Abstract
Cervix cancer is the fourth most common cancer globally but the second most cancer in women in resource-limited countries. It has remained a clinically-staged neoplasm as per the International Federation of Gynecology and Obstetrics staging classification. As the imaging machines are becoming more available worldwide, the resource-stratified guidelines recommended the inclusion of imaging whenever possible to guide treatment planning. In this report, the utility of imaging in low- and middle-income countries for diagnosis and treatment of cancer of the cervix will be reviewed.
Keywords: Cervical cancer, Staging, Ultrasound, Computed tomography, Low and middle-income countries
Highlights
-
•
Imaging should be included to guide diagnosis and treatment planning.
-
•
Role of ultrasound and computerized tomography in LMIC was reviewed.
-
•
Cross-sectional imaging is important in planning for radiotherapy.
1. Introduction
There are extraordinary disparities in the diagnosis and treatment of cancer of the cervix. Mortality is 18 times higher in low and middle income countries (LMIC) than it is in wealthy nations (Ferlay et al., 2015). The absence of screening programs results in a dramatically higher incidence in sub-Saharan Africa, Central and South America, the Caribbean, Southern Asia and parts of Eastern Europe. Cervix cancer remains a leading cause of death among women with 530,000 new cases annually and 270,000 deaths globally (Centers for Disease Control and Prevention, n.d.). There has been little new progress in the early detection and treatment of cancer of the cervix. The National Cancer Institute (NCI) alert that indicated a survival improvement with the addition of cisplatin-based chemotherapy to radiation therapy was published >19 years ago (Morris et al., 1999; Rose et al., 1999). With access to care, many women can be cured without advanced technologies. An almost century-old study demonstrated survival rates of 75% for women with early-stage cervix cancer (Regaud, 1932). This determination was made well before the advent of cross-sectional imaging. Cervix cancer has remained a clinically-staged neoplasm because most or many patients do not have access to imaging and accurate assessment of the extent of disease. The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) have published resource-stratified guidelines that indicate the highest level of care, including imaging, should be provided whenever possible (Chuang et al., 2016; Network NCC, 2016). In this report, we will describe the utility of imaging in low- and middle-income countries for diagnosis and treatment of cancer of the cervix.
2. History of staging of cervix cancer
Nearly one hundred years ago it was recognized that staging systems should be valid, reliable, and practical. Cervix cancer staging had its origins in the Radiological Sub-Commission of the Cancer Commission of the Health Organization of the League of Nations (Odicino et al., 2008). Drs. J. Heyman (Radiumhemmet, Stockholm), A. Lacassagne (Radium Institute for the University of Paris), and F. Voltz (Munich) argued for information to be collected more consistently. They discussed the necessity of a uniform method to describe the extent of disease. These recommendations, adopted and published in 1929, became known as the League of Nations Classification for Cervical Cancer; however, widespread use did not immediately occur (Tropé et al., 2001). Annual Reports were published in the ensuing years, and in 1958 the International Federation of Gynecology and Obstetrics (FIGO) became the official patron of the Annual Report. In 1976 the American Joint Committee for Cancer Staging and End Results Reporting accepted the FIGO stage grouping for gynecological cancers (Annual Report on the Results of Treatment in Gynecological Cancer, n.d.). There have been eight changes to the FIGO staging system since its adoption 60 years ago with the most recent update in 2009.
3. Rules for staging of cervix cancer
Cervix cancer continues to be a clinically-staged disease as a result of the lack of surgical and radiographic expertise in areas of the world where cervix cancer predominates; however, efforts continue in advancing these modalities (Pecorelli et al., 2009). The optional staging techniques are palpation, inspection, colposcopy, endocervical curettage, hysteroscopy, cystoscopy, proctoscopy, intravenous urography, and x-ray examination of the lungs and skeleton. A visible lesion confined to the cervix, a microscopic lesion greater than IA1 or IA2, or both and <4 cm, is stage IB1. Inaccuracies exist between clinical staging and surgical staging in IB and stage II disease (FIGO (International Federation of Gynecology and Obstetrics), 2006). Tumor size has been recognized as an important prognostic factor and is more challenging to assess accurately in advanced-stage disease (Narayan et al., 2003). Cross-sectional imaging has not been endorsed because of its limited availability. If available, these techniques should be utilized to assess the primary tumor and direct treatment. If nodal involvement is discovered, the methodology by which the diagnosis was identified should be noted: pathologic or radiologic. If pathologic details are identified, they are not permitted to change the clinical stage, yet they should be recorded and used to develop an accurate treatment plan. Lymph node metastases strongly impact survival but are not included in the clinical staging system. This is the principle shortcoming of the clinical staging system. Regional lymph nodes include parametrial, obturator, internal and external iliac, common iliac, and presacral. Para-aortic lymph nodes are deemed metastatic, yet many reports document that a subset of these patients can be cured (Grigsby et al., 2001). In a series of 560 patients evaluated by a pre-treatment positron-emission tomography (PET) scan, Kidd et al., describe markedly worse outcomes for lymph node-positive patients. Additionally, patients with para-aortic involvement had a lower survival rate compared to pelvic-only involvement, and all patients with para-aortic involvement had PET avid pelvic lymph nodes. Of the 560 patients, para-aortic involvement was present in 17% and the disease-specific 5-year survival rate in this cohort was lower than 35% (Kidd et al., 2010).
4. Ultrasound in diagnosis in low- and middle-income countries
Ultrasound (US) has been an essential first-line imaging modality in the detection and characterization of gynecological disorders since the advent of real-time ultrasonography in the mid-1970's (Campbell, 2013). Although magnetic resonance (MR) is considered the gold standard imaging modality in the detection, characterization, and local staging of cervical cancer in industrialized nations, the worldwide acceptance of US in the assessment of cervical cancer has significantly increased over the past two decades (Fischerova et al., 2008; Testa et al., 2009; Gaurilcikas et al., 2011). Some relatively recent prospective studies have demonstrated an accuracy of transvaginal or transrectal US comparable to MR. (Epstein et al., 2013; Testa et al., 2014) Ultrasound is fast, widely available, requires minimal patient preparation, and is far more affordable than other imaging modalities. Therefore, it is the most attractive imaging modality for the detection and characterization of cervical cancer in low- and middle-income countries. Transvaginal and transrectal US probes can be positioned in close proximity to the cervix, providing detailed high-resolution imaging of cervical neoplasms (Testa et al., 2009; Gaurilcikas et al., 2011). In a recent European multicenter study of 182 women with early-stage cervical cancer (FIGO IA2-IIA), preoperative transvaginal and transrectal ultrasound examination provided 96% accuracy for tumor detection, with 90% sensitivity and 97% specificity (Epstein et al., 2013).
