Abstract
Aim
Obtain initial estimates of the change in brachial artery endothelial function and maximal oxygen uptake (VO2peak) with 8 weeks of low‐frequency electrical muscle stimulation (LF‐EMS) or sham in patients with advanced chronic heart failure.
Methods and results
Using a double blind, randomized design, 35 patients with chronic heart failure (New York Heart Association class III–IV) were assigned to 8 weeks (5 × 60 min per week) of either LF‐EMS (4 Hz, continuous) or sham (skin level stimulation only) of the quadriceps and hamstrings muscles. Four of the five sessions were at home and one under supervision. Ultrasound images of resting brachial artery diameter and post 5 min occlusion to determine flow‐mediated dilation (FMD), a marker of vascular function and peak oxygen uptake (VO2peak) during cardiopulmonary exercise test, were measured before and after LF‐EMS (n = 20) and sham (n = 15) interventions. FMD improved by 2.56% (95% confidence interval: 0.69 to 3.80) with LF‐EMS compared with sham (P = 0.07). There were no notable changes in VO2peak.
Conclusions
Improvements in FMD with LF‐EMS may have a clinically meaningful effect as higher FMD is associated with better prognosis. This is a preliminary finding, and a larger trial is warranted.
Keywords: Advanced heart failure, Cardiac rehabilitation, Endothelial function electrical muscle stimulation, Neuromuscular electrical stimulation, Flow‐mediated dilation
Introduction
New York Heart Association (NYHA) class III/IV chronic heart failure (CHF) patients are unable to perform simple activities of daily living.1 Low fitness2 and endothelial dysfunction3 are predictors of mortality in CHF and are useful targets for treatment. Low‐frequency electrical muscle stimulation (LF‐EMS) has been explored as a potential therapy in patients with mild CHF with positive outcomes.4, 5, 6 Improvements in exercise capacity and endothelial function with LF‐EMS in patients with CHF NYHA class III/IV could reduce the incidence of all‐cause mortality7 and improve overall quality of life. We have reported previously that a randomized control trial is feasible in this patient group, but minimal improvements in quality of life and functional capacity were evident.8 Here, we aimed to obtain initial estimates of the change in brachial artery endothelial function and maximal oxygen uptake (VO2peak) with LF‐EMS in a subset of patients with CHF class III/IV from our larger feasibility trial.8 We hypothesized that 8 weeks of LF‐EMS would enhance endothelial function and cardiorespiratory fitness.
Methods
Research design
Fifty‐six participants with stable CHF NYHA functional class III–IV symptoms (ejection fraction <40% on echocardiography, Table 1) were randomized to either LF‐EMS (n = 28) or ‘sham’ placebo (n = 28) for a period of 8 weeks in a double blind, parallel group, randomized controlled feasibility trial, which is reported elsewhere.8 A subset of participants (LF‐EMS n = 20, sham n = 15, Figure 1 ) from the original trial were able to tolerate the additional research measurement (withdrawn, n = 10; unable to tolerate tests, n = 5; refused tests due to illness, n = 6) and underwent assessment of endothelial function and maximal oxygen uptake. Participants with implantable cardiac devices, life‐threatening cardiac arrhythmias, neurological disorders, or previous stroke were excluded. The study received ethical approval and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Table 1.
Baseline demographic and clinical characteristics of the LF‐EMS and sham placebo groups
| EMS intervention (n = 20) | Sham (n = 15) | P value | |
|---|---|---|---|
| Demographics | |||
| N, Male | 13 (65%) | 10 (66.6%) | 0.92 |
| Age (years) | 68.6 ± 9.4 | 66.7 ± 6.8 | 0.59 |
| BMI (kg/m2) | 29.5 ± 4.7 | 27.8 ± 5.4 | 0.1 |
| Clinical | |||
| NT‐proBNP (pg/mL) | 3052 ± 3398 | 2132 ± 2012 | 0.23 |
| Creatinine (μmol/L) | 101 ± 47 | 109 ± 41 | 0.45 |
| LVEF % | 39 ± 11 | 22 ± 12 | 0.42 |
| BPsys (mmHg) | 116 ± 19 | 123 ± 14 | 0.16 |
| BPdia (mmHg) | 67 ± 11 | 70 ± 8 | 0.23 |
| NYHA III | 14 (70%) | 11 (73.3%) | 0.83 |
| NYHA IV | 6 (30%) | 4 (26.7%) | 0.83 |
| Co‐morbidities | |||
| Prev MI/PCI/CABG | 13 (65%) | 8 (53.3%) | 0.49 |
| Diabetes | 10 (50%) | 7 (46.6%) | 0.84 |
| COPD | 5 (25%) | 3 (20%) | 0.73 |
| AF | 14 (68%) | 9 (60%) | 0.62 |
| Hypertension | 9 (45%) | 7 (46.6%) | 0.92 |
| CKD | 5 (25%) | 9 (60%) | 0.03 |
AF, atrial fibrillation; BMI, body mass index; BPdia, diastolic blood pressure; BPsys, systolic blood pressure; CABG, coronary artery bypass graft surgery; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LF‐EMS, low‐frequency electrical muscle stimulation; LVEF, left ventricular ejection fraction; NT‐proBNP, N terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Data presented as mean ± standard deviation or absolute number and per cent.
