Abstract
Exertional rhabdomyolysis is a metabolic event characterized by the release of muscle content into the circulation due to exercise-driven breakdown of skeletal muscle. Recurrent exertional rhabdomyolysis has been associated with metabolic myopathies and mitochondrial disorders, a clinically and genetically heterogeneous group of predominantly autosomal recessive, monogenic conditions. Although genetics factors are well recognized in recurrent rhabdomyolysis, the underlying causes and mechanisms of exercise-driven muscle breakdown remain unknown in a substantial number of cases. We present clinical and genetic study results from seven adult male subjects with recurrent exertional rhabdomyolysis. In all subject, whole exome sequencing identified multiple heterozygous variants in genes associated with monogenic metabolic and/or mitochondrial disorders. These variants consisted of known pathogenic and/or new likely pathogenic variants in combination with other rare deleterious alleles. The presence of heterozygous pathogenic and rare deleterious variants in multiple genes suggests an oligogenic inheritance for exertional rhabdomyolysis etiology. Our data imply that exertional rhabdomyolysis can reflect cumulative effects or synergistic interactions of deleterious variants in multiple genes that are likely to compromise muscle metabolism under the stress of exercise.
Keywords: Exertional rhabdomyolysis, Metabolic disorders, Mitochondrial diseases, Multiple genetic variants, Synergistic heterozygosity, Oligogenic inheritance
1. Introduction
Rhabdomyolysis is a metabolic event characterized by the release of muscle contents into circulation due to skeletal muscle breakdown. It can lead to elevations in urinary myoglobin (myoglobinuria), renal failure, and a potentially life-threatening metabolic crisis [1, 2]. The development of rhabdomyolysis is associated with a wide variety of diseases, injuries and medications, and there is little consensus on clinical signs and diagnostic criteria [[1], [2], [3]]. Generally, rhabdomyolysis manifests as muscle pain and transient elevation of serum creatine kinase (CK) with or without myoglobinuria [1, 2]. Although the diagnostic CK level has not been agreed upon due to large variability by race, gender, and other factors, a CK level of 5–10 times normal (>1000 U/L) is widely used as a cut-off for detection of rhabdomyolysis [2].
Exertional rhabdomyolysis is rhabdomyolysis triggered by strenuous physical exercise. Exercise intensity, metabolic state (fed or fasted), unaccustomed exercise, motivation, exercising while dehydrated, and exercising under extreme environmental conditions are all known risk factors for the development of exertional rhabdomyolysis [[1], [2], [3]]. After these risk factors are eliminated, exertional rhabdomyolysis is self-limiting. However, when exertional rhabdomyolysis occurs episodically in an adult, signs and symptoms persist, no causative risk factors can be identified, the affected individual may need an extensive clinical work-up, including muscle biopsy and enzyme analyses. Unfortunately, even thorough workups often result in non-specific findings with no clear explanations for the patient's experience.
Episodic exertional rhabdomyolysis is the hallmark of defects in cellular energy metabolism. Deficiencies in energy supply due to impaired glycogenolysis, glycolysis, fatty acid oxidation (FAO), and/or oxidative phosphorylation are well-known causes of recurrent exertional rhabdomyolysis and exercise intolerance [2, [4], [5], [6], [7]]. These deficiencies are a clinically and genetically heterogeneous group of metabolic and mitochondrial disorders inherited predominantly as monogenic disease with childhood onset. Most of these disorders are inherited in an autosomal recessive fashion and a few are X linked. A notable exception is Malignant Hyperthermia (MH), a disease of skeletal muscle calcium (Ca 2+) dysregulation due largely to dominant mutations in RYR1 or CACNA1S, that are also associated with exertional rhabdomyolysis, exertional heat illness or a combination of both [8, 9]. Although these genetic factors are well recognized in recurrent rhabdomyolysis, underlying causes and mechanisms of exercise-driven muscle breakdown remain unknown in a substantial number of cases.
Metabolic myopathies sometimes arise from coexisting partial enzyme deficiencies caused by heterozygous mutations in multiple genes associated with monogenic forms of metabolic disorders [[10], [11], [12]]. These multiple deleterious genetic variants interact synergistically to cause disease or increase disease risk. This disease mechanism, termed synergistic heterozygosity, has been elucidated in patients with metabolic myopathies and studies of animal models with enzyme deficiencies for mitochondrial FAO [10, 13]. Synergistic heterozygosity is oligogenic inheritance that, in its simplest form, entails synergistic effects or interactions between two mutations in different genes as the disease etiology. However, it is not yet clear whether oligogenic inheritance is involved in sporadic, non-familial cases of exertional rhabdomyolysis.
