Abstract
Aims
We evaluated the effectiveness of a multidisciplinary transition plan to reduce early readmission among heart failure patients.
Methods and results
We conducted a before‐and‐after study in a tertiary internal medicine department, comparing 3 years of retrospective data (pre‐intervention) and 13 months of prospective data (intervention period). Intervention was the introduction in 2013 of a transition plan performed by a multidisciplinary team. We included all consecutive patients hospitalized with symptomatic heart failure and discharged to home. The outcomes were the fraction of days spent in hospital because of readmission, based on the sum of all days spent in hospital, and the rate of readmission. The same measurements were used for those with potentially avoidable readmissions. Four hundred thirty‐one patients were included and compared with 1441 patients in the pre‐intervention period. Of the 431 patients, 138 received the transition plan while 293 were non‐completers. Neither the fraction of days spent for readmissions nor the rate of readmission decreased during the intervention period. However, non‐completers had a higher rate of the fraction of days spent for 30 day readmission (19.2% vs. 16.1%, P = 0.002) and for potentially avoidable readmission (9.8% vs. 13.2%, P = 0.001). The rate of potentially avoidable readmission decreased from 11.3% (before) to 9.9% (non‐completers) and 8.7% (completers), reaching the adjusted expected range given by SQLape® (7.7–9.1%).
Conclusions
A transition plan, requiring many resources, could decrease potentially avoidable readmission but shows no benefit on overall readmission. Future research should focus on potentially avoidable readmissions and other indicators such as patient satisfaction, adverse drug events, or adherence.
Keywords: Readmission, Heart failure, Transitional care, Discharge plan, Potentially avoidable readmission
Introduction
Heart failure (HF) is a well‐defined but complex clinical syndrome characterized by typical signs and symptoms and caused by structural or functional impairment of ventricular filling or ejection of blood. Its prevalence varies between 10 and 20 per 1000 individuals worldwide. Healthcare expenditure attributed to HF approaches 1–2% in Europe and North America.1 HF is a chronic disease associated with a high risk of hospitalization and the highest risk of early readmission, reaching 25%.2, 3, 4, 5 In the last decade, reduction of early readmission, defined as a new hospital admission within 30 days after discharge, has become a major goal for healthcare systems as a quality and cost indicator.6 Moreover, the lack of communication between hospital and community care providers causes ineffective and unsafe discharge.7, 8
Transition from hospital to home care is recognized as critical to reduce readmission. Transitional care is defined as a set of actions designed to ensure the coordination and continuity of healthcare.9 Many interventions on transitional care have been evaluated, such as follow‐up call or telemonitoring. Recent reviews showed that no single intervention implemented alone was regularly associated with reduced risk for 30 day readmission. Effective interventions are complex and should reinforce patient self‐care. They tend to be more comprehensive, extend beyond the hospital stay, and have the flexibility to respond to individual patient's needs.10, 11, 12
Reviews focusing on readmission of patients with HF conclude that structured telephone support, multidisciplinary HF clinics, and home‐visiting programmes can potentially reduce readmission, but strength of evidence is low or lacking for 30 day readmission and of varying degrees for 3–6 months' readmissions.13 Currently, the major limitations and weaknesses of the literature on transitional care are the variations in definition of the interventions, the need for better evidence, and the high variability of healthcare settings. It makes a broad implementation of specific interventions difficult.11, 13
It is clear, however, that even the best intervention could not prevent all readmissions. Halfon et al. defined potentially avoidable readmissions (PARE) as unforeseen readmissions for a previously known affliction. They validated the SQLape® (Striving for Quality Level and Analyzing of Patient Expenditures) algorithm for identification of PARE with a sensitivity and specificity up to 96%. On the basis of the Swiss national healthcare register, SQLape® allows estimating the range of adjusted expected rate of PARE in a defined population.14 As for all‐cause readmissions, HF condition is also associated with a high risk of PARE.15 Readmission is expressed as a rate: the number of readmissions divided by the number of hospitalizations. However, the burden of readmission is better assessed by the length of stay (LOS). Higher LOS is associated with higher costs and complications such as hospital‐acquired infections or deconditioning.16
Objectives
Our objective was to assess the effectiveness of a multimodal care transition plan to reduce the burden of 30 day all‐cause readmissions among adult patients hospitalized with HF in a general internal medicine department. We chose the fraction of days spent in hospital because of a readmission (days spent in readmission over all days spent in hospital during the study periods) as primary outcome (Figure A1 ). The secondary outcomes evaluated the effectiveness of this transition plan on the PARE.
Methods
Our study followed a before‐and‐after design with a retrospective pre‐intervention cohort of HF patients and a prospective intervention cohort of HF patients. We added a non‐equivalent non‐intervention control group composed of all other hospitalized patients during the same period and planned a sensitivity analysis.
The study was conducted in the Department of Internal Medicine at the University Hospital of Lausanne, Switzerland. Between 2011 and 2013, the department discharged, on average, 3842 patients annually. They were mainly admitted from the emergency department (92.3%) and mostly discharged to home (57.3%).
