of probability (P < 0.05) (Table 4). The contents of lactate dehydrogenase decreased with the addition of EOC, and the other means are not significantly different as indicated by a probability level greater than 5%.
Table 4.
Effects of the EOC on serum physiological and biochemical of goat.
| Item | Group | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 mg | 52 mg | 91 mg | |||
| Leukocyte (109/L) | 16.06 | 16.5 | 15.85 | 0.6 | 0.91 |
| Erythrocyte (1012/L) | 4.73 | 4.93 | 4.91 | 0.15 | 0.85 |
| Hemoglobin (g/L) | 144.16 | 138.83 | 147.5 | 2.18 | 0.27 |
| Lymphocyte percentage (%) | 55.41 | 53.51 | 54.1 | 1.6 | 0.89 |
| Calcium (mmoL/L) | 2.35a | 2.43b | 2.46b | 0.01 | 0.01 |
| Magnesium (mmol/L) | 1.04a | 0.93ab | 0.88b | 0.03 | 0.06 |
| Phosphorus (mmol/L) | 2.48 | 2.36 | 2.01 | 0.1 | 0.10 |
| Ferrum (mmol/L) | 38.41 | 28.81 | 32.21 | 2.3 | 0.23 |
| Creatinine (umol/L) | 63.13a | 46.23b | 62.13b | 2.79 | 0.01 |
| Carbon dioxide-combining Power (mmol/L) | 20.88a | 25.35b | 24.96b | 0.74 | 0.01 |
| Bilirubin acid (umol/L) | 3.23a | 1.88a | 2.33b | 0.2 | 0.01 |
| Total protein (g/L) | 76.48a | 71.06ab | 73.13ab | 0.88 | 0.03 |
| Albumin (g/L) | 35.50a | 32.95a | 33.73b | 0.36 | 0.01 |
| Globulin (g/L) | 40.98 | 38.11 | 39.4 | 0.82 | 0.38 |
| AST (u/L) | 17.83 | 18 | 16 | 1.11 | 0.74 |
| ALT (u/L) | 89.5 | 73.16 | 85.66 | 3.75 | 0.18 |
| γ-glutamyl trans peptidase (µ/L) | 54.33 | 52 | 61.5 | 2.35 | 0.24 |
| HDL (mmol/L) | 2.02 | 1.8 | 2.16 | 0.09 | 0.25 |
| Creatine kinase (µ/L) | 197.16 | 235.5 | 233.83 | 12.08 | 0.36 |
| Lactate dehydrogenase (µ/L) | 372.66 | 367.66 | 342.33 | 19.94 | 0.82 |
SEM = standard error measurement. a,bMeans with different superscripts differ significantly (P < 0.05).
Characters of Hair fibers
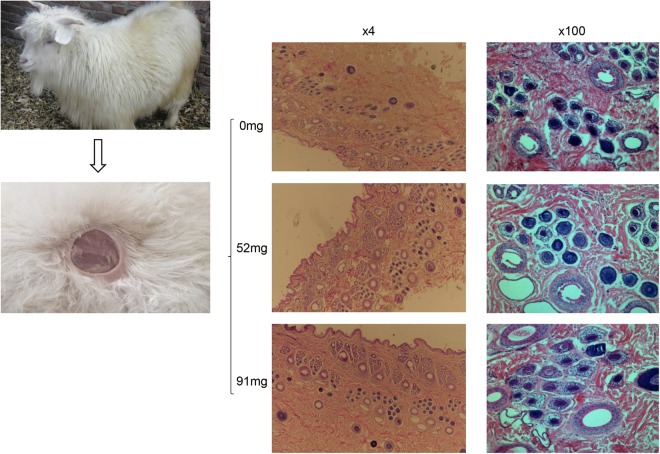
The crack extension, EYS1.5 and elongation per unit length of the different treatment groups were significantly different (P < 0.05) (Table 5). Hair fineness was considered a very important economic trait of the cashmere goat included in the study reported herein. With the addition of essential oils, fineness tended to be thinner, but differences were not significant between the treatment groups (P > 0.05). In addition, the primary and secondary HF diameter was significant different (P < 0.05). Also, the diameter ratio of primary HF number to secondary HF (S/P ratio) was significantly different (P < 0.05) (Fig. 2).
Table 5.