Cervical cancers generally present as solid, predominantly hypoechoic masses on US relative to the background cervical stroma (Fig. 1-b) (Testa et al., 2014). However, cervical cancers may rarely present as isoechoic or hyperechoic lesions (Testa et al., 2014). It has been suggested that the echogenicity of the tumor may correlate with the histologic subtype of the cervical cancer. In a multicenter study of 55 women with cervical cancer, isoechoic tumors were more commonly seen in adenocarcinomas (13 out of 19 [68%]), whereas hypoechoic tumors were more commonly seen in squamous cell carcinoma (11 out of 15 [73%]) (Epstein et al., 2010). Tumor growth characteristics have been described in the literature based on lesion morphology, with “mushroom” lesions corresponding to an exophytic growth pattern, whereas “ovoidal” or “conical” lesions correspond to an endophytic growth pattern (Testa et al., 2014). An advantage of intracavitary US is the ability to dynamically compress the region of interest during real-time evaluation. Tumors of the cervix have been described as relatively noncompressible on transvaginal or transrectal US examination relative to the normal background cervical stroma (Testa et al., 2014).
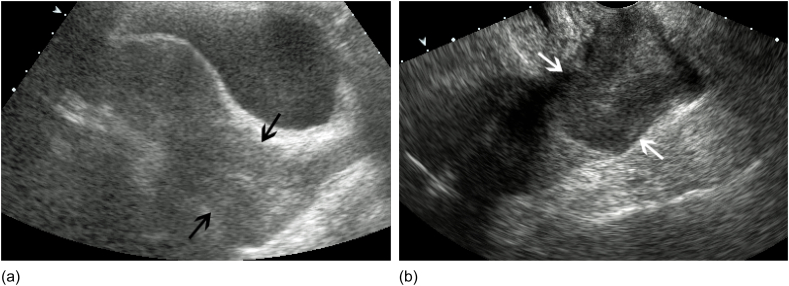
Fig. 1.
a. Cervical Carcinoma Transabdominal US sagittal view of the uterus in a 38 year old female with dysfunctional uterine bleeding demonstrates nonspecific enlargement of the cervix consistent with cervical carcinoma (black arrows). b. Transvaginal sagittal view of the same patient in 1A shows a better delineated hypoechoic mass (white arrows) expanding the cervix consistent with cervical carcinoma.
Color and power Doppler interrogation frequently demonstrate increased vascularity of cervical tumors compared to the unaffected background cervical stroma (Fig. 2) (Testa et al., 2014). Some investigators have suggested that color Doppler may aid in predicting the aggressiveness of cervical tumors. In a study by Cheng et al., 104 women with early-stage cervical carcinoma (stage IB-IIA) underwent preoperative transvaginal US assessment. Tumors with detectable blood flow had higher histologically proven vascular density, and were associated with higher risk of stromal and parametrial invasion and metastatic lymphadenopathy (Cheng et al., 1999). In a study by Alcazar et al., increased vascularity was found more frequently in squamous carcinoma, moderately or poorly differentiated tumors, large tumors, and advanced-stage tumors (Alcazar et al., 2003).
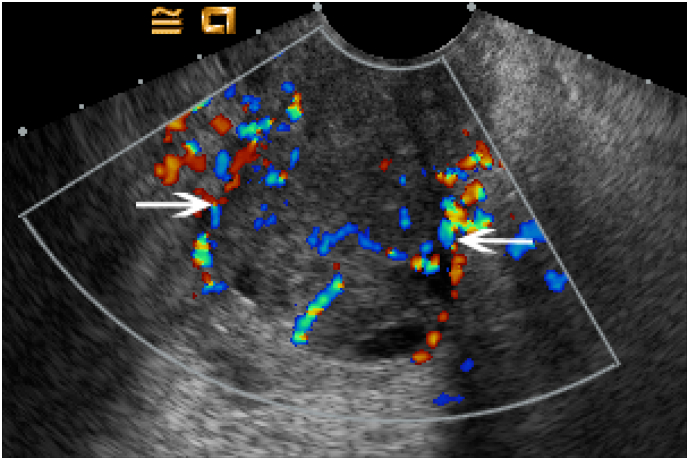
Fig. 2.
Color Doppler Evaluation of Cervical Carcinoma Transverse transvaginal view of the cervix in a 43 year old woman with cervical carcinoma demonstrates diffusely increased color flow throughout the heterogeneously enlarged cervix (white arrows).
5. Computed tomography in diagnosis in low- and middle-income countries
Computed tomography (CT) is a widely utilized imaging modality that is accepted as the “workhorse” modality of oncologic imaging. CT is more cost-effective than MR and more widely available worldwide, particularly in developing nations. It can provide a comprehensive evaluation of an oncologic patient with the rapid acquisition of high-spatial resolution images. However, in spite of recent technological developments in intravenous contrast-enhanced CT, the soft-tissue contrast resolution remains inferior to that of MR. (Testa et al., 2014)
Following intravenous administration of iodinated contrast material, the normal cervix demonstrates a variable enhancement pattern that can persist over several minutes (Kaur et al., 1998). Cervical tumors can be either hypoattenuating or isoattenuating to normal cervical stroma after administration of intravenous contrast material. Unfortunately, 50% of stage IB cancers have been described as isoattenuating to normal background cervical stroma, severely limiting tumor detection (Hricak and Yu, 1996). Even large tumors may present as nonspecific cervical enlargement (Testa et al., 2014). However, cervical enlargement in the setting of underlying malignancy can be of prognostic significance; cervical enlargement > 3.5 cm (cm) with an anteroposterior size of the cervix > 6 cm correlates with a poorer outcome (Walsh, 1992; Ogino et al., 1997). In certain instances, large tumors often show a rim of high attenuation with central areas having low attenuation. (Fig. 3). Such low attenuation areas of tumor involvement may be seen as a result of necrosis, ulceration, or reduced vascularity leading to increased conspicuity (Hricak, 1991). Tumors of the cervix frequently obstruct the endocervical canal and distention of the endometrial cavity with blood, serous fluid, or pus (Walsh, 1992).
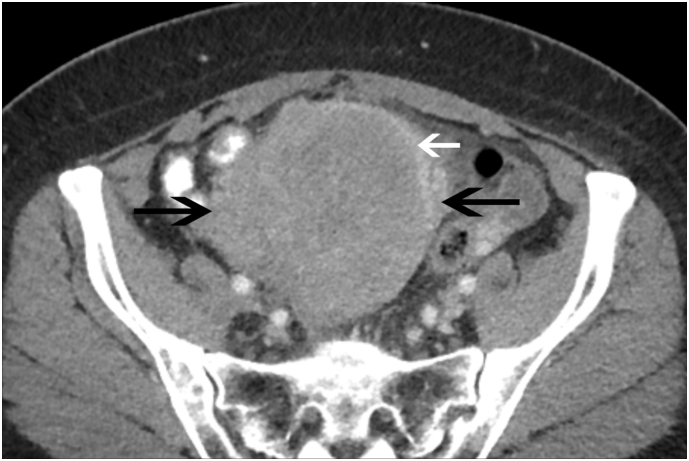
Fig. 3.