Enrolment began 1 November 2013 and ended 30 November 2014. Patients could be enrolled at each hospitalization. Patients were eligible if they were at least 18 years old, had HF as an active diagnosis, and were discharged to home. HF was defined according to the European Cardiology Society definition17 or the presence of the appropriate International Classification of Diseases, Tenth Revision codes in the medical record (I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5 to I42.9, I43.x, I50.x, and P29.0). The study excluded patients undergoing chronic haemodialysis, asymptomatic HF stated as New York Heart Association functional class 1, and hospitalizations rejected by the SQLape® algorithm (i.e. living abroad).
The local human research ethics committee reviewed and authorized the protocol (reference number: 278/13). In case of failure to obtain a signed consent form, the patient received usual care.
Transition plan
A multidisciplinary team of senior physicians, clinical pharmacists, and experienced nurses developed the 11 interventions composing the transition plan. None was part of the usual care, and the team operated the transition plan independently from physicians in charge. The details are given in Table 1 and illustrated in Figure A2 .
Table 1.
Transition plan
| Components | Description | Comment and references |
|---|---|---|
|
Targeted therapeutic education |
On the basis of existing material of the Swiss Heart Foundation for the patient,18 the nurse provided a structured focused education on self‐monitoring, instability signs, and compliance. |
Self‐monitoring as weight or breath is associated with less hospitalization. Self‐care improves outcomes and should be promoted.19, 20, 21
|
|
Caregiver therapeutic education |
The nurse gave the same education to the caregivers, if the patients had dementia or language issue. |
Caregivers have specific demands related to HF patients: physical limitations, medication, symptoms monitoring, regime, or disturbed sleep.22
|
|
Medication reconciliation at admission |
The clinical pharmacist collected three sources of information to build the best available list of home medication, certified during an interview with the patients. |
Pharmacist's interventions reduce the morbidity and mortality associated with heart failure by extending his role from professional guidance to the delivery of continuity of care.23
|
|
Medication reconciliation at discharge |
The clinical pharmacist reviewed and proposed improvement of the discharge prescription, on the basis of the medication reconciliation on admission. The patients, the outpatient pharmacy, and the GP received a commented medication plan. |
|
|
Set‐up of an appointment with the GP |
The nurse strongly encouraged the patients to visit their GP within 7 days after discharge, by helping them and reminding them during follow‐up calls. |
Follow‐up appointment prior to discharge strongly protects against readmission.24
|
|
Notification of the GP |
The nurse sent a message including discharge date, diagnosis, and medication to the GPs to improve their awareness. |
Communication with the GP is central for continuity of care and to reduce the risk of rehospitalization.25
|
|
Community nurses notification |
If the patients benefited from community nurses services, they were informed about the transition plan either in writing or by phone. |
Poor communication between community nurse and physician is associated with an increased risk of hospital readmission among high‐risk patients.26
|
|
Patient‐centred discharge instructions |
Before discharge, patient's awareness was challenged with three questions: What is my diagnosis? What is my medication? When and where is my next appointment? |
Interactive communication strategy improves the comprehension of the patient.27, 28
|
|
Follow‐up call |
The nurse called the patients at the 3rd, 7th, and 18th days after discharge, using a structured interview to identify instability signs, motivate the patients to self‐monitor, and, if needed, to call their GPs. The calls were supervised by the senior physician. |
Counselling and monitoring through frequent telephone follow‐up reduce significantly admissions for heart failure.29
|
|
Optional consultation |
To overcome unavailability of GPs, the patients might ask for a follow‐up visit at hospital, within the week after discharge. |
Early follow‐up visit after discharge may be effective to reduce all‐cause readmission, emergency department visits, and mortality.30
|
| Hotline | During office hours, the patients could call the nurse for any reason. | Patient hotline provides an effective complementary intervention to follow‐up calls.31 |
GP, general practitioner; HF, heart failure.
The transition plan was provided by a trained nurse acting as a coordinator. The medication reconciliations were performed by a pharmacist. All cases were discussed with a senior physician as clinical supervisor.
Data collection
We collected patient characteristics, setting of hospitalization, and diagnosis from the medico‐administrative database of the hospital. We ensured identical inclusion and analysis of before and after groups by using diagnosis coded after discharge from the discharge summary. We therefore avoided a selection bias. The transition team had no access to the statistical analysis results until the end of the study.
Outcomes
The primary outcome measure was the fraction of days spent in hospital because of a readmission within 30 days after discharge, based on the sum of all days of hospitalization (illustrated in Figure A1 ). In case of a second readmission, the first readmission was analysed as an index hospitalization and the patient was included twice. We also measure the 30 day all‐cause readmission rate.
The secondary outcomes were (i) the fraction of days spent in hospital because of a PARE based on the sum of all days of hospitalization, (ii) the rate of PARE and the comparison to the adjusted expected interval calculated by SQLape®, and (iii) the readmission‐free survival estimates.
Data analysis
We defined two cohorts of HF patients, one for the pre‐intervention period and one for the intervention period. Within the intervention period cohort, we also distinguished the hospitalizations of HF patients who received the transition plan (‘receivers’) from those who did not complete the plan (‘non‐completers’).
The SQLape® software version 2014 (SQLape SARL, Switzerland), including gender, age, admission mode, LOS, diagnosis, procedures, and other variables—was used to identify PARE and calculate adjusted expected range of PARE.32
Descriptive measures of variables were calculated and presented as means and standard deviations. χ2 test and Wilcoxon tests were used to compare patients' characteristics between pre‐intervention and intervention periods. We used a multivariate logistic regression to compare readmissions and PARE. We used STATA 14 (Stata Corp, College Station, TX, USA) to perform the analysis.
