Abstract
Aims
This study aimed to evaluate the impact of aerobic exercise training with vascular occlusion in patients with chronic heart failure.
Methods and results
Thirty patients with post‐infarction heart failure were randomized to an interventional exercise group (IG; n = 15) or a control exercise group (CG; n = 15). Exercise was performed at an intensity of 40–70% of the peak VO2/W for 6 months. Patients in the IG remained seated on the saddle of the cycle ergometer with their feet on the pedals. Pneumatic tourniquets were applied to the proximal ends of their thighs with appropriate pressure resulting in a 40–80 mmHg increase in the systolic blood pressure that is required for vascular occlusion (208.7 ± 7.4 mmHg). We evaluated the safety and efficacy of the intervention and its effect on exercise capacity and serum BNP levels. There were no significant differences between the IG and CG in patient characteristics at study entry. Peak VO2/W in the IG significantly increased compared with that in the CG; the change in the serum BNP levels was significantly larger in the IG than in the CG.
Conclusions
These results suggest that aerobic exercise training with vascular occlusion can improve exercise capacity and serum BNP levels in patients with chronic heart failure.
Keywords: Heart failure, Aerobic exercise training, Vascular occlusion
Introduction
Despite major progress in pharmacological and device therapy, patients with chronic heart failure (CHF) are left with reduced exercise capacity and poor quality of life, resulting in muscular atrophy. Indeed, muscular atrophy in patients with CHF is a major determinant of exercise capacity.1 In general, aerobic exercise training is an important adjunct to medical therapy in patients with CHF. However, some patients with CHF do not have enough muscular strength to get the aerobic exercise even at low exercise intensity because of muscular atrophy‐induced exercise intolerance and fatigue.
Neurohormonal activation is a hallmark of CHF.2 The production of brain natriuretic peptide (BNP) by ventricular cardiomyocytes correlates with left ventricular dysfunction.3, 4 Thus, the assay of BNP has been proposed as diagnostic and prognostic tool.4
Aerobic exercise training with vascular occlusion is effective in inducing muscular hypertrophy and concomitant increase in strength.5 The effect of aerobic exercise training with vascular occlusion in patients with CHF on improving exercise capacity is unknown. In the present study, we tested the hypothesis that in patients with CHF, aerobic exercise training with vascular occlusion compared with aerobic exercise training is more effective in improving submaximal exercise capacity. In addition, BNP levels and safety of the intervention were assessed.
Methods
Study protocol
The protocol was approved by the local ethics committee. Patients signed an informed consent form. Patients were randomized to an interventional exercise group (IG; n = 15) or a control exercise group (CG; n = 15). The randomization code was developed with a computer random number generator to select random permuted blocks.
Patients and interventions
We enrolled 30 consecutive patients (30 men; mean age, 60.7 ± 11.1 years) with post‐infarction heart failure from the Department of Cardiovascular Internal Medicine, Yodogawa Christian Hospital, Osaka, Japan, from 2012 to 2014 (Table 1). None of the patients had a myocardial infarction in the 12 months preceding the study. Eight patients were 70 to 80 years old, 10 were 60 to 70 years old, 5 were 50 to 60 years old, and 7 were 40 to 50 years old. Four patients were >70% of left ventricular ejection fraction (LVEF), 6 were 60% to 70%, 8 were 50% to 60%, 2 were 40% to 50%, and 8 were <40% of LVEF. LVEF was evaluated with biplane transthoracic echocardiography by the modified Simpson rule. Inclusion criteria were as follows: clinical stability during the last 3 months and an ability to perform exercise. Exclusion criteria were as follows: recently diagnosed acute coronary syndrome or a recent coronary intervention or both, renal insufficiency (estimated glomerular filtration rate < 30 mL/min), liver abnormalities, uncontrolled hypertension, arteriosclerosis obliterans, and orthopaedic and/or neurological limitations. None of the patients had atrial fibrillation or an artificial cardiac pacemaker.
Table 1.