Cervical Carcinoma Axial contrast-enhanced CT image in a 53 year old female with profuse vaginal bleeding and clinically palpable cervical mass shows marked, heterogeneous enlargement of the cervix (black arrows). A rim of increased attenuation is noted along the left anterolateral aspect of the mass (white arrow).
6. US and CT for evaluation of extent of disease
Sonographic assessment of cervical stromal infiltration is optimally assessed using a transvaginal or transrectal probe. The degree of cervical stromal invasion is a major prognostic factor which directly correlates with the degree of nodal involvement. The degree of cervical stromal invasion can be reliably quantified by transvaginal sonography, with superficial tumor stromal invasion involving <2/3 of the stroma, and deep stromal invasion involving ≥2/3 of the stroma (Epstein et al., 2013). Both transvaginal and transrectal US examination can provide high sensitivity and specificity for the assessment of the depth of stromal invasion (Testa et al., 2009; Epstein et al., 2010). The relationship of tumor to the internal os should likewise be documented. Tumor infiltration of the vagina can be assessed by placing the transvaginal probe within the vaginal fornices (Fig. 4) (Testa et al., 2014).
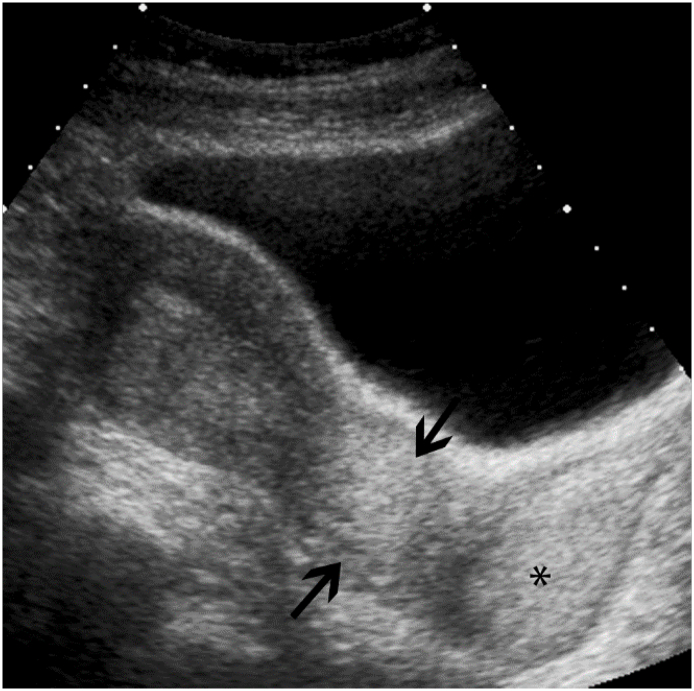
Fig. 4.
Cervical Carcinoma Transabdominal sagittal view of the uterus shows diffuse, echogenic enlargement of the cervix (black arrows), consistent with cervical carcinoma. Diffuse, echogenic expansion of the vaginal fornices (*) are likewise noted, consistent with tumoral involvement of the proximal vagina.
In the setting of full-thickness cervical stromal invasion, the anterior and posterior compartments and lateral parametria should be carefully assessed for local tumor extension. The anterior compartment is comprised of the bladder wall, vesico-vaginal septum and anterior parametria. The posterior compartment is comprised of the rectum, recto-vaginal septum and posterior parametria, including the uterosacral ligaments. The parametrium is the connective tissue between the leaves of the broad ligament (Vick et al., 1984). The parametrium is predominantly comprised of fat, through which course uterine vessels, nerves, fibrous tissues, and lymphatic channels (Vick et al., 1984). Sonographic findings of parametrial tumor involvement include irregular, generally hypoechoic elements extending into adjacent paracervical regions. Transvaginal and transrectal US examination can provide high specificity and moderate sensitivity in evaluating parametrial involvement (Fischerova et al., 2008; Testa et al., 2009; Epstein et al., 2013).
In a study by Epstein et al., US was significantly better than MR in the detection of residual tumors, and in the assessment of parametrial invasion in women with early-stage cervical cancer (Epstein et al., 2013). The greater accuracy of US in the detection of residual cancer is likely attributable to recent technical improvements, including high-frequency endoluminal probes which provide detailed visualization of the cervix.
CT is not indicated for local staging of cervical cancer (Testa et al., 2014). In certain instances, areas of decreased attenuation may be seen within the adjacent uterine corpus, indicative of contiguous tumor extension (Pannu et al., 2001). Tumor involvement of the vagina is inherently difficult to assess by CT, because tumor bulging into the vagina is often indistinguishable from vaginal wall invasion (Pannu et al., 2001). Multiplanar reformatted images in sagittal and coronal planes is essential to ascertain the superior and inferior extent of the tumor in suspected uterine or vaginal involvement (Pannu et al., 2001).
Early parametrial extension of tumor has been described on CT as poorly defined cervical margins, a relatively nonspecific finding (Hricak, 1991). In certain instances, parametrial tumor extension may be seen on CT as soft tissue infiltration of the parametrial fat (Fig. 5) (Pannu and Fishman, 2003). However, parametrial extension is suboptimally visualized by CT. In one study by Bipat et al., the sensitivity of CT for the detection of parametrial infiltration was reported at 55% (Bipat et al., 2003). Parametrial inflammation may likewise coexist in the setting of cervical cancer as a result of instrumentation, ulceration, and infection of the cervical tumor, and prior pelvic surgery (Vick et al., 1984). Parametrial infiltration is frequently indistinguishable from tumoral invasion on CT (Pannu et al., 2001).
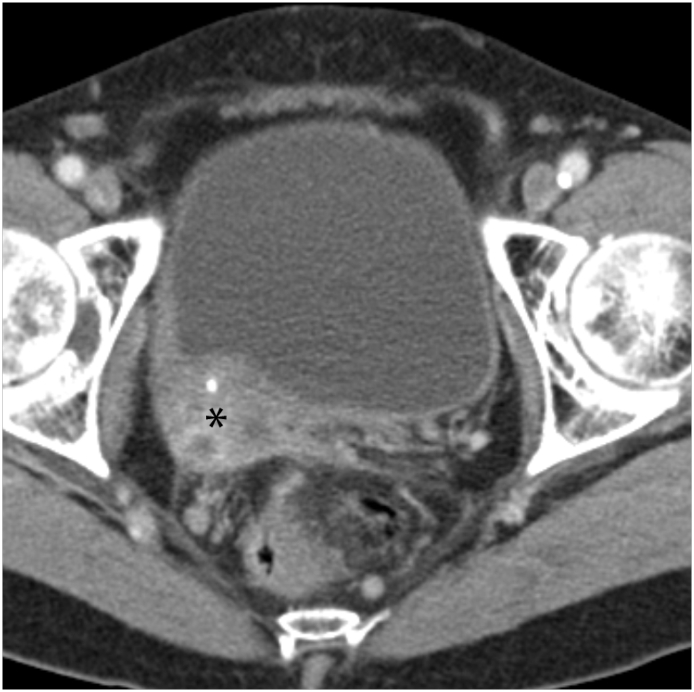
Fig. 5.