Readmission data from all patients hospitalized in our department of internal medicine were collected during the study period to detect general trend of readmission. A sensitivity analysis comparing patients who benefited from the transition plan (receivers vs. non‐completers) was performed.
Results
Figure 1 shows the flow diagram of screening and enrolment of participants. In the pre‐intervention period, 1441 hospitalizations were eligible. In the intervention period, 431 hospitalizations were included for analysis. In 293 out of 431 hospitalizations, patients (non‐completers) did not complete the transition plan: 130 were diagnosed for HF after screening, 111 were discharged before enrolment, and 52 refused to give their consent. The remaining 138 patients received the transition plan.
Figure 1.
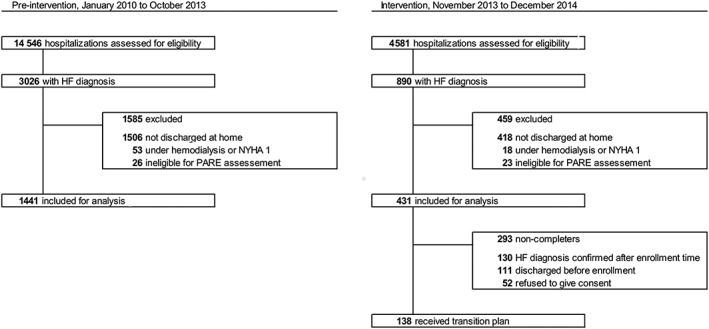
Flow diagram of screening and enrolment of participants. Medical records were screened according to discharge date and diagnosis codes. Hospitalizations rejected by the SQLape algorithm were excluded. HF, heart failure; NYHA 1, New York Heart Association functional class 1 (asymptomatic HF); PARE, potentially avoidable readmission.
Table 2 shows characteristics of patients and hospitalizations during the pre‐intervention and intervention periods. Patients were similar except for age: patients in the intervention period were significantly older (+1.8 years, P‐value 0.016). However, the multivariate analysis did not identify age as a significant variable.
Table 2.
Characteristics of the 1441 hospitalizations studied during the pre‐intervention period and 431 in the intervention period
| Pre‐intervention period | Intervention period | ||||
|---|---|---|---|---|---|
| % | % | P‐value | |||
| Number of hospitalizations (n) | 1441 | 431 | |||
| Patient characteristics | |||||
| Mean age (years old ± SD) | 76.4 ± 12.6 | 78.2 ± 12.4 | 0.016a | ||
| Female | 661 | 45.9 | 203 | 47.1 | 0.653 |
| Married | 669 | 46.4 | 186 | 43.2 | 0.232 |
| General insuranceb | 1299 | 90.1 | 397 | 92.1 | 0.220 |
| Lives in the same sanitary region as hospital | 1172 | 81.3 | 344 | 79.8 | 0.481 |
| Incident hospitalization | |||||
| Admission | |||||
| Unplanned admission | 1373 | 95.3 | 407 | 94.4 | 0.474 |
| Need of urgent care (E.S.T score ≤ 2) | 907 | 66.0c | 264 | 65.0c | 0.726 |
| Admission on weekend | 270 | 18.7 | 81 | 18.8 | 0.979 |
| Admission by night | 435 | 30.2 | 147 | 34.1 | 0.123 |
| First complaint at admission | |||||
| Chest pain | 154 | 10.7 | 48 | 11.1 | 0.792 |
| Dyspnoea | 748 | 51.9 | 210 | 48.7 | 0.246 |
| Arrhythmia | 94 | 6.5 | 19 | 4.4 | 0.106 |
| Shock | 33 | 2.3 | 6 | 1.4 | 0.252 |
| Other | 346 | 24.0 | 123 | 28.5 | 0.057 |
| Unavailable | 66 | 4.6 | 25 | 5.8 | 0.301 |
| Hospitalization | |||||
| Average length of stay (days ± SD) | 15.4 ± 12.4 | 15.5 ± 12.4 | 0.487 | ||
| >8 medications at discharge | 1044 | 75.4 | 324 | 77.9 | 0.305 |
| ≥4 hospitalizations within the last year | 357 | 24.8 | 121 | 28.1 | 0.168 |
| Principal diagnosis | |||||
| Congestive heart failure | 624 | 43.3 | 186 | 43.2 | 0.957 |
| Acute myocardial infarct | 95 | 6.6 | 28 | 6.5 | 0.944 |
| Pneumonia | 42 | 2.9 | 9 | 2.1 | 0.355 |
| Respiratory failure | 90 | 6.2 | 21 | 4.9 | 0.290 |
| Atrial fibrillation or flutter | 64 | 4.4 | 18 | 4.2 | 0.814 |
| COPD | 40 | 2.8 | 11 | 2.6 | 0.802 |
| Sepsis | 24 | 1.7 | 7 | 1.6 | 0.953 |
COPD, chronic obstructive pulmonary disease.
P‐value considered as significant.
Non‐general insurances include private and semi‐private insurance, more expensive, allowing daily senior attending physician visit and a single‐bed room.