Patient characteristics and medication use
| IG | CG | P | |
|---|---|---|---|
| Age, years | 58.5 ± 11.2 | 62.9 ± 11.0 | NS |
| Male/female | 15/0 | 15/0 | NS |
| Height, cm | 167.8 ± 7.7 | 167.1 ± 4.5 | NS |
| Weight, kg | 69.7 ± 10.6 | 71.5 ± 11.7 | NS |
| BMI | 24.7 ± 3.5 | 25.6 ± 3.9 | NS |
| Previous cardiac disease | OMI 15 | OMI 15 | NS |
| NYHA functional class I/II | 7/8 | 10/5 | NS |
| BNP, pg/mL | 148.1 ± 118.7 | 144.5 ± 174.1 | NS |
| BUN, mg/dL | 16.6 ± 6.6 | 17.6 ± 8.8 | NS |
| Creatinine, mg/dL | 1.0 ± 0.3 | 1.0 ± 0.3 | NS |
| eGFR, mL/min/1.73m2 | 62.0 ± 19.2 | 65.0 ± 15.4 | NS |
| Glucose, mg/dL | 123.9 ± 24.0 | 131.4 ± 30.3 | NS |
| HbA1c, % | 6.0 ± 0.6 | 6.7 ± 1.2 | <0.05 |
| TG, mg/dL | 134.0 ± 44.9 | 163.2 ± 109.7 | NS |
| HDL‐C, mg/dL | 44.0 ± 21.6 | 46.7 ± 9.0 | NS |
| LDL‐C, mg/dL | 104.4 ± 42.4 | 140.6 ± 57.3 | <0.05 |
| Uric acid, mg/dL | 6.8 ± 2.1 | 7.6 ± 2.4 | NS |
| C‐reactive protein, mg/dL | 0.5 ± 0.6 | 0.4 ± 0.7 | NS |
| EF, % | 49.3 ± 15.8 | 54.4 ± 16.3 | NS |
| E/A | 1.1 ± 0.8 | 0.9 ± 0.2 | NS |
| Peak VO2/W, mL/kg/min | 16.3 ± 3.6 | 16.1 ± 3.4 | NS |
| ACE‐I/ARB (n) | 3/5 | 9/3 | <0.05 |
| Beta‐blocker (n) | 13 | 9 | NS |
| Aldosterone antagonist (n) | 6 | 3 | NS |
| Statin (n) | 10 | 13 | NS |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; BMI, body mass index; BUN, blood urea nitrogen; CG, control exercise group; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; IG, interventional exercise group; LDL‐C, low density lipoprotein cholesterol; NS, not significant; NYHA, New York Heart Association; TG, triglyceride; OMI, old myocardial infarction.
Data are mean ± standard deviation or number of patients.
Exercise in IG and CG was performed on a cycle ergometer (Aerobike 75XL III, Combi Wellness) at an intensity of 40–70% of the peak VO2/W for 15 min three times a week for 6 months. Heart rate was monitored during exercise sessions in all patients. On study entry and at 6 months, all patients underwent cardiopulmonary exercise testing, blood chemistry, and thigh circumference evaluation.
Interventional exercise group patients remained seated on the saddle of the cycle ergometer with their feet on the pedals. Pneumatic tourniquets (width: 90 mm; length: 700 mm) were applied to the proximal ends of their thighs with appropriate pressure resulting in a 40–80 mmHg increase in the systolic blood pressure that is required for vascular occlusion (208.7 ± 7.4 mmHg). No subject was allowed to participate in a training session without 2 days recovery from the prior session. Each training session began with a 5 min warm‐up period, followed by adjustment of the cycle ergometer resistance for each individual. The researcher instructed the patients about when to begin and when to stop pedalling during each training session. The cuffs remained inflated on the patients in the IG only during the cycle ergometer session.
Blood chemistry
In all patients, blood was sampled from an antecubital vein before cardiopulmonary exercise testing. Serum BNP levels was measured with standard procedures at the Yodogawa Christian Hospital, Osaka, Japan.
Thigh circumference
In all patients, thigh circumference was measured in the supine position at a point 15 cm above the patella before cardiopulmonary exercise testing.