In this report, we present the results of clinical and genetic studies of seven subjects, who presented with a history of repeated exertional rhabdomyolysis with muscle pain and fatigue. In all cases, whole exome sequencing (WES) identified the presence of multiple heterozygous pathogenic and rare deleterious variants in genes associated with monogenic metabolic and/or mitochondrial disorders, suggesting oligogenic inheritance for exertional rhabdomyolysis etiology. Our data demonstrates that subjects with clinically significant exertional rhabdomyolysis can sometimes be explained by the cumulative effects of two or more genetic variants coupled with the stress of exercise.
2. Material and methods
The study protocol was approved by the Institutional Review Board of Uniformed Services University. Clinical study included evaluation of clinical history; muscle histopathology; and laboratory test results for muscle enzymes, serum CK, and myoglobin.
2.1. Study subjects and samples
Seven unrelated subjects with a history of recurrent exertional rhabdomyolysis were enrolled in this study. Inclusion criteria were recurrent events of muscle pain, weakness, and/or swelling with transient elevation of CK in the setting of physical exertion. The cut-off value for peak CK value was >1000 U/L. All subjects were males between the ages 21 and 50 with diverse ethnic backgrounds: one Asian, two African Americans, three Caucasians, and one of mixed Asian and Caucasian descent. All subjects had been referred for further evaluation, including muscle biopsy, and subsequently enrolled in the genetic study after providing informed consent. Genomic DNA were extracted from blood or muscle samples using standard methods.
2.2. Muscle biopsy
Open biopsies were performed for diagnostic purposes. Muscle tissues for histopathology and enzyme analysis were obtained from the vastus lateralis six to 12 months after episodes. Muscle enzymes were analyzed by using a commercial myoglobinuria panel (Athena Diagnostic or Robert Guthrie Biochemical & Molecular Genetics Laboratory). Histopathology, consisting of routine histochemical analyses, was performed at the Joint Pathology Center, Defense Health Agency.
2.3. Genetic analysis
WES was performed and analyzed as described previously [14, 15]. Briefly, variants were filtered for minor allele frequency of <0.01. Nonsynonymous, splice, stop gain and stop loss variants were prioritized. A number of in silico algorithms including SWIFT, PolyPhen, and Mutation Tester was utilized to predict the effect of identified variants. To interpret WES results, American College of Medical Genetics (ACMG) guidelines were followed [16]. ACMG classifies genetic variants into 5 categories: pathogenic, likely pathogenic, variant of unknown significance (VUS), likely benign and benign. Conventional Sanger sequencing was performed for a variant validation of WES results.
The insertion and deletion mutation in the PCCB gene, encoding for beta propionyl-CoA carboxylase, was analyzed by cloning. A fragment of the human PCCB transcript comprising amino acids from 301 to 539 was generated using mRNA extracted from affected muscle. Reverse transcription was conducted and PCR products were inserted into the pCR2.1-TOPO vector (Thermo-Fisher Scientific). After transformation, positive colonies were analyzed by sequencing.
3. Results
3.1. Clinical history
Clinical characteristics of subjects are summarized in Table 1. All subjects had a personal history of recurrent exertional rhabdomyolysis with two or more documented episodes. All denied the use of performance-enhancing drugs and/or prescription drugs known to be associated with exertional rhabdomyolysis. They also denied a family history of muscle disease, exercise and/or heat intolerance, or complications to anesthetics. Intense muscle pain, fatigue and/or muscle weakness during or after episodes were major complaints in all subjects, although clinical manifestations of episodes were variable. Peak CK values ranged from 2081 to 155,000 U/L, myoglobinuria was noted in one subject. Two African Americans, R279 and R302, had Sickle Cell Trait; one of them (R302) had been diagnosed with compartment syndrome. Three subjects had four to five episodes of exertional rhabdomyolysis. Subject R410 experienced five episodes of exertional rhabdomyolysis and complained of being unable to exercise without muscle pain and fatigue. His last three episodes occurred within 14 months after mild exercise, and the latest event was essentially unprovoked. Subject R462 had two episodes of exertional rhabdomyolysis with exertional heat illness. During one episode, he had syncope with exercise and a rectal temperature of 106.4 °F. His syncope accompanied by headache, pallor, sweating and lethargy. Subject R465, who experienced his first episode in high school and subsequently had three episodes associated with upper-body exercise but not running. He also complained of severe pain and tightness in his chest muscles.