Data were available for 1375 (pre‐intervention period) and 406 (intervention period) hospitalizations. Echelle Suisse de Triage (E.S.T) Swiss triage scale from 1 to 4. Level 2 patients must be treated in <20 min.
Completion of the transition plan
Actions taking place right before discharge had a lower rate of completion. On average, the transition team needed 3.25 h of work to complete one transition plan. The medication reconciliation was the most time‐consuming task. Moreover, overhead time (administration, visiting wards, screening, meeting, and supervision of team) accounted for 5 to 6 h per opening day. Table A1 shows the different rates of realization of the components of the transition plan.
Thirty‐day readmissions of heart failure patients and general population
For HF patients, the per cent of days spent in hospital because of a 30 day readmission during the intervention period reached 18.1% (1213/6689 days), significantly higher than that in the pre‐intervention period, which was at 15.5% (3451/22 235 days, P‐value < 0.001; Table 3). The rate of hospitalizations followed by a readmission within 30 days was higher during the intervention period (21.1% vs. 19.2%, P‐value 0.368).
Table 3.
Outcomes
| Pre‐intervention period | Intervention period | P‐value | |
|---|---|---|---|
| Sum of days spent in hospital by HF patients | 22 235 | 6689 | |
| Days due to any readmission within 30 days | 3451 (15.5%) | 1213 (18.1%) | <0.001 |
|
Days due to a PARE within 30 days |
2553 (11.5%) |
805 (12.0%) |
0.520 |
| Hospitalizations of HF patients | 1441 | 431 | |
| Followed by any readmission within 30 days | 276 (19.2%) | 91 (21.1%) | 0.368 |
| Followed by a PARE within 30 days | 163 (11.3%) | 41 (9.5%) | 0.293 |
| Range of adjusted expected rate of PARE | 7.6–9.0% | 7.8–9.1% | N/A |
N/A, not applicable; PARE, potentially avoidable readmission.
The ranges of adjusted expected rate of PARE are calculated with the SQLape® (Striving for Quality Level and Analyzing of Patient Expenditures) algorithm.
However, during the same periods, 30 day readmission also increased in the general population hospitalized in the department of internal medicine. The per cent of days spent in hospital by patients without HF because of a 30 day readmission was 17.6% (13 617/77 461 days) in the pre‐intervention period and significantly increased to 18.9% (4763/25 188, P‐value < 0.001) during the intervention period. The rate of readmission of patients without HF increased as well (18.4% vs. 19.9%, P‐value 0.134) but was not significant (Figure 2 ).
Figure 2.
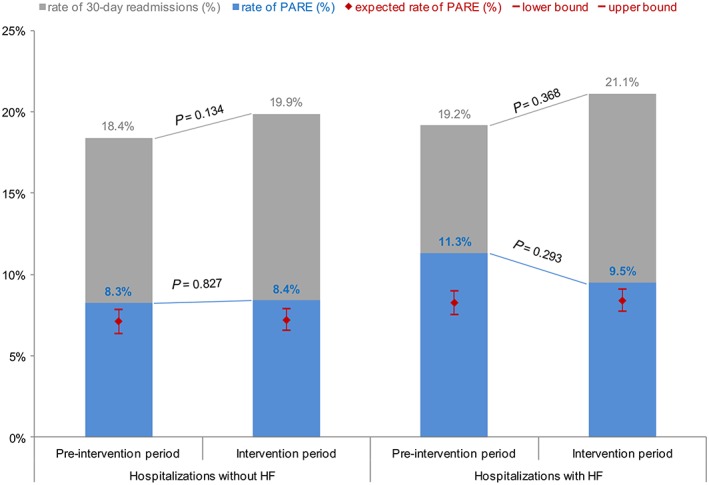
Rates of hospitalizations followed by a 30 day readmission: comparison of the pre‐intervention and intervention periods among hospitalizations of patients with HF (on the right) and hospitalizations in our internal medicine department of patients without HF (on the left) after being discharged to home. Hospitalizations of patients with HF show an increased rate of all cause readmissions and a decreasing rate of PARE. This leads to a significant reduction of the ratio PARE/total readmissions (P‐value 0.020). During the study period, overall readmission rate increased, while PARE rate remains stable. Red range stands for adjusted expected range of PARE in each group, according to the SQLape® algorithm. PARE, potentially avoidable readmission.
Potentially avoidable readmissions of heart failure patients
The per cent of days spent in hospital because of a PARE was similar in the pre‐intervention and intervention periods (11.5 vs. 12.0%, P‐value 0.520; Table 3). The rate of hospitalizations followed by a PARE decreased from 11.3% (163 PARE/1441 hospitalizations) in the pre‐intervention cohort to 9.5% (41 PARE/431 hospitalizations, P‐value 0.293) in the intervention cohort. Although this difference is not significant, this rate approached the adjusted expected range of 7.8–9.1% PARE, calculated by SQLape® (Figure 2 ). Moreover, the ratio of PARE over total readmissions is significantly reduced, meaning—after adjustment for age, sex, sanitary region, and frequency of hospitalization—the risk of PARE is reduced to half (adjusted odds ratio 0.55, P‐value 0.020).