Cardiopulmonary exercise testing
A symptom‐limited cardiopulmonary exercise test was performed on a cycle ergometer, and the work rate was increased in a ramp pattern. All exercise tests were performed in the morning ≥3 h after a light breakfast. Expired gases and volumes were analysed on a breath‐by‐breath basis using an AE‐300S Aeromonitor (Minato Medical Science, Osaka, Japan). The heart rate and blood pressure were measured every minute during increasing work rate exercise and recovery. The exercise test was stopped when ≥1 of the following criteria were present: fatigue, dyspnoea, excessive systemic blood pressure increase (>230/130 mmHg), >2 mm ST depression in >2 adjacent leads, and/or angina. The anaerobic threshold was measured by the V‐slope method.6 Peak VO2/W was the mean oxygen uptake during the last 15 s of exercise. Ventilation (VE) and carbon dioxide output (VCO2) were measured, and the gradient of the VE–VCO2 relationship (VE vs. VCO2 slope) was determined. All exercise tests were conducted at the Yodogawa Christian Hospital, Osaka, Japan.
Statistical analysis
Data were analysed using repeated‐measures two‐way ANOVAs with a between‐participant factor of group (IG or CG) and a within‐participant factor of time points of measurements (initial, after 6 months). Greenhouse–Geisser corrections were applied when appropriate to adjust for non‐sphericity. Post hoc analysis used the Bonferroni correction for multiple comparisons. A significance threshold of P < 0.05 was chosen for all tests. All statistical analyses were performed with SPSS 25.0 for Mac (SPSS Inc., Chicago, IL).
Result
Table 1 summarizes patient characteristics and medication use in IG and CG. There were significant differences in haemoglobin A1c (HbA1c) and low density lipoprotein cholesterol (LDL‐C) levels between the IG and CG at baseline (Table 1). Patients were treated with angiotensin‐converting enzyme inhibitor (ACE‐I) or angiotensin receptor blocker (ARB) (IG: 53.3%, CG: 80.0%, P < 0.05), beta‐blockers (IG: 86.7%, CG: 60.0%, P = NS), and aldosterone antagonists (IG: 40.0%, CG: 33.3%, P = NS). The treatment did not change during the intervention period. There were no significant difference in the measured exercise intensity between the IG and CG during the training sessions (Figure 1 ).
Figure 1.
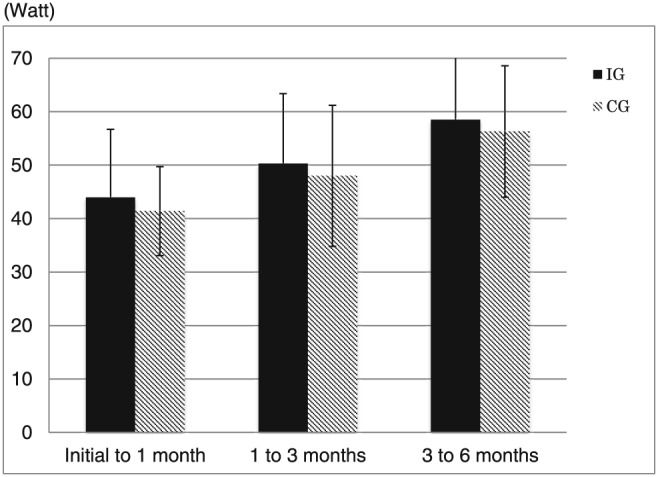
The measured exercise intensity of initial to 1 month, 1 to 3 months, and 3 to 6 months. There were no significant differences in the measured exercise intensity between the interventional exercise group (IG) and control exercise group (CG) during the training sessions.
Clinical follow‐up
After 6 months of exercise training, the serum triglyceride, high‐density lipoprotein, LDL‐C, total cholesterol, glucose, and HbA1c levels did not change (Table 2). No adverse effects of exercise training were noted.
Table 2.
