Abstract
Aims
Patients with fulminant myocarditis (FM) often present with cardiogenic shock and require mechanical circulatory support, including extracorporeal membrane oxygenation (ECMO) and ventricular assist device (VAD) implantation. This study sought to clarify the determinants of successful weaning from ECMO in FM patients.
Methods and results
We studied 37 consecutive FM patients supported by ECMO as the initial form of mechanical circulatory support between January 1995 and December 2014 in our hospital. Twenty‐two (59%) patients were successfully weaned from ECMO, while 15 (41%) were not. There were significant differences in levels of peak creatine kinase and those of its MB isoform (CK‐MB), left ventricular posterior wall thickness (LVPWT), and prevalence of cardiac rhythm disturbances. Receiver operating characteristic curve analysis revealed that a peak CK‐MB level of 185 IU/L and LVPWT of 11 mm were the optimal cut‐off values for predicting successful weaning from ECMO (areas under the curve, 0.89 and 0.85, respectively). During the follow‐up [median 48 (interquartile range 8–147) months], 83% of FM patients who were weaned from ECMO survived, with preserved fractional shortening based on echocardiography. Of the 15 FM patients who were not weaned from ECMO, nine bridged to VAD, and only two were successfully weaned from VAD and survived.
Conclusions
These results indicate that myocardial injury, as evidenced by CK‐MB and LVPWT, and prolonged presence of cardiac rhythm disturbances are important clinical determinants of successful weaning from ECMO.
Keywords: Fulminant myocarditis, Extracorporeal membrane oxygenation, Ventricular assist device
Introduction
Fulminant myocarditis (FM) is an inflammatory disease of the myocardium that causes acute onset of heart failure. FM has a rapidly progressive course, resulting in cardiogenic shock.1, 2 Treatment involves full supportive care, including aggressive pharmacological therapy and mechanical circulatory support (MCS).1 One type of MCS, percutaneous extracorporeal membrane oxygenation (ECMO), has often been used as an initial therapy because it is less invasive than ventricular assist device (VAD) implantation.3, 4, 5, 6
Increased clinical experience and recent advances in the treatment of FM have resulted in heart transplantation being considered the therapy of choice for severe heart failure in patients under age 65.7 However, the number of heart donors has reached a plateau despite an increasing number of potential recipients.8 The situation is more serious in Japan, where the wait time for heart transplantation is the longest (mean 2.4 years in 2013) among countries with available data, and approximately 90% of candidates who are registered for heart transplantation require VAD while waiting.8
Previous studies have advocated midterm circulatory support to stabilize patients with ECMO as a bridge to decision about transplantation in order to improve outcomes in this high‐risk population of FM.4, 9 However, it is unknown what factors predict successful weaning and survival after ECMO support in terms of duration of support and cardiac function improvement.4, 9, 10, 11 Therefore, the present study sought to investigate prompt and clear strategies of ECMO support to optimize outcomes with transitions to more durable devices in FM patients.
Methods
Clinical classification of myocarditis and fulminant myocarditis
The diagnosis of myocarditis was based on the following findings: (i) a recent medical history consistent with a viral infection within 4 weeks; (ii) signs of inflammation based on high fever (>38°C) with increased white blood cell count and C‐reactive protein levels; (iii) evidence of myocardial damage defined as significant changes in electrocardiographic and echocardiographic parameters and elevations in serum creatine kinase and its MB isoform (CK‐MB); and (iv) signs of cardiac dysfunction and haemodynamic instability of recent onset not due to myocardial ischaemia, as determined by coronary angiography.4, 9 Patients with FM were defined as those who required percutaneous ECMO or a VAD for cardiogenic shock and who were not responsive to intensive medical treatments, such as high doses of intravenous catecholamines, or who had refractory ventricular tachyarrhythmia.1, 5, 6 Patients supported by intra‐aortic balloon pumping, inotropic agents, or vasopressor agents, or those with stable chronic post‐myocarditis heart failure, were excluded. We also studied patients with acute myocardial infarction who required ECMO support due to cardiogenic shock.12 The investigations conforms to the principles outlined in the Declaration of Helsinki. This study was approved by the National Cerebral and Cardiovascular Center Institutional Review Board for Clinical Research (M25‐046‐2).
Mechanical circulatory support
Percutaneous extracorporeal membrane oxygenation system
A centrifugal ECMO pump was used to drain venous blood. After membrane oxygenation, the blood was returned to the systemic arterial circulation. Intravenous unfractionated heparin was used for anticoagulation with adjusted activated clotting time controlled to a range of 180–200 s. During the ECMO support, we measured left ventricular (LV) ejection time using echocardiography, which was corrected for the RR interval. When corrected LV ejection time improved to more than 200 ms, the ECMO flow rate was gradually decreased to 1.5 L/min, and ECMO was subsequently discontinued if haemodynamic status did not deteriorate.4, 12, 13 In the present study, the survival after veno‐arterial ECMO score was calculated, as previously reported.11
Ventricular assist device system
Three types of left ventricular assist devices (LVADs) were used for patients with FM: Nipro‐Toyobo LVAD (Extracorporeal, Nipro, Osaka, Japan), DuraHeart (Implantable, Terumo Heart, Ann Arbor, MI, USA), and HeartMate II (Implantable, Thoratec Corporation, Pleasanton, CA, USA).6, 8, 14, 15 The Nipro‐Toyobo LVAD is an extracorporeal, pneumatic, diaphragm‐type device that is used in all patients during the acute phase of FM and is subsequently switched to an implantable VAD (DuraHeart or HeartMate II).14, 15
Echocardiographic and electrocardiogram measures
Standard two‐dimensional echocardiography was performed by a cardiologist to measure the LV diastolic dimension, LV systolic dimension, LV interventricular septum thickness, and LV posterior wall thickness (LVPWT). Electrocardiogram was recorded to identify rhythm disturbances such as advanced atrioventricular block, high‐grade ventricular ectopy (frequent isolated monomorphic or polymorphic ventricular beats or couplets deemed to be of high grade by the cardiologist), and ventricular tachycardia or ventricular fibrillation.16
Endomyocardial biopsy
Endomyocardial biopsy (EMB) was performed from the right ventricular wall or the apical portion of the LV in patients who required VAD implantation. These samples were examined by pathologists after being fixed in formalin and embedded in paraffin. Following haematoxylin–eosin and Masson trichrome staining, the presence of myocarditis or borderline myocarditis was determined according to the Dallas Criteria.17 In microscopic examination, myocarditis was diagnosed by the presence of an inflammatory infiltrate and associated myocyte necrosis or damage not characteristic of an ischaemic event. Borderline myocarditis was diagnosed by the findings of a less intense inflammatory infiltrate and no light microscopic evidence of myocyte destruction.17
Follow‐up and statistical analysis
During the chronic phase after FM, echocardiography was performed to evaluate LV function. Standard two‐dimensional echocardiography was performed to evaluate fractional shortening (FS). Follow‐up data were obtained from medical records and by telephone, letter, or interview. Continuous values for normally distributed variables were compared using the t‐test and are displayed as the mean ± standard deviation. Non‐normally distributed variables were compared using the Mann–Whitney U‐test, and medians [interquartile range (IQR)] are presented. Categorical baseline variables were compared using the χ2 test as appropriate. Comparison of clinical outcomes was performed with the log‐rank test for trend, and repeated measure analysis was used for estimating values. All analyses were performed using the statistical software STATA, version 13 (College Station, TX, USA). A two‐sided P < 0.05 was considered to be statistically significant.