Sensitivity analysis
Table 4 details the results of the sensitivity analysis in the intervention cohort, between receivers and non‐completers. The per cent of days spent in hospital because of a 30 day readmission was significantly lower in the receivers group (16.1% vs. 19.2%, P‐value 0.002). The two groups show no significant difference in rate of readmission (8.7% vs. 9.9%), but the PARE rate of the receivers group lies in the adjusted expected range given by SQLape® (7.8–9.1%; Figure 3 ).
Table 4.
Sensitivity analysis: comparison of the ‘non‐completers’ vs. ‘receivers’ groups in the intervention period
| Non‐completers | Receivers | P‐value | |
|---|---|---|---|
| Sum of days spent in hospital by HF patients | 4435 | 2254 | |
| Days due to any readmission within 30 days | 851 (19.2%) | 362 (16.1%) | 0.002 |
|
Days due to a PARE within 30 days |
585 (13.2%) |
220 (9.8%) |
<0.001 |
| Hospitalizations of HF patients | 293 | 138 | |
| Followed by any readmission within 30 days | 60 (20.5%) | 31 (22.5%) | 0.637 |
| Followed by a PARE within 30 days | 29 (9.9%) | 12 (8.7%) | 0.692 |
| Range of adjusted expected rate of PARE | 7.8–9.2% | 7.7–9.1% | N/A |
N/A, not applicable; PARE, potentially avoidable readmission.
The ranges of adjusted expected rate of PARE are calculated with the SQLape® (Striving for Quality Level and Analyzing of Patient Expenditures) algorithm.
Figure 3.
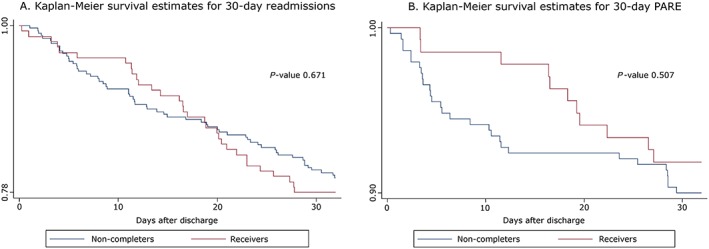
Readmission‐free survival estimates between the receivers and non‐completers groups. Both (A) and (B) show no significant difference at 30 days. PARE, potentially avoidable readmission.
Discussion
Our before‐and‐after study aimed to evaluate the impact of a transition plan for HF patients on 30 day readmissions and on PARE. The results highlighted that the transition plan for HF patients discharged from hospital to home failed to reduce 30 day readmissions, for either the primary outcome (per cent of days spent in readmission) or the rate of readmission. However, the rate of PARE decreased from 11.3% to 9.5% but did not reach significance. Furthermore, we found in the comparison of receivers versus non‐completers group that the PARE rate significantly decreased from 11.1% to 8.7% in the group who received the transition plan.
As seen in other studies, reaching significance on readmissions rate in transition care studies is challenging.33 We identified other studies evaluating similar interventions, although transition care highly depends on local healthcare systems. A randomized controlled trial of an outpatient inter‐professional management programme for HF patients in Switzerland had a non‐significant increased rate of readmission in the intervention group.34 In the BEAT‐HF study, a large randomized controlled trial, the combination of telemonitoring and transition care also failed to reduce 30 day readmission.35 The systematic review of Feltner et al. found little evidence in favour of interventions reducing early readmission but confirms the benefit of multidisciplinary HF clinics on 6 month readmission.13
The outcome measure for transition care is usually the readmission rate, because it is an indicator of the disease and symptoms control, and the efficiency of care. However, not all readmissions are preventable: Unavoidable chronic conditions, such as HF, socio‐economic status, elderly, or external factors, lead to repeated admissions.36, 37 For example, an overcrowded medical unit (and overloaded staff) could press for anticipation of discharge before complete clinical recovery and favour readmissions. For this reason, it is important to evaluate the impact of transition care on PARE. Given the increasing pressure to improve quality and the financial incentives associated with quality measures in general, a beneficial effect on PARE is particularly welcome. Patients at risk for PARE may be identified before discharges using the HOSPITAL score.38, 39 However, to date, no intervention studies have been able to demonstrate a 30 day decrease in PARE. Our transition plan was able to demonstrate a decrease of PARE that reached the adjusted expected range given by SQLape®. Furthermore, the time to readmission for PARE was delayed. This information is important only if the quality systems focus on readmissions at 15 or 20 days and not at 30 days. Recently, an intervention (during and/or following hospital discharge) targeting patients at high risk of readmission has demonstrated a decrease risk of 15 day readmission.40 However, to obtain such a benefit, money and time must be invested. We describe later the difficulties we have encountered.
The selection of patients is very demanding. The transition team identified each new HF patient at admission and also screened every single medical record every day to identify new diagnosis during hospitalization, based on European Society of Cardiology criteria for HF.17 We included patients hospitalized with HF and going home. Unfortunately, orientation at discharge was subject to sudden changes: Even if a stay in a rehabilitation centre was planned during the entire stay, the physician in charge might decide on the last day—with the patient and the family—to let the patient go home; we had no chance to provide a discharge plan in a short time. Conversely, if a patient is suddenly not going home because he or she was transferred to surgery, or even died, we lost the efforts put in the discharge plan. We spent many resources by providing a complete or partial transition plan for a surplus of 53 enrolled patients who could not be included afterwards because of late decision to not discharge to home,28 death before discharge,7 ineligibility for PARE assessment,5 and initiation of haemodialysis.2
The second lesson learned is that the transition plan, tailored to fit patients' needs, presented a high rate of completion and therefore confirmed its feasibility, although this complexity was inevitable. Time is the critical resource for our plan. For example, we performed complete medication reconciliation, at admission and discharge, but it required ~1.5 h per patients. Overall, patients gave a positive feedback about the plan, particularly the follow‐up phone calls.