Figure 1.
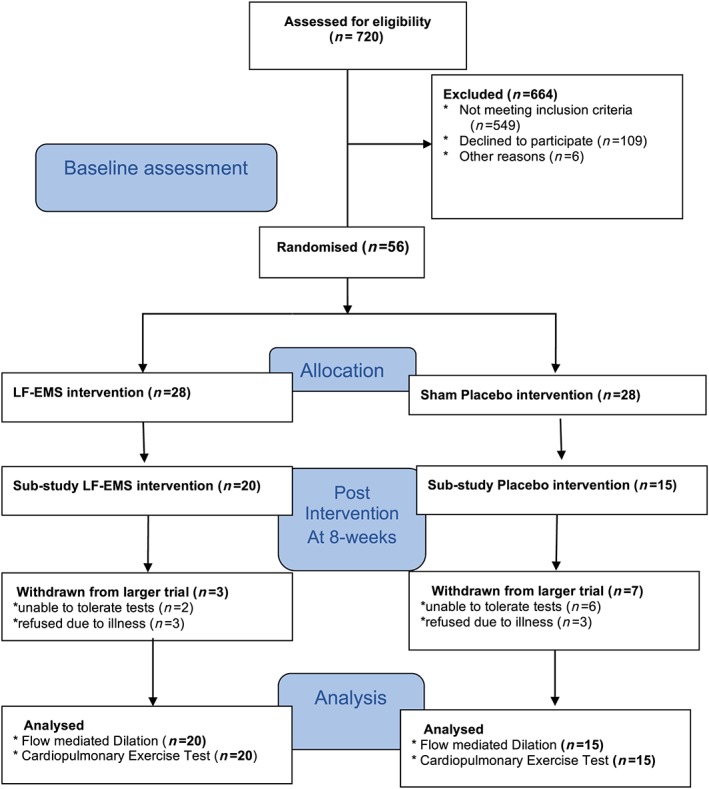
Consort diagram of the study; low‐frequency electrical muscle stimulation (LF‐EMS) vs. sham placebo in severe heart failure patients.
Low‐frequency electrical muscle stimulation/sham stimulation
Detailed description of the LF‐EMS and sham interventions is described previously.8 Briefly, the LF‐EMS equipment (Biomedical Research Limited, Galway, Ireland) containing built‐in adhesive gel electrodes was worn on the upper legs. The LF‐EMS group received stimulation at a pulse frequency of 4 Hz (pulse width: 620 μs, maximum current amplitude: 140 mA). The sham group received a very low level of stimulation (frequency: 99 Hz, pulse width: 150 μs, maximum current amplitude: 7.3 mA). Participants used the LF‐EMS or sham for 1 h, five times a week, for 8 weeks. Four of the five sessions were at home and one under supervision.
Measurements
Brachial artery endothelium‐dependent vasodilation was assessed using the flow‐mediated dilation (FMD) technique following recommended methods.9 Briefly, the brachial artery was imaged using high‐resolution ultrasound (Tersaon t3000, Aloka, UK) to detect the change in arterial diameter in response to a 5 min ischaemic stimulus induced by forearm cuff inflation to 220 mmHg.9 Diameter, flow, and shear stress were measured prior to and following 5 min of forearm cuff inflation. Analysis was performed using custom‐designed edge‐detection and wall‐tracking software by a single person who was blinded to treatment allocation. Allometric scaling for baseline diameter was performed.10 Maximal cardiopulmonary exercise testing was performed in accordance with American Thoracic Society guidelines11 on an upright cycle ergometer with an exercise respiratory gas analysis system (Oxycon Pro, Jaeger, Warwick, Warwickshire, UK). All assessments were performed prior to and following LF‐EMS or sham.
Statistical analysis
Given that this is a feasibility study, no a priori sample size was calculated. The sample size of each group in this current sub‐study provides 47% power to detect a between‐group difference in FMD of 1.0% (equivalent to a 20% decreased mortality in patients with CHF3) assuming a standard deviation of 1.5% for within group change scores and using a two‐sided independent t‐test (G*Power 3.1.5).12 This sample size was deemed appropriate to enable an estimate of sample size for a larger trial.