Table 1.
Clinical characteristics of subjects with recurrent exertional rhabdomyolysis.
| Subject ID | Age | Ethnicity | Maximum creatine kinase (U/L) | Number of events | Symptoms during events | EMGa results | Sickle cell trait | Muscle work up |
|
|---|---|---|---|---|---|---|---|---|---|
| Histopathology | Muscle enzymes | ||||||||
| R279 | 22 | African American | 36,000 | 3 | Muscle pain and fatigue | N.A. | Positive | Non-diagnostic | Within normal range |
| R302 | 50 | African American | 16,000 | 2 | Muscle pain and swelling | Abnormal | Positive | Non-diagnostic | Within normal range |
| R410 | 21 | Caucasian | 100,000 | 5 | Muscle pain, fatigue and weakness after minimal exertion | N.A. | Negative | Non-diagnostic | Within normal range |
| R462 | 32 | Asian/Caucasian | 2081 | 2 | Muscle pain, exertional heat illness, syncope, pallor, lethargy, diaphoresis | Normal | Negative | Non-diagnostic | Within normal range |
| R465 | 24 | Caucasian | 155,000 | 4 | Chest pain and tightness, myoglobinuria | Normal | Negative | Non-diagnostic | 63% of normal CPT2 activity |
| R469 | 28 | Asian | 65,000 | 2 | Muscle pain and weakness after minimal exertion | Normal | Negative | Non-diagnostic | Elevations of Long, Very-long chain Acyl-CoAs |
| R470 | 26 | Caucasian | 5000 | 4 | Muscle pain, and stiffness | Normal | Negative | Non-diagnostic | Within normal range |
EMG - Electromyography; N.A. - Not available.
3.2. Muscle biopsy and electromyography results
Muscle histopathology results were unremarkable in all subjects. Representative images are shown in the Supplementary Data (Fig. S1). No findings diagnostic of congenital myopathy, muscular dystrophy, or metabolic myopathy were observed. Immunocytochemical analyses for periodic acid-Schiff and oil-red-O stains demonstrated normal distribution of glycogen and lipids. Myophosphorylase, phosphofructokinase, and AMP-deaminase activities were present in all muscles. The Gomori trichrome stain is negative for ragged red fibers and sarcoplasmic inclusions. The nicotinamide adenine dinucleotide-tetrazolium reductase and succinate dehydrogenase stains showed no indications of mitochondrial disorders.
Activities of muscle enzymes were within reference ranges in all subjects except for subject R465, who showed only 63% of normal activity of the carnitine palmitoyltransferase II (CPTII) enzyme. Abnormal acylcarnitine profiles were noted in the clinical history of R469, however, more detailed test results were not available. Results of electromyography were available for five subjects (Table 1). Subject R302 showed an absence of the right superficial peroneal sensory nerve action potential consistent with peroneal mononeuropathy, likely due to his compartment syndrome. Electromyography results were normal in four remaining subjects.
3.3. Genetic studies
WES findings are presented in Table 2. Among the seven subjects, a total of 19 variants in 16 genes was found. Detailed analyses of all variants are given in the Supplementary Data (Supplementary Table, Fig. S2. A, B). Most of the variants were missense; eight were loss of function (LoF) variants, including four frame-shift, three nonsense and one splice variant. As shown in Table 2, four subjects carried two variants; three subjects carried three to four variants. Importantly, all subjects were heterozygous carriers of one to two pathogenic or likely pathogenic variants in genes known to be associated with metabolic and mitochondrial disorders.
Table 2.
