Abstract
Purpose
To demonstrate the dangers associated with the BrightOcular iris implant, a model that had initially been touted as safer than its predecessors.
Observations
A 41-year-old male presented with decreased vision in both eyes, approximately two years following bilateral BrightOcular cosmetic iris implantation performed in Mexico. On initial consultation, he was found to have bilateral corneal decompensation with stromal edema and a significantly reduced endothelial cell count (ECC). On follow up 5 weeks later, his vision and corneal edema had further detriorated. In the following month, he underwent explantation of the cosmetic iris implants in both eyes. Significant corneal edema persisted in the right eye several months post-operatively, to the point of necessitating endothelial keratoplasty.
Conclusions and importance
Despite numerous reports in the literature of the significant ocular complications that can arise secondary to cosmetic iris implantation, individuals continue to willingly undergo this surgery. Our intention with presenting this case to the ophthalmologic community is two-fold: to highlight the ongoing clinical risk that BrightOcular devices pose, despite being marketed as safer than the older NewColourIris models, and to stress the urgency with which cosmetic iris implants should be removed from the eye.
Keywords: Cosmetic iris implants, BrightOcular, Iris implant removal, Corneal decompensation, Endothelial keratoplasty
1. Introduction
Implantation of iris prostheses has been shown to be a safe and effective method of decreasing photophobia for a variety of ocular pathologies, including aniridia, ocular albinism and traumatic iris defects.1,2 More recently, elective surgery for cosmetic iris implants has emerged as a means to change iris colour in the absence of ocular pathology. These devices are advertised by manufacturers as being relatively benign, permanent alternatives to coloured contact lenses (BrightOcular company website: http://www.brightocular.com).
Over the last decade, several case reports describing complications secondary to cosmetic iris implants have been published in the literature.3, 4, 5, 6 NewColorIris, one of the earliest manufacturers, produced an implant that was found to be associated with anterior uveitis, glaucoma, corneal edema, hyphema, and decreased visual acuity.3,4 As a result, the use of these particular devices has fallen out of favour and has largely been replaced by BrightOcular iris implant insertion. In 2015, Mansour et al. published a case series of 12 patients with bilateral BrightOcular implants highlighting very similar ocular morbidities to those initially found with the NewColorIris products. While corneal decompensation was reported in several of the cases in the series, none of the patients went on to require corneal transplantation. In contrast, there are numerous reports in the literature of Descemet Stripping Automated Endothelial Keratoplasy (DSAEK) following NewColourIris devices.3,5
Several mechanisms have been proposed to explain the etiology of these complications.7, 8, 9 Scanning electron microscopy (SEM) performed on cosmetic iris implants has revealed highly irregular borders, which may increase contact with native iris, lead to pigment dispersion, and raise intraocular pressure (IOP).7 Other theories include endothelial damage during implantation, as well as direct mechanical irritation of adjacent structures, including the endothelial surface and corneoscleral trabecular meshwork.8,9 Regardless of the mechanism by which damage occurs, the consensus is that cosmetic iris implants are a source of significant ocular dysfunction and should be explanted.3,4 While removal of the implants can help stabilize the eye, many patients go on to require secondary surgeries in order to address the lingering and progressive complications, such as uncontrolled glaucoma and corneal decompensation.3, 4, 5
Here, we describe a case of a patient who presented with corneal decompensation 2 years following BrightOcular cosmetic iris implantation. We bring this case to the attention of the ophthalmologic community to highlight the fact that despite the well-established risks associated with these implants, individuals are still having this surgery performed, and by willing ophthalmologists. Additionally, the following case demonstrates that corneal decompensation is not unique to the NewColourIris model, but can be equally as severe with the BrightOcular model, to the point of requiring corneal transplantation.