Should we drop transition care because of the difficulty to show the effectiveness of a transition plan? We believe that the benefit cannot just be a marginal reduction of readmission rate. Decrease of stress related to hospitalization, better adherence to medication, and reduction of post‐discharge adverse drug event could also be indicators of success.41
Strengths and limitations
Our study resents a major effort to improve transition care at discharge of HF patients. Because of consecutive inclusion with few exclusion criteria, the transition team worked in real conditions. Investigators were blinded until the end of the intervention period, because outcome measures (readmission) were extracted from medico‐administrative database afterwards. Furthermore, physicians in charge of the patient were independent of the transition team.
Our study has limitations. Despite daily review of all medical record, one‐third (138) only of the targeted population received the intervention, potentially explaining the non‐significant outcome. As discussed earlier, screening and identifying patients for the transition plan were challenging, like in other studies.42 Less autonomous patients, confused patients, and foreign language‐speaking patients were more likely to refuse to give consent, inducing a selection bias. It would likely have been the same issue while implementing a permanent transition team. Secondly, the before‐and‐after study design is subject to bias: The population characteristic and the overall readmission rate varied across time. However, although it increased significantly during the study, age was not identified as a significant variable in a multivariate analysis. It might be that older people receive more support and therefore compensate the risk for 30 day readmission. Moreover, age is not part of the HOSPITAL score predicting readmission.39 Still, we cannot exclude coding and calculation variation.
Thirdly, readmissions in another hospital, death outside hospital, and patient moving abroad could not be identified in the retrospective pre‐intervention cohort, because our outcome measurements were based on the available medico‐administrative database.
Finally, we performed our study in a single hospital, so there is a limit to external validity. Some systematic reviews already recognized this limitation.10 However, an effective transition plan would have to be adapted to local healthcare system, while being based on clinical guidelines related to HF.
Conclusions
A transition plan is feasible and is likely to improve transition to home for HF patients. It requires, however, many resources, and the benefit represented by the reduction of readmissions is not clear. Future research should focus more on PARE than on readmission rate, and on other indicators like stress related to hospitalizations, patient satisfaction, adverse drug events, or adherence.
Conflict of interest
None declared.
Author contributions
Conception or design of the work: A.G., O.L., N.R., C.N., P.V., A‐C.G., M.U.
Obtainment of funding: A.G.
Data collection: A.G., C.N., N.R., D.G.
Data analysis and interpretation: A.G., O.L.
Drafting of article: A.G.
Critical revision of the article: A.G., N.R., D.G., C.N., P.V., A‐C.G., M.U., G.W., O.L.
Final approval of the version to be published: A.G., N.R., D.G., C.N., P.V., A‐C.G., M.U., G.W., O.L.
Funding
This work was supported by the Swiss General Internal Medicine‐Foundation through a competitive fund. It had no influence on the protocol, on the realization or the analysis of the results. The University Hospital of Lausanne substantially contributed to financing the project.
Data sharing statement
Requests should be sent to A. Garnier (antoine.garnier@chuv.ch) and are conditional on a signed data transfer agreement.
Acknowledgements
The investigators thank the following people for their contribution to the study: Aude Giger, Sylvie Furrer, Nicole Bonvin, Véronique Prudent, Jacqueline Ghosn, and Lilli Herzig.
Figure A1.
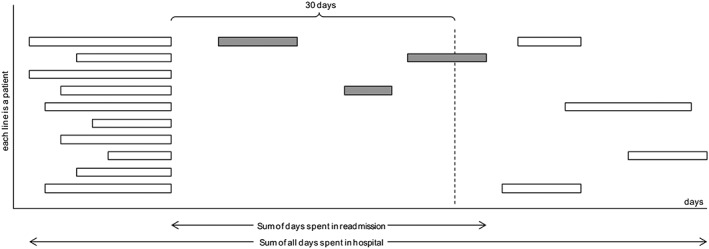
Burden of readmission: more than just a readmission rate, primary outcome takes lengths of stay into account. We considered the ratio of days spent in readmission over all days spent in hospital during the study periods. Each line corresponds to a patient, and each box is a hospitalization with various lengths of stay. Grey boxes are hospitalizations considered as 30 day readmission.
Figure A2.
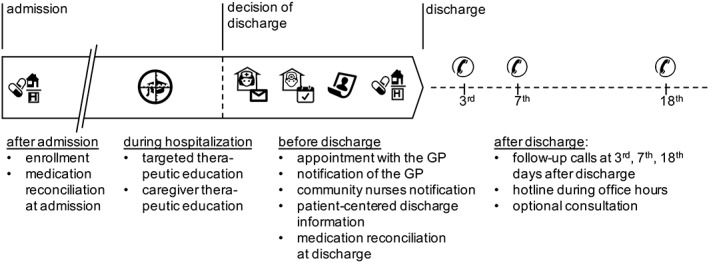
Timeline of the transition plan. Each intervention is described in the Table 1. Many are taking place after decision of discharge and are therefore challenging to provide in time. GP, general practitioner.