Exome sequencing results of subjects with recurrent exertional rhabdomyolysis.
| Genes and variants | Pathogenicitya | Inheritance | Gene function, affected pathway | Disease association, mode of disease inheritanceb | |
|---|---|---|---|---|---|
| R279 | NDUFA10: N288RfsTer20 | Likely Pathogenic | Heterozygous | Respiratory chain-electron transport | Mitochondrial complex I deficiency, AR |
| PYGM: R387C | VUS | Heterozygous | Muscle glycogen metabolism | Glycogen storage disease type V, AR | |
| TIMM50: R342W | VUS | Heterozygous | Mitochondrial protein transport | Mitochondrial 3-methylglutaconic aciduria, AR | |
| R302 | HMBS: R175W | Likely Pathogenic | Heterozygous | Heme biosynthesis | Acute intermittent porphyria, AD |
| GBE1: D413N | VUS | Heterozygous | Glycogen synthesis and storage | Glycogen storage disease type IV, AR | |
| RYR1: T4823 M | VUS | Heterozygous | Muscle calcium regulation | Malignant hyperthermia, AD | |
| Core myopathy, AD/AR | |||||
| PHKA1: L718F | VUS | Hemizygous | Muscle glycogen metabolism | Glycogen storage disease type IX, X linked | |
| R410 | GBE1: R524Ter | Pathogenic | Heterozygous | Glycogen synthesis and storage | Glycogen storage disease type IV, AR |
| PCCB: G407RfrTer14 | Pathogenic | Heterozygous | Catabolism of fatty acids, aminoacids | Propionic acidemia type II, AR | |
| R462 | HMBS: R225Q | Pathogenic | Heterozygous | Heme biosynthesis | Acute intermittent porphyria, AD |
| CACNA1S: R498L | VUS | Heterozygous | Muscle calcium regulation | Malignant hyperthermia, AD | |
| Hypokalemic periodic paralysis, AD | |||||
| NDUFS8: I126V | VUS | Heterozygous | Respiratory chain-electron transport | Mitochondrial complex I deficiency, AR | |
| R2C | Likely benign | Heterozygous | |||
| R465 | CPT2: K457Ter | Likely pathogenic | Heterozygous | Catabolism of fatty acid | Carnitine palmitoyltransferase II deficiency, AR |
| ELAC2: Q92RfsTer9 | Likely pathogenic | Heterozygous | tRNA maturation in mitochondria | Combined oxidative phosphorylation deficiency, AR | |
| R469 | NDUFA6: I120KfrTer44 | Likely pathogenic | Heterozygous | Respiratory chain-electron transport | Member of mitochondrial complex I, AR |
| ACADVL: S110Y | VUS | Heterozygous | Catabolism of fatty acid | Very long-chain Acyl-CoA dehydrogenase deficiency, AR | |
| R470 | NUBPL: IVS8DS | Pathogenic | Heterozygous | Respiratory chain-electron transport | Mitochondrial complex I deficiency, AR |
| OAT: Y299Ter | Pathogenic | Heterozygous | Ornithine catabolism | Ornithine keto acid aminotransferase deficiency, AR |
Variants previously reported as pathogenic are in bold.
AD - autosomal dominant, AR - autosomal recessive.
3.4. Variant analysis and incidental findings
Of the 19 variants identified in this study, four were previously reported as pathogenic disease causing variants: 1) GBE1 R524Ter; 2) PCCB G407RfrTer14; 3) NUBPL IVS8DC; 4) OAT Y299Ter [[17], [18], [19], [20]]. Two additional variants, p. T4823 M in RYR1 and p. R498L in CACNA1S, were also previously reported in association with Core myopathy and Malignant Hyperthermia Susceptibility (MHS), respectively [21, 22]. However, the pathogenic impacts of these variants are unknown.
The remaining variants, with exceptions of variants in HMBS, have never been described in association with any disease, although the genes containing them have been shown to cause a variety of metabolic disorders and defects in the mitochondrial respiratory chain. Among these variants, four were LoF and eight were missense changes. The null variants in CPT2, ELAC2, NDUFA6 and NDUFA10 were determined as likely pathogenic as LoF is a known mechanism of pathogenicity in FAO and oxidative phosphorylation deficiencies by these genes. In contrast, interpretation of missense variants was challenging in the absence of functional studies and family analyses with detailed phenotypic data. Missense variants were determined as variants of uncertain significance (VUS) due to an absence of functional studies and other supporting evidence. It is worth noting that most of those missense variants affect highly conserved residues, they are very rare or not reported in the general population, and predicted to have deleterious effects by various analyses (Supplementary Table).
Two variants, likely pathogenic p. R175W and pathogenic p. R225Q, in HMBS were found in two subjects. Pathogenic variants in HMBS are associated with Acute Intermittent Porphyria [23]. We consider HMBS variants as incidental findings that are likely unrelated to phenotype.