2. Case report
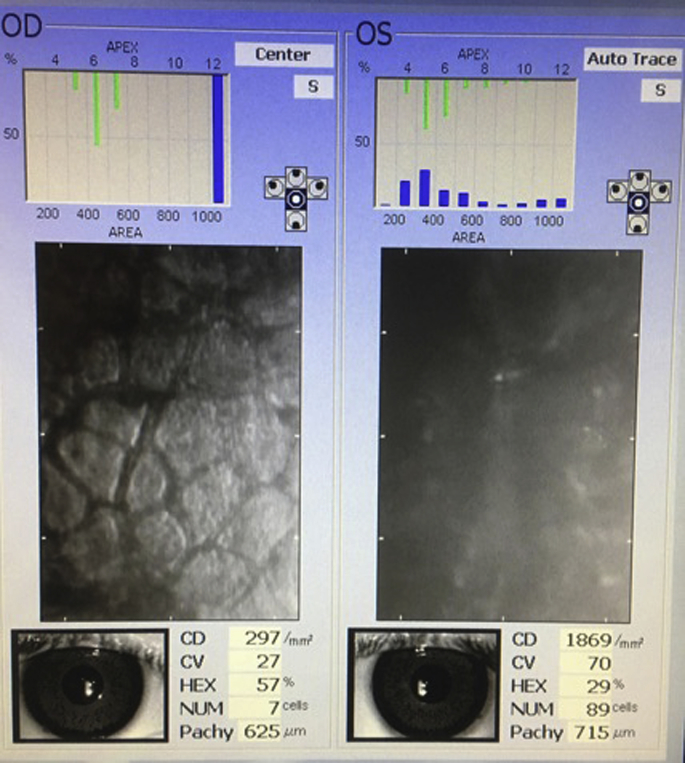
A 41-year-old male was referred to the cornea service at the Toronto Western Hospital in September 2017 with a 1-week history of blurred vision, particularly in the left eye. At that time, he was taking Muro 128 drops four times daily as well as Muro 128 ointment nightly. His past ocular history was significant for bilateral BrightOcular iris implantation in April 2015, which was performed in Mexico. On initial presentation, his uncorrected visual acuity (UCVA) was 20/80-2 in the right eye and 20/60 in the left eye. With pinhole, his visual acuity improved to 20/50-2 and 20/30 in the right and left eyes, respectively. His intraocular pressure (IOP) was 10 mmHg in both eyes. Slit lamp examination revealed trace to 1 + stromal edema in the right eye and microcystic edema and 2 + stromal edema in the left eye. His anterior chamber was deep and quiet, and gray iris implants were noted bilaterally (Fig. 1). Specular microscopy of the right eye showed a corneal thickness of 625 μm and a markedly reduced endothelial cell count (ECC) of 297 per mm2 (Fig. 2). The left cornea measured 715 μm in thickness and was too edematous to obtain an ECC value.
Fig. 1.

Presenting anterior segment photos. Clinical photos of the patient's left eye upon initial presentation to our clinic. 1a) Gross view of the gray model of the BrightOcular iris implant. 2b) High magnification slit beam view of the patients anterior chamber, revealing the iris implant and secondary corneal edema.
Fig. 2.
Specular microscopy. Endothelial imaging upon initial presentation to our clinic reveals significant endothelial cell loss in the right eye and is obscured in the left eye due to the significant degree of corneal edema.
Evidence of bilateral corneal decompensation necessitated a discussion regarding surgical removal of the iris implants. The patient was hesitant to proceed with surgery at that time. Five weeks later, the patient returned to clinic with further visual decline. His UCVA had quickly deteriorated to 20/300 in the right eye and 20/400 in the left eye. His IOP remained stable at 12 mmHg bilaterally. Slit lamp examination revealed 2 + and 3 + corneal edema in the right and left eye, respectively. Specular microscopy was now unattainable in the right eye as well, given the rapid progression of the corneal edema. At this visit, the patient agreed to proceed with urgent removal of the iris implants, beginning with the left eye.
One week later, the patient returned for surgical removal of the left cosmetic iris implant. The right iris implant was subsequently removed two weeks later.