Metabolic parameters
| IG | CG | |||
|---|---|---|---|---|
| Initial | 6 months | Initial | 6 months | |
| BNP, pg/mL | 148.1 ± 118.7 | 75.3 ± 70.6* | 144.5 ± 174.1 | 129.3 ± 124.0 |
| BUN, mg/dL | 16.6 ± 6.6 | 19.0 ± 5.8 | 17.6 ± 8.8 | 18.9 ± 7.9 |
| Creatinine, mg/dL | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.2 |
| eGFR, mL/min/1.73m2 | 62.0 ± 19.2 | 63.4 ± 18.9 | 65.0 ± 15.4 | 62.3 ± 12.8 |
| Glucose, mg/dL | 123.9 ± 24.0 | 113.6 ± 12.3 | 131.4 ± 30.3 | 151.6 ± 90.8 |
| HbA1c, % | 6.0 ± 0.6 | 6.1 ± 0.5 | 6.7 ± 1.2 | 6.6 ± 1.5 |
| TG, mg/dL | 134.0 ± 44.9 | 139.9 ± 109.7 | 163.2 ± 109.7 | 147.4 ± 92.1 |
| HDL‐C, mg/dL | 44.0 ± 21.6 | 47.5 ± 14.5 | 46.7 ± 9.0 | 46.5 ± 12.2 |
| LDL‐C, mg/dL | 104.4 ± 42.4 | 81.0 ± 17.7 | 140.6 ± 57.3 | 98.5 ± 28.3 |
| Uric acid, mg/dL | 6.8 ± 2.1 | 6.8 ± 1.0 | 7.6 ± 2.4 | 6.4 ± 1.5 |
| C‐reactive protein, mg/dL | 0.5 ± 0.6 | 0.1 ± 0.1* | 0.4 ± 0.7 | 0.2 ± 0.2 |
BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CG, control exercise group; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; IG, interventional exercise group; LDL‐C, low density lipoprotein cholesterol; TG, triglyceride.
Data are mean ± standard deviation.
Different from initial, P < 0.05.
Exercise capacity
After 6 months of exercise training, the peak VO2/W in the IG significantly increased compared with that in the CG [IG: 16.3 ± 3.6 to 22.1 ± 3.7, F(1, 28) = 18.55; P = 0.0002; η 2 = 0.398; CG: 16.1 ± 3.4 to 18.1 ± 3.5, F(1, 28) = 2.598; P = 0.118; η 2 = 0.085; Table 3]. After 6 months of exercise training, there were significant differences in changes of peak VO2/W between the IG and CG [38.7 ± 24.2 vs. 13.5 ± 12.4%, F(1, 28) = 12.798; P = 0.002; η 2 = 0.314; Figure 2 ].
Table 3.
Exercise parameters
| IG | CG | |||
|---|---|---|---|---|
| Initial | 6 months | Initial | 6 months | |
| Weight, kg | 69.7 ± 10.6 | 70.5 ± 9.3 | 69.8 ± 11.3 | 69.7 ± 11.8 |
| BMI, kg/m2 | 24.7 ± 3.5 | 25.0 ± 2.5 | 25.6 ± 3.9 | 25.7 ± 4.1 |
| Thigh circumference (R), cm | 47.3 ± 6.1 | 49.7 ± 4.4 | 47.8 ± 4.7 | 47.6 ± 4.3 |
| Thigh circumference (L), cm | 48.1 ± 6.3 | 50.0 ± 4.6 | 47.8 ± 4.7 | 47.7 ± 4.2 |
| PeakVO2/W, mL/kg/min | 16.3 ± 3.6 | 22.1 ± 3.7* | 16.1 ± 3.4 | 18.1 ± 3.5 |
| AT, mL/kg/min | 13.0 ± 2.3 | 15.3 ± 2.0* | 12.1 ± 2.3 | 13.7 ± 2.2 |
| VE vs. VCO2 slope | 31.7 ± 4.8 | 29.7 ± 4.5 | 31.0 ± 3.0 | 30.8 ± 4.6 |
AT, anaerobic threshold; BMI, body mass index; CG, control exercise group; IG, interventional exercise group; L, left; R, right; VE vs. VCO2 slope, ventilation and carbon dioxide output relationship.
Data are mean ± standard deviation.
Different from initial, P < 0.05.
Figure 2.