Locally Invasive Cervical Carcinoma Coronal oblique contrast-enhanced CT image shows diffuse, heterogeneous enlargement of the cervix (*). Diffusely decreased, heterogeneous attenuation is seen throughout the uterine corpus with distortion of the endometrial cavity (white arrow), consistent with tumoral infiltration of both endometrium and myometrium. Nodular, soft-tissue stranding is seen within the parametrium (black arrows), consistent with parametrial extension of tumor.
The distal ureters traverse the parametrium, generally located 2 cm lateral to the cervix. Ureteral encasement is a fairly common indicator of parametrial invasion. Ureteral encasement with an associated parametrial soft tissue mass are specific signs of parametrial invasion (Hricak, 1991). Ureteral encasement with resultant hydronephrosis is consistent with stage IIIb disease (Fig. 6). Pelvic sidewall invasion can be reliably diagnosed on CT when the tumor is <3 mm (mm) from the sidewall (Hricak and Yu, 1996). More extensive direct sidewall invasion can be seen in locally advanced tumors with heterogenous enlargement of the piriformis and obturator internus muscles (Hricak, 1991). Locally advanced cervical tumors may likewise result in iliac vessel encasement, and infiltration of the adjacent osseous pelvis (Kim et al., 1987).
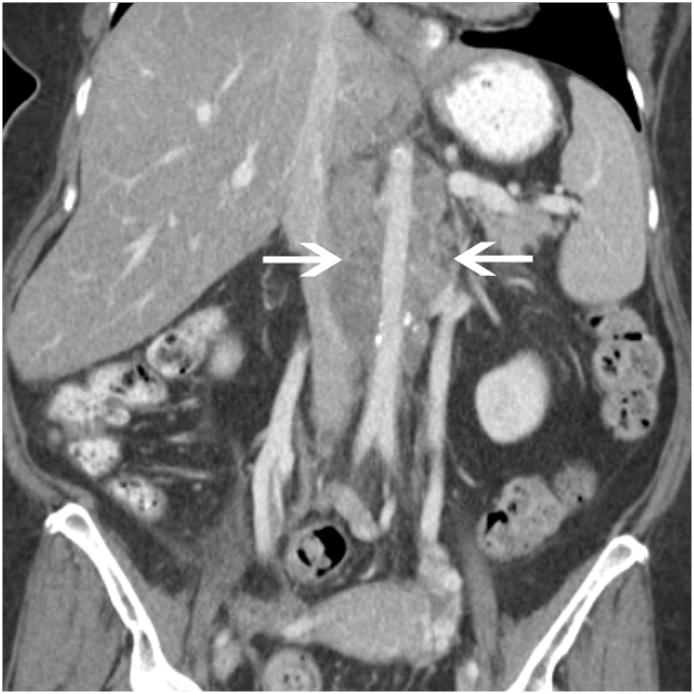
Fig. 6.
Locally Invasive Cervical Carcinoma Coronal contrast-enhanced CT shows a large, heterogeneous mass arising from the cervix (black *). Extensive tumoral infiltration of the right pelvic side wall is noted (white arrow). Bilateral hydronephrosis is seen (white *) as a result of tumoral encasement of the distal ureters.
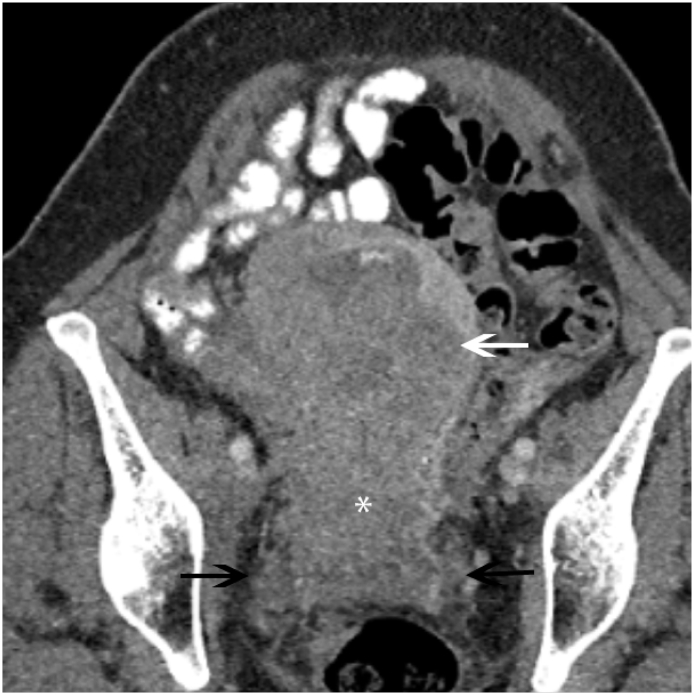
Tumor infiltration of the bladder and rectum constitutes stage IVa disease. CT signs of visceral invasion include loss of the perivesical or perirectal fat plane, asymmetric nodular thickening of the bladder or rectal wall (Fig. 7), an intraluminal mass, or formation of a fistula with air in the bladder (Walsh, 1992). The sensitivity of CT is low for mucosal invasion of the bladder (Massad et al., 2000). Suspected invasion is generally confirmed with cystoscopy or proctoscopy (Massad et al., 2000).
Fig. 7.
Cervical Carcinoma with Bladder Wall Invasion Axial contrast-enhanced CT shows a poorly marginated, heterogeneous cervical mass (*) that is inseparable from the right posterolateral bladder wall, consistent local bladder wall invasion.
Lymphatic spread of cervical malignancies can be readily assessed by CT. Nodes >1 cm in short-axis diameter are considered abnormal (Hricak and Yu, 1996). The nodes in the parametrium are the first to be involved by tumor by three lymphatic pathways (Hricak, 1991). The lateral route is along the external iliac vessels, the hypogastric route is along the internal iliac vessels, and the presacral route is along the uterosacral ligament (Park et al., 1994). All three routes lead to common iliac nodes, which can eventually involve para-aortic nodes.