3.5. Variant segregation in subjects
All subjects were heterozygous carriers of pathogenic or deleterious variants in genes known to affect various enzymatic pathways of cellular energy. Five subjects (R279, R410, R465, R469 and R470) carried pathogenic and deleterious variants in genes known to affect glycogen metabolism (GBE1, PYGM), FAO (ACADVL and CPT2), fatty acid and amino acid catabolism (PCCB), oxidative phosphorylation (ELAC2, NDUFA6, NDUFA10 and NUBPL), mitochondrial matrix enzymes (OAT and TIMM50).
Two subjects (R302 and R462) had variants in genes involved in Ca 2+ regulation (RYR1 and CACNA1S), glycogen metabolism (GBE1 and PHKA1) and oxidative phosphorylation (NDUFS8). Mutations in PHKA1 cause Glycogen Storage Disease type IX, X-linked phosphorylase kinase (PHK) enzyme deficiency, characterized by high muscle glycogen content and severe reduction of muscle PHK activity [5, 24]. However, none of these signs were evident from metabolic work of the patient with PHKA1 L718F, thus ruling out pathogenic significance of this variant. Pathogenic effects of GBE1 D413N and NDUFS8 I126V variants remain unknown. It is important to note that these variants changed amino acids that are highly conserved in species from human down to bacteria (data not shown).
Because dominant mutations in RYR1 and CACNA1S are associated with MHS, we evaluated MH diagnostic test results from clinical history of these two subjects. Subject R302 was diagnosed as MH negative, so we ruled out a pathogenic role of the RYR1 p.T4823 M variant in MH. Subject R462 was diagnosed as MHS, which appeared to correlate with CACNA1S p. R498L, previously reported in a single MHS subject [22]. However, the frequency of this variant in the general population is about 20-fold higher than the frequencies of pathogenic CACNA1S variants associated with MHS. It is also important to note that the diagnostic test for MH has a high false-positive rate of 22% [25], which raises the possibility that MHS diagnosis in subject R462 may be false. Based on these results, we also ruled out a pathogenic role of the CACNA1S p. R498L variant in MH.
4. Discussion
We performed clinical and molecular genetic studies on seven subjects who had a personal history of recurrent exertional rhabdomyolysis: all had experienced at least two episodes. Although their clinical manifestations varied, all had intense muscle pain, muscle weakness, and significant increase in serum CK value. The severity of symptoms varied from subject to subject. Some had experienced up to five episodes of rhabdomyolysis after mild exercise. This episodic occurrence of rhabdomyolysis upon physical exertion was suggestive of genetic risk factors associated with defects in cellular energy metabolism.
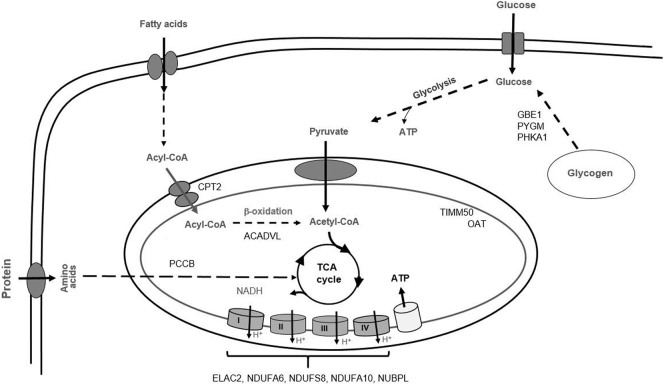
WES results revealed that each subject had pathogenic and/or rare deleterious variants in multiple genes: this suggests oligogenic inheritance for exertional rhabdomyolysis. Importantly, all subjects were heterozygous carriers of one to two pathogenic variants and/or multiple deleterious variants. Consistent with heterozygous carrier status, muscle metabolic work up of subjects were either non-diagnostic or suggestive of partial enzyme defects in the genes identified. Variants were primarily detected in the genes involved in FAO, glycogen metabolism, and oxidative phosphorylation, three major sources of cellular energy supply (Fig. 1.). These findings suggest that individuals who carry deleterious variants in multiple genes involved in energy metabolism could be at risk for recurrent rhabdomyolysis. Multiple gene variants are expected to result in clinically significant rhabdomyolysis through various mechanisms of actions, such as direct interactions between affected genes or the combined effects of partial enzyme deficiencies caused by pathogenic variants. For example, double heterozygosity of GBE1 and PCCB null variants expected to lead to partial deficiencies of glycogen debranching and propionyl-CoA carboxylase enzymes, respectively [5, 20]. These enzymes participate in the renewal of the tricarboxylic acid (TCA) cycle intermediates by contributing to the acetyl-CoA and succinyl-CoA pools [5, 26, 27]. The cumulative effect of partial GBE1 and PCCB deficiencies will likely result in poor energy production through reduced TCA intermediates. Similarly, the combined effects or direct interactions between oxidative phosphorylation and mitochondrial matrix enzymes or FAO could be postulated in heterozygous carriers of pathogenic and deleterious variants. Direct interactions between FAO and oxidative phosphorylation have been demonstrated in both animal models and patients with metabolic myopathies [10, 11].