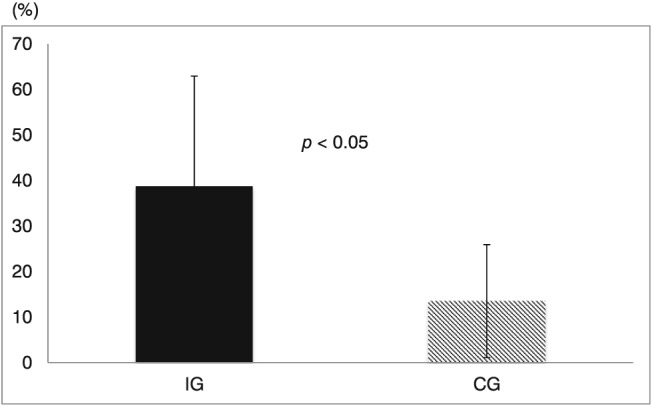
Change from initial of peak VO2/W. Probability values inside figures indicate within‐group differences. There were significant differences in changes of peak VO2/W between the interventional exercise group (IG) and control exercise group (CG).
Brain natriuretic peptide
After 6 months of exercise training, the change in serum BNP levels was significantly larger in the IG than in the CG [−72.8 ± 76.7 vs. −13.3 ± 91.0 pg/mL, F(1, 28) = 3.747; P = 0.032; η 2 = 0.118; Figure 3 ]. There were significant inverse correlations between changes in BNP levels and exercise capacity in the IG compared with the CG (IG: R = 0.74; CG: R = 0.33; Figure 4 ).
Figure 3.
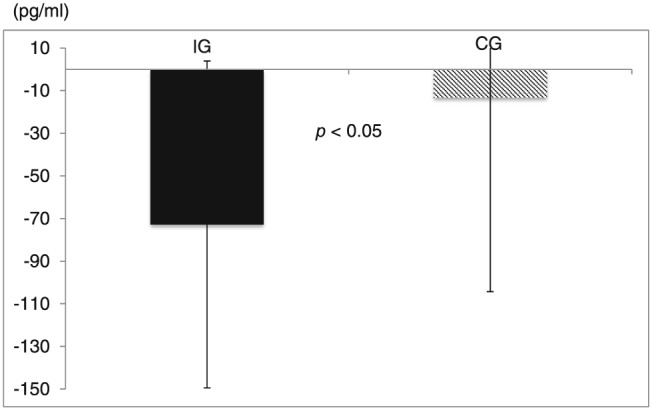
Change from initial of serum BNP levels. Probability values inside figures indicate within‐group differences. The change in serum BNP levels was significantly larger in the interventional exercise group (IG) than in the control exercise group (CG).
Figure 4.
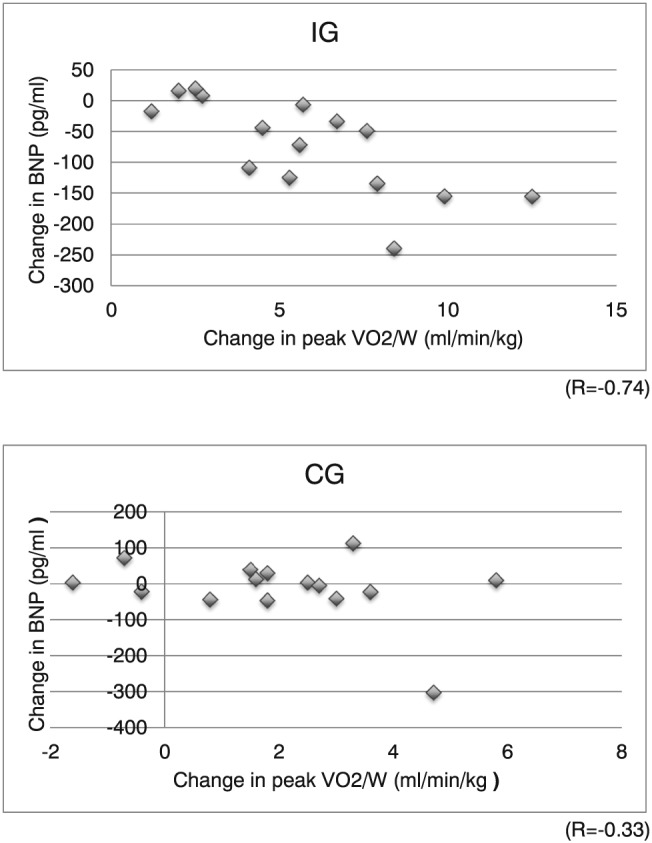
Relation between the changes in peak VO2/W and in plasma concentration of BNP in both groups. CG, control exercise group; IG, interventional exercise group.