Results
Clinical outcome of extracorporeal membrane oxygenation support in patients with fulminant myocarditis
From 1995 to 2014, there were 37 consecutive patients diagnosed with FM who received peripheral veno‐arterial ECMO as first‐line haemodynamic support. Eleven patients (30%) suffered from cardiac arrest before ECMO implantation. As shown in Figure 1, 22 of the 37 patients (59%) were successfully weaned from ECMO (weaned group) after a median of 6.5 (IQR, 5–10) days of support. Among the 22 patients, one died of sepsis 35 days after being weaned from ECMO, and one died of pulmonary embolism 13 days after being weaned. The remaining 15 patients were not weaned from ECMO (unweaned group). Supporting Information, Table S1 shows the comparative data between the first and second halves of the study (from 1995 to 2004 and 2005 to 2014, respectively), and Supporting Information, Table S2 summarizes the data of acute myocardial infarction patients (n = 68) who were hospitalized between 1995 and 2014 and required ECMO due to cardiogenic shock.
Figure 1.
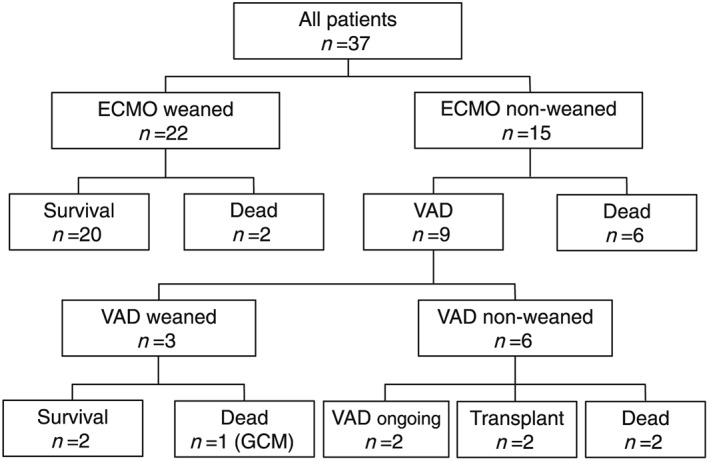
Flow chart of the study patients. ECMO, extracorporeal membrane oxygenation; GCM, giant cell myocarditis; VAD, ventricular assist device.
Comparison of clinical characteristics between patients who were and were not weaned from extracorporeal membrane oxygenation
The patient characteristics of the weaned and unweaned groups are summarized in Tables 1, 2, 3. There were no significant differences in age, gender, symptoms, or clinical background. The median time from onset of FM to the initiation of ECMO, ECMO driving periods, and median doses of dopamine, dobutamine, and norepinephrine was also comparable. Intra‐aortic balloon pumping was used in 15 (68%) patients in the weaned group, compared with 13 (87%) patients in the unweaned group. Renal replacement therapy was performed in 9 patients in the weaned group and in 10 patients in the unweaned group. Glucocorticoid treatment was performed in 12 patients (four in the weaned group and eight in the unweaned group), and intravenous immunoglobulin was administered to 15 patients (six in the weaned group and nine in the unweaned group).
Table 1.
Baseline characteristics of patients who were weaned and not weaned from ECMO
| Weaned from ECMO (n = 22) | Not weaned from ECMO (n = 15) | P value | |
|---|---|---|---|
| Age (years) | 44 [24–64] | 40 [25–57] | 0.50 |
| Male, n (%) | 13 (59) | 8 (53) | 0.73 |
| BMI (kg/m2) | 21 [19–22] | 22 [20–24] | 0.27 |
| Hypertension, n (%) | 4 (18) | 1 (7) | 0.31 |
| Diabetes mellitus, n (%) | 2 (9) | 0 (0) | 0.23 |
| Fever, n (%) | 20 (91) | 13 (87) | 0.68 |
| Dyspnoea, n (%) | 11 (50) | 10 (67) | 0.32 |
| Syncope, n (%) | 4 (18) | 0 (0) | 0.08 |
| Symptom onset to hospitalization (days) | 4 [3–5] | 3 [2–5] | 0.61 |
| Symptom onset to ECMO (days) | 5 [4–8] | 4 [3–7] | 0.41 |
| PR on admission (beats/min) | 106 [81–130] | 98 [70–120] | 0.39 |
| SBP on admission (mmHg) | 84 [74–90] | 86 [70–94] | 0.82 |
| CAVB, n (%) | 6 (27) | 5 (33) | 0.69 |
| CPA before ECMO, n (%) | 4 (18) | 7 (47) | 0.06 |
| Duration of hospital stay (days) | 75 [42–121] | 24 [9–243] | 0.47 |
BMI, body mass index; CAVB, complete atrioventricular block; CPA, cardiopulmonary arrest; ECMO, extracorporeal membrane oxygenation; PR, pulse rate; SBP, systolic blood pressure.
