Abstract
Background
Aetiology of heart failure (HF) often remains obscure. We therefore evaluated the usefulness of a combined diagnostic approach including cardiac magnetic resonance imaging (CMRI) and endomyocardial biopsy (EMB) to assess the cause of unexplained cardiomyopathy underlying HF.
Methods and results
We retrospectively investigated 100 consecutive patients (36% women, mean age 53.6 ± 18.8 years) presenting with unexplained cardiomyopathy (HF with reduced ejection fraction or left ventricular hypertrophy; excluding ischaemic and valvular heart disease; left ventricular ejection fraction 31.6 ± 13.9%, Left ventricular end‐diastolic pressure 18.2 ± 9.3 mmHg, heart rate 89 ± 26.6 b.p.m.; mean ± SEM) at the University Medical Center Mainz. We performed electrocardiography, echocardiography, CMRI, and cardiac catheterization with EMB analysed at a Food and Drug Administration‐approved reference centre in 100%, 94%, 69%, and 100% of patients, respectively. On the basis of CMRI findings, electrocardiography, echocardiography, and medical history, the exact cause of cardiomyopathy remained uncertain in 37 of 69 cases (53.6%). In EMB, 25% of patients had viral replication, 23% had inflammation defined as lymphocytic infiltrations without active virus replication, 1% had giant cell myocarditis, and 1% had eosinophilic myocarditis. After diagnostic workup including EMB findings, the cause of cardiomyopathy remained unidentified in 14% of the cases, classified as idiopathic dilated cardiomyopathy or hypertrophic cardiomyopathy in 10% or 4%, respectively. EMB helped to discuss a causal treatment strategy of HF involving immunosuppression or antiviral treatment in 53% of patients, which was opted for in 12% of the patients.
Conclusions
A comprehensive workup including imaging and EMB in an all‐comer population of patients with HF may help physicians to improve diagnostics of unexplained cardiomyopathy in the majority of cases.
Keywords: Endomyocardial biopsy, All‐comers with heart failure, CMRI
Introduction
Heart failure (HF) is a major cause of morbidity and mortality worldwide.1 While coronary artery disease and primary valve disease are two causes of HF that can be identified with high probability, the aetiology of HF and/or underlying cardiomyopathy remains unexplained in many cases.
Various non‐invasive diagnostic procedures, like electrocardiogram (ECG), echocardiography, and cardiac magnetic resonance imaging (CMRI) offer important information about the anatomy and the functionality of heart structures, the extent of impairment, and in many cases the underlying cause of HF. Despite the fact that the above‐mentioned modalities and especially CMRI offer great diagnostic accuracy, endomyocardial biopsy (EMB) is referred to as the gold standard of cardiomyopathy diagnostic by some authors. EMB integrates pathologic information such as description of tissue damage and assessment of the presence of inflammation as well analysis of active or subacute infection (viral or other pathogens). Thereby, EMB can help further to identify the exact cause of the disease.
In a common scientific statement in 2007, the American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC) tried to help physicians decide in which cases an EMB should be performed, presenting suitable clinical scenarios.2
Two more recent position papers published by the Working Group on Myocardial and Pericardial Diseases of the ESC provide expert opinion on how to perform diagnostic workup of patients with cardiomyopathy3 and in particular how to diagnose and treat myocarditis.4 While the role of EMB in the management of suspected myocarditis is supported, its role in diagnosing HF with cardiomyopathy of unknown origin in general remains elusive, and recommendations are still based on the 2007 guidelines. Furthermore, there is ongoing uncertainty of how inflammation could contribute to different phenotypes of HF, and whether it is causal or secondary in the disease process.
The purpose of this retrospective analysis was to assess the methodology listed above and especially the EMB to explore causes of unexplained cardiomyopathy in an all‐comer population with HF admitted to the University Medical Center Mainz. Furthermore, we aimed to evaluate how often a combined diagnostic workup may help to uncover to a specific aetiology and how often this information may guide therapeutic decisions.
Material and methods
Patient population
One hundred all‐comer patients presenting with unexplained cardiomyopathy [HF with reduced ejection fraction or left ventricular (LV) hypertrophy] at the University Medical Center Mainz between March 2013 and April 2014 were enrolled in this retrospective analysis. The decision to obtain EMBs in the selected patients was made according to the guidelines published of the AHA, the ACC, and the ESC in 2007.2 Causes of cardiac dysfunction like coronary artery disease, primary valve disease, and systemic disease with known cardiac involvement were ruled out before the EMB.
The analysis of the patients' medical records involved the following data: personal history, clinical presentation, laboratory values, ECG, echocardiography, magnetic resonance imaging (MRI), and the finding of the EMB.
Echocardiography
Transthoracic echocardiography was carried out in the echocardiography lab in our department using a Philips ie33, a GE E9, or Siemens Acuson s2000 machine. The exam included two‐dimensional and M‐mode imaging as well as continuous‐wave, pulsed‐wave, and colour flow Doppler to assess LV ejection fraction (LVEF), the size of the left ventricle, the presence of diastolic dysfunction or valve disease, the thickness of the interventricular septum and the posterior LV wall, the gradient across the tricuspid valve, and the presence of pericardial effusion (colour flow Doppler was not included in one case). In 6% of cases, echocardiography was performed at another site prior to admission to our centre.
Cardiac magnetic resonance imaging
CMR was performed with a 1.5 T MAGNETOM® Sonata® MRI scanner (Maestro Class; Siemens Healthcare, Erlangen, Germany) using a six‐channel phased‐array cardiac coil and integrated spine array coil elements for signal detection. For imaging, all patients were positioned in the supine position.
The MRI protocol comprised cine, oedema‐sensitive, and late gadolinium enhancement (LGE) imaging.
Global and regional ventricular function was assessed by cine imaging using a segmented steady‐state free precession pulse sequence, in horizontal and vertical long‐axis views as well as in multiple short‐axis views every 10 mm, covering the entire left ventricle. Typical in‐plane resolution was 2.0 × 1.5 mm2, with a section thickness of 6.0 mm and section gap of 4.0 mm (TR/TE = 3.02/1.51 ms, flip angle 60°, temporal resolution 33.22 ms, parallel imaging using the GRAPPA algorithm, acceleration factor of 2, 33 reference lines).
Oedema‐sensitive imaging consisted of a triple inversion recovery turbo spin‐echo sequence [turbo inversion recovery magnitude (TIRM)] that was acquired in the same long‐axis and short‐axis planes (TE = 60 ms, TR = 2 × RR‐interval, TI 170 ms, slice thickness 10 mm, flip angle 180°, pixel size 2.3 × 1.3 mm2). For signal detection of this sequence, the integrated body coil was used.
Each CMR examination was enhanced by 0.2 mmol/kg body weight of gadopentetate dimeglumine (Magnevist®, Bayer Vital, Leverkusen, Germany). Ten minutes after contrast application, late enhancement images were acquired using a segmented T1‐weighted inversion recovery TurboFLASH sequence in identical long‐axis and short‐axis planes (TE = 4.38 ms, TR = 2 × RR‐interval, flip angle 25°, pixel size 1.4 × 1.8 mm2; section thickness 8 mm, section gap 2 mm). TI was adjusted for each patient after acquisition of a TI scout in order to optimize the nullification of normal myocardium; it ranged between 260 and 320 ms and was increased during the acquisition approximately every minute by 10 ms to optimally ‘null’ the normal myocardium.