Discussion
Exercise capacity
The primary findings of this study are as follows: aerobic exercise training with vascular occlusion can improve exercise capacity. Vascular occlusion training is an exercise practice developed to increase skeletal muscle mass and strength in normal, healthy individuals.7, 8, 9 Popovici demonstrated that combining partial vascular occlusion with low loads during a complex high‐speed task such as sprinting on a cycle ergometer can improve peak power as effectively as traditional sprint training with high resistance.10 Furthermore, Takarada et al. demonstrated that blood flow through the superficial femoral artery after occlusive low‐intensity cycle exercise reached a level comparable with that observed in a preliminary study of moderate‐intensity (65% of HRmax) cycle exercise without occlusion. This resulted in a marked increase in the change in diameter, with elevated nitric oxide production that probably accounted for the improvement in vasodilatation.11 The enhancement of post‐exercise hyperaemia by low‐intensity cycle exercise with vascular occlusion could be directly related to the occlusion and reperfusion involved in the occlusive exercise, leading to a greater change in superficial femoral artery diameter. In skeletal muscle, nitric oxide has been suggested to be involved in the expression of cyclooxygenase 2.12 The COX enzymes catalyse production of prostaglandins from arachidonic acid; one of these, prostaglandin F2a, may regulate skeletal muscle hypertrophy by activating both cell fusion and protein synthesis.13 The results presented previously suggest that a low‐intensity cycle exercise with vascular occlusion brings about acute responses similar to those observed in low‐intensity resistance exercise aimed at muscular hypertrophy.
Although there are numerous studies demonstrating the muscular benefits of aerobic exercise training with vascular occlusion, studies investigating the cardiovascular effects of vascular occlusion are sparse.14
The results of this study indicate that aerobic exercise training with vascular occlusion can improve exercise capacity in patients with CHF.
In addition, aerobic exercise training in male patients with CHF has been postulated to be useful for cardiovascular mortality compared with female patients.15 In this context, we enrolled male patients with CHF.
Brain natriuretic peptide
BNP is a useful diagnostic tool for heart failure in Japan, as well as other countries.16, 17, 18, 19
Passino et al. reported that exercise training decreases plasma BNP levels in patients with moderate CHF.20 Our results are consistent with those of that study. The significant inverse correlation that we found between changes in BNP levels and exercise capacity is also consistent with previous reported results.20
Study limitations
There are some limitations in the present study that should be emphasized. First, the study population was relatively small. Therefore, there were significant differences between IG and CG on HbA1c, LDL‐C, and ACE‐I/ARB. However, the sample size is in line with most CHF training studies.21, 22 Second, all the patients in the present study were clinically stable. Therefore, beta‐blocker, ACE‐I/ARB, and aldosterone antagonist therapy were not standardized across the study participants. Further studies are necessary to address these issues. Third, we used young patients for the present investigation. Haemodynamic responses to aerobic exercise training with vascular occlusion may be different in older patients.
Conclusions
These results suggest that aerobic exercise training with vascular occlusion can improve exercise capacity and BNP levels in patients with CHF without serious adverse events.
Conflict of interest
None declared.
Tanaka, Y. , and Takarada, Y. (2018) The impact of aerobic exercise training with vascular occlusion in patients with chronic heart failure. ESC Heart Failure, 5: 586–591. 10.1002/ehf2.12285.