Continuous variables are presented as medians and interquartile ranges and categorical variables as percentages.
Table 2.
Laboratory data and medical treatment of patients who weaned and not weaned from ECMO
| Weaned from ECMO (n = 22) | Not weaned from ECMO (n = 15) | P value | |
|---|---|---|---|
| LVDd on admission (mm) | 49.5 [45–53] | 43 [40–53] | 0.11 |
| %FS on admission (%) | 14 [7–18] | 15.5 [5–20] | 0.99 |
| PWT on admission (mm) | 10 [8–11] | 13 [12–14] | 0.005 |
| T‐Bil on admission (mg/dL) | 0.95 [0.6–1.5] | 1.2 [0.6–2.4] | 0.44 |
| BUN on admission (mg/dL) | 32.5 [22–46] | 34 [20–39] | 0.75 |
| Cr on admission (mg/dL) | 1.0 [0.9–1.6] | 1.1 [0.7–1.9] | 0.88 |
| CK on admission (IU/L) | 1071.5 [588–1732] | 2026 [749–3063] | 0.24 |
| CK‐MB on admission (IU/L) | 70 [38–106] | 101 [67–241] | 0.10 |
| Peak BUN (mg/dL) | 45 [32–58] | 62 [35–101] | 0.11 |
| Peak Cr (mg/dL) | 1.25 [1.1–2.4] | 2.3 [1.2–4.1] | 0.09 |
| Peak CK (IU/L) | 1803 [967–3691] | 5717 [2784–14 341] | 0.01 |
| Peak CK‐MB (IU/L) | 77 [53–131] | 491.5 [195–641] | 0.001 |
| Dopamine at ECMO (μg/kg/min) (n = 25) | 5 [4.5–10] | 6 [5–11.3] | 0.55 |
| Dobutamine at ECMO (μg/kg/min) (n = 32) | 5 [5–9.9] | 5 [4–8.2] | 0.39 |
| Noradrenaline at ECMO (μg/kg/min) (n = 16) | 0.17 [0.08–0.40] | 0.2 [0.1–0.4] | 0.86 |
| Renal replacement therapy, n (%) | 9 (41) | 10 (67) | 0.12 |
| Glucocorticoid, n (%) | 4 (18) | 8 (53) | 0.03 |
| IVIG, n (%) | 6 (27) | 9 (60) | 0.05 |
| EMB, n (%) | 17 (77) | 12 (80) | 0.84 |
| EMB during the acute phase (<7 days), n (%) | 8 (36) | 11 (73) | 0.03 |
%FS, % fractional shortening; BUN, serum blood urea nitrogen; Cr, serum creatinine; CK, creatine kinase; CK‐MB, creatine kinase‐MB isoform; ECMO, extracorporeal membrane oxygenation; EMB, endomyocardial biopsy; IVIG, intravenous immunoglobulin; LVDd, left ventricular diastolic dimension size; PWT, posterior wall thickness; T‐Bil, total bilirubin.
Continuous variables are presented as medians and interquartile ranges and categorical variables as percentages.
Table 3.
Mechanical support relating data of patients who weaned and not weaned from ECMO
| Weaned from ECMO (n = 22) | Not weaned from ECMO (n = 15) | P value | |
|---|---|---|---|
| ECMO duration (days) | 6.5 [5–10] | 7 [2–9] | 0.63 |
| IABP, n (%) | 15 (68) | 13 (87) | 0.20 |
| IABP duration (days) | 8 [4–18] | 8 [3–18] | 0.85 |
| Size of ECMO cannula, artery (Fr) (n = 12) | 21 [21–21] | 21 [21–21] | 0.67 |
| Size of ECMO cannula, vein (Fr) (n = 12) | 15 [15–16] | 16.5 [16.5–16.5] | 0.49 |
| Maximal ECMO flow (L/min) | 3.5 [2.6–3.9] | 3.3 [3–3.7] | 0.96 |
| Distal limb perfusion, n (%) | 1 (5) | 2 (13) | 0.34 |
| Retroperitoneal haemorrhage, n (%) | 4 (18) | 2 (13) | 0.69 |
| Intracranial haemorrhage, n (%) | 1 (5) | 1 (7) | 0.78 |
| Gastrointestinal bleeding, n (%) | 2 (9) | 4 (27) | 0.16 |
| Respiratory bleeding, n (%) | 2 (9) | 3 (20) | 0.35 |
| Arterial blood gas | |||
| Lactate at ECMO (mg/dL) (n = 25) | 28.2 [16.3–47.8] | 13.5 [6.8–60.2] | 0.59 |
| Lactate at 24 h (mg/dL) (n = 25) | 15 [10.5–22] | 19 [3.9–28] | 0.54 |
| Lactate at weaning (mg/dL) (n = 14) | 11.9 [8.9–13.9] | N/A | N/A |
| pH at ECMO, (n = 34) | 7.47 [7.39–7.51] | 7.46 [7.38–7.50] | 0.52 |
| pH at 24 h, (n = 30) | 7.45 [7.42–7.53] | 7.36 [7.25–7.43] | 0.01 |
| pH at weaning, (n = 19) | 7.47 [7.42–7.52] | N/A | N/A |
| SAVE score | 0 [−2.3 to 3] | 1 [−4 to 3] | 0.59 |
ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pumping; N/A, not applicable; SAVE, survival after veno‐arterial extracorporeal membrane oxygenation.
Continuous variables are presented as medians and interquartile ranges and categorical variables as percentages.