Fig. 1.
Metabolic pathways affected in subjects with recurrent exertional rhabdomyolysis.
Pathogenic and/or rare deleterious variants in genes associated with glycogen metabolism (GBE1, PYGM, and PHKA1), fatty acid oxidation (ACADVL, and CPT2), fatty acid and amino acid catabolism (PCCB), mitochondrial inner membrane and matrix enzymes (TIMM50 and OAT) and mitochondrial respiratory chain complex (ELAC2, NDUFA6, NDUFS8, NDUFA10, and NUBPL). Straight lines denote one step, dotted lines indicate multiple steps of metabolic pathways.
WES results were challenging to interpret in two cases (R302 and R462) with multiple variants in multiple genes. Based on essentially negative clinical data, pathogenic effects of dominant variants in RYR1 and CACNA1S as well as hemizygous variant in PHKA1 were excluded. However, in the absence of functional data, the significance of variants in GBE1 and NDUFS8 remains unknown. It is possible that additive effect of multiple variants in the genes involved in Ca2+ regulation and energy production, and the stress of exercise may be responsible for recurrent episodes of exertional injuries. But, it is also possible that other missing pathogenic variants exist in these two cases. Given adequate coverage of WES for all cases, we ruled out variants in genes known to be implicated in rhabdomyolysis, although the existence of new genes associated with energy metabolism cannot be excluded. In these or similar cases, future studies to determine the functional consequences of gene variants and large scale studies are needed to conclusively establish association between genetic variants and individual's susceptibility to recurrent rhabdomyolysis.
The presence of hundreds of genetics variants is to be expected in any individual exome. However, we used a stringent approach to determine and select pathogenic and likely pathogenic variants in genes associated with metabolic energy defects, the hallmark of episodic rhabdomyolysis. In addition, subjects had other rare deleterious variants in genes affecting the same pathways. The recurrent exertional rhabdomyolysis experienced by the subjects described in this study suggest that deficits in energy metabolism, traditionally approached as a single-gene disorder, may be a backdrop for multiple heterozygous pathogenic and rare deleterious variants in genes associated with monogenic metabolic disorders. It is known that metabolic myopathies sometimes arise from partial enzyme deficiencies caused by heterozygous mutations in multiple genes within functionally relevant pathways [10, 11]. This disease mechanism, termed synergistic heterozygosity, has been elucidated in studies of inborn error metabolism and the mice model of mitochondrial FAO [10, 13]. In keeping with its implications, we conclude that recurrent exertional rhabdomyolysis can reflect cumulative effects or synergistic interactions of pathogenic and rare deleterious variants in multiple genes that are likely to compromise muscle metabolism under the stress of exercise, as this case series indicates.
This study had a limited number of enrolled subjects. We were also unable to interrogate variants that might exists in gene regulatory regions and/or deep in introns due to inadequate coverage of these regions by WES. Another limitation of this study is a lack of functional studies to elucidate pathogenic effects of the new variants, specifically the missense changes, first described here. Further studies of their pathogenic effects on protein function and/or their role in energy metabolism are warranted.
5. Conclusions
We present clinical and genetic study results from seven adult male subjects with recurrent exertional rhabdomyolysis. In all subject, multiple heterozygous variants in genes associated with monogenic metabolic and/or mitochondrial disorders were identified. This finding suggest oligogenic inheritance in recurrent exertional rhabdomyolysis, which is conventionally approached as a metabolic disease of monogenic origin. Co-inheritance of pathogenic mutations or enrichment of rare deleterious variants in metabolic disease genes associated with energy defects should be considered as potential causes of exertional rhabdomyolysis, especially in recurrent cases. Large-scale case-control studies with mutation burden analysis will be important to further understand the complexity of genetic risk factors in exertional rhabdomyolysis.