Effects of the EOC on hair fibers characters of cashmere goat.
| Item | Group | SEM | P-value | ||
|---|---|---|---|---|---|
| 0 mg | 52 mg | 91 mg | |||
| Degree of curling | 1.39 | 1.44 | 1.6 | 0.05 | 0.31 |
| Recovery rate | 78.13 | 75.92 | 75.44 | 2.04 | 0.86 |
| Crack extension (mm) | 3.94a | 5.21b | 5.02b | 0.21 | 0.02 |
| Intension (CN/dT) | 25.41 | 23.97 | 19.1 | 1.92 | 0.40 |
| EYS1.5 (mm) | 3.05a | 4.29b | 4.22b | 0.23 | 0.04 |
| Elongation per unit length (%) | 39.41a | 52.11b | 49.82b | 2.17 | 0.02 |
| Fineness degree | 17.99 | 20.19 | 20.08 | 0.52 | 0.16 |
| Natural length (cm) | 3.53 | 4.11 | 3.64 | 0.30 | 0.72 |
| Level of stretch (cm) | 4.04 | 5.02 | 4.38 | 0.29 | 0.42 |
| Whiteness | 26.04 | 33.11 | 41.01 | 4.02 | 0.33 |
| Primary HF diameter (mm) | 314a | 335a | 365b | 7.43 | 0.01 |
| Secondary HF diameter (mm) | 94a | 117b | 120b | 4.60 | 0.03 |
| S/P | 9.37a | 10.8b | 14.65b | 1.06 | 0.04 |
SEM = standard error measurement. a,bMeans with different superscripts differ significantly (P < 0.05).
Figure 2.
Longitudinal sections (×4 and ×100) H&E staining of cashmere goat skins.
Transcriptome profiling of 0 mg group, 52 mg group and 91 mg group of skin and liver
With regard to dietary supplementation of EOC affects, the growth performance, physiological, and biochemical indexes of the blood and the meat quality of the cashmere goats were affected. To elucidate the consequences these changes at genetic level, skin and liver were analyzed for RNA-seq.
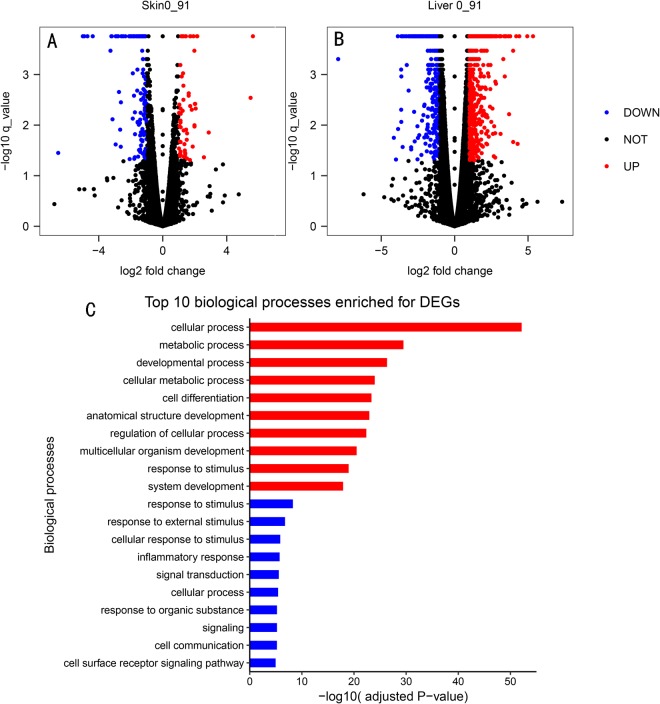
To quantify the gene expression patterns of skin and liver tissues from these three groups, we constructed three RNA-seq libraries and then subjected them to deep sequencing using Illumina HiSeq. 2000. In total, in liver samples, we obtained 52,343,437, 58,562,606 and 68,157,431 reads from 0 mg,52 mg and 91 mg group, respectively, among them, 50,670,031, 56,320,741, and 65,948,002 short reads could be mapped to the goat reference genome (Table S1). In skin samples, researchers in this study obtained 63,703,887, 66,245,924 and 55,055,371 reads from 0 mg, 52 mg and 91 mg group, respectively. Among them, 61,632,490, 64,065,321, and 53,495,041 short reads could be mapped to the goat reference genome. Of the total reads, the rate of match reads was more than 96%, and the remaining reads were unmatched (Table S2). Of the total reads, the rate of match reads was more than 96%, and the remaining reads were unmatched. Expression levels of a distinct gene from two groups were compared to give an expression difference using the Cuffdiff (Table S1). The number of DEG in skin_0 mg, skin_91 mg. liver_0 mg, liver_91 mg, respectively, for transcripts detected with |log (fold change)| >1 and q value < 0.05, A total, 1127 DEGs were found in liver_0mg vs liver_91mg, 191 DEGs were found in skin_0 mg vs skin_91 mg.
DEGs in goat skin and liver (0 vs 91 mg)
In skin samples, we identified 190 genes that were expressed at least two-fold differently between the two types of EOC (Table S3). 58 genes from 91 mg were upregulated compared with 0 mg and 132 were downregulated (Fig. 3A). In liver samples, we identified 1125 genes that were expressed at least two-fold differently between the two types of EOC. 613 genes from 91 mg were upregulated compared with 0 mg and 512 were downregulated (Fig. 3B). The specific genetic information is shown in Table S4.