Compared with the weaned group, the unweaned group was characterized by higher LVPWT [10 (IQR 8–11) vs. 13 (IQR 12–14) mm, respectively, P = 0.005] as determined by echocardiography, higher peak CK levels [1803 (IQR 967–3691) vs. 5717 (IQR 2784–14 341) IU/L, respectively, P = 0.01), and higher peak CK‐MB levels [74 (IQR 53–131) vs. 492 (IQR 195–641) IU/L, respectively, P = 0.001], whereas the prevalences of complete atrioventricular block and %FS on admission were comparable between the two groups. The 17 patients (77%) in the weaned group underwent EMB at a median of 10 (IQR 0–37) days after admission, while the 12 patients (80%) in the unweaned group underwent EMB at a median of 0 (IQR 0–2) day after admission. One patient in the unweaned group underwent EMB at 15 days after the admission. In particular, EMB was performed during the acute phase (within 7 days) in 8 patients (36%) in the weaned group and 11 (73%) patients in the unweaned group. Among the patients with biopsy‐proven myocarditis, six of 14 (43%) patients with lymphocytic myocarditis and zero of four (0%) patients with giant cell myocarditis (all treated with glucocorticoid) were weaned from ECMO (P = 0.10).
Determinants of weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis
Comparisons of CK‐MB levels between the two groups on admission and at 1, 2, and 3 days after the start of ECMO support are shown in Figure 2 A. From admission to Day 3, there was a significant increase (β = 111.58, P = 0.001) in CK‐MB levels in the unweaned group, whereas there was a non‐significant decrease (β = −5.70, P = 0.059) in CK‐MB levels in the weaned group. CK‐MB levels were higher at all time points in the unweaned patients.
Figure 2.
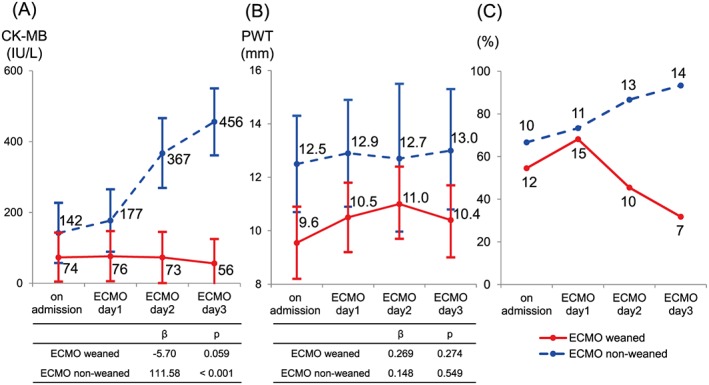
Clinical courses of patients weaned and unweaned from extracorporeal membrane oxygenation (ECMO). (A) Creatine kinase (CK)‐MB levels, (B) left ventricular posterior wall thickness (PWT), and (C) prevalence of cardiac rhythm disturbances on admission and on Days 1, 2, and 3 after ECMO implantation in patients with fulminant myocarditis. Blue lines show data from patients weaned from ECMO. Red lines show data from patients who were not weaned from ECMO. The central dots in (A) and (B) show the estimated values based on repeated measure analysis, while the lines show the 95% confidence intervals.
The LVPWT on admission and at 1, 2, and 3 days after ECMO are shown based on ECMO weaning status in Figure 2 B. No significant temporal changes in LVPWT were seen in either group (weaned, β = 0.269, P = 0.274; unweaned, β = 0.148, P = 0.549). However, the two groups showed significant differences in LVPWT on admission [LVPWT difference, −2.956 mm; 95% confidence interval (CI), −5.185 to −0.726 mm, P = 0.009], Day 1 (LVPWT difference, −2.439 mm; 95% CI, −4.823 to −0.055 mm, P = 0.045), and Day 3 (LVPWT difference, −2.679 mm; 95% CI, −5.268 to −0.091 mm, P = 0.042). Of note, none of the patients with LVPWT greater than 12.5 mm at any point were weaned from ECMO.
The prevalences of cardiac rhythm disturbances, including complete atrioventricular block, left bundle branch block, and ventricular tachycardia, are shown in Figure 2 C for the two groups on admission and at 1, 2, and 3 days after the initiation of ECMO support. There was a significantly lower prevalence of cardiac rhythm disturbances in both groups on Day 2 (odds ratio, 0.0288; 95% CI, 0.0014 to 0.5883) and Day 3 (odds ratio, 0.0046; 95% CI, 0.0001 to 0.1771). We performed receiver operating characteristic curve analysis to determine whether peak CK‐MB and LVPWT could predict weaning from ECMO in patients with FM. As shown in Figure 3 A, the optimal peak CK‐MB and LVPWT cut‐off values were 183 IU/L and 11 mm, respectively. The areas under the receiver operating characteristic curve for peak CK‐MB and LVPWT were 0.89 (sensitivity 86% and specificity 71%) and 0.85 (sensitivity 89% and specificity 80%), respectively.
Figure 3.
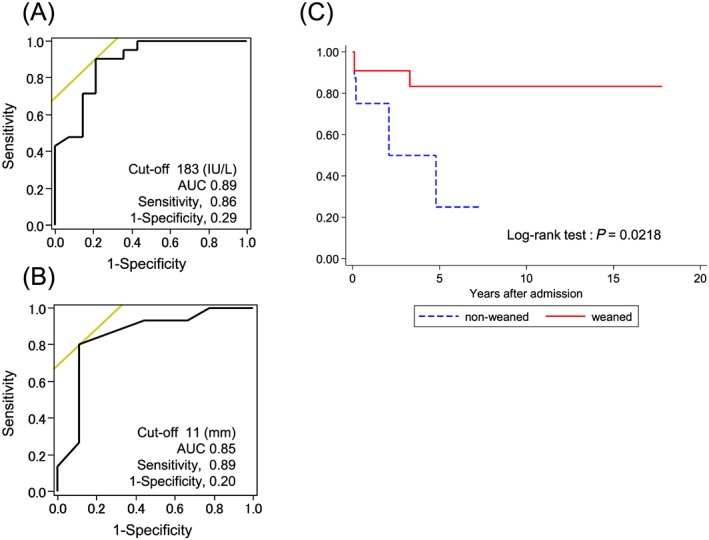
Receiver operating characteristic curve analyses and Kaplan–Meier curves for all causes of death in patients with fulminant myocarditis (FM). Receiver operating characteristic curve analyses predicting successful weaning from extracorporeal membrane oxygenation (ECMO) with (A) peak creatine kinase‐MB levels, (B) left ventricular posterior wall thickness, and (C) Kaplan–Meier curves for all causes of death in patients with FM. (A) A peak creatine kinase‐MB level of 183 U/L was identified as the best cut‐off level, with 86% sensitivity and 71% specificity; the calculated area under the curve (AUC) was 0.89 (95% confidence interval, 0.77–1.00). (B) left ventricular posterior wall thickness of 11 mm on admission was identified as the best cut‐off level, with 89% sensitivity and 80% specificity; the calculated AUC was 0.85 (95% confidence interval, 0.66–1.00). (C) The survival rate was 83% in FM patients who were successfully weaned from ECMO (n = 22) and 25% in those who were not weaned from ECMO (n = 15). The blue line shows data from patients weaned from ECMO. The red line shows data from patients who were not weaned from ECMO.