Table A1.
Rate of completion of the transition plan
| Interventions | Performed | % |
|---|---|---|
| Number of transition plan performed (n) | 138 | |
| Early during hospitalization | ||
| Targeted therapeutic education | 137 | 99 |
| Caregiver therapeutic education (if present) | 25 | 18 |
| Medication reconciliation at admission | 111 | 80 |
| Before discharge | ||
| Medication reconciliation at discharge | 101 | 73 |
| Set‐up of an appointment with the primary care physician | 123 | 89 |
| Notification of the primary care physician | 134 | 97 |
| Community nurse notification (if present) | 80 | 58 |
| Patient‐centred discharge instructions | 125 | 91 |
| After discharge | ||
| Follow‐up call at Day 3 | 133 | 96 |
| Follow‐up call at Day 7 | 135 | 98 |
| Follow‐up call at Day 18 | 133 | 96 |
| Optional follow‐up consultation | 1 | 1 |
Breakdown of the components of the transition plan. Hotline utilization is not reported. Caregiver therapeutic education and community nurse notification were only performed when a partner was present.
Garnier, A. , Rouiller, N. , Gachoud, D. , Nachar, C. , Voirol, P. , Griesser, A.‐C. , Uhlmann, M. , Waeber, G. , and Lamy, O. (2018) Effectiveness of a transition plan at discharge of patients hospitalized with heart failure: a before‐and‐after study. ESC Heart Failure, 5: 657–667. 10.1002/ehf2.12295.
Trial registration: ISRCTN53184579.Study performed in University Hospital of Lausanne, Switzerland.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cowie MR, Anker SD, Cleland JGF, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López‐Sendón J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Failure 2014; 1: 110–145. [DOI] [PubMed] [Google Scholar]
- 3. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hines AL, Barrett ML, Jiang HJ, Steiner CA. Conditions With the Largest Number of Adult Hospital Readmissions by Payer, 2011: Statistical Brief #172. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med 2009; 360: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 6. Kristensen SR, Bech M, Quentin W. A roadmap for comparing readmission policies with application to Denmark, England, Germany and the United States. Health Policy 2015; 119: 264–273. [DOI] [PubMed] [Google Scholar]
- 7. Hesselink G, Schoonhoven L, Barach P, Spijker A, Gademan P, Kalkman C, Liefers J, Vernooij‐Dassen M, Wollersheim H. Improving patient handovers from hospital to primary care a systematic review. Ann Intern Med 2012; 157: 417–481. [DOI] [PubMed] [Google Scholar]
- 8. Garnier A, Uhlmann M, Griesser AC, Lamy O. Discharge from hospital: how to improve continuity of medical care? Rev Med Suisse 2015; 11: 2064, 2066‐2069. [PubMed] [Google Scholar]
- 9. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc 2003; 51: 549–555. [DOI] [PubMed] [Google Scholar]
- 10. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med 2011; 155: 520–528. [DOI] [PubMed] [Google Scholar]
- 11. Kansagara D, Chiovaro JC, Kagen D, Jencks S, Rhyne K, O'Neil M, Kondo K, Relevo R, Motu'apuaka M, Freeman M, Englander H. So many options, where do we start? An overview of the care transitions literature. J Hosp Med 2016; 11: 221–230. [DOI] [PubMed] [Google Scholar]
- 12. Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, Wang Z, Erwin PJ, Sylvester T, Boehmer K, Ting HH, Murad MH, Shippee ND, Montori VM. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med 2014; 174: 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feltner C, Jones CD, Cene CW, Zheng ZJ, Sueta CA, Coker‐Schwimmer EJL, Arvanitis M, Lohr KN, Middleton JC, Jonas DE. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med 2014; 160: 774–784. [DOI] [PubMed] [Google Scholar]
- 14. Halfon P, Eggli Y, van Melle G, Chevalier J, Wasserfallen JB, Burnand B. Measuring potentially avoidable hospital readmissions. J Clin Epidemiol 2002; 55: 573–587. [DOI] [PubMed] [Google Scholar]
- 15. Donze J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ 2013; 347: f7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Philbin EF, Roerden JB. Longer hospital length of stay is not related to better clinical outcomes in congestive heart failure. Am J Manag Care 1997; 3: 1285–1291. [PubMed] [Google Scholar]
- 17. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA,, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Tridade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 18. Swiss Heart Foundation . Training Kit for Heart Failure Patients Bern: Swiss Heart Foundation,; 2012. https://www.swissheart.ch/fr/shop/produit/produktdetail/detail/23/kit-de-formation-pour-les-insuffisants-cardiaques.html (27 November 2015). [Google Scholar]
- 19. Jones CD, Holmes GM, Dewalt DA, Erman B, Broucksou K, Hawk V, Cene CW, Wu JR, Pignone M. Is adherence to weight monitoring or weight‐based diuretic self‐adjustment associated with fewer heart failure‐related emergency department visits or hospitalizations? J Card Fail 2012; 18: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dickson VV, Riegel B. Are we teaching what patients need to know? Building skills in heart failure self‐care. Heart Lung 2009; 38: 253–261. [DOI] [PubMed] [Google Scholar]