Figure 3.
(A) Schematic representation of the differentially expressed genes between 0 mg group and 91 mg group of skins. (B) Schematic representation of the differentially expressed genes between 0 mg group and 91 mg group of livers. (C) Top 10 biological processes enriched for DEGs. Red bars indicate biological process enriched by DEGs in liver, blue bars indicate biological process enriched by DEGs in skin.
GO-term analysis of DEGs
In skin samples, 192 DEGs were categorized into three gene ontology categories: cellular component, biological process and molecular function. The top five functional categories of DEGs in 0 mg vs 91 mg included cell, cell part, binding, cellular process, biological adhesions (Fig. S1). The top five biological processes enriched for DEGs included response to stimulus, response to external stimulus, cellular response to stimulus, inflammatory response, signal transduction. (Fig. 3C). Under the cellular component category, a large number of up-regulation DEGs, as well as down-regulation DEGs, was categorized as extracellular region, for example, the FGG, MUCL1, ADIPOQ, ASGR1, AGT, TNFRSF11B, TNFAIP6, COL6A5, HSPA6 and EGFL6 were enriched in the extracellular region and SYNDIG1 was enriched in the synapse (Fig. 4).
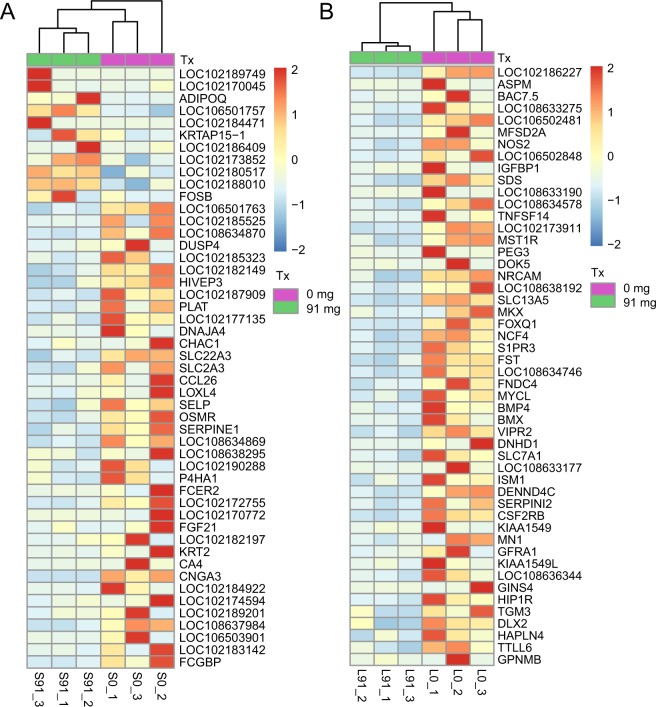
Figure 4.
The FPKM heatmap of differentially expressed genes between 0 mg group and 91 mg group of skin (A) and liver (B).
In liver samples, 1,127 DEGs were categorized into three gene ontology categories: cellular component, biological process and molecular function. The top five functional categories of DEGs in 0 mg vs 91 mg included cell, cell part, binding, cellular component organization, biological regulation (Fig. S1). The top five biological processes enriched for DEGs included cellular process, metabolic process, developmental process, cellular metabolic process, cell differentiation (Fig. 3C). Under the cellular component category, a large number of up-regulation DEGs, as well as down-regulation DEGs, was categorized as extracellular region, for example, the TSKU, LYPD8, SEMA3C, PLA2G2F, MMRN1, TFF2 and IHH were enriched in the extracellular region (Fig. 4).
KEGG pathway analysis of DEGs
In order to explore the mechanism of EOC, we performed KEGG pathway analysis of the dysregulated genes between 0 mg and 91 mg. In liver samples, the results indicated that “MAPK signaling pathway” (26 DEGs, 8.07%) was the first highly significantly enriched (P value = 0.001), the MAPK signaling pathway promotes cell survival by a dual mechanism comprising the posttranslational modification and inactivation of a component of the cell death machinery and the increased transcription of pro-survival genes30; “Rap1 signaling pathway” (18 DEGS, 5.59%) was the second significantly highly enriched (P value = 0.03), the Rap1 is activated by extracellular signals through several regulatory proteins, and it might function in diverse processes, ranging from modulation of growth and differentiation to secretion, integrin-mediated cell adhesion and morphogenesis (Fig. S2)31.
Discussion
Improvement of productive traits with dietary supplementary of EOC
In this study, we show that diets supplemented with EOC are capable of significantly improve production performance including animal growth, carcass weight, physiological parameters, meat quality, and fiber traits in cashmere goats.