Comparison of clinical characteristics between patients who underwent ventricular assist device implantation and those who did not
Of the 15 patients who were not weaned from ECMO (Figure 1 and Table 3), six died of multi‐organ failure following cardiogenic shock before bridging to VAD, with a median of 6 (IQR 2–11) days of ECMO support. Of note, in the unweaned group, the incidence of cardiopulmonary arrest before ECMO in patients who did not undergo VAD implantation was 83%. Three of nine patients with LVADs were also supported by right VADs. Of the nine patients who switched from ECMO to LVAD, two were weaned from VAD at 61 and 137 days after VAD implantation, respectively, and survived for 1.3 years and 7.4 years after discharge, respectively. One patient with giant cell myocarditis had to discontinue VAD support because of intracranial haemorrhage and subsequently died of recurrent heart failure. Among the six patients who were not weaned from VAD, two died of biventricular failure and systemic bleeding, two underwent heart transplantation, and two remained on implantable VAD support (DuraHeart, n = 1; HeartMate II, n = 1). We also performed subgroup analysis to compare the clinical characteristics of patients who underwent VAD implantation and those who did not. The patients who did not undergo VAD implantation were characterized by older age and a higher prevalence of cardiopulmonary arrest on admission (Table 4). EMB was performed in all patients with VAD implantation. Lymphocytic myocarditis was noted in six patients (67%), giant cell myocarditis in two (22%), and borderline myocarditis in one (11%). Biopsy was performed in three (50%) patients who did not undergo VAD implantation and demonstrated lymphocytic myocarditis (n = 1) and giant cell myocarditis (n = 2).
Table 4.
Comparison of clinical variables between patients with and without VAD implantation
| VAD implantation (n = 9) | No VAD implantation (n = 6) | P value | |
|---|---|---|---|
| Age (years) | 26 [19–40] | 55 [51–67] | 0.0132 |
| Male, n (%) | 6 (67) | 2 (33) | 0.21 |
| BMI (kg/m2) | 21 [20–23] | 22.5 [21–24] | 0.59 |
| Symptom onset to hospitalization (days) | 4 [3–5] | 3 [0–4] | 0.31 |
| PR (beats/min) | 86 [72–111] | 112 [58–140] | 0.56 |
| SBP (mmHg) | 85 [65–106] | 88 [76–98] | 0.81 |
| CAVB, n (%) | 4 (44) | 1 (17) | 0.26 |
| CPA before ECMO, n (%) | 2 (22) | 5 (83) | 0.02 |
| Symptom onset to ECMO (days) | 5 [3–6] | 4 [4–7] | 1.00 |
| ECMO duration (days) | 7 [5–9] | 6 [2–9] | 0.72 |
| IABP, n (%) | 9 (100) | 4 (67) | 0.06 |
| IABP duration (days) | 8 [5–18] | 7 [3–14] | 0.64 |
| %FS on admission (%) | 7 [5–19] | 18 [14–26] | 0.12 |
| %FS at ECMO (%) | 5.5 [4–12.5] | 14 [5–16] | 0.56 |
| T‐Bil on admission (mg/dL) | 1.8 [0.6–5.0] | 0.75 [0.6–1.2] | 0.15 |
| BUN on admission (mg/dL) | 34 [20–37] | 31.5 [23–39] | 0.64 |
| Cr on admission (mg/dL) | 1.1 [0.9–1.2] | 1.3 [0.7–2.1] | 0.81 |
| CK on admission (IU/L) | 2240 [1828–3063] | 841.5 [508–2026] | 0.10 |
| CK‐MB on admission (IU/L) | 106 [67–241] | 78 [77–98] | 0.39 |
| Peak BUN (mg/dL) | 53 [35–87] | 95.5 [34–101] | 0.56 |
| Peak Cr (mg/dL) | 1.4 [1.2–2.3] | 3.6 [2.9–5.9] | 0.05 |
| Peak CK (IU/L) | 5166 [2784–7819] | 10 190 [3764–22 675] | 0.29 |
| Peak CK‐MB (IU/L) | 279 [195–500] | 607 [524–641] | 0.21 |
| Retroperitoneal haemorrhage, n (%) | 1 (11) | 1 (17) | 0.76 |
| Duration of hospital stay (days) | 177 [80–314] | 6 [2–11] | 0.003 |
| EMB during the acute phase (<7 days), n (%) | 8 (89) | 3 (50) | 0.09 |
| Lymphocytic myocarditis, n (%) | 5 (63) | 1 (33) | 0.38 |
| Giant cell myocarditis, n (%) | 2 (25) | 2 (67) | 0.20 |
| Borderline myocarditis, n (%) | 1 (13) | 0 (0) | 0.41 |
%FS, % fractional shortening; BMI, body mass index; BUN, serum blood urea nitrogen; CAVB, complete atrioventricular block; CPA, cardiopulmonary arrest; Cr, serum creatinine; CK, creatine kinase; CK‐MB, creatine kinase‐MB isoform; ECMO, extracorporeal membrane oxygenation; EMB, endomyocardial biopsy; IABP, intra‐aortic balloon pumping; PR, pulse rate; PWT, posterior wall thickness; SBP, systolic blood pressure; T‐Bil, total bilirubin; VAD, ventricular assist device.