2.1. Surgical steps
The patient was brought to the operating room and was given a retrobulbar injection comprised of 1% lidocaine without preservative. A 4mm temporal wound was performed using a keratome. An inferior and superior sideport was made using an MVR blade. Miochol was injected into the anterior chamber. Viscoelastic was injected both behind and above the iris implant. MST scissors were used to create two radial cuts into the peripheral nasal aspects of the implant. The MST forceps were then used to grasp the distal nasal part of the iris implant and remove it. The viscoelastic was removed using the irrigation aspiration unit. The wound was closed with a single 10-0 Nylon suture (Link to Video 1). The same procedure was performed on the right eye several weeks later.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.07.001.
The following is the supplementary data related to this article:
Final Iris Prosthesis Video2
One week post-operatively, the left eye's visual acuity was still measuring 20/400 but improved to 20/60 two months post-operatively. In the right eye, visual acuity was 20/100 one week post-operatively, and at six weeks remained at 20/100. At the patient's most recent follow-up visit, at 3 months postoperatively, visual acuity was 20/100 in the right eye and 20/70 in the left eye. The corneal edema was consistent with the reduced vision in the right eye but had the edema had cleared substantially in the left eye (Fig. 3).
Fig. 3.

Post-operative anterior segment photos. Clinical photos of the patient's right eye (3a) and left eye (3b) at their most recent follow-up visit following explantation of the iris implant. Corneal edema persists in the right eye, while the left cornea is relatively clear.
3. Discussion
Iris implants for the treatment of aniridia, iris pathology and aphakia have been developed by several companies, including HumanOptics (Erlangen, Germany), Ophtec (Groningen, Netherlands) and Morcher (Stuttgart, Germany). These implants have received the Conformitee Europeenne (CE) label, indicating compliance with European Union healthcare standards. These implants are typically inserted into the capsular bag or are part of an intraocular lens (IOL) complex. The safety of these models has been relatively well-established and their purpose is for treating ocular pathology. This is in contrast to the BrightOcular and NewColorIris implants, whose safety has not been adequately demonstrated and whose purpose is primarily elective and cosmetic.
The BrightOcular model became more popular following the serious adverse events reported with the NewColorIris implant. The BrightOcular implant incorporated several features thought to make the prosthesis safer for implantation. The NewColorIris implant is comprised of a silicone plate measuring 15mm in diameter, a pupillary aperture of 3.5mm, a thickness of 0.16mm and six anchoring hinges. The BrightOcular implant improved on this model in several ways. The diameter can be customized between 11.5 and 13.5mm and five rounded triangular edges are used to hinge the implant in place. The company puts particular emphasis on its patented posterior surface grooves and ribs, which purportedly improve aqueous flow between the implant and iris, and thus reduce the risks of iris atrophy, uveitis and glaucoma.
The NewColorIris was widely denounced based on the multiple reports of serious ocular complications. Fewer series have reported on the dangers of BrightOcular implantation. Mansour et al. published a series of 12 cases that led to uveitis, glaucoma and/or corneal decompensation. Following explantation of the device, however, none of the patients in their series went on to require corneal transplantation. The present case is the first to report on a patient who developed advanced corneal decompensation from the BrightOcular implant requiring endothelial keratoplasty for visual rehabilitation.
Of note, the corneal decompensation progressed quite rapidly in our patient. Just five weeks after his first visit, his vision declined from 20/60 to 20/300 in the right eye and from 20/40 to 20/400 in the left eye. The mechanism of endothelial damage is unclear, but it appears that once the process of the corneal damage is initiated, it can proceed quite rapidly. Endothelial damage during the iris implantation has been implicated as a cause of corneal decompensation, as has intermittent direct contact with the endothelial surface.8,9
Phakic intraocular lenses (PIOLs) are known to lead to endothelial loss. Much of the damage has been shown to occur in the early post-operative period, consistent with endothelial trauma caused by any anterior segment procedure.10 Other purported causes of endothelial damage include intermittent endothelial-lens touch and persistent inflammation. It can be extrapolated that such mechanisms would be at play with The BrightOcular implant, which is even closer to the endothelium than a PIOL. Our patient did not have significant inflammation, so that was unlikely a prominent factor in endothelial damage. While the hinges of the implant embed into the peripheral iris and supposedly do not contact the cornea, intermittent iris-cornea touch is certainly a possibility for the damage observed. Unlike the designs of the artificial irises used to treat pathology, the hinge mechanism in the anterior chamber is both more anterior and less secure than the sulcus-fixation, enclavation or in-the-bag techniques used for other more proven models.