7. Para-aortic evaluation in in low- and middle income countries
In spite of continued refinements in US and MR, CT remains the most cost-effective imaging modality for the detection and characterization of metastatic lymphadenopathy and distant metastases in gynecologic malignancies (Fig. 8). Overall, 14.8% of patients across all stages have para-aortic nodes that are positive for tumor (Scheidler et al., 1997). However, patients with cervical cancer may have a secondary infection that results in adenopathy. Enlarged malignant and hyperplastic nodes cannot be reliably distinguished at CT (Hricak, 1991). Tumor may be present in normal-sized nodes, resulting in a sensitivity of 44% for the detection of metastatic lymphadenopathy by CT (Hricak and Yu, 1996).
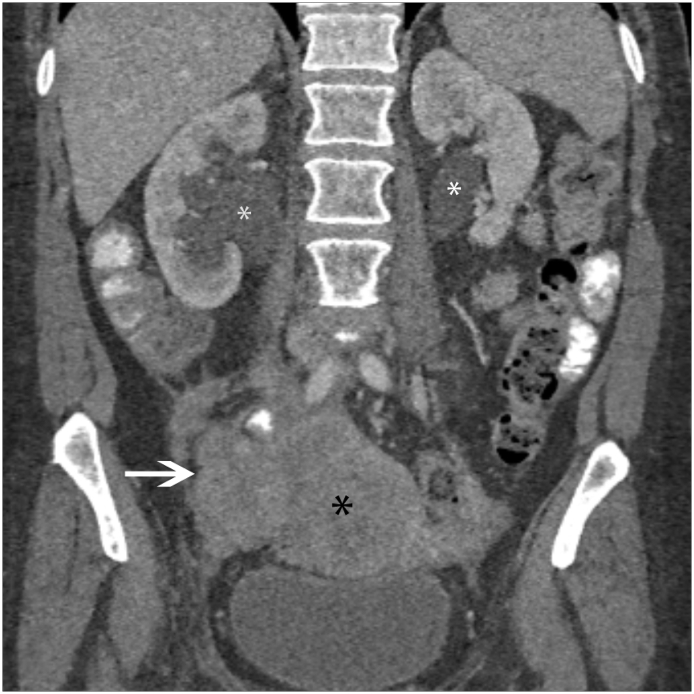
Fig. 8.
Cervical Carcinoma with Retroperitoneal Lymphadenopathy Coronal contrast-enhanced image of a 57 year old female with metastatic cervical carcinoma shows bulky, confluent retroperitoneal aortocaval and para-aortic lymphadenopathy (white arrows).
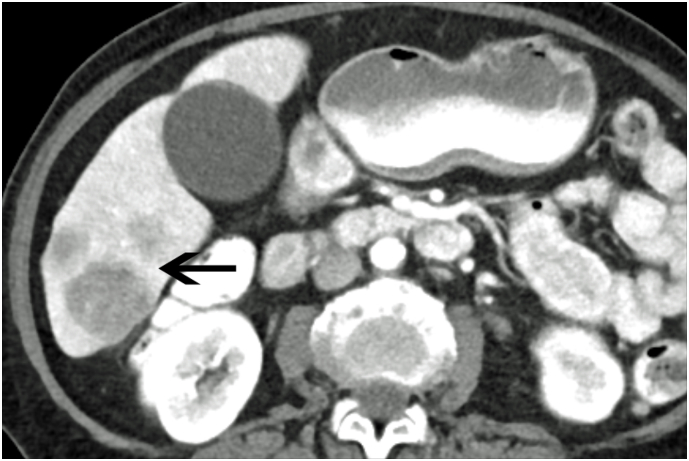
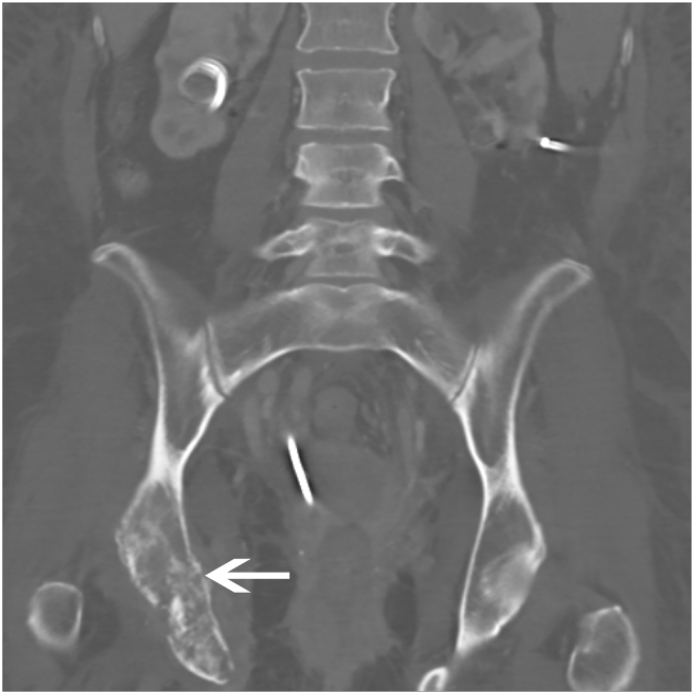
Distant metastases from cervical cancer are usually a result of recurrent disease, most frequently involving the liver, lung, and bone (Fulcher et al., 1999). Liver metastases generally present as poorly marginated, solid hypodense masses (Fig. 9) (Fulcher et al., 1999). Lung metastases generally manifest as multiple pulmonary nodules, occurring in 33%–38% of patients (Fulcher et al., 1999). Mediastinal or hilar adenopathy and pleural masses or effusion are present in up to one-third of patients with metastatic disease of the thorax (Shin et al., 1995). Osseous metastases usually occur secondary to direct extension from adjacent nodes, most commonly involving the lumbar spine (Fulcher et al., 1999). (See Fig. 10.)
Fig. 9.
Metastatic Cervical Cancer with Liver Metastases Axial contrast-enhanced CT of the abdomen in a patient with widely metastatic cervical carcinoma shows several ill-defined, poorly marginated masses within the inferior right lobe (black arrow) consistent with liver metastases.
Fig. 10.
Cervical Carcinoma with Osseous Metastases Coronal CT in a 37 year old female with widely metastatic cervical carcinoma shows lytic, destructive changes of the right acetabulum and inferior pubic ramus (white arrow). A right nephroueteral stent is partially visualized due to tumoral infiltration of the right collecting system.