Continuous variables are presented as medians and interquartile ranges and categorical variables as percentages.
Midterm survival and functional changes assessed by echocardiography in the chronic phase
The midterm survival of patients with FM are shown in Figure 3 C. In the weaned group, 1 patient died from a non‐cardiac cause 33 months after discharge, while the remaining 21 remained alive for a median of 5.7 (1.2–12.2) years after discharge. Among the patients who were not weaned from ECMO and underwent VAD implantation, two were weaned from VAD, two underwent cardiac transplantation, and two remained on VAD support. During a median of 48 (8–147) months of follow‐up, the patients with FM who were weaned from ECMO had favourable midterm survival compared with those who were not. In the chronic phase, echocardiographic data were obtained from 15 patients in the weaned group and 4 patients in the unweaned group (Figure 4 ). During a median of 80 days after admission, %FS recovered from 9% to 28%. After discharge, %FS improved further to 31% after a median of 27 months of follow‐up in the weaned group. Of the patients in the unweaned group, two who were successfully weaned from VAD showed improvement in %FS, from a median of 4% to 18% during a median of 243 admission days. Two other patients with implantable VADs still had low %FS, even after discharge.
Figure 4.
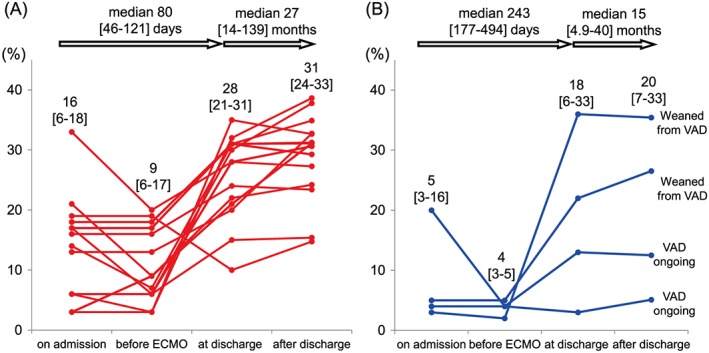
Serial changes in % fractional shortening in patients with fulminant myocarditis: (A) 15 patients who were weaned from extracorporeal membrane oxygenation (ECMO) and (B) 4 patients who were switched from ECMO to ventricular assist device (VAD) but did not undergo transplantation.
Discussion
The major findings of this study are as follows: (i) excessive myocardial injury and prolonged cardiac rhythm disturbance after MCS support are important clinical determinants of successful weaning from ECMO; (ii) during a median follow‐up period of 48 months, the survival rate of FM patients who were weaned from ECMO was 83%, with almost full recovery of LV function as assessed by echocardiography; and (iii) among 15 FM patients who were not weaned from ECMO, nine bridged to VAD, and only two were successfully weaned from VAD and survived.
Mechanical circulatory support for fulminant myocarditis
The two most common mechanical devices available for treatment of FM patients with cardiogenic shock are ECMO and VAD. FM patients are characterized by biventricular failure and impaired oxygenation. ECMO is a highly effective technology against haemodynamic instability and systemic hypoperfusion that can be used as first‐line mechanical support for FM patients and may be considered if patients are candidates for surgical VAD implantation as a bridge to transplantation.3, 4, 18 In this study, the median duration of ECMO support was 6.5 (IQR, 5–10) days, which is comparable with that in the Extracorporeal Life Support Organization registry (median 138 h; IQR, 86–189).10 A recent meta‐analysis showed that the lowest and highest rates of survival to hospital discharge were 60% and 88%, respectively.19 In this study, the survival rate was approximately 65%, which may be due to the relatively older age of patients supported by ECMO. Pages et al. reported that a biventricular assist device was as efficient as ECMO in FM patients with cardiogenic shock,20 whereas Acker demonstrated that survival rates were lower in patients supported by VAD than in those supported by ECMO.21 Although VAD can provide adequate blood flow support for a longer period and facilitate renal and hepatic recovery, its invasiveness is likely a trade‐off for these advantages in severely ill patients with FM. Taken together, surgical MCS may be considered when patients continue to deteriorate despite initial percutaneous intervention. According to the Registry Report of Heart Transplantation in Japan, the number of heart transplantations from brain dead donors remains limited. The average waiting period for candidates in 2013 was 855 days and that for candidates supported by extracorporeal or implantable VAD was 896 days.8
Clinical parameters for extracorporeal membrane oxygenation weaning or transition from extracorporeal membrane oxygenation to ventricular assist device
Extracorporeal membrane oxygenation can be introduced quickly and easily to prevent haemodynamic deterioration in FM patients with cardiogenic shock.1, 3, 4, 5 Indeed, the Extracorporeal Life Support Organization registry demonstrated that early ECMO deployment prior to cardiac arrest may be associated with better outcomes.10 Recently, research has been performed to identify clinical factors related to survival after ECMO support. The survival after veno‐arterial ECMO study of 3846 patients with cardiogenic shock treated with ECMO identified the following pre‐ECMO prognostic factors for in‐hospital survival: age, weight, chronic renal failure, time with mechanical ventilation before initiation of ECMO, extra‐cardiac organ failure, cardiac arrest, congenital heart disease, cause of cardiogenic shock, haemodynamic data, serum bicarbonate value, and peak inspiratory pressure.11 However, there is little information about good prognostic factors for weaning and survival after ECMO support in terms of duration of support and cardiac function improvement. In particular, biomarkers have not been used to evaluate the strategy of switching from ECMO to VAD. Hsu et al. reported that the timing of peak troponin levels was an important predictor of myocardial recovery.5 In this study, as shown in Table 2, elevated cardiac enzyme levels, increased LVPWT on admission, and the development of cardiac rhythm disturbances were more frequently noted in FM patients who were not weaned from ECMO compared with those who were. Furthermore, both Figures 2 and 3 indicate that patients with increased LVPWT (>11 mm) during the first 3 days of mechanical support or CK‐MB greater than 183 IU/L were at higher risk of failing to maintain haemodynamics with ECMO alone. Progressive (hours ~ days) and severe inflammatory‐induced myocardial damage and oedema may manifest as significant LV wall thickening by cardiac ultrasound. Of note, in the unweaned group, the incidence of cardiopulmonary arrest before ECMO in patients who could not undergo VAD implantation was high (83%) (Table 4). Once haemodynamic status has deteriorated to the point of cardiopulmonary arrest, there seems to be a low probability of subsequent switching from ECMO to VAD. Given our findings and those of previous reports, conversion from ECMO to VAD support should be considered if myocardial recovery has not occurred and if severe myocardial injury, more significant LV wall thickening, or continuous cardiac rhythm disturbance has been noted within 3–5 days after the initiation of ECMO.6, 22 Use of these numeric variables along with electrocardiographic evaluation could facilitate appropriate decision‐making regarding ECMO weaning vs. transition from ECMO to VAD at the early phase of FM.