Cosmetic iris implants, including the newer generation of BrightOcular devices, have been shown to have severely detrimental effects on corneal integrity. These devices should not be implanted, and removal on an urgent basis should be recommended to mitigate risks to ocular health and preserve vision.
Patient consent
The patient consented to publication of the case in writing.
Acknowledgements and disclosures
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: Z.M, T.B, D.B, A.S.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.07.001.
Contributor Information
Zale Mednick, Email: zalemednick@gmail.com.
Devin Betsch, Email: dv307044@dal.ca.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Burk S.E., DaMata A.P., Snyder M.E., Cionni R.J., Cohen J.S., Osher R.H. Prosthetic iris implantation for congenital, traumatic, or functional iris deficiencies. J Cataract Refract Surg. 2001;27(11):1732–1740. doi: 10.1016/s0886-3350(01)01124-5. [DOI] [PubMed] [Google Scholar]
- 2.Mayer C.S., Reznicek L., Hoffman A.E. Pupillary reconstruction and outcome after artificial iris implantation. Ophthalmology. 2016;123(5):1011–1018. doi: 10.1016/j.ophtha.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Hoguet A., Ritterband D., Koplin R., Wu E., Raviv T., Aljian J., Seedor J. Serious ocular complications of cosmetic iris implants in 14 patients. J Cataract Refract Surg. 2012;38:387–393. doi: 10.1016/j.jcrs.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Sikder S., Davis S.W., Holz H., Moshirfar M. Complications of NewColorIris implantation in phakic eyes: a review. Clin Ophthalmol. 2011;5:435–438. doi: 10.2147/OPTH.S15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansour A.M., Ahmed II., Eadie B. Iritis, glaucoma and corneal decompensation associated with BrightOcular cosmetic iris implant. Br J Ophthalmol. 2016;100:1098–1101. doi: 10.1136/bjophthalmol-2015-307295. [DOI] [PubMed] [Google Scholar]
- 6.Arthur S.N., Wright M.M., Kramarevsky N., Kaufman S.C., Grajewski A.L. Uveitis-glaucoma-hyphema syndrome and corneal decompensation in association with cosmetic iris implants. Am J Ophthalmol. 2009;148:790–793. doi: 10.1016/j.ajo.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Castanera F., Fuentes-Paez G., Pen T., Pinalla B., Guevara O. Scanning electron microscopy of explanted cosmetic iris implants. Clinl Experiment Ophthalmol. 2010;38:648–651. doi: 10.1111/j.1442-9071.2010.02315.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Pous M., Udaondo P., Garcia-Delpech S., Salom D., Diaz- Llopis M. Acute endothelial failure after cosmetic iris implants. Clin Ophthalmol. 2011;5:721–723. doi: 10.2147/OPTH.S18569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson J.E., Grippo T.M., Sbeity Z., Rich R. Serious complications of cosmetic NewColorIris implantation. Acta Ophthalmol. 2010;88:700–704. doi: 10.1111/j.1755-3768.2008.01499.x. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Alfaro I., Benitez del Castillo J.M., Garcia-Feijoo J., Gil de Bernabe J.G., Serrano de La Iglesia J.M. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens impantation. Ophthalmology. 2001;108(1):90–99. doi: 10.1016/s0161-6420(00)00403-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Final Iris Prosthesis Video2