The addition of EOC significantly increased FCR, resulting in increased ADG of goats (Table 1). The main reason is that EOC was used as a feed additive derived from plants. It has a spicy, aromatic taste, stimulates appetite, and increases feed intake32, and thereby resulted in increased ADG and improved finishing and carcass performance. Previous studies reported that EO is able to significantly improve FCR, for instance, an inclusion level of 400 mg/kg of EO enhanced FCR by approximately 12%33. In line with previous effects to improve meat traits with essential oils, it is plausible that addition of EOC enhanced ADG and eventually promoted finishing and carcass performance in goats.
In terms of blood physiological and biochemical indexes, the change of nutritional level of diets may cause metabolite changes related to available nutrients circulation in blood. For example, the addition of EOC significantly increased the proportion of calcium in the blood, reducing the proportion of magnesium (Table 4), calcium and phosphorus are essential mineral elements in animals and play important roles in maintaining the body’s acid-base balance and regulating osmotic pressure34. These changes can also affect the efficiency of growth and development of food and fiber producing animals and their subsequent products in food and fiber output. The effects of diets on the growth and development of goats were studied by measuring the changes of serum biochemical indexes considering dietary nutritional levels, which was of great significance to the preparation of diets in goat breeding practices. Blood biochemical indicators reflect the healthy status of growth and development in goats. This is an important measurement of goat body conditions35. Serum protein concentration reflects the feed status and animal physiology and growth and development level. The results of this study showed that the total protein levels in the 91 mg and 52 mg groups were significantly higher than those in the 0 mg group (P < 0.05). This may be due to EOC promotion of increased liver synthesis as serum total protein levels increased.
We further found that the perirenal fat and marbling score significantly increased (P < 0.01), indicating that the addition of EOC significantly enhanced the fat deposition. Further examination of cooked meat percentage found that the addition of EOC significantly improved the quality traits of LM. Our results show that the addition of EOC to feeds can alleviate the tendency of muscle acidification caused by muscle glycolysis and reduce the possibility of PSE meat formation. After adding EOC, the muscle water loss was significantly reduced, and the water retention performance of muscles was significantly improved. This would be beneficial to maintaining tenderness, juiciness, quality of meat products, and an improved meat quality36. Previous study also suggested that the addition of 1000 ppm OEO to pig diet could be recommended to produce meat of good quality and minimum lipid oxidation37. Additionally, water holding capacity is an important meat quality indicator. It refers to the muscles by external forces such as pressure, heating, freezing, chopping to maintain water capacity. Water holding capacity directly affect the flesh, tenderness and nutritional value38,39. In the present study, 91 mg group and 52 mg group of cooked meat rate was significantly higher than 0 mg group (P < 0.05), with water loss and cooked meat rate to measure the mutton water holding capacity whereas the lower the water loss, indicated higher rates of meat and water retention suggesting improved meat quality related to water retention of mutton22. We showed that the effects of the EOC on total fatty acid concentration and composition, the results found that the myristic (C14:0) was significant increased with EO supplementation (Table 3). It is reported that medium-chain FA (e.g. myristic) biosynthesis relys on low activity and mRNA abundance of lipogenic enzymes (e.g. acetyl CoA carboxylase and fatty acid synthase)40, we assume that the decrease of C14:0 may results from the inhibition of acetyl CoA carboxylase and fatty acid synthase after dietary EOC supplementation. Moreover, the flavor in meat is related to stearic (C18:0) and linoleic (C18:2) acid levels41, we show that the addition of EOC has not influenced meat flavor in this study (Table 3). The FA composition of ruminant edible fats is mostly determined by complex interactions between dietary factors and rumen metabolism42. The effect of the EO supplementation on meat FA profile recorded in the current study is in agreement with the results of previous study43, which observed that the supplementation of artemisia EO affected lamb meat FA profile but rosemary essential oils did not have effect. The present study also confirms the importance of EO by-products diet in protecting and maintaining higher FA levels in animal tissues due to their content in polyphenol compounds44,45. Therefore, EOC supplementary provides an alternative way to modify the FA composition of meat in food animals.
Early studies described that EOs, from slow-growing trees such as C. obtuse, have antimicrobial and antifungal effects46. Such EO was used in commercial hair care products since it promotes hair growth since a positive regulatory gene VEGF was involved47. EOs are found to enhance hair growth and promote HF proliferation48,49. In this study, the 91 mg group and 52 mg group exhibited an increased diameter of HFs and S/P ratios (Fig. 2 and Table 5), indicating that the addition of EOC also improved hair fiber output (P < 0.05). We assume that dietary supplement of EOC has a positive effect to dietary energy intake with increased ADG, and therefore resulted in cumulative hair diameter and HF density.