Midterm prognosis of fulminant myocarditis and functional changes assessed by echocardiography
In the previous studies cited previously, echocardiographic findings were limited to those obtained 6–12 months after discharge.3, 4, 14 Importantly, this study used echocardiographic measurements to address the midterm survival of FM patients who were weaned from ECMO.4 Although %FS was as low as 9% in the acute phase, it recovered to 28% during a 27‐month median follow‐up (Figure 4 ). Freedom from all‐cause death was high, at 83% during a 48‐month median follow‐up (Figure 3 C), which was comparable with the 65% 5‐year survival demonstrated in a recent multi‐institutional study by Lorusso et al.9 These findings suggest that the midterm prognosis of FM patients is favourable when LV function is preserved after survival from haemodynamic deterioration in the acute phase of the disorder. Recent advances in MCS have led to a population of FM patients who are not weaned from ECMO but survive with additional LVAD support. Freedom from all‐cause death was low (25%) during a 48‐month median follow‐up (Figure 3 C). There were some patients supported by VAD who had low %FS without functional recovery (Figure 4 ). Release of cytokines in response to immune responses may play a significant role in the development of heart failure.23 Indeed, Nishii et al. reported that serum IL‐10 level on admission is a prognostic predictor in FM patients.24 Percutaneous ECMO and VAD favourably alter ventricular geometry, reduce wall stress, decrease cytokine activation, and improve myocyte contractile function.1 During a median follow‐up of 27 months, patients with low %FS in the weaned group may be in the chronic phase of myocarditis or have developed dilated cardiomyopathy (Figure 4 ). Follow‐up EMB in patients with low %FS may be useful in evaluating the presence of inflammatory cells, myocardial remodelling, and fibrosis.25
Study limitations
Several limitations of the present study should be mentioned. First, this was a single‐centre, retrospective cohort study with a small number of patients and events. Second, not all patients underwent EMB in the acute phase. Thus, we were unable to determine an accurate aetiology or make a pathological diagnosis in all patients. Also, there remains a possibility that patients with giant cell myocarditis, who are often asymptomatic and do not have a history of preceding viral illness, were not included on the basis of the present criteria. Third, although treatment of myocarditis should focus on the causal pathophysiology, aetiology‐based therapies for FM are still under development. Modulation of viral‐mediated and immune‐mediated myocardial injuries or both might be an essential target to improve the clinical outcome in FM patients requiring acute MCS treatments.26, 27 Fourth, although troponin is a sensitive biomarker to detect myocardial injury, it was not systematically measured in this study. Its serial measurement may provide information on disease severity and stage. Further studies are needed to evaluate the predictive potential of troponin for ECMO weaning.
Conclusions
Myocardial injury as evaluated by necrosis (CK‐MB) and swelling (LVPWT) is an important clinical determinant of successful weaning from ECMO.
Conflict of interest
None declared.
Funding
The present work was supported in part by a grant from the Ministry of Health, Labour and Welfare of Japan (H26‐Ippan‐001) (S.Y.).
Supporting information
Table S1. Comparative data between 1995–2004 and 2005–2014.
Table S2. Comparison of clinical characteristics between patients with FM and those with AMI who were hospitalized between 1995 and 2014 and required ECMO support.
Matsumoto, M. , Asaumi, Y. , Nakamura, Y. , Nakatani, T. , Nagai, T. , Kanaya, T. , Kawakami, S. , Honda, S. , Kataoka, Y. , Nakajima, S. , Seguchi, O. , Yanase, M. , Nishimura, K. , Miyamoto, Y. , Kusano, K. , Anzai, T. , Noguchi, T. , Fujita, T. , Kobayashi, J. , Ishibashi‐Ueda, H. , Shimokawa, H. , and Yasuda, S. (2018) Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Failure, 5: 675–684. 10.1002/ehf2.12291.