Disclaimer and conflict of interest
The information presented represents the opinions of the authors and not those of the Department of Defense or the Uniformed Services University.
We have no financial interests or relationships to disclose.
Acknowledgments
The authors are grateful to the Collaborative Health Initiative Research Program, USU, for exome sequencing and the Biomedical Instrumental Center, USU, for Sanger sequencing and oligo synthesis. We thank Drs. Georgirene Vladutiu and Kristin Heitman for critical comments and Ms. Maria Voelkel for a technical assistance. The study was supported by National Heart, Lung and Blood Institute grant HU0001-14-1-0060 to Dr. P. Deuster, and by a grant PPG-ANE-80-3397 from USU's Intramural Research Program made to Dr. F. O'Connor.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2018.07.007.
Appendix A. Supplementary data
Supplementary material
References
- 1.O'Connor F.G., Deuster P.A. Goldman's Cecil Medicine. 24th edn. Saunders Elsevier; Philadelphia: 2012. Rhabdomyolysis; pp. 700–705. [Google Scholar]
- 2.Nance J.R., Mammen A.L. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve. 2015;51(6):793–810. doi: 10.1002/mus.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scalco R.S., Snoeck M., Quinlivan R., Treves S., Laforet P., Jungbluth H., Voermans N.C. Exertional rhabdomyolysis: physiological response or manifestation of an underlying myopathy? BMJ Open Sport Exerc. Med. 2016;2(1) doi: 10.1136/bmjsem-2016-000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinlivan R., Jungbluth H. Myopathic causes of exercise intolerance with rhabdomyolysis. Dev. Med. Child Neurol. 2012;54(10):886–891. doi: 10.1111/j.1469-8749.2012.04320.x. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey R., Quinlivan R. Skeletal muscle disorders of glycogenolysis and glycolysis. Nat. Rev. Neurol. 2016;12(7):393–402. doi: 10.1038/nrneurol.2016.75. [DOI] [PubMed] [Google Scholar]
- 6.Scalco R.S., Gardiner A.R., Pitceathly R.D., Zanoteli E., Becker J., Holton J.L., Houlden H., Jungbluth H., Quinlivan R. Rhabdomyolysis: a genetic perspective. Orphanet J. Rare Dis. 2015;10:51. doi: 10.1186/s13023-015-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Gharbawy A., Vockley J. Inborn errors of metabolism with myopathy: defects of fatty acid oxidation and the carnitine shuttle system. Pediatr. Clin. N. Am. 2018;65(2):317–335. doi: 10.1016/j.pcl.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg H., Sambuughin N., Riazi S., Dirksen R. Malignant hyperthermia susceptibility. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., LJH Bean, Stephens K., Seattle Amemiya A., editors. GeneReviews((R)). edn. 1993. WA. [Google Scholar]
- 9.Anandan C., Cipriani M.A., Laughlin R.S., Niu Z., Milone M. Rhabdomyolysis and fluctuating asymptomatic hyperCKemia associated with CACNA1S variant. Eur. J. Neurol. 2018;25(2):417–419. doi: 10.1111/ene.13528. [DOI] [PubMed] [Google Scholar]
- 10.Vockley J., Rinaldo P., Bennett M.J., Matern D., Vladutiu G.D. Synergistic heterozygosity: disease resulting from multiple partial defects in one or more metabolic pathways. Mol. Genet. Metab. 2000;71(1–2):10–18. doi: 10.1006/mgme.2000.3066. [DOI] [PubMed] [Google Scholar]
- 11.Vockley J. Metabolism as a complex genetic trait, a systems biology approach: implications for inborn errors of metabolism and clinical diseases. J. Inherit. Metab. Dis. 2008;31(5):619–629. doi: 10.1007/s10545-008-1005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vladutiu G.D. Heterozygosity: an expanding role in proteomics. Mol. Genet. Metab. 2001;74(1–2):51–63. doi: 10.1006/mgme.2001.3240. [DOI] [PubMed] [Google Scholar]