Taken together, we demonstrate that that the addition of EOC had a significant effect on the performance, meat quality, blood physiological and biochemical indexes and HF of tested goats. We designed the transcription analysis of skin and liver, to further study the important economic traits affected by the regulation of genetic networks of supplementation with EOC.
Differentially expressed genes of liver samples
Liver plays an important role in regulating the supply of animal nutrients50. The liver transcriptomic profiles thus may lead to the identification of genes that are important for regulating feed efficiency51. In the 91 mg group, the top five upregulated DEGs were NKG2D, HRASLS2, LOC102179132, ACTA1, and SIM1 (Table S7). Ectopic expression of NKG2D ligands causes rejection of transfected tumor cells by natural killer cells and primed cytotoxic T cells in syngeneic mice. Immunity is induced against subsequent challenges with tumor cells that lack NKG2D ligands52. The NKG2D-ligand system, stimulation through the receptor can lead to the enhancement of innate immune functions, mediated by NK cells and myeloid cells, and the enhancement of adaptive immunity53. The HRASLS2 gene belongs to the H-REV107 gene family involved in the regulation of cell growth and differentiation. HRASLS2 is expressed at high levels in normal tissues of the small intestine, kidney, and trachea54. Arachidonates have esterified cholesterol, to increase blood vessel elasticity, reduce blood viscosity, and regulate blood cell function in a series of physiological activities55,56. Combined with GO-term analysis of DEGs, we found that these genes are mainly involved in the cellular process, metabolic process, developmental process, and cellular metabolic process, and cell differentiation metabolism (Fig. 3C). We hypothesize that the increase of feed conversion rates and improvement of meat quality, which may be related to the promotion of changes in these genes at the cellular and metabolic levels.
In the 91 mg group, the top five differential genes were significantly downregulated of TFF2 (Table S8), trefoil factor family (TFF) domain peptides consist of three members that play a role in intestinal mucosal defense and repair, and in tumorigenesis57,58. Recent study supported the notion that Tff3 peptide is included in the hepatic glucose and glycogen metabolism. Tff3 knockout animals had more glycogen positive cells and those cells had more glycogen accumulated in them when compared to wild type and Tff2 knockout animals59. We believe that the down-regulation of TFF gene may be related to energy metabolism in goats.
Differentially expressed genes of skin samples
In the 91 mg group, the top three DEGs in skins were significantly upregulated of Odorant-binding, HRAS-like, BOLA. Odorant binding protein (OBP) is the major odorant binding component of mammalian nasal mucosa60,61. Previous investigators have verified that OBPs play a role in insect olfactory responses. Odor-binding proteins in the lymph fluid surrounding olfactory neurons play an important role in promoting the binding of odor molecules to odorant receptors and the sensitivity of olfactory responses. Combined with the GO-term analysis of DEGs, we found that these genes are mainly involved in the response to stimulus, response to external stimulus, cellular response to stimulus, inflammatory response, signal transduction (Fig. 3C). However, the physiological functions of OBPs are roughly summarized as follows: recognition and binding of specific odor molecules62; recognition and binding of specific odor molecules63; rapid inactivation of odor molecules that complete stimulation. This is consistent with GO analysis-related pathways.
HRAS-like is the same gene as the liver tissue upregulated gene. In the 91 mg group, the first two differential genes were significantly downregulated of lipopolysaccharide-binding, MUCL1 (Table S5), lipopolysaccharide-binding was descripted lipopolysaccharide-binding, lipopolysaccharide binding protein may control the response to lipopolysaccharides under physiologic conditions by forming high-affinity complexes with lipopolysaccharides that bind to monocytes and macrophages, which then secrete tumor necrosis factor64. MUCL1 is an attractive tumor associated antigen as a potential therapeutic target. However, very little is known about the cellular location and biological functions of the MUCL1 protein65. Through these gene-related functions, we found that these genes play a role in the changes of the skin’s HF at cellular response to stimulus, inflammatory response, and signal transduction.
Correlation analysis of MAPK and Rap1 signaling pathway
HF development and periodic growth process by a variety of signaling pathways. Some of these signaling molecules belong to the MAPK family. The genetic mutations, epigenetic modifications, and posttranslational modifications of ligands, receptors, intermediate signaling molecules, downstream transcription factors and their target genes in these signaling pathways affect the development of animal HF and lead to hair growth and hair quality changes16. The mitogen-activated protein kinase (MAPK) cascade is a highly-conserved module that is involved in various cellular functions, including cell proliferation, differentiation and migration66. We found that the addition of EOC led to an increase in the number of skin HF in cashmere goats, and the major regulatory genes were on the MAPK signaling pathway, and previous studies have shown that MAPK is an extracellular signal protein kinase transduction pathway, which Participate in pathogenesis of many skin diseases67,68.