References
- 1. Gupta S, Markham DW, Drazner MH, Mammen PP. Fulminant myocarditis. Nat Clin Pract Cardiovasc Med 2008; 5: 693–706. [DOI] [PubMed] [Google Scholar]
- 2. McCarthy RE, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long‐term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. New Engl J Med 2000; 342: 690–695. [DOI] [PubMed] [Google Scholar]
- 3. Chen YS, Yu HY, Huang SC, Chiu KM, Lin TY, Lai LP, Lin FY, Wang SS, Chu SH. Myocarditis with shock: what mechanical support should be considered first? J Heart Lung Transplant 2005; 24: 81–87. [DOI] [PubMed] [Google Scholar]
- 4. Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, Sasako Y, Nakatani T, Nonogi H, Miyazaki S. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J 2005; 26: 2185–2192. [DOI] [PubMed] [Google Scholar]
- 5. Hsu KH, Chi NH, Yu HY, Wang CH, Huang SC, Wang SS, Ko WJ, Chen YS. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: analysis of a single center's experience. Eur J Cardiothoracic Surg 2011; 40: 682–688. [DOI] [PubMed] [Google Scholar]
- 6. Atluri P, Ullery BW, MacArthur JW, Goldstone AB, Fairman AS, Hiesinger W, Acker MA, Woo YJ. Rapid onset of fulminant myocarditis portends a favourable prognosis and the ability to bridge mechanical circulatory support to recovery. Eur J Cardiothoracic Surg 2013; 43: 379–382. [DOI] [PubMed] [Google Scholar]
- 7. Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation 2010; 122: 173–183. [DOI] [PubMed] [Google Scholar]
- 8. Nakatani T, Fukushima N, Ono M, Saiki Y, Matsuda H, Yozu R, Isobe M. The Registry Report of Heart Transplantation in Japan (1999–2013). Circ J 2014; 78: 2604–2609. [DOI] [PubMed] [Google Scholar]
- 9. Lorusso R, Centofanti P, Gelsomino S, Barili F, Di Mauro M, Orlando P, Botta L, Milazzo F, Actis Dato G, Casabona R, Casali G, Musumeci F, De Bonis M, Zangrillo A, Alfieri O, Pellegrini C, Mazzola S, Coletti G, Vizzardi E, Bianco R, Gerosa G, Massetti M, Caldaroni F, Pilato E, Pacini D, Di Bartolomeo R, Marinelli G, Sponga S, Livi U, Mauro R, Mariscalco G, Beghi C, Miceli A, Glauber M, Pappalardo F, Russo CF. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: a 5‐year multi‐institutional experience. Ann Thorac Surg 2016; 101: 919–926. [DOI] [PubMed] [Google Scholar]
- 10. Diddle JW, Almodovar MC, Rajagopal SK, Rycus PT, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med 2015; 43: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno‐arterial‐ECMO (SAVE)‐score. Eur Heart J 2015; 36: 2246–2256. [DOI] [PubMed] [Google Scholar]
- 12. Aiba T, Nonogi H, Itoh T, Morii I, Daikoku S, Goto Y, Miyazaki S, Sasako Y, Nakatani T. Appropriate indications for the use of a percutaneous cardiopulmonary support system in cases with cardiogenic shock complicating acute myocardial infarction. Circ J 2001; 65: 145–149. [DOI] [PubMed] [Google Scholar]
- 13. Nakatani T, Takano H, Beppu S, Noda H, Taenaka Y, Kumon K, Kito Y, Fujita T, Kawashima Y. Practical assessment of natural heart function using echocardiography in mechanically assisted patients. ASAIO Trans 1991; 37: M420–M421. [PubMed] [Google Scholar]
- 14. Saito S, Matsumiya G, Sakaguchi T, Fujita T, Kuratani T, Ichikawa H, Sawa Y. Fifteen‐year experience with Toyobo paracorporeal left ventricular assist system. J Artifi Organs 2009; 12: 27–34. [DOI] [PubMed] [Google Scholar]
- 15. Yoshioka D, Sakaguchi T, Saito S, Miyagawa S, Nishi H, Yoshikawa Y, Fukushima S, Ueno T, Kuratani T, Sawa Y. Initial experience of conversion of Toyobo paracorporeal left ventricular assist device to DuraHeart left ventricular assist device. Circ J 2012; 76: 372–376. [DOI] [PubMed] [Google Scholar]
- 16. Miyake CY, Teele SA, Chen L, Motonaga KS, Dubin AM, Balasubramanian S, Balise RR, Rosenthal DN, Alexander ME, Walsh EP, Mah DY. In‐hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J of Cardiol 2014; 113: 535–540. [DOI] [PubMed] [Google Scholar]
- 17. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987; 18: 619–624. [DOI] [PubMed] [Google Scholar]
- 18. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T, Society for Cardiovascular A , Interventions HFSoASoTSAHA , American College of C . 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; Affirmation of value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol 2015; 65: 2140–2141. [DOI] [PubMed] [Google Scholar]
- 19. Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B. Clinical outcomes in fulminant myocarditis requiring extracorporeal membrane oxygenation: a weighted meta‐analysis of 170 patients. J Card Fail 2014; 20: 400–406. [DOI] [PubMed] [Google Scholar]
- 20. Pages ON, Aubert S, Combes A, Luyt CE, Pavie A, Leger P, Gandjbakhch I, Leprince P. Paracorporeal pulsatile biventricular assist device versus extracorporal membrane oxygenation‐extracorporal life support in adult fulminant myocarditis. J Thorac Cardiovasc Surg 2009; 137: 194–197. [DOI] [PubMed] [Google Scholar]
- 21. Acker MA. Mechanical circulatory support for patients with acute‐fulminant myocarditis. Ann Thorac Surg 2001; 71: S73–S76, discussion S82–S85. [DOI] [PubMed] [Google Scholar]
- 22. Sezai A, Hata M, Niino T, Yoda M, Takayama T, Saito S, Ayusawa M, Minami K. Mechanical circulatory support for fulminant myocarditis. Surg Today 2008; 38: 773–777. [DOI] [PubMed] [Google Scholar]
- 23. Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol 2008; 3: 127–155. [DOI] [PubMed] [Google Scholar]
- 24. Nishii M, Inomata T, Takehana H, Takeuchi I, Nakano H, Koitabashi T, Nakahata J, Aoyama N, Izumi T. Serum levels of interleukin‐10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol 2004; 44: 1292–1297. [DOI] [PubMed] [Google Scholar]
- 25. Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, Salvi A, Perkan A, Di Lenarda A, Bussani R, Bartunek J, Sinagra G. Long‐term evolution and prognostic stratification of biopsy‐proven active myocarditis. Circulation 2013; 128: 2384–2394. [DOI] [PubMed] [Google Scholar]
- 26. Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, Baker AL, Perez‐Atayde AR, Newburger JW. Gamma‐globulin treatment of acute myocarditis in the pediatric population. Circulation 1994; 89: 252–257. [DOI] [PubMed] [Google Scholar]
- 27. Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L, Tresoldi M. Treating life‐threatening myocarditis by blocking interleukin‐1. Crit Care Med 2016; 44: e751–e754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparative data between 1995–2004 and 2005–2014.
Table S2. Comparison of clinical characteristics between patients with FM and those with AMI who were hospitalized between 1995 and 2014 and required ECMO support.