Delta changes (∆) from pre‐intervention were calculated for each group and entered as the dependent variable in a linear mixed model (Statistical Package for the Social Sciences, Version 20: SPSS Inc., Chicago, IL) with pre‐intervention data entered as a covariate. Data are presented in the text as mean and 95% confidence intervals with exact P values.
Results
Brachial artery FMD improved by 2.56% (95% confidence interval: 0.69 to 3.80) with LF‐EMS compared with sham (Figure 2 ), which approached statistical significance (P = 0.07). Based on this outcome, it was estimated that 86 participants per group would be required to have 80% power to detect a statistically significant (P < 0.05) between‐group differences in FMD.
Figure 2.
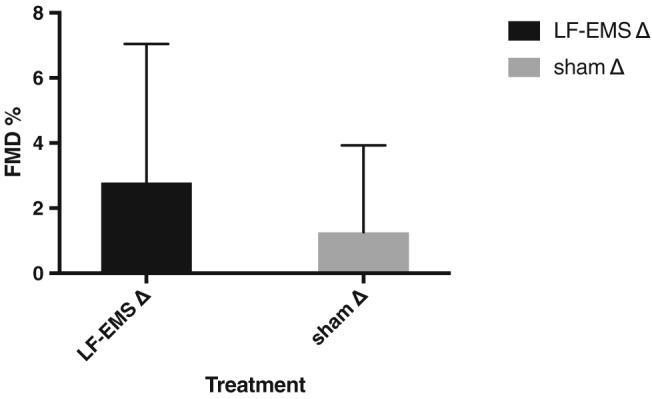
Mean flow‐mediated dilation (FMD) change after 8‐week low‐frequency electrical muscle stimulation (LF‐EMS) or sham. Error bars are standard deviations.
There was also a trend towards a smaller arterial diameter (Table 2) with sham vs. LF‐EMS (P = 0.08). There were no notable intervention‐mediated changes in peak arterial diameter or shear rate and time to peak. There were negligible changes in VO2peak in both groups following the 8 week intervention period (Table 2).
Table 2.
Changes in endothelial function and CPET performance after 8‐week EMS or sham intervention
| Pre‐LF‐EMS | LF‐EMS Δ change | Pre‐sham | Sham Δ change | P | |
|---|---|---|---|---|---|
| Endothelial function | |||||
| FMD (%) | 5.48 (4.34 to 6.32) | 2.79 (0.8 to 1.99) | 5.43 (3.47 to 7.39) | 1.26 (−0.22 to 2.7) | 0.075 |
| Baseline diameter(cm) | 0.43 (0.39 to 0.47) | 0.00(−0.02 to 0.01) | 0.46 (0.42 to 0.5) | −0.01 (−0.04 to 0.02 | 0.086 |
| Peak diameter (cm) | 0.46 (0.42 to 0.49) | 0.01 (−0.01 to 0.03) | 0.48 (0.45 to 0.52) | 0.00 (−0.03 to 0.03′) | 0.268 |
| Shear rateAUC | 14 048.71 (10 127.90 to 17 969.52 | −2735.94 (−7148.93 to 1677.07) | 12 700.18 (7269.49 to 18 130.87) | 1127.39 (−5262.84 to 7517.63′) | 0.953 |
| Time to peak (s) | 73.45 (57.52 to 89.37) | −11.00 (−31.83 to 9.82) | 70.04 (48.94 to 91.13) | 4.09 (−28.16 to 36.34) | 0.887 |
| Maximal O2 uptake | |||||
| VO2 peak (mL/kg/min) | 13.87 (12.47 to 15.26) | −0.19 (−1.05 to 0.69) | 12.87 (10.99 to 14.75) | 0.06 (−0.75 to 0.87) | 0.922 |
| Max watts output | 67.25 (56.12 to 78.38) | −1.70 (−9.01 to 5.61) | 69.12 (53.94 to 84.29) | −2.29 (−7.64 to 3.05) | 0.999 |
| Anaerobic threshold (mL/kg/min) | 8.84 (7.31 to 10.38) | −0.11 (−0.2 to 2.3) | 8.05 (6.21 to 9.89) | 0.64 (0.18 to 0.32) | 0.893 |
CPET, cardiopulmonary exercise testing; FMD, flow‐mediated dilation; LF‐EMS, low‐frequency electrical muscle stimulation.
Data was analysed using general estimating equations and presented as mean (95% CI). Delta (Δ) change from baseline values (95%CI).
Discussion
We provide preliminary evidence towards enhanced endothelial function following LF‐EMS compared with sham in patients with CHF NYHA III/IV. Despite no notable changes in VO2peak, these data suggest that the impact of LF‐EMS on endothelial function should be explored in a larger trial.