Cardiac magnetic resonance image analysis
Left ventricular ejection fraction, LV mass, and volumes were measured on short‐axis stack cine imaging, using semi‐automated software (Argus 2.3, Siemens Medical Systems). All ventricular volumes were indexed for body surface area.
Cine images were reviewed with assessment of regional wall thickness and wall motion abnormalities. Fat‐suppressed TIRM images were reviewed for areas of high signal intensity suggesting oedema, and by measuring the ratio of myocardial signal intensity to that of skeletal muscle. A ratio > 1.9 was considered as a significant increase in signal intensity.12 Finally, LGE images were assessed for the presence of enhancing areas, the location within the myocardial tissue (e.g. subendocardial, subepicardial, mid‐wall, and transmural), and their segmental distribution.
On the basis of image analysis, patients presenting with focal or diffuse areas of oedema not related to the territory of a coronary artery and LGE in at least one segment in the subepicardial or mid‐ventricular layers of the myocardium were diagnosed to have acute myocarditis.5 In addition, pericardial effusion and enhancement were interpreted as perimyocarditis. The absence of a myocardial oedema in combination with typical spots of late enhancement was characterized as chronic (post‐) myocarditis status.
Dilated cardiomyopathy (DCM) was diagnosed in patients with increased normalized volumes and reduced systolic function but without evidence of significant oedema on fat‐suppressed images. LGE—if present—showed a predominantly mid‐wall distribution mostly in the interventricular septum.6
Patients presenting with an end‐diastolic wall thickness of the interventricular septum ≥13 mm and preserved or only mildly reduced global systolic LV function were suspected to have hypertensive heart disease. This was sometimes accompanied by diffuse LV oedema and foci of mid‐ventricular or subepicardial enhancement on LGE images.
A wall thickness ≥ 18 mm and an increased normalized myocardial mass lead to the diagnosis of hypertrophic cardiomyopathy (HCM). Cases with hypertrophied myocardium and LGE distribution predominantly subendocardially, and not confined to one clear vascular territory, were considered to represent cardiac amyloid deposition.7, 8
Left or right heart catheterization with biopsy
Endomyocardial biopsies were either taken from the right ventricular septum or from the lateral wall of the left ventricle. The procedure was mostly carried out through the right femoral artery (or right femoral vein in case of a right heart biopsy). In some cases, a new method of transradial cardiac catheterization was used.9 The procedures were carried out with the following biopsy forceps: Medwork bioptom, 180 cm, 1.8 mm, Cat.‐No. BIO‐C4‐18‐180. The EMB specimens were immediately stabilized in an intermediary solution to preserve ribonucleic acid (RNA) integrity (RNAlater™, a trademark of Ambion, Inc., Austin, Texas) and were sent for further examination to a specialized laboratory approved by Food and Drug Administration [Institut Kardiale Diagnostik und Therapie (IKDT), Berlin, Germany].
Examination of the biopsy specimens
The histology, immunohistochemistry, and virus detection were carried out at the IKDT as part of the routinely performed workup of EMBs. The histological examination required paraffin wax embedding and staining. For the evaluation of the morphological features of the myocardium and the detection of myocarditis, four standard stains were used (Heidenhain's AZAN trichrome stain, haematoxylin and eosin stain, elastic Van Gieson stain, and periodic acid‐Schiff stain). In case of a suspected storage disease, additional stains were necessary. The histological evaluation was made according to the Dallas criteria.
Immunohistochemistry tests were used to identify inflammatory processes. Through detection of specific antibodies, it was possible to identify immune cell infiltrations and the expression of cell adhesion molecules as signs of an active inflammation. The specimens of our patients were tested for CD3‐positive lymphocytes, lymphocyte function‐associated antigen 1‐positive cells, macrophages (Mac‐1), cytotoxic T‐cells (perforin), and the expression of adhesion molecules (Hl‐A class I, ICAM‐1).10
Virus detection
PCR methods were used to detect the genomic sequences of viruses that most commonly cause myocarditis (enterovirus, adenovirus, human cytomegalovirus, herpes simplex virus, Epstein–Barr virus, human herpesvirus 6, parvovirus B19 [B19V], and influenza A and B viruses). After sequencing of the virus‐positive probes, the viral load is being calculated through quantitative PCR methods. Serological examinations for cardiotropic viruses were performed in order to diagnose a systemic viral infection.11
Statistics
IBM Statistical Package for the Social Sciences was used for the statistical analysis of the patients' data, and χ2 test was applied as appropriate. A probability value < 0.05 was considered statistically significant.
Results
Patient characteristics, electrocardiogram, and laboratory findings
Table 1 presents the most common symptoms including severity of symptoms according to the New York Heart Association classification at the time of the first hospital admission. Most of the patients had only mild dyspnoea and sinus rhythm at the time of first presentation (82%). Fifteen per cent of the patients were diagnosed with atrial fibrillation and 1% with atrial flutter. The QRS complex was normal in 71% of the patients, whereas 22% had a left bundle branch block and 2% had a right bundle branch block. T‐wave inversion appeared in 36% of the patients, and only 11% had an ST depression. High‐sensitivity troponin I was measured in 62 of our patients. Elevated troponin levels were found in 15 patients (24%).
Table 1.
Demographic and clinical characteristics
| Values | |
|---|---|
| Age, year; mean ± SEM | 53.6 ± 8.8 |
| Female sex, no. (%) | 36 (36%) |
| Weight; mean (range) | 85.6 (40–185) kg |
| BMI; mean (range) | 27.8 (17–42) kg/m2 |
| Diagnosed hypertension, no. (%) | 27 (27%) |
| Diabetes, no. (%) | 12 (12%) |
| History of alcohol abuse, no. (%) | 5 (5%) |
| History of smoking, no. (%) | 23 (23%) |
| History of infection, no. (%) | 29 (47.5%) |
| History of amphetamine abuse, no. (%) | 1 (1%) |
| History of cardiotoxic chemotherapy, no. (%) | 2 (2%) |
| Medical treatment for heart failure | |
| ACE inhibitor, no. (%) | 74 (74%) |
| AT1 blocker, no. (%) | 15 (15%) |
| Beta‐blockers, no. (%) | 82 (82%) |
| Ivabradine, no. (%) | 24 (24%) |
| Loop diuretics, no. (%) | 65 (65%) |
| Thiazides, no. (%) | 31 (31%) |
| Potassium sparing diuretics, no. (%) | 67 (67%) |
| Phenotype based on echocardiography | |
| DCM, no. (%) | 89 (89%) |
| HCM, no. (%) | 7 (7%) |
| Normal, no. (%) | 4 (4%) |
| Time since onset of symptoms | |
| <2 weeks, no. (%) | 34 (34%) |
| >2 weeks, <3 months, no. (%) | 24 (24%) |
| >3 months, no. (%) | 41 (41%) |
| Acute decompensated heart failure, no. (%) | 13 (13%) |
| Atrial fibrillation, no. (%) | 15 (15%) |
| Atrial flutter, no. (%) | 1 (1%) |
| History of VTs, no. (%) | 7 (7%) |
| Family history of congenital heart disease, no. (%) | 1 (1%) |
| Symptoms in cardiomyopathy patients | |
| Dyspnoea, no. (%) | 77 (77%) |
| Chest pain, no. (%) | 32 (32%) |
| Oedema, no. (%) | 26 (26%) |
| Palpitations, no. (%) | 11 (11%) |
| Cough, no. (%) | 8 (8%) |
| Nausea, no. (%) | 3 (3%) |
| Syncope, no. (%) | 3 (3%) |
| No symptoms, no. (%) | 5 (5%) |
| Severity of symptoms according to the NYHA classification | |
| NYHA I, no. (%) | 19 (19.8%) |
| NYHA II, no. (%) | 39 (40.6%) |
| NYHA III, no (%) | 16 (16%) |
| NYHA IV, no (%) | 22 (22.9%) |
ACE, angiotensin‐converting enzyme; BMI, body mass index; NYHA, New York Heart Association; VT, ventricular tachycardia.