- 13.Schuler A.M., Gower B.A., Matern D., Rinaldo P., Vockley J., Wood P.A. Synergistic heterozygosity in mice with inherited enzyme deficiencies of mitochondrial fatty acid beta-oxidation. Mol. Genet. Metab. 2005;85(1):7–11. doi: 10.1016/j.ymgme.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Toro C., Olive M., Dalakas M.C., Sivakumar K., Bilbao J.M., Tyndel F., Vidal N., Farrero E., Sambuughin N., Goldfarb L.G. Exome sequencing identifies titin mutations causing hereditary myopathy with early respiratory failure (HMERF) in families of diverse ethnic origins. BMC Neurol. 2013;13:29. doi: 10.1186/1471-2377-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambuughin N., Zvaritch E., Kraeva N., Sizova O., Sivak E., Dickson K., Weglinski M., Capacchione J., Muldoon S., Riazi S. Exome analysis identifies Brody myopathy in a family diagnosed with malignant hyperthermia susceptibility. Mol. Genet. Genomic Med. 2014;2(6):472–483. doi: 10.1002/mgg3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kevelam S.H., Rodenburg R.J., Wolf N.I., Ferreira P., Lunsing R.J., Nijtmans L.G., Mitchell A., Arroyo H.A., Rating D., Vanderver A. NUBPL mutations in patients with complex I deficiency and a distinct MRI pattern. Neurology. 2013;80(17):1577–1583. doi: 10.1212/WNL.0b013e31828f1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashima Y., Murakami A., Weleber R.G., Kennaway N.G., Clarke L., Shiono T., Inana G. Nonsense-codon mutations of the ornithine aminotransferase gene with decreased levels of mutant mRNA in gyrate atrophy. Am. J. Hum. Genet. 1992;51(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno C., van Diggelen O.P., Cassandrini D., Gimpelev M., Giuffre B., Donati M.A., Introvini P., Alegria A., Assereto S., Morandi L. Clinical and genetic heterogeneity of branching enzyme deficiency (glycogenosis type IV) Neurology. 2004;63(6):1053–1058. doi: 10.1212/01.wnl.0000138429.11433.0d. [DOI] [PubMed] [Google Scholar]
- 20.Ugarte M., Perez-Cerda C., Rodriguez-Pombo P., Desviat L.R., Perez B., Richard E., Muro S., Campeau E., Ohura T., Gravel R.A. Overview of mutations in the PCCA and PCCB genes causing propionic acidemia. Hum. Mutat. 1999;14(4):275–282. doi: 10.1002/(SICI)1098-1004(199910)14:4<275::AID-HUMU1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Stenson P.D., Ball E.V., Mort M., Phillips A.D., Shiel J.A., Thomas N.S., Abeysinghe S., Krawczak M., Cooper D.N. Human gene mutation database (HGMD): 2003 update. Hum. Mutat. 2003;21(6):577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 22.Gillies R.L., Bjorksten A.R., Du Sart D., Hockey B.M. Analysis of the entire ryanodine receptor type 1 and alpha 1 subunit of the dihydropyridine receptor (CACNA1S) coding regions for variants associated with malignant hyperthermia in Australian families. Anaesth. Intensive Care. 2015;43(2):157–166. doi: 10.1177/0310057X1504300204. [DOI] [PubMed] [Google Scholar]
- 23.Lenglet H., Schmitt C., Grange T., Manceau H., Karboul N., Bouchet-Crivat F., Robreau A.M., Nicolas G., Lamoril J., Simonin S. From a dominant to an oligogenic model of inheritance with environmental modifiers in acute intermittent porphyria. Hum. Mol. Genet. 2018;27(7):1164–1173. doi: 10.1093/hmg/ddy030. [DOI] [PubMed] [Google Scholar]
- 24.Orngreen M.C., Schelhaas H.J., Jeppesen T.D., Akman H.O., Wevers R.A., Andersen S.T., ter Laak H.J., van Diggelen O.P., Dimauro S., Vissing J. Is muscle glycogenolysis impaired in X-linked phosphorylase b kinase deficiency? Neurology. 2008;70(20):1876–1882. doi: 10.1212/01.wnl.0000289190.66955.67. [DOI] [PubMed] [Google Scholar]
- 25.Allen G.C., Larach M.G., Kunselman A.R. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the north American malignant hyperthermia registry. The north American malignant hyperthermia registry of MHAUS. Anesthesiology. 1998;88(3):579–588. doi: 10.1097/00000542-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wongkittichote P., Ah Mew N., Chapman K.A. Propionyl-CoA carboxylase - a review. Mol. Genet. Metab. 2017;122(4):145–152. doi: 10.1016/j.ymgme.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowtell J.L., Marwood S., Bruce M., Constantin-Teodosiu D., Greenhaff P.L. Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Med. 2007;37(12):1071–1088. doi: 10.2165/00007256-200737120-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material