In lower eukaryotes, Rap1 is implicated in processes that involve either the polarity of cells or the proper functioning of cells, rather than processes that are related to cell proliferation and differentiation. In mammalian cells, Rap1 has also been implicated in various cellular processes31. Rap1 is a small GTPase that controls diverse processes, such as cell adhesion, cell-cell junction formation and cell polarity69. Like all G proteins, Rap1 cycles between an inactive GDP-bound and an active GTP-bound conformation (Fig. S3). A variety of extracellular signals control this cycle through the regulation of several unique guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Rap1 plays a dominant role in the control of cell-cell and cell-matrix interactions by regulating the function of integrin’s and other adhesion molecules in various cell types. Rap1 also regulates MAP kinase (MAPK) activity in a manner highly dependent on the context of cell types (Fig. S3). Therefore, the addition of EOC may activate RAP1 and MAPK signaling pathway at the level of cell differentiation, thus affecting the growth performance of goats.
Conclusions
In summary, by investigating the effects of dietary EOC (0, 52 mg and 91 mg daily) in cashmere goats, we demonstrate the supplement of EOC resulted in a promoted average daily gain and improved phenotypes (cashmere fiber traits, carcass weight, and meat quality). RNA-seq using skin and liver tissues further revealed DEGs that are important to understand the molecular basis conferring the phenotypic changes. We found that EOC mainly stimulated the body’s immune system, including blood and cell levels related physiological changes.
Electronic supplementary material
Acknowledgements
This work was supported by grants from Special Fund for Agro-scientific Research in the Public Interest (No. 201303059, 201503134), China Agriculture Research System (CARS-39-18 as well as projects of Lanzhou Science & Technology Bureau (No. 2012-2-159).
Author Contributions
Conceived and designed the experiments: Z.L., J.W., D.D., D.C., H.J. Performed the experiments: Z.L., K.Z., C.L., T.J., J.W. Analyzed the data: Z.L., K.Z., C.L., X.W., J.W. Prepared the samples: Z.L., J.W. Wrote the manuscript: K.Z., X.W., D.D., D.C. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gunal M, Ishlak A, AbuGhazaleh A, Khattab W. Essential oils effect on rumen fermentation and biohydrogenation under in vitro conditions. Czech J Anim Sci. 2014;59:450–459. doi: 10.17221/7708-CJAS. [DOI] [Google Scholar]
- 2.Becerril R, Nerín C, Gómez-Lus R. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathogens & Disease. 2012;9:699. doi: 10.1089/fpd.2011.1097. [DOI] [PubMed] [Google Scholar]
- 3.Patra AK, Yu Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Applied and Environmental Microbiology. 2012;78:4271–4280. doi: 10.1128/AEM.00309-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodas R, et al. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Animal Feed Science and Technology. 2012;176:78–93. doi: 10.1016/j.anifeedsci.2012.07.010. [DOI] [Google Scholar]
- 5.Simitzis P, et al. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Science. 2008;79:217–223. doi: 10.1016/j.meatsci.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Ranucci D, et al. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat science. 2015;100:319–326. doi: 10.1016/j.meatsci.2014.09.149. [DOI] [PubMed] [Google Scholar]
- 7.Tsigarida E, Skandamis P, Nychas GJ. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 C. Journal of Applied Microbiology. 2000;89:901–909. doi: 10.1046/j.1365-2672.2000.01170.x. [DOI] [PubMed] [Google Scholar]
- 8.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food chemistry. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- 9.Shahidi F. Antioxidants in food and food antioxidants. Molecular Nutrition & Food Research. 2000;44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Vokou D, Kokkini S, Bessiere J-M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochemical Systematics and Ecology. 1993;21:287–295. doi: 10.1016/0305-1978(93)90047-U. [DOI] [Google Scholar]
- 11.Dorman H, Deans S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of applied microbiology. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 12.Hostetler CE, Kincaid RL, Mirando MA. The role of essential trace elements in embryonic and fetal development in livestock. The Veterinary Journal. 2003;166:125–139. doi: 10.1016/S1090-0233(02)00310-6. [DOI] [PubMed] [Google Scholar]
- 13.Pepper, M. R. & Black, M. M. Seminars in cell & developmental biology 22, 619–623 (2011). [DOI] [PubMed]
- 14.Oro AE, Scott MP. Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell. 1998;95:575–578. doi: 10.1016/S0092-8674(00)81624-4. [DOI] [PubMed] [Google Scholar]
- 15.Hardy MH. The secret life of the hair follicle. Trends in Genetics. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-D. [DOI] [PubMed] [Google Scholar]
- 16.Yang C-C, Cotsarelis G. Review of hair follicle dermal cells. Journal of dermatological science. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature reviews genetics. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, et al. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poultry science. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- 20.Honikel KO. Reference methods for the assessment of physical characteristics of meat. Meat science. 1998;49:447–457. doi: 10.1016/S0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Li L, Zhu Y, Wang X, He Y, Cao B. Effects of different dietary energy and protein levels and sex on growth performance, carcass characteristics and meat quality of F1 Angus × Chinese Xiangxi yellow cattle. Journal of animal science and biotechnology. 2014;5:21. doi: 10.1186/2049-1891-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. Journal of lipid research. 1986;27:114–120. [PubMed] [Google Scholar]
- 24.Carter H, Clarke W. Hair follicle group and skin follicle population of Australian Merino sheep. Crop and Pasture Science. 1957;8:91–108. doi: 10.1071/AR9570091. [DOI] [Google Scholar]
- 25.Auber L. VII.—The Anatomy of Follicles Producing Wool-Fibres, with special reference to Keratinization. Transactions of the Royal Society of Edinburgh. 1952;62:191–254. doi: 10.1017/S0080456800009285. [DOI] [Google Scholar]
- 26.Trapnell C, et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature Biotechnology. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics, 1165–1188 (2001).