Brain natriuretic peptide (BNP) was used to assess the severity of HF. This marker was measured in 45 of the patients. Eight of them had no BNP elevation at the time of admission to hospital (18%). In 24 patients (53%), the BNP levels were found to be >900 pg/mL.
Echocardiography
Transthoracic echocardiography was performed in 94 patients (in 93 of them including colour Doppler) at the Center for Cardiology of the University Medical Center Mainz. In six patients, the transthoracic examination was carried out at another site and the exact value of LVEF could not be determined.
Of the patients who underwent echocardiography, 57.5% had a severely reduced LV function; in 22.3%, the LVEF was moderately decreased and in 10.6% mildly decreased, and only nine patients (9.6%) had an ejection fraction > 55% (Figure 1 ). In cases of normal LVEF, EMB was carried out because of severe symptoms and clinical features of myocarditis (i.e. episodes of ventricular tachycardia) despite the normal echocardiography findings or because of a hypertrophic phenotype in order to rule out storage disease.
Figure 1.
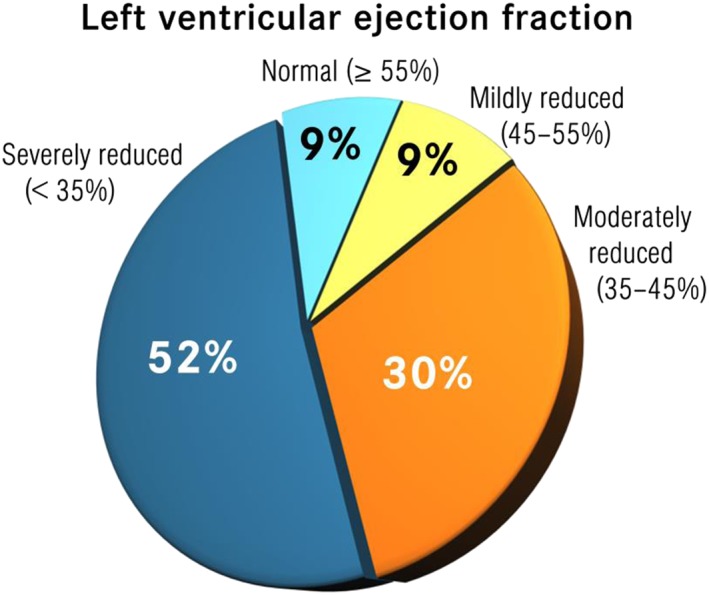
Left ventricular systolic and diastolic function in our patients' population based on the transthoracic echocardiography.
Diastolic function was assessed in 57 patients during the transthoracic echocardiography; 82.5% of the patients had an LV diastolic dysfunction; 35.1% had a diastolic dysfunction I°, a pseudonormalization (II°) appeared in 15.8% of the patients, and 31% presented a restrictive pattern (III°).
End‐diastolic thickness of the ventricular septum was measured in 75 patients. Forty three of them had normal thickness (<1.1 cm). Thirty two of them (42%) had LV hypertrophy. Left ventricular end‐diastolic diameter (LVEDD) in the parasternal long axis was used to assess LV size. LVEDD measurement was carried out in 74 patients. Thirty two of them had a normal ventricular size (43%). Left ventricular dilation was present in 55% of male and in 60% of female patients. Because of the differences in the reference range, the two genders were analysed separately. Secondary (functional) mitral valve regurgitation was very common in patients who underwent myocardial biopsy. Only 13% had no significant regurgitation, whereas 16.1% suffered from severe mitral valve regurgitation (Table 2).
Table 2.
Echocardiography findings
| n | Total | % | |
|---|---|---|---|
| LVEF | |||
| Normal (>55%) | 9 | 94 | 9.6 |
| Mildly reduced (45–54%) | 10 | 94 | 10.6 |
| Moderately reduced (30–44%) | 21 | 94 | 22.3 |
| Severely reduced (<30%) | 54 | 94 | 57.5 |
| Diastolic functiona | |||
| Normal | 10 | 57 | 17.5 |
| Diastolic dysfunction I° (E < A) | 20 | 57 | 35.1 |
| Diastolic dysfunction II° (pseudonormalization) | 9 | 57 | 15.8 |
| Diastolic dysfunction III° (restrictive profile) | 18 | 57 | 31 |
| Secondary mitral valve regurgitationb | |||
| None | 12 | 93 | 13 |
| Mild | 46 | 93 | 49.5 |
| Moderate | 20 | 93 | 21.5 |
| Severe | 15 | 93 | 16.1 |
Assessment of the diastolic function was included in the echocardiography of 57 patients.
Assessment of the mitral valve regurgitation was included in the echocardiography of 93 patients.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance was used to assess the presence of myocardial inflammation or storage disease. As mentioned before, the diagnosis of myocarditis was based on the presence of myocardial oedema and LGE, in combination with the clinical history; T1 or T2 mapping criteria were not included in the analysis at that time. CMRI was performed in 69 patients. LGE was found in 46 (66.6%), and myocardial oedema was present in 25 (36.2%). Nineteen of the patients had both LGE and fluid retention (27.5%). The causes of cardiomyopathy based on the CMRI findings appear in Table 3.
Table 3.
Diagnosis based on cardiac magnetic resonance imaging findings/clinical information
| Diagnosis | Frequency | % |
|---|---|---|
| Dilated phenotype | 61 | 88.4 |
| Myocarditis | 12 | 17.4 |
| Perimyocarditis | 9 | 13 |
| Post‐myocarditis | 6 | 8.7 |
| Sarcoidosis | 1 | 1.45 |
| Idiopathic dilated cardiomyopathy | 33 | 47.8 |
| Hypertrophic phenotype | 8 | 11.6 |
| Pericarditis constrictiva | 1 | 1.45 |
| Amyloidosis | 3 | 4.35 |
| Idiopathic hypertrophic cardiomyopathy | 4 | 5.8 |
| Total | 69 | 100 |
As shown in Table 3, 21 patients (30.4%) were diagnosed with myocarditis or perimyocarditis according to CMRI criteria. In six patients (8.7%), there were no signs of inflammation in CMRI, compatible with previous but no longer active myocarditis. A total of 39% of the patients' cardiomyopathy was myocarditis associated, according to CMRI findings. Amyloidosis was diagnosed in three patients (4.3%). One of the 69 patients presented signs of cardiac sarcoidosis, and one was diagnosed with constrictive pericarditis.