8. Options of surgery concerning tumor size and nodal status
Women with cervical carcinoma at clinical stages IB1 and IIA1 can be cured by either radical hysterectomy (RH), pelvic lymphadenectomy with and without para-aortic lymph node biopsy or concurrent chemoradiation therapy (CCRT) (Landoni et al., 1997). Both approaches offer compatible 5-year survival rates of between 83% to 92%. The selection of treatment modalities depends on factors such as tumor size and histology, lymph-vascular space involvements, comorbid conditions, and patient preference. Tumor size is an important prognostic factor and has been used to guide this decision-making process. CCRT or, when indicated in LMIC, neoadjuvant chemotherapy (NACT) are preferred over primary surgery for patients with tumor size > 4 cm. Among the women who underwent primary RH, those with larger cervical tumor sizes are more likely to present with high-risk factors for recurrence, including positive lymph nodes, parametrial invasion, or positive surgical margins which warrant adjuvant chemoradiation (Peters III et al., 2000). The additional CCRT carries a 16% risk of grade 3 or higher gastrointestinal complications for these women. Lymphedema risk is increased for women who received CCRT after RH (Monk et al., 2005). Assessment of tumor size clinically or radiographically prior to surgery may assist in the selection of treatment modality for patients with early cervical cancers.
Compared to surgical staging, the FIGO clinical staging for cervical cancer based on examination understages 30% of stage IB patients with risk factors including positive lymph nodes (Lagasse et al., 1980). Preoperative assessment with imaging studies including abdominopelvic CT may assist in assessing lymph node metastasis, thus minimizing the need for adding adjuvant therapies after RH. For these patients with suspicious lymph nodes on CT, the options of CCRT or, in LMIC, RH may be considered in selected cases who have excellent responses following NACT (Chuang et al., 2016). A Cochrane review evaluating 1078 women who participated in 6 trials comparing NACT plus RH versus RH alone for locally-advanced cervical cancer has shown improved overall and progression-free survival with NACT (Rydzewska et al., 2012). Significant benefit of NACT was shown in the resolution of the positive lymph node (OR 0.54, 95% CI 0.40 to 0.73, P ≤ .0001). Presence of positive PAN or intra-abdominal spread on CT scan should deter surgeons from contemplating surgical management of these patients. In September 2017, Gupta reported the first phase III randomized trial comparing NACT followed by RH versus CCRT for 633 patients with stage IB2 to IIB squamous cell carcinoma of the cervix (Shrivastava et al., 2018). Pretreatment radiological imaging identified 14% of the patients with a positive pelvic lymph node. In the NACT followed by RH group, 68 patients (21.5%) received CCRT as a result of presurgery crossover and unresectable diseases. There was a 7% improvement (69.3% and 76.7%, P = .038) in disease-free interval in favor of CCRT. In the patients with stage IIB cervical cancer, the hazard ratio for survival was superior in the group that received CCRT. There was, however, no difference in overall survival between the two groups. Despite the notion that CCRT should remain the standard treatment for locally-advanced cervical cancer, radiation capacities including teletherapy and brachytherapy are not readily available for women with cervical cancer in LMIC. In India for example, where 26% of global cervical cancer deaths occur, there were only 0.4 high energy teletherapy units per million inhabitants in 2014 (WHO, India, 2017). This is in contrast to the availability of 5 high-energy teletherapy units per million inhabitants units in the United Kingdom and United States where the incidence of cervical cancer is three times lower than that in India (WHO, United Kingdom, 2018). In LMIC where radiation is lacking or not available, NACT followed by RH may be an alternative option for women with locally invasive diseases with no suspicious pelvic lymph node metastasis on imaging after completion of NACT. The results of a recently closed randomized controlled trial called EORTC 55994, which compares NACT followed by RH with primary CCRT, will be released next year.
9. Radiation therapy in low- and middle-income countries
Approximately 740 deaths occur daily as a result of cervical cancer (Small Jr. et al., 2017), making it the second most common cause of cancer death in women. In portions of Africa and South Asia, cervical cancer remains the most common cancer in women, and the number one cause of death (Chabra, 2016). The entire continent of Africa is functioning at about 25% of its potential capacity to treat cervical cancer (Abdel-Wahab et al., 2013). Women in low resource settings present with late stage disease. In one African nation, 48% of patients presented with stage III to IV disease (Einck et al., 2014). Of 139 countries defined as low- and middle-income by the World Bank criteria, Datta et al., indicated that only four were meeting their current radiotherapy needs (Datta et al., 2014). Furthermore, 55 nations had no RT, and for 80 nations only 37% of the population had access to RT. In the developed world, countries average one radiotherapy unit to 130,000 people, and this ratio is ten times less favorable in developing nations: approximately 1 unit to 1.4 million people in LMIC (Datta et al., 2014).
There are significant disparities in rapidly developing nations such as Brazil. There are an estimated 596,000 cancer cases annually in Brazil, and about half of cancer patients require RT at some point during their illness. Approximately 111,000 of Brazilian patients (37%) who required RT did not receive it because of lack of access. Furthermore, in Brazil the highest proportional cause of death was cervix cancer (Mendez et al., 2018).
Cervix cancer care should be tailored to each specific country or region. The ASCO, the American Society of Brachytherapy, and the NCCN have published resource stratified guidelines (Chuang et al., 2016; Suneja et al., 2017).Imaging should be utilized depending on local availability. Cross-sectional imaging should be utilized to obtain an accurate assessment of nodal burden if surgical staging is not performed.
Important considerations for external beam include simplified beam arrangements and standard fields. Cobalt-60 machines have been demonstrated to be reliable and effective in low-resource settings. Maintaining a high throughput is an important parameter, and hence imaging may need to be limited. Many centers use standard fields, if a 2- or 4-field approach is utilized. Physics support is often limited and a simplified planning approach may be preferred. Hypofractionation is one consideration to maintain a high throughput and permit more patients to access RT.
Ideally low- and middle-income countries will invest in external beam and brachytherapy because both modalities are required to cure women with advanced disease. In many developing nations only one modality is available requiring that treatments be palliative rather than curative (Einck et al., 2014). If available, cross-sectional imaging (CT or MRI) should be utilized if possible for brachytherapy planning. Even US can be utilized for brachytherapy planning (van Dyk et al., 2016). Brachytherapy planning may be facilitated by using fixed applicator configurations with a plan library and using radio-opaque applicators (Einck et al., 2014; Suneja et al., 2017). Fewer fractions per patient may permit increased throughput in busy facilities. Some modeling experiments have indicated that improving RT access has the potential to increase survival in cervix cancer more than in any other neoplasm (Atun et al., 2015).