- 29.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research. 2017;45:D353. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonni A, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 31.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nature reviews Molecular cell biology. 2001;2:369. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 32.Hippenstiel F, Abdel-Wareth AAA, Kehraus S, Südekum KH. Effects of selected herbs and essential oils, and their active components on feed intake and performance of broilers - a review. Archiv Fur Geflugelkunde. 2011;75:226–234. [Google Scholar]
- 33.Ciftci, M., Guler, T., Dalkilic, B. & Ertas, O. N. The Effect of Anise Oil (Pimpinella anisum L.) On Broiler Performance. International Journal of Poultry Science (2005).
- 34.Soetan K, Olaiya C, Oyewole O. The importance of mineral elements for humans, domestic animals and plants-A review. African journal of food science. 2010;4:200–222. [Google Scholar]
- 35.Stanley C, et al. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves. Journal of dairy science. 2002;85:2335–2343. doi: 10.3168/jds.S0022-0302(02)74313-0. [DOI] [PubMed] [Google Scholar]
- 36.Zou Y, Xiang Q, Wang J, Wei H, Peng J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livestock Science. 2016;192:33–38. doi: 10.1016/j.livsci.2016.08.005. [DOI] [Google Scholar]
- 37.Alarcon-Rojo AD, Peña-Gonzalez E, Janacua-Vidales H, Santana V, Ortega JA. Meat quality and lipid oxidation of pork after dietary supplementation with oregano essential oil. World Applied Sciences Journal. 2013;21:665–673. [Google Scholar]
- 38.Asimwe L, et al. Effect of days in feedlot on growth performance, carcass and meat quality attributes of Tanzania shorthorn zebu steers. Tropical animal health and production. 2015;47:867–876. doi: 10.1007/s11250-015-0801-z. [DOI] [PubMed] [Google Scholar]
- 39.Bidanel JP, Caritez JC, Gruand J, Legault C. Growth, carcass and meat quality performance of crossbred pigs with graded proportions of Meishan genes. Genetics Selection Evolution. 1993;25:83–99. doi: 10.1186/1297-9686-25-1-83. [DOI] [Google Scholar]
- 40.Kim SC, Adesogan AT, Badinga L, Staples CR. Effects of dietary n-6:n-3 fatty acid ratio on feed intake, digestibility, and fatty acid profiles of the ruminal contents, liver, and muscle of growing lambs. Journal of Animal Science. 2007;85:706. doi: 10.2527/jas.2006-289. [DOI] [PubMed] [Google Scholar]
- 41.Knapik, J., Ropkamolik, K. & Pieszka, M. Genetic and nutritional factors determining the production and quality of sheep meat – a review. Annals of Animal Science17 (2017).