Taking into account the additional information obtained from clinical data available to the radiologist (in particular medical history of arterial hypertension, cardiotoxic drugs, alcohol abuse, or tachycardia), electrocardiography, and echocardiography, the cause of cardiomyopathy remained unknown in 37 patients (53.6%) after having performed CMRI. In those 37 patients, CMRI performed as described earlier without T1 or T2 mapping revealed unspecific alterations in cardiac morphology, leading to the diagnosis of either idiopathic DCM or idiopathic HCM.
Endomyocardial biopsy
In 92% of the patients, EMB was taken from the lateral LV wall; 8% of the patients underwent a right ventricular biopsy (Table 4). According to EMB findings, an active myocardial disease was found in 53% of the patients. We use the term ‘active myocardial disease’ to differentiate patients with an abnormal inflammatory infiltrate, a clinically relevant virus presence, or a storage disease from those with no aforementioned signs of in the biopsy specimens. Three of them were diagnosed with a storage disease; all of them had amyloidosis. An inflammatory cardiomyopathy with no evidence for virus replication and no myocardial cell necrosis (‘borderline myocarditis’ according to the Dallas criteria) was found in 23 patients. Two patients had special forms of myocardial inflammation: One was diagnosed with giant cell myocarditis and one with the eosinophilic myocarditis on the basis of EMB. Active viral replication was detected in 25 patients. In four of them, viral presence was combined with an inflammation. Sensitivity of the EMB in the detection of inflammation improved when histological findings were combined with immunohistochemistry: solely on the basis of histological findings, active inflammation would have been detected in only three patients. In most of them, active inflammation could only be diagnosed on the basis of immunohistochemical criteria as proposed in the position statement of the ESC (≥14 leucocytes/mm2 including up to 4 monocytes/mm2 with the presence of CD3‐positive T‐lymphocytes ≥ 7 cells/mm2).4
Table 4.
Diagnosis based only on endomyocardial biopsy findings
| Diagnosis | Frequency | % |
|---|---|---|
| Virus‐associated cardiomyopathya , b | 25 | 25 |
| Parvovirus B19 | 21 | 21 |
| Coxsackie virus | 2 | 2 |
| Combined B19V /coxsackie | 1 | 1 |
| Combined B19V/HHV | 1 | 1 |
| Inflammation‐associated cardiomyopathyb | 47 | 47 |
| Inflammatory cardiomyopathy, virus negative | 23 | 23 |
| Giant cell myocarditis | 1 | 1 |
| Eosinophilic myocarditis | 1 | 1 |
| Post‐inflammatory cardiomyopathyc | 22 | 22 |
| Amyloidosisb | 3 | 3 |
| Unknown causec | 25 | 25 |
| Total | 100 | 100 |
B19V, parvovirus B19; HHV, human herpes virus.
Active replication of the respective virus; compare with Figure 2 . Please note that the individual with active replication of HHV6 listed in Figure 2 had a replication of only 43 copies, considered too low to qualify for virus‐associated cardiomyopathy. The individual with active replication of Epstein–Barr virus had cardiac amyloidosis and was therefore not considered to have virus‐associated cardiomyopathy as the leading diagnosis.
In total, 30 patients had any form of active inflammation. Twenty five had inflammatory cardiomyopathy, giant cell myocarditis, or eosinophilic myocarditis; active virus replication plus inflammation was present in four of the 30 patients, filed under virus‐associated cardiomyopathy; one had amyloidosis plus inflammation, filed under amyloidosis.
No active myocardial disease, for example, virus negative, no active inflammation; sum of ‘post‐inflammatory cardiomyopathy’ and ‘unknown cause’ equals n = 47.
In 47 patients, no active myocardial disease was detected (Table 4). In 22 of these 47 cases, findings such as myocardial hypertrophy, interstitial fibrosis/scarring, and only marginal presence of macrophages or CD3‐positive T‐ lymphocytes led the pathologist to the diagnosis of a post‐inflammatory DCM. This could possibly originate from viral infection and also from arterial hypertension, metabolic disorders, or other causes that are known to cause inflammation. Seven patients received EMB because of a suspected amyloidosis. The diagnosis could be confirmed in three of them (3%), in accordance with the CMRI finding (Table 3).
The parvovirus genome was found in 72 patients (72%): in 11 of them combined with human herpes virus 6 (HHV6) DNA (11%), in 3% combined with Epstein–Barr virus DNA, and in 1% with coxsackie virus genome (in this case ssRNA). A combination of three viruses was found in one patient (coxsackie, HHV6, and B19V). In three patients, HHV6 was found, without the presence of any other viruses; and three patients had a single coxsackie virus infection (Figure 2 ).
Figure 2.
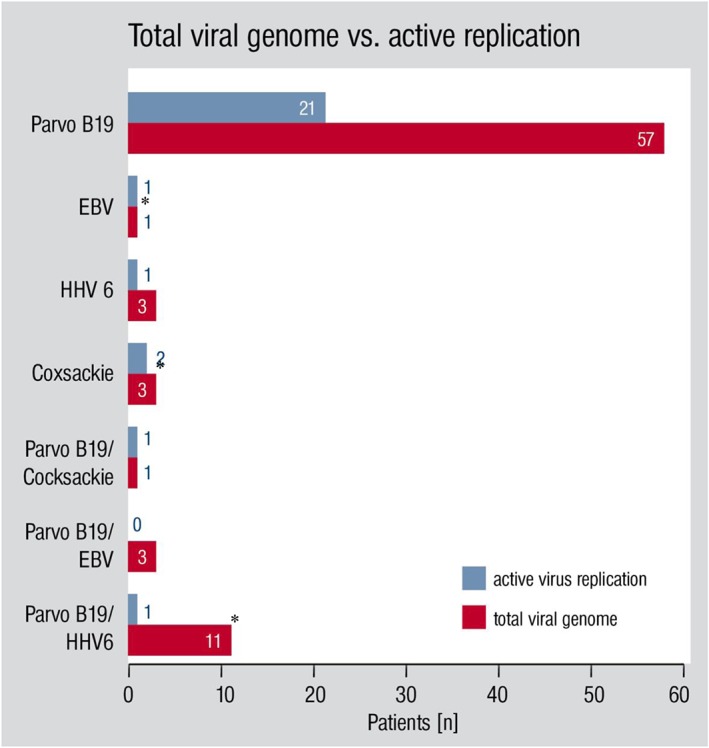
Viral presence in biopsy specimens. Total viral genome vs. active virus replication (see also Table 4, where only endomyocardial biopsy findings with actively replicating viruses are listed). Asterisk indicates that in some patients of these groups, genome of other viruses was found but without clinical significance or replication. EBV, Epstein–Barr virus; HHV6, human herpes virus 6.