10. Conclusions
Eighty-five percent of global cervical cancer incidence and deaths occur to women in LMIC. This has prompted efforts to increase HPV vaccination and improve cervical cancer screening to reduce the number of new cases and downstage the disease at initial diagnosis. Meaningful changes in the outcome of locally-advanced cervical cancer in LMIC would depend on the availability of surgeons who can perform RH, radiotherapy availability and expertise, and chemotherapy. The FIGO staging for cervical cancer that is based on clinical examinations frequently understages these patients and hence is not providing optimal guidance in the choices of treatments. Clinical examination based on the tumor size and local extensions limit the treatment planning and survival outcomes and fails to provide prognostic information to the patients and their families, since it does not include the most important prognostic factor: lymph node status. As CT scans and radiologists reading those studies become more available in LMIC, imaging studies provide information in addition to that obtained from clinical examination for the assessments and treatment selections for women with cervical cancer. The cervical cancer treatment guidelines published by ASCO and NCCN have recommended the use of imaging studies as part of the pretreatment assessments. This review discusses the roles of US and CT in the planning of the management of cervical cancer patients in LMIC and how the optimization of treatment with surgery or CCRT may be enhanced with these imaging technologies in LMIC. When available, imaging studies should be utilized to help in the planning of the primary tumor and direct treatments to all sites of disease. We advocate the identification of these nodal involvements as being pathologic or radiologic findings. As the survival of patients with cervical cancer depends on the lymph node status, patients with positive pelvic and para-aortic nodes carry a poorer prognosis than those without lymph node metastasis. With appropriate planning, CCRT have been shown to improve survivals even in the settings of positive para-aortic lymph nodes. In most cases when radiation therapy is available, CCRT should be recommended to patients with positive lymph nodes. Should NACT be considered in locally advanced cervical cancer in settings where radiation machines are limited, a follow-up CT after NACT may yield important information for gynecologic oncologists to make the decision on whether RH may or may not be of benefit. For patients undergoing CCRT, cross-sectional imaging (US, CT, or MRI) is important in planning for external radiation and brachytherapy. It is hoped that this review will guide oncologists in utilizing imaging as part of their treatment planning. Additional research is needed to assess the application of imaging in guiding cervical cancer treatment in LMIC.
Authors's contribution
Olpin J: concept development and writing.
Chuang L: concept development and writing.
Berek J: concept development and writing.
Gaffney D: concept development and writing.
References
- Abdel-Wahab M., Bourque J.M., Pynda Y. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- Alcazar J.L., Castillo G., Jurado M. Intratumoral blood flow in cervical cancer as assessed by transvaginal color doppler ultrasonography: correlation with tumor characteristics. Int. J. Gynecol. Cancer. 2003;13:510–514. doi: 10.1046/j.1525-1438.2003.13302.x. [DOI] [PubMed] [Google Scholar]
- Annual Report on the Results of Treatment in Gynecological Cancer, in HL K (ed), FIGO, 1982.
- Atun R., Jaffray D.A., Barton M.B. Expanding global access to radiotherapy. Lancet Oncol. 2015;16:1153–1186. doi: 10.1016/S1470-2045(15)00222-3. [DOI] [PubMed] [Google Scholar]
- Bipat S., Glas A.S., van der Velden J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: a systematic review. Gynecol. Oncol. 2003;91:59–66. doi: 10.1016/s0090-8258(03)00409-8. [DOI] [PubMed] [Google Scholar]
- Campbell S. A short history of sonography in obstetrics and gynaecology. Facts Views Vis Obgyn. 2013;5:213–229. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Global Cancer Statistics, Centers for Disease Control and Prevention, pp http://www.cdc.gov/cancer/international/statistics.htm.
- Chabra S. Cervical cancer preventable, treatable, but continues to kill women | OMICS International. J. HPV Cerv. Cancer. 2016;1 [Google Scholar]
- Cheng W.F., Wei L.H., Su Y.N. The possible use of colour flow Doppler in planning treatment in early invasive carcinoma of the cervix. Br. J. Obstet. Gynaecol. 1999;106:1137–1142. doi: 10.1111/j.1471-0528.1999.tb08138.x. [DOI] [PubMed] [Google Scholar]
- Chuang L.T., Temin S., Camacho R. Management and care of women with invasive cervical cancer: American society of clinical oncology resource-stratified clinical practice guideline. J. Glob. Oncol. 2016;2:311–340. doi: 10.1200/JGO.2016.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N.R., Samiei M., Bodis S. Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:448–457. doi: 10.1016/j.ijrobp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- van Dyk S., Narayan K., Bernshaw D. Clinical outcomes from an innovative protocol using serial ultrasound imaging and a single MR image to guide brachytherapy for locally advanced cervix cancer. Brachytherapy. 2016;15:817–824. doi: 10.1016/j.brachy.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Einck J.P., Hudson A., Shulman A.C. Implementation of a high-dose-rate brachytherapy program for carcinoma of the cervix in Senegal: a pragmatic model for the developing world. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:462–467. doi: 10.1016/j.ijrobp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Epstein E., Di Legge A., Masback A. Sonographic characteristics of squamous cell cancer and adenocarcinoma of the uterine cervix. Ultrasound Obstet. Gynecol. 2010;36:512–516. doi: 10.1002/uog.7638. [DOI] [PubMed] [Google Scholar]
- Epstein E., Testa A., Gaurilcikas A. Early-stage cervical cancer: tumor delineation by magnetic resonance imaging and ultrasound - a European multicenter trial. Gynecol. Oncol. 2013;128:449–453. doi: 10.1016/j.ygyno.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- FIGO (International Federation of Gynecology and Obstetrics) 26th annual report on the results of treatment in gynecological cancer. Int. J. Gynaecol. Obstet. United States. 2006:S1–257. doi: 10.1016/S0020-7292(06)60025-8. [DOI] [PubMed] [Google Scholar]
- Fischerova D., Cibula D., Stenhova H. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int. J. Gynecol. Cancer. 2008;18:766–772. doi: 10.1111/j.1525-1438.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- Fulcher A.S., O'Sullivan S.G., Segreti E.M. Recurrent cervical carcinoma: typical and atypical manifestations. Radiographics. 1999;19:103–116. doi: 10.1148/radiographics.19.suppl_1.g99oc19s103. (19 Spec No:S103-16; quiz S264-5, The PMID: 10517448) [DOI] [PubMed] [Google Scholar]