- 21. Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007; 116: 1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molloy GJ, Johnston DW, Witham MD. Family caregiving and congestive heart failure. Review and analysis. Eur J Heart Fail 2005; 7: 592–603. [DOI] [PubMed] [Google Scholar]
- 23. Ponniah A, Anderson B, Shakib S, Doecke CJ, Angley M. Pharmacists' role in the post‐discharge management of patients with heart failure: a literature review. J Clin Pharm Ther 2007; 32: 343–352. [DOI] [PubMed] [Google Scholar]
- 24. Baky V, Moran D, Warwick T, George A, Williams T, McWilliams E, Marine JE. Obtaining a follow‐up appointment before discharge protects against readmission for patients with acute coronary syndrome and heart failure: a quality improvement project. Int J Cardiol 2018; 257: 12–15. [DOI] [PubMed] [Google Scholar]
- 25. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med 2002; 17: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pesko MF, Gerber LM, Peng TR, Press MJ. Home health care: nurse–physician communication, patient severity, and hospital readmission. Health Serv Res 2018; 53: 1008–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong‐Grotz K, Castro C, Bindman AB. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163: 83–90. [DOI] [PubMed] [Google Scholar]
- 28. Louis‐Simonet M, Kossovsky MP, Sarasin FP, Chopard P, Gabriel V, Perneger TV, Gaspoz JM. Effects of a structured patient‐centered discharge interview on patients' knowledge about their medications. Am J Med 2004; 117: 563–568. [DOI] [PubMed] [Google Scholar]
- 29. Investigators G. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005; 331: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Health Quality O. Effect of early follow‐up after hospital discharge on outcomes in patients with heart failure or chronic obstructive pulmonary disease: a systematic review. Ont Health Technol Assess Ser 2017; 17: 1–37. [PMC free article] [PubMed] [Google Scholar]
- 31. Rennke S, Kesh S, Neeman N, Sehgal NL. Complementary telephone strategies to improve postdischarge communication. Am J Med 2012; 125: 28–30. [DOI] [PubMed] [Google Scholar]
- 32. Halfon P, Eggli Y, Pretre‐Rohrbach I, Meylan D, Marazzi A, Burnand B. Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care 2006; 44: 972–981. [DOI] [PubMed] [Google Scholar]
- 33. Jayakody A, Bryant J, Carey M, Hobden B, Dodd N, Sanson‐Fisher R. Effectiveness of interventions utilising telephone follow up in reducing hospital readmission within 30 days for individuals with chronic disease: a systematic review. BMC Health Serv Res 2016; 16: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leventhal ME, Denhaerynck K, Brunner‐La Rocca HP, Burnand B, Conca‐Zeller A, Bernasconi AT, Mahrer‐Imhof R, Froelicher ES, De Geest S. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM‐HF): a randomised controlled trial study of an outpatient inter‐professional management programme for heart failure patients in Switzerland. Swiss Med Wkly 2011; 141: w13171. [DOI] [PubMed] [Google Scholar]
- 35. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, de Marco T, Escarce JJ, Evangelista LS, Hanna B, Ganiats TG, Greenberg BH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M, Tong K, Fonarow GC, for the Better Effectiveness After Transition–Heart Failure (BEAT‐HF) Research Group . Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: The Better Effectiveness After Transition‐Heart Failure (BEAT‐HF) randomized clinical trial. JAMA Intern Med 2016; 176: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fischer C, Lingsma HF, Marang‐van de Mheen PJ, Kringos DS, Klazinga NS, Steyerberg EWI. the readmission rate a valid quality indicator? A review of the evidence. PLoS One 2014; 9: e112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fischer C, Steyerberg EW, Fonarow GC, Ganiats TG, Lingsma HF. A systematic review and meta‐analysis on the association between quality of hospital care and readmission rates in patients with heart failure. Am Heart J 2015; 170: 1005–1017, e2. [DOI] [PubMed] [Google Scholar]
- 38. Donze J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30‐day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med 2013; 173: 632–638. [DOI] [PubMed] [Google Scholar]
- 39. Donze JD, Williams MV, Robinson EJ, Zimlichman E, Aujesky D, Vasilevskis EE, Kripalani S, Metlay JP, Wallington T, Fletcher GS, Auerbach AD, Schnipper JL International validity of the HOSPITAL score to predict 30‐day potentially avoidable hospital readmissions. JAMA Intern Med 2016; 176: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorajoo SR, See V, Chan CT, Tan JZ, Tan DS, Abdul Razak SM, Ong TT, Koomanan N, Yap CW, Chan A. Identifying potentially avoidable readmissions: a medication‐based 15‐day readmission risk stratification algorithm. Pharmacotherapy 2017; 37: 268–277. [DOI] [PubMed] [Google Scholar]
- 41. Dharmarajan K, Chaudhry SI. New approaches to reduce readmissions in patients with heart failure. JAMA Intern Med 2016; 176: 318–320. [DOI] [PubMed] [Google Scholar]
- 42. Dedhia P, Kravet S, Bulger J, Hinson T, Sridharan A, Kolodner K, Wright S, Howell E. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc 2009; 57: 1540–1546. [DOI] [PubMed] [Google Scholar]