This is the first study to assess the impact of LF‐EMS on FMD in patients with advanced CHF. Our data suggest that a sample size of 86 patients per group would be required to show statistical improvement in FMD with LF‐EMS. We show preliminary evidence of a clinically relevant improvement in FMD greater than 1%.3 An improvement of similar or greater magnitude in FMD with a fully powered, larger study would be important for this group of high‐risk patients, given that (i) a 1% increase in FMD is associated with a 20% decreased mortality in CHF patients3, 7 and (ii) this group of patients are physically debilitated and generally contradicted for exercise‐based cardiac rehabilitation. Therefore, LF‐EMS may offer an alternative means to conventional exercise in altering blood flow (shear) stress patterns against the artery walls to improve vascular function. A noteworthy observation to support the endothelial function data was evidence of a decrease in artery size in the sham intervention. A change in artery size may suggest that the health of the artery is deteriorating possibly related to persistent physical inactivity,13 but a positive impact of LF‐EMS on artery size may maintain or augment endothelial function.
The measurement of VO2peak is challenging in this population. Many participants were unable to meet the requirements for peak oxygen uptake including respiratory exchange ratio < 1.10 and test duration <8 min,13 suggesting musculoskeletal issues rather than oxygen uptake were limiting exercise capacity. Given these issues, together with the findings from the 6 min walk test previously reported, measuring VO2peak in any larger study may not be appropriate.
Participants were deemed eligible for the study based on the judgement of experienced heart failure clinicians using available knowledge. This may have led to greater variability in disease severity/limitation between groups; this potential confounding can be factored into the randomization procedure of any large trial.
In summary, LF‐EMS could be useful in improving FMD in patients with CHF NYHA III/IV, which could improve mortality and should be explored in a larger trial.
Conflict of interest
None declared.
Author contributions
S.E., G.M., and P.B. conceived the work; S.E., G.M., P.B., B.M., H.J., and R.S. designed the work; S.E. and G.M. acquired the data; S.E., G.M., P.B., B.M., H.J., R.J., and A.T. analysed and interpreted the data; S.E. and H.J. drafted the manuscript; P.B., B.M., H.J., R.S., and A.T revised the manuscript; All gave final approval and agree to be accountable for integrity and accuracy of this work.
Ennis, S. , McGregor, G. , Shave, R. , McDonnell, B. , Thompson, A. , Banerjee, P. , and Jones, H. (2018) Low frequency electrical muscle stimulation and endothelial function in advanced heart failure patients. ESC Heart Failure, 5: 727–731. 10.1002/ehf2.12293.
References
- 1. Kop WJ, Synowski SJ, Gottlieb SS. Depression in heart failure: biobehavioral mechanisms. Heart Fail Clin. 2011; 7: 23–38. [DOI] [PubMed] [Google Scholar]
- 2. Aslanger E, Assous B, Bihry N, Beauvais F, Logeart D, Cohen‐Solal A. Effects of cardiopulmonary exercise rehabilitation on left ventricular mechanical efficiency and ventricular‐arterial coupling in patients with systolic heart failure. J Am Heart Assoc 2015; 4: e002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 2005; 111: 310–314. [DOI] [PubMed] [Google Scholar]
- 4. Smart NA, Dieberg G, Giallauria F. Functional electrical stimulation for chronic heart failure: a meta‐analysis. Int J Cardiol 2013; 167: 80–86. [DOI] [PubMed] [Google Scholar]
- 5. Dobsák P, Nováková M, Siegelová J, Fiser B, Vítovec J, Nagasaka M, Kohzuki M, Yambe T, Nitta S, Eicher JC, Wolf JE, Imachi K. Low‐frequency electrical stimulation increases muscle strength and improves blood supply in patients with chronic heart failure. Circ J 2006; 70: 75–82. [DOI] [PubMed] [Google Scholar]
- 6. Nuhr MJ, Pette D, Berger R, Quittan M, Crevenna R, Huelsman M, Nuhr MJ. Beneficial effects of chronic low‐frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J 2004; 25: 136–143. [DOI] [PubMed] [Google Scholar]
- 7. Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail 2009; 11: 588–593. [DOI] [PubMed] [Google Scholar]
- 8. Ennis S, McGregor G, Hamborg T, Jones H, Shave R, Singh SJ, Banerjee P. Randomised feasibility trial into the effects of low‐frequency electrical muscle stimulation in advanced heart failure patients. BMJ Open 2017; 7: e016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300: H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atkinson G, Batterham AM. The percentage flow‐mediated dilation index: a large‐sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med (London, England) 2013; 18: 354–365. [DOI] [PubMed] [Google Scholar]
- 11. Society AT, Physicians ACoC . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277. [DOI] [PubMed] [Google Scholar]
- 12. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 13. de Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT. Rapid and extensive arterial adaptations after spinal cord injury. Arch Phys Med Rehabil 2006; 87: 688–696. [DOI] [PubMed] [Google Scholar]