On the basis of all the aforementioned findings, Table 4 presents the most frequent causes of cardiomyopathy only on the basis of the EMB findings. Viral DNA in EMB specimens can also be found in the myocardium of patients with no myocarditis or DCM.12 Therefore, the presence of viral replication in EMB specimens is required for the diagnosis of viral myocarditis.13, 14, 15 The final EMB diagnosis was a combined assessment by the pathologist based on histological and immunohistochemical findings, as well as those of the mRNA expression analysis by PCR.
Virus‐negative inflammatory cardiomyopathy was the most common cause of cardiomyopathy. None of the 100 patients that underwent EMB were found to have an acute myocarditis according to the Dallas criteria.16
Parvovirus B19‐associated cardiomyopathy was the second most frequent finding among our patients. Active parvovirus replication was found in 21 patients (21%). In three of them, parvovirus reactivation was accompanied by inflammation. The rest (18%) presented no inflammatory cell infiltrations or increased expression of adhesion molecules.
Coxsackie virus genome was amplified by PCR in three patients (3%), in one of them with concomitant inflammation and in one with B19V. One patient was diagnosed with a combined parvovirus/HHV6 reactivation without inflammation.
Proposed causes of cardiomyopathy on the basis of the complete diagnostic workup
Table 5 displays the summary of cardiomyopathy aetiologies, taking into account the findings of the complete diagnostic workup. In 37 from 69 patients (53.6%), the cause of disease remained unexplained after CMRI, taking also into account the patients' history. Proposing a diagnosis was possible in 61 of 69 patients (88%) who underwent the diagnostic workup including both CMRI and EMB, a significantly higher proportion than in patients without EMB (χ2 test, P < 0.05; also Table 6). Irrespective of imaging modalities, putative diagnosis was proposed in 86 out of 100 patients who obtained EMBs in total. The remaining 14 cases were termed idiopathic cardiomyopathy (DCM or HCM; Table 5). It should be noted, that in some cases, the suspected diagnosis was based solely on patient's history and therefore not assured. For example, if a patient had a history of alcohol abuse, chemotherapy, or amphetamines and no other obvious cause (no myocarditis in EMB), it was assumed that these potentially cardiotoxic agents were the underlying cause of cardiomyopathy, which is in accordance with current guidelines and position statements.
Table 5.
Diagnosis based on the complete diagnostic workup
| Cause of cardiomyopathy | Frequency | % |
|---|---|---|
| Virus‐related disease | 25 | 25 |
| Parvovirus B19‐associated cardiomyopathy | 21 | 21 |
| Coxsackie virus‐associated cardiomyopathy | 2 | 2 |
| Combined B19V/coxsackie | 1 | 1 |
| Combined B19V/HHV | 1 | 1 |
| Inflammation‐associated disease with no virus presence | 49 | 49 |
| Inflammatory cardiomyopathy | 23 | 23 |
| Eosinophilic myocarditis | 1 | 1 |
| Giant cell myocarditis | 1 | 1 |
| Post‐inflammatory cardiomyopathya | 24 | 24 |
| Toxic damage | 5 | 5 |
| Alcohol‐related cardiomyopathy | 3 | 3 |
| Amphetamine‐related cardiomyopathy | 1 | 1 |
| Chemotherapy‐related cardiomyopathy | 1 | 1 |
| Storage disease | 3 | 3 |
| Amyloidosis | 3 | 3 |
| Secondary cardiomyopathy of other causes | 4 | 4 |
| Hypertensive heart disease | 2 | 2 |
| Tachycardia‐induced cardiomyopathy | 2 | 2 |
| Idiopathic cardiomyopathyb | 14 | 14 |
| HCM | 4 | 4 |
| IDCM | 10 | 10 |
| Total | 100 | 100 |
B19V, parvovirus B19; HCM, hypertrophic cardiomyopathy; HHV, human herpes virus; IDCM, idiopathic dilated cardiomyopathy.
Including individuals with signs of suspected active or previous myocarditis in CMRI, in which diagnosis could not be confirmed through EMB. This group may therefore possibly include old myocarditis.
This entity may include familial/hereditary forms of cardiomyopathy.
Table 6.
Addition on endomyocardial biopsy to the diagnostic workup in the 69 patients who underwent cardiac magnetic resonance imaging
| Cause found | Specific therapy initiated | Total | |
|---|---|---|---|
| Diagnostic workup with CMRI | 31 | 0 | 69 |
| Diagnostic workup with CMRI and EMB | 61 | 9 | 69 |
Analysis of the group of 69 patients who underwent both CMRI and EMB. The addition of EMB helped us to find a cause of cardiomyopathy in 61 out of 69 patients, compared with 38 out of 69 patients without it (χ2 test, P < 0.05). With this information, a specific therapy was initiated in nine patients. No patients received specific therapy on the basis of only CMRI findings (χ2 test, P < 0.002). See also Table 7.
In three of the five patients with history of alcohol abuse, no other cause could be found, which makes an alcohol‐related cardiomyopathy very likely (3%). One patient had a history of amphetamine abuse (1%), and one had been treated with potentially cardiotoxic chemotherapeutic agents (1%). Two patients (2%) had atrial fibrillation with an increased heart rate at the time of admission. Tachycardia‐induced cardiomyopathy was the most likely diagnosis in these cases. In some patients with suspected active or previous myocarditis in CMRI, the diagnosis could not be confirmed through EMB. These cases were classified according to the CMRI diagnosis and are included into the group ‘post‐inflammatory cardiomyopathy’ (Table 5).
Disease‐modifying therapy
In 53 patients, EMB prompted us to evaluate appropriateness for disease‐modifying therapy. In 12 patients (12%), a disease‐modifying therapy was initiated on the basis of complete workup including the EMB findings (Table 7). Five patients with virus‐negative inflammatory cardiomyopathy were treated with a combination of prednisolone (1 mg/kg/day for 4 weeks followed by 0.33 mg/kg/day for 5 months) and azathioprine (2 mg/kg/day for 6 months), on the basis of the protocol published by Frustaci et al.17 The patient with the giant cell myocarditis reported in this study still is on treatment with cyclosporine 2 mg/kg/day and prednisolone 1 mg/kg/day at the time of writing and has a stable course of disease over the last 40 months. The patient with the eosinophilic myocarditis received prednisolone (1 mg/kg/day for 4 weeks followed by 0.33 mg/kg/day for 5 months). Three patients diagnosed with parvovirus‐related cardiomyopathy were treated with the thymidine analogue telbivudine after 3 months on standard HF therapy without recovery. Two patients were sent for further oncological treatment after the diagnosis of an amyloid light‐chain amyloidosis through EMB. It should be noted that no specific treatment could be initiated on the basis of only CMRI findings (χ2 test, P < 0.002; Table 6).
Table 7.
Decision to initiate a disease‐modifying therapy based on endomyocardial biopsy findings
| % | |
|---|---|
| Recommendation to evaluate appropriateness for disease‐modifying therapy based on EMB findingsa | 53 |
| Treatment: optimal medical treatment combined with | |
| No disease‐modifying therapy | 88 |
| Immunosuppression | 7 |
| Azathioprine, prednisolone based on the TIMIC study17 | 5 |
| Cyclosporine, prednisolone (giant cell myocarditis) | 1 |
| Prednisolone (eosinophilic myocarditis) | 1 |
| Antiviral therapy | 3 |
| Telbivudineb | 3 |
| Amyloidosis‐directed therapy | 2 |
| Chemotherapy in multiple myeloma with AL‐amyloidosis | 2 |
AL, amyloid light‐chain.