- Gaurilcikas A., Vaitkiene D., Cizauskas A. Early-stage cervical cancer: agreement between ultrasound and histopathological findings with regard to tumor size and extent of local disease. Ultrasound Obstet. Gynecol. 2011;38:707–715. doi: 10.1002/uog.9037. [DOI] [PubMed] [Google Scholar]
- Grigsby P.W., Siegel B.A., Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J. Clin. Oncol. 2001;19:3745–3749. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- Hricak H. Role of imaging in the evaluation of pelvic cancer. Important Adv. Oncol. 1991:103–133. [PubMed] [Google Scholar]
- Hricak H., Yu K.K. Radiology in invasive cervical cancer. AJR Am. J. Roentgenol. 1996;167:1101–1108. doi: 10.2214/ajr.167.5.8911159. [DOI] [PubMed] [Google Scholar]
- Kaur H., Loyer E.M., Minami M. Patterns of uterine enhancement with helical CT. Eur. J. Radiol. 1998;28:250–255. doi: 10.1016/s0720-048x(97)00173-3. [DOI] [PubMed] [Google Scholar]
- Kidd E.A., Siegel B.A., Dehdashti F. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J. Clin. Oncol. 2010;28:2108–2113. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
- Kim R.Y., Weppelmann B., Salter M.M. Skeletal metastases from cancer of the uterine cervix: frequency, patterns, and radiotherapeutic significance. Int. J. Radiat. Oncol. Biol. Phys. 1987;13:705–708. doi: 10.1016/0360-3016(87)90288-4. [DOI] [PubMed] [Google Scholar]
- Lagasse L.D., Creasman W.T., Shingleton H.M. Results and complications of operative staging in cervical cancer: experience of the gynecologic oncology group. Gynecol. Oncol. 1980;9:90–98. doi: 10.1016/0090-8258(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Landoni F., Maneo A., Colombo A. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- Massad L.S., Calvello C., Gilkey S.H. Assessing disease extent in women with bulky or clinically evident metastatic cervical cancer: yield of pretreatment studies. Gynecol. Oncol. 2000;76:383–387. doi: 10.1006/gyno.1999.5714. [DOI] [PubMed] [Google Scholar]
- Mendez L.C., Moraes F.Y., Fernandes G.D.S. Cancer deaths due to lack of universal access to radiotherapy in the Brazilian public health system. Clin. Oncol. (R Coll. Radiol.) 2018;30(1):e29–e36. doi: 10.1016/j.clon.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Monk B.J., Wang J., Im S. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a gynecologic oncology group/southwest oncology group/radiation therapy oncology group trial. Gynecol. Oncol. 2005;96:721–728. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Morris M., Eifel P.J., Lu J. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- Narayan K., McKenzie A.F., Hicks R.J. Relation between FIGO stage, primary tumor volume, and presence of lymph node metastases in cervical cancer patients referred for radiotherapy. Int. J. Gynecol. Cancer. 2003;13:657–663. doi: 10.1046/j.1525-1438.2003.13026.x. [DOI] [PubMed] [Google Scholar]
- Network NCC . In: NCCN Framework for Resource Stratification of NCCN Guidelines: Cervical Cancer. NCCN, editor. NCCN; 2016. (NCCN Framework). (ed 2.2015) [Google Scholar]
- Odicino F., Pecorelli S., Zigliani L. History of the FIGO cancer staging system. Int. J. Gynaecol. Obstet. 2008;101:205–210. doi: 10.1016/j.ijgo.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Ogino I., Okamoto N., Andoh K. Analysis of prognostic factors in stage IIB-IVA cervical carcinoma treated with radiation therapy: value of computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 1997;37:1071–1077. doi: 10.1016/s0360-3016(96)00599-8. [DOI] [PubMed] [Google Scholar]
- Pannu H.K., Fishman E.K. Evaluation of cervical cancer by computed tomography: current status. Cancer. 2003;98:2039–2043. doi: 10.1002/cncr.11684. [DOI] [PubMed] [Google Scholar]
- Pannu H.K., Corl F.M., Fishman E.K. CT evaluation of cervical cancer: spectrum of disease. Radiographics. 2001;21:1155–1168. doi: 10.1148/radiographics.21.5.g01se311155. [DOI] [PubMed] [Google Scholar]
- Park J.M., Charnsangavej C., Yoshimitsu K. Pathways of nodal metastasis from pelvic tumors: CT demonstration. Radiographics. 1994;14:1309–1321. doi: 10.1148/radiographics.14.6.7855343. [DOI] [PubMed] [Google Scholar]
- Pecorelli S., Zigliani L., Odicino F. Revised FIGO staging for carcinoma of the cervix. Int. J. Gynaecol. Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Peters W.A., III, Liu P.Y., Barrett R.J., II Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- Regaud C. Comparaison des valeurs curatives de l'hysterectomie et des methodes radiotherapiques dans le traitement des epitheliomas cervicouterins du premier degre. Bull. Acad. de rned. 1932;107:611–625. [Google Scholar]
- Rose P.G., Bundy B.N., Watkins E.B. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- Rydzewska L., Tierney J., Vale C.L. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst. Rev. 2012;12:Cd007406. doi: 10.1002/14651858.CD007406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidler J., Hricak H., Yu K.K. Radiological evaluation of lymph node metastases in patients with cervical cancer. A meta-analysis. Jama. 1997;278:1096–1101. [PubMed] [Google Scholar]
- Shin M.S., Shingleton H.M., Partridge E.E. Squamous cell carcinoma of the uterine cervix. Patterns of thoracic metastases. Investig. Radiol. 1995;30:724–729. doi: 10.1097/00004424-199512000-00006. [DOI] [PubMed] [Google Scholar]
- Shrivastava S., Mahantshetty U., Engineer R. Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: a randomized clinical trial. JAMA Oncol. 2018;4:506–513. doi: 10.1001/jamaoncol.2017.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small W., Jr., Bacon M.A., Bajaj A. Cervical cancer: a global health crisis. Cancer. 2017;123:2404–2412. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- Suneja G., Brown D., Chang A. American brachytherapy society: brachytherapy treatment recommendations for locally advanced cervix cancer for low-income and middle-income countries. Brachytherapy. 2017;16:85–94. doi: 10.1016/j.brachy.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Testa A.C., Ludovisi M., Manfredi R. Transvaginal ultrasonography and magnetic resonance imaging for assessment of presence, size and extent of invasive cervical cancer. Ultrasound Obstet. Gynecol. 2009;34:335–344. doi: 10.1002/uog.7325. [DOI] [PubMed] [Google Scholar]
- Testa A.C., Di Legge A., De Blasis I. Imaging techniques for the evaluation of cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014;28:741–768. doi: 10.1016/j.bpobgyn.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Tropé C., Kristensen G., Onsrud M. Controversies in cervical cancer staging. CME J. Gynecol. Oncol. Jan 2001;6(2):240–245. [Google Scholar]
- Vick C.W., Walsh J.W., Wheelock J.B. CT of the normal and abnormal parametria in cervical cancer. AJR Am. J. Roentgenol. 1984;143:597–603. doi: 10.2214/ajr.143.3.597. [DOI] [PubMed] [Google Scholar]
- Walsh J.W. Computed tomography of gynecologic neoplasms. Radiol. Clin. N. Am. 1992;30:817–830. [PubMed] [Google Scholar]
- WHO, India . WHO, World Health Organization; 2017. Country Profiles. [Google Scholar]
- WHO, United Kingdom . World Health Organization; 2018. WHO. [Google Scholar]