- 42.Bessa RJ, et al. Effect of lipid supplements on ruminal biohydrogenation intermediates and muscle fatty acids in lambs. European Journal of Lipid Science and Technology. 2007;109:868–878. doi: 10.1002/ejlt.200600311. [DOI] [Google Scholar]
- 43.Ponnampalam EN, et al. Muscle antioxidant (vitamin E) and major fatty acid groups, lipid oxidation and retail colour of meat from lambs fed a roughage based diet with flaxseed or algae. Meat Science. 2016;111:154–160. doi: 10.1016/j.meatsci.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Nieto MG. Incorporation of by-products of rosemary and thyme in the diet of ewes: effect on the fatty acid profile of lamb. European Food Research & Technology. 2013;236:379–389. doi: 10.1007/s00217-012-1892-7. [DOI] [Google Scholar]
- 45.Smeti S, Hajji H, Mekki I, Mahouachi M, Atti N. Effects of dose and administration form of rosemary essential oils on meat quality and fatty acid profile of lamb. Small Ruminant Research. 2017;158:62–68. doi: 10.1016/j.smallrumres.2017.10.007. [DOI] [Google Scholar]
- 46.Hong EJ, Na KJ, Choi IG, Choi KC, Jeung EB. Antibacterial and antifungal effects of essential oils from coniferous trees. Biological & Pharmaceutical Bulletin. 2004;27:863. doi: 10.1248/bpb.27.863. [DOI] [PubMed] [Google Scholar]
- 47.Lee GS, et al. The essential oils of Chamaecyparis obtusa promote hair growth through the induction of vascular endothelial growth factor gene. Fitoterapia. 2010;81:17–24. doi: 10.1016/j.fitote.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Orafidiya LO, et al. A study on the effect of the leaf essential oil of Ocimum gratissimum Linn. on cyclophosphamide-induced hair loss. International Journal of Aromatherapy. 2004;14:119–128. doi: 10.1016/j.ijat.2004.06.006. [DOI] [Google Scholar]
- 49.Yoon JI, Al-Reza SM, Sun CK. Hair growth promoting effect of Zizyphus jujuba essential oil. Food & Chemical Toxicology. 2010;48:1350–1354. doi: 10.1016/j.fct.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 50.Drackley JK, Overton TR, Douglas GN. Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. Journal of Dairy Science. 2001;84:E100–E112. doi: 10.3168/jds.S0022-0302(01)70204-4. [DOI] [Google Scholar]
- 51.Salleh M, et al. RNA-Seq transcriptomics and pathway analyses reveal potential regulatory genes and molecular mechanisms in high-and low-residual feed intake in Nordic dairy cattle. BMC Genomics. 2017;18:258. doi: 10.1186/s12864-017-3622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 53.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nature Reviews Immunology. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 54.Golczak M, et al. Structural basis for the acyltransferase activity of lecithin: retinol acyltransferase-like proteins. Journal of Biological Chemistry. 2012;287:23790–23807. doi: 10.1074/jbc.M112.361550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niehaus W, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 56.Bell R, Kennerly DA, Stanford N, Majerus PW. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proceedings of the National Academy of Sciences. 1979;76:3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farrell JJ, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. The Journal of clinical investigation. 2002;109:193–204. doi: 10.1172/JCI0212529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machado JC, Nogueira AM, Carneiro F, Reis CA, Sobrinho‐Simões M. Gastric carcinoma exhibits distinct types of cell differentiation: an immunohistochemical study of trefoil peptides (TFF1 and TFF2) and mucins (MUC1, MUC2, MUC5AC, and MUC6) The Journal of pathology. 2000;190:437–443. doi: 10.1002/(SICI)1096-9896(200003)190:4<437::AID-PATH547>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 59.Rođak, E. et al. A histological analysis of glycogen content in hepatocytes of trefoil factor family 2 and trefoil factor family 3 knock-out mice[C]// Multinational Congress on Microscopy: Book of. (2017).
- 60.Pikielny C, Hasan G, Rouyer F, Rosbash M. Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron. 1994;12:35–49. doi: 10.1016/0896-6273(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 61.Bianchet MA, et al. The three-dimensional structure of bovine odorant binding protein and its mechanism of odor recognition. Nature Structural & Molecular Biology. 1996;3:934–939. doi: 10.1038/nsb1196-934. [DOI] [PubMed] [Google Scholar]
- 62.Steinbrecht RA. Odorant-Binding Proteins: Expression and Function. Ann NY Acad Sci. 2010;855:323–332. doi: 10.1111/j.1749-6632.1998.tb10591.x. [DOI] [PubMed] [Google Scholar]
- 63.Franco M, et al. Sensory Cell Proliferation within the Olfactory Epithelium of Developing Adult Manduca sexta (Lepidoptera) Plos One. 2007;2:e215. doi: 10.1371/journal.pone.0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schumann RR, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1432. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 65.Conley SJ, et al. Abstract 4364: Characterization of mucin-like 1 (MUCL1) in breast cancer and its novel role as a potent activator of cell proliferation. Cancer Research. 2015;75:4364–4364. doi: 10.1158/1538-7445.AM2015-4364. [DOI] [Google Scholar]
- 66.Cuevas B, Abell A, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 67.Ge X, et al. Inhibition of MAPK signaling pathways enhances cell death induced by 5-Aminolevulinic acid-photodynamic therapy in skin squamous carcinoma cells. European Journal of Dermatology. 2016;26:164–172. doi: 10.1684/ejd.2015.2725. [DOI] [PubMed] [Google Scholar]
- 68.Li D, et al. A novel lipopeptide from skin commensal activates TLR2/CD36-p38 MAPK signaling to increase antibacterial defense against bacterial infection. Plos One. 2013;8:e58288. doi: 10.1371/journal.pone.0058288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature reviews Molecular cell biology. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.