Comprising patients with virus‐related disease (25%), amyloidosis (3%) as well as virus‐negative inflammatory cardiomyopathy (23%), giant cell myocarditis (1%), and eosinophilic myocarditis (1%; Table 5).
Expanded access in cases of B19V‐associated cardiomyopathy with high replication and clinical impairment (New York Heart Association III and/or LVEF < 30%), individualized decision based on expert opinion and scientific proceedings publications;45, 46 600 mg telbivudine was given p.o. for 6 months.
Discussion
With this work, we provide evidence from a single‐centre retrospective analysis of an all‐comer population with HF and/or cardiomyopathy of unknown origin, which encourages the combined use of imaging modalities and EMBs in the diagnostic workup. One main finding is that in a subset of patients, EMB helped to initiate an anti‐inflammatory or antiviral therapy that would otherwise not have been feasible. In addition, our study gives insight into the distribution pattern of viral pathogens in the 2010s in south‐west Germany.
A great variety of viral infections can cause cardiomyopathy. The predominant viruses vary in different parts of the world as well as in different periods in the same region. The prevalence of viral myocarditis caused by specific viruses like coxsackie or influenza, for example, might be particularly high in times of viral prevalence.18, 19, 20 Traditionally, coxsackie viruses of group A or B are regarded as the most common cause of myocarditis, followed by enteric cytopathic human orphan virus type 6 and adenovirus types 3 and 7.21 The most common virus in our samples was B19V. Its DNA was detected in 72% of our patients as a single virus infection or combined with other viruses, with active virus replication detected in only 23%. No adenovirus or influenza genome was found in any of our patients (Figure 2 and Table 4).
Various studies have demonstrated that B19V may persist without replication in solid tissues, but also in the bone marrow of asymptomatic patients.12 The findings of Norja et al. suggest a lifelong persistence of erythrovirus DNA genome in human tissue, which represents a source of information about our past infectious encounters, the ‘bioportfolio’.22 Kuethe et al. demonstrated a high prevalence of erythrovirus DNA in myocardial tissue with no evidence of DCM or myocarditis.23 In a study of Lindner et al., no discrepancies in the B19V could be demonstrated in cardiomyopathy patients as compared with controls. The authors suggest the complementation of additional virological and immunological parameters in order to associate B19V with cardiomyopathy.24
All the aforementioned findings point out that the detection of parvovirus DNA alone is not necessarily related to cardiomyopathy.
Nevertheless, according to the findings of a later study from Kühl et al., parvovirus reactivation from latency appears to be a key factor in the pathogenesis of cardiomyopathy. The authors found significant differences in molecular level between patients with transcriptionally active vs. latent virus and suggest that transcriptional mapping should be a part of the evaluation of EMBs.25 With this study, as well as a later study of Bock et al.,26 we regarded the presence of transcriptional activity to be related to cardiomyopathy, whereas the detection of viral DNA is regarded only as a sign of previous erythrovirus infection.27
Interestingly, patients with parvovirus‐induced cardiomyopathy tended to have a relatively low LVEF in our retrospective cohort. The subgroups are too small to yield statistically significant differences between groups. This observation though is interesting, as B19V has been associated with isolated diastolic dysfunction.28
In a study of Bowles et al. reporting on EMB findings of both myocarditis and DCM‐like patients enrolled between 1988 and 2000, viral genome was found in 38% of myocarditis patients and in 30% of DCM patients. Adenovirus was the most common virus in both groups, followed by enterovirus. Parvovirus was found only in six patients.29 These findings differ greatly from ours, as well as from those of Kühl et al. (with EMBs carried out between 2001 and 2003),11 both focusing on cardiomyopathy of unknown origin and not on myocarditis and showing the highest prevalence for B19V.
A common finding of all three studies is that coxsackie virus was not a common cause of myocarditis or cardiomyopathy. This suggests that the virus spectrum of myocarditis may have changed since the 1970s and 1980s, with adenovirus being the most common cause of myocarditis or DCM in the USA in the 1990s and parvovirus being the most prevalent in Germany from the early 2000s until now.11, 29 This would have broad implications for translational research, because the most frequently used animal model of myocarditis is a coxsackie viral myocarditis mouse model.30, 31 It is questionable, though, whether the shift is real. Although the patients in the Bowles study were enrolled between 1988 und 2000 and were also examined for parvovirus, other findings suggest that the B19V bioportfolio existed also before 2000.22, 23, 32
The aforementioned findings are similar to those of Stewart et al. published in 2011. In their study, B19V was the only virus found among 100 patients with HF who underwent EMB and none of the parvovirus‐positive patients met the Dallas histopathologic criteria for active or borderline myocarditis. According to the authors of this article, their findings do not support a causative role of B19V in cardiomyopathy.33 However, these findings can also be explained by an alternative pathomechanism of B19V‐related cardiomyopathy. On the basis of an animal model, in which B19V causally impaired endothelial regeneration with spreading of the virus in bone marrow‐derived circulating angiogenic cells, Schmidt‐Lucke et al. support the theory of a primary bone marrow disease with secondary end‐organ damage, caused by dysfunctional endogenous vascular repair.34 This could explain why parvovirus infection often mimics acute myocardial infarction.35
Interestingly, there was no correlation between active viral replication and the presence of inflammatory infiltrates in our patients' selection. On the contrary, active virus replication was found more often in patients with no active inflammation (see footnote to Table 4). This contributed to the decision to initiate antiviral therapy only in individuals with high virus replication and the presence of inflammation (Table 7). Importantly, there were six patients with no signs of inflammation in the EMB but have typical findings in the CMRI. This could be a result of sampling error. In HHV6 myocarditis, for instance, LGE is frequently observed in the mid‐wall of the interventricular septum,36 whereas most of the EMBs were obtained from the LV lateral wall in our study. A contrast enhancement in this location is frequently found in most B19V patients.37
Yilmaz et al. found that a combined EMB of both ventricles may optimize the diagnostic yield, but an increase in the number of positive findings through referential biopsy in regions showing LGE on CMRI could not be demonstrated.38 EMB and CMRI are no opposing diagnostic tools but should rather be combined synergistically to improve the chance of detecting a treatable cause of cardiomyopathy.39
CMR is a powerful tool for myocardial characterization as it enables the differentiation between acute and chronic diseases (via T2‐weighted imaging or mapping) and the detection of myocardial fibrosis (LGE imaging, T1 mapping).6 The pattern‐based approach of LGE was described in the literature,40 allowing characterization of ischaemic and non‐ischaemic cardiomyopathies by the distribution of enhancement. Nevertheless, there is an LGE imaging overlap between different forms of non‐ischaemic DCM, as, for example, mid‐wall interventricular enhancement can be found in post‐myocarditis DCM (together with epicardial enhancement) as in other secondary forms of disease, related, for example, to drug toxicity and alcohol abuse or idiopathic DCM. In conclusion, differentiation between different forms of non‐ischaemic DCM is actually still a complex issue under investigation.6 It will be interesting to evaluate the correlation between the biopsy and the CMRI findings in more detail and to find out how sensitive CMRI really is when it comes to chronic inflammation or viral persistence. For this type of analysis, more advanced CMRI features such as T1 and T2 mapping will be necessary.39, 41 On the basis of our data, a CMRI without routine T1 and T2 mapping that does not reveal the presence of inflammation does not justify the decision not to perform an EMB.