References
- 1. Harrington D, Anker SD, Chua TP, Webb‐Peploe KM, Ponikowski PP, Poole‐Wilson PA, Coats AJS. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol 1997; 30: 1758–1764. [DOI] [PubMed] [Google Scholar]
- 2. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 1992; 20: 248–254. [DOI] [PubMed] [Google Scholar]
- 3. Clerico A, Emdin M. Diagnostic accuracy and prognostic relevance of the measurement of cardiac natriuretic peptides: a review. Clin Chem 2004; 50: 33–50. [DOI] [PubMed] [Google Scholar]
- 4. Emdin M, Passino C, Prontera C, Iervasi A, Ripoli A, Massini S, Zucchelli GC, Clerico A. Cardiac natriuretic hormones, neuro‐hormones, thyroid hormones and cytokines in normal subjects and patients with heart failure. Clin Chem Lab Med 2004; 42: 627–636. [DOI] [PubMed] [Google Scholar]
- 5. Takarada Y, Takazawa H, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 2000; 88: 2097–2106. [DOI] [PubMed] [Google Scholar]
- 6. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 7. Abe T, Fujita S, Ishii N, Nakajima T, Sakamaki M, Ozaki H, Ogasawara R, Sugaya M, Kudo M, Kurano M, Yasuda T, Sato Y, Ohshima H, Mukai C. Effects of low‐intensity cycle training with restricted leg blood flow on thigh muscle volume and VO2MAX in young men. J Sports Sci Med 2010; 9: 452–458. [PMC free article] [PubMed] [Google Scholar]
- 8. Credeur DP, Hollis BC, Welsch MA. Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exerc 2010; 42: 1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowery RP, Joy JM, Loenneke JP, de Souza EO, Machado M, Dudeck JE, Wilson JM. Practical blood flow restriction training increases muscle hypertrophy during a periodized resistance training programme. Clin Physiol Funct Imaging 2014; 34: 317–321. [DOI] [PubMed] [Google Scholar]
- 10. Popovici C. The impact of low-load training with partial vascular occlusion on cycle ergometer peak power. (Unpublished masters thesis). State University of New York College, Cortland, 2013.
- 11. Takarada Y, Ito M. Endocrine and hyperemic responses to low‐intensity aerobic exercise with vascular occlusion. Sports Science Research 2012; 9: 350–365. [Google Scholar]
- 12. Soltow A, Betters L, Criswell S, Sellman JE, Lira VA, Long JHD. Ibuprofen inhibits skeletal muscle hypertrophy in rats. Med Sci Sports Exerc 2006; 38: 840–846. [DOI] [PubMed] [Google Scholar]
- 13. Horsley V, Pavlath K. Prostaglandin F2α stimulates growth of skeletal muscle cells via an NFATC2‐dependent pathway. J Cell Biol 2003; 161: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takano H, Morita T, Nakajima T, Iida H, Asada K‐i, Kato M, Uno K, Herose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y. Haemodynamic and hormonal responses to a short‐term low‐intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 2005; 95: 65–73. [DOI] [PubMed] [Google Scholar]
- 15. Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004; 328: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 17. Okuda S, Yano M. Guidelines for treatment of chronic heart failure (JCS 2010). Nihon Rinsho 2011; 69(Suppl 9): 595–604. [PubMed] [Google Scholar]
- 18. Japanese Circulation Society . Guidelines for treatment of acute heart failure (JCS 2011). Circ J 2013; 77: 2157–2201. [DOI] [PubMed] [Google Scholar]
- 19.2013 ACCF/AHA Guideline for the Management of Heart Failure, authors. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines
- 20. Passino C, Severino S, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. Aerobic training decreases B‐type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 2006; 47: 1835–1839. [DOI] [PubMed] [Google Scholar]
- 21. Wisløff U, Støylen A, Skjærpe T, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007; 115: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 22. Spee R, Niemeijer V, Wijn P, Doevendans P, Kemps H. Effects of high‐intensity interval training on central haemodynamics and skeletal muscle oxygenation during exercise in patients with chronic heart failure. Eur J Prev Cardiol 2016; 23: 1943–1952. [DOI] [PubMed] [Google Scholar]