It is recommended by current guidelines for HF that CMRI should be involved in the diagnostic workup of every newly diagnosed cardiomyopathy.42 In patients with no response to conventional treatment, EMB should be performed. In case of suspected giant cell myocarditis, EMB should be performed immediately.
The scientific statement of 2007 regarding EMB indications2 was published at a time when EMB diagnostics mainly involved a histological analysis of the specimens. The addition of immunohistochemistry and PCR enhanced the sensitivity of EMB. In our opinion, the high rate of cases with no specific diagnosis after CMRI (53.6%) as well as the relatively high rate of patients who received specific therapy based on the EMB findings (12%) among the patients in our study supports a more routine use of EMB in patients with HF and cardiomyopathy of unknown origin.
The perfect time point to perform EMB is mostly uncertain and a potential cause for bias. An ‘active myocardial disease’ defined as evidence of abnormal inflammatory infiltrate, a clinically relevant virus presence, or a storage disease was found in EMBs of 53 cases among the patients in our study and prompted us to evaluate a disease‐modifying therapy. With these findings, a tailored treatment consisting of immunosuppression or antiviral therapy was initiated in 12 of them (Table 7). The remaining patients improved with regard to clinical and echocardiographic findings during the first months after the EMB and after optimization of medical therapy, so that a disease‐modifying therapy was not regarded as necessary. This raises the argument that EMB in some of these cases might have been carried out too early. On the other hand, 22 patients presented signs of previous inflammation, suggesting that the EMB might have been carried out too late. A specific antiviral therapy or immunosuppression was initiated only when standard therapy failed. In some cases, as in giant cell myocarditis, the immediate initiation of a disease‐modifying therapy is of vital importance for the patient and is only possible after the confirmation of the diagnosis through EMB.43, 44 As it is hard to estimate in advance, if the underlying cause of cardiomyopathy is one that requires immediate treatment, it is more preferable to carry out an EMB too early than to withhold therapy in a patient who requires it immediately. With this consideration, the rate of initiating an anti‐inflammatory or antiviral therapy might have even been higher than 12%.
After a complete diagnostic workup, the cause of cardiomyopathy remained unknown in 14 cases. Among them there were four of the nine patients with HCM phenotype (44%) and 10 of the 91 patients with DCM phenotype (11%). As no other explanation could be found, a familial disease could be causative of cardiomyopathy in these cases. The patients in our study did not systematically undergo molecular genetic analysis. Addition of molecular genetic analysis to the diagnostic workup may help clinicians find a specific diagnosis in even more patients with unexplained cardiomyopathy. Furthermore, there might also be a considerable overlap of patients with genetic traits permissive for HF and overt myocardial inflammation detected by EMB.
We therefore conclude that imaging modalities alone cannot specify the cause of cardiomyopathy in most patients and no causal treatment may be initiated on the basis of only CMRI. Our findings point out the need for a less restrictive approach regarding EMB in patients with unexplained cardiomyopathy to allow disease‐modifying treatment in selected cases. Ideally, a holistic approach involving CMRI, EMB, and molecular genetic testing is desirable to understand the causes of HF and unexplained cardiomyopathy.
Conflict of interest
None declared.
Acknowledgements
We acknowledge the expert graphical work of Margot Neuser. M.B. and P.W. were supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF 01EO1503). This work was supported by the Center for Translational Vascular Biology (CTVB), University Medical Center Mainz.
Sotiriou, E. , Heiner, S. , Jansen, T. , Brandt, M. , Schmidt, K. H. , Kreitner, K.‐F. , Emrich, T. , Schultheiss, H.‐P. , Schulz, E. , Münzel, T. , and Wenzel, P. (2018) Therapeutic implications of a combined diagnostic workup including endomyocardial biopsy in an all‐comer population of patients with heart failure: a retrospective analysis. ESC Heart Failure, 5: 630–641. 10.1002/ehf2.12296.
References
- 1. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013; 15: 808–817. [DOI] [PubMed] [Google Scholar]
- 2. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kühl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007; 50: 1914–1931. [DOI] [PubMed] [Google Scholar]
- 3. Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno‐Blanes J, Helio T, Linhart A, Mogensen J, Pinto Y, Ristic A, Seggewiss H, Sinagra G, Tavazzi L, Elliott PM. Diagnostic work‐up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 1448–1458. [DOI] [PubMed] [Google Scholar]
- 4. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on M , Pericardial D. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648, 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 5. Friedrich MG, Sechtem U, Schulz‐Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel‐Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009; 53: 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Francone M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRN Radiol 2014; 2014: 365404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, Lehmkuhl L, Gutberlet M. Value of cardiovascular MR in diagnosing left ventricular non‐compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol 2012; 22: 2699–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, Vidal V, Bartoli JM, Habib G, Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non‐compaction. Eur Heart J 2010; 31: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 9. Schulz E, Jabs A, Gori T, Hink U, Sotiriou E, Tschope C, Schultheiss HP, Munzel T, Wenzel P. Feasibility and safety of left ventricular endomyocardial biopsy via transradial access: technique and initial experience. Catheter Cardiovasc Interv 2015; 86: 761–765. [DOI] [PubMed] [Google Scholar]
- 10. Noutsias M, Seeberg B, Schultheiss HP, Kühl U. Expression of cell adhesion molecules in dilated cardiomyopathy: evidence for endothelial activation in inflammatory cardiomyopathy. Circulation 1999; 99: 2124–2131. [DOI] [PubMed] [Google Scholar]
- 11. Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005; 111: 887–893. [DOI] [PubMed] [Google Scholar]
- 12. Schenk T, Enders M, Pollak S, Hahn R, Huzly D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol 2009; 47: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pauschinger M, Phan MD, Doerner A, Kuehl U, Schwimmbeck PL, Poller W, Kandolf R, Schultheiss HP. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 1999; 99: 889–895. [DOI] [PubMed] [Google Scholar]
- 14. Koepsell SA, Anderson DR, Radio SJ. Parvovirus B19 is a bystander in adult myocarditis. Cardiovasc Pathol: the official journal of the Society for Cardiovascular Pathology 2012; 21: 476–481. [DOI] [PubMed] [Google Scholar]
- 15. Bock CT, Duchting A, Utta F, Brunner E, Sy BT, Klingel K, Lang F, Gawaz M, Felix SB, Kandolf R. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J Cardiol 2014; 6: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987; 18: 619–624. [DOI] [PubMed] [Google Scholar]
- 17. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus‐negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009; 30: 1995–2002. [DOI] [PubMed] [Google Scholar]
- 18. Xuemin F. Discussion on viral myocarditis prevalence survey during influenza pandemic period. Zhonghua Xin Xue Guan Bing Za Zhi 1984; 1984: 177–179. [Google Scholar]
- 19. Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail 2006; 12: 293–298. [DOI] [PubMed] [Google Scholar]
- 20. Dechkum N, Pangsawan Y, Jayavasu C, Saguanwongse S. Coxsackie B virus infection and myopericarditis in Thailand, 1987‐1989. Southeast Asian J Trop Med Public Health 1998; 29: 273–276. [PubMed] [Google Scholar]
- 21. Lv S, Rong J, Ren S, Wu M, Li M, Zhu Y, Zhang J. Epidemiology and diagnosis of viral myocarditis. Hellenic J Cardiol: HJC = Hellenike kardiologike epitheorese 2013; 54: 382–391. [PubMed] [Google Scholar]
- 22. Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, Kiviluoto O, Davidkin I, Leivo T, Eis‐Hübinger AM, Schneider B, Fischer HP, Tolba R, Vapalahti O, Vaheri A, Söderlund‐Venermo M, Hedman K. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci U S A 2006; 103: 7450–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuethe F, Sigusch HH, Hilbig K, Tresselt C, Gluck B, Egerer R, Figulla HR. Detection of viral genome in the myocardium: lack of prognostic and functional relevance in patients with acute dilated cardiomyopathy. Am Heart J 2007; 153: 850–858. [DOI] [PubMed] [Google Scholar]
- 24. Lindner J, Noutsias M, Lassner D, Wenzel J, Schultheiss HP, Kuehl U, Modrow S. Adaptive immune responses against parvovirus B19 in patients with myocardial disease. J Clin Virol: the official publication of the Pan American Society for Clinical Virology 2009; 44: 27–32. [DOI] [PubMed] [Google Scholar]
- 25. Kühl U, Lassner D, Dorner A, Rohde M, Escher F, Seeberg B, Hertel E, Tschope C, Skurk C, Gross UM, Schultheiss HP, Poller W. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res Cardiol 2013; 108: 372. [DOI] [PubMed] [Google Scholar]
- 26. Bock CT, Klingel K, Kandolf R. Human parvovirus B19‐associated myocarditis. N Engl J Med 2010; 362: 1248–1249. [DOI] [PubMed] [Google Scholar]
- 27. Corcioli F, Zakrzewska K, Rinieri A, Fanci R, Innocenti M, Civinini R, De Giorgi V, Di Lollo S, Azzi A. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J Med Virol 2008; 80: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 28. Sabella C, Goldfarb J. Parvovirus B19 infections. Am Fam Physician 1999; 60: 1455–1460. [PubMed] [Google Scholar]
- 29. Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss H‐P, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 2003; 42: 466–472. [DOI] [PubMed] [Google Scholar]
- 30. Yang F, Wu WF, Yan YL, Pang Y, Kong Q. Alteration of interleukin‐17/interferon‐gamma double positive cells in mice with Coxsackie virus induced myocarditis. Zhonghua Xin Xue Guan Bing Za Zhi 2011; 39: 1135–1139. [PubMed] [Google Scholar]
- 31. Miteva K, Haag M, Peng J, Savvatis K, Becher PM, Seifert M, Warstat K, Westermann D, Ringe J, Sittinger M, Schultheiss HP, Tschope C, Van Linthout S. Human cardiac‐derived adherent proliferating cells reduce murine acute Coxsackievirus B3‐induced myocarditis. PLoS One 2011; 6: e28513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rohrer C, Gartner B, Sauerbrei A, Bohm S, Hottentrager B, Raab U, Thierfelder W, Wutzler P, Modrow S. Seroprevalence of parvovirus B19 in the German population. Epidemiol Infect 2008; 136: 1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stewart GC, Lopez‐Molina J, Gottumukkala RV, Rosner GF, Anello MS, Hecht JL, Winters GL, Padera RF, Baughman KL, Lipes MA. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ Heart Fail 2011; 4: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt‐Lucke C, Zobel T, Schrepfer S, Kühl U, Wang D, Klingel K, Becher PM, Fechner H, Pozzuto T, Van Linthout S, Lassner D, Spillmann F, Escher F, Holinski S, Volk HD, Schultheiss HP, Tschope C. Impaired endothelial regeneration through human parvovirus B19‐infected circulating angiogenic cells in patients with cardiomyopathy. J Infect Dis 2015; 212: 1070–1081. [DOI] [PubMed] [Google Scholar]
- 35. Kühl U, Pauschinger M, Bock T, Klingel K, Schwimmbeck CP, Seeberg B, Krautwurm L, Poller W, Schultheiss HP, Kandolf R. Parvovirus B19 infection mimicking acute myocardial infarction. Circulation 2003; 108: 945–950. [DOI] [PubMed] [Google Scholar]
- 36. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006; 114: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 37. Olimulder M, van Es J, Galjee MA. The importance of cardiac MRI as a diagnostic tool in viral myocarditis‐induced cardiomyopathy. Neth Heart J 2009; 17: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M, Klingel K, Kandolf R, Bohm M, Sechtem U. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010; 122: 900–909. [DOI] [PubMed] [Google Scholar]
- 39. Spieker M, Katsianos E, Gastl M, Behm P, Horn P, Jacoby C, Schnackenburg B, Reinecke P, Kelm M, Westenfeld R, Bonner F. T2 mapping cardiovascular magnetic resonance identifies the presence of myocardial inflammation in patients with dilated cardiomyopathy as compared to endomyocardial biopsy. Eur Heart J Cardiovasc Imaging 2017. 10.1093/ehjci/jex230. [DOI] [PubMed] [Google Scholar]
- 40. Cummings KW, Bhalla S, Javidan‐Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern‐based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics 2009; 29: 89–103. [DOI] [PubMed] [Google Scholar]
- 41. Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M, Radziwolek L, Avanesov M, Tahir E, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging 2017; 18: 744–751. [DOI] [PubMed] [Google Scholar]
- 42. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 43. Elamm CA, Al‐Kindi SG, Bianco CM, Dhakal BP, Oliveira GH. Heart transplantation in giant cell myocarditis: analysis of the United Network for Organ Sharing Registry. J Card Fail 2017; 23: 566–569. [DOI] [PubMed] [Google Scholar]
- 44. Ekstrom K, Lehtonen J, Kandolin R, Raisanen‐Sokolowski A, Salmenkivi K, Kupari M. Incidence, risk factors, and outcome of life‐threatening ventricular arrhythmias in giant cell myocarditis. Circ Arrhythm Electrophysiol 2016; 9: e004559. [DOI] [PubMed] [Google Scholar]
- 45. Dominguez F, Kühl U, Pieske B, Garcia‐Pavia P, Tschope C. Update on myocarditis and inflammatory cardiomyopathy: reemergence of endomyocardial biopsy. Rev Esp Cardiol (Engl Ed) 2016; 69: 178–187. [DOI] [PubMed] [Google Scholar]
- 46. Pawlak A, Gil KE, Dorr M. Viral heart disease—treatment. Kardiol Pol 2017; 75: 1300–1306. [DOI] [PubMed] [Google Scholar]


