Abstract
Aims
This study aims to assess subclinical changes in functional and morphological myocardial magnetic resonance parameters very early into an anthracycline treatment, which may predict subsequent development of anthracycline‐induced cardiomyopathy (aCMP).
Methods and results
Thirty sarcoma patients with planned anthracycline‐based chemotherapy (360–400 mg/m2 doxorubicin‐equivalent) were recruited. Median treatment time was 19.1 ± 2.1 weeks. Enrolled individuals received three cardiovascular magnetic resonance studies (before treatment, 48 h after first anthracycline treatment, and upon completion of treatment). Native T1 mapping (modified Look–Locker inversion recovery 5s(3s)3s), T2 mapping, and extracellular volume maps were acquired in addition to a conventional cardiovascular magnetic resonance with steady‐state free precession cine imaging at 1.5 T. Patients were given 0.2 mmol/kg gadoteridol for extracellular volume quantification and late gadolinium enhancement imaging. Development of relevant aCMP was defined as drop of left ventricular ejection fraction (LVEF) by >10%. For analysis, 23 complete data sets were available. Nine patients developed aCMP with LVEF reduction >10% until end of chemotherapy. Baseline LVEF was not different between patients with and without subsequent aCMP. When assessed 48 h after first dose of antracyclines, patients with subsequent aCMP had significantly lower native myocardial T1 times compared with before therapy (1002.0 ± 37.9 vs. 956.5 ± 29.2 ms, P < 0.01) than patients who did not develop aCMP (990.9 ± 56.4 vs. 978.4 ± 57.4 ms, P > 0.05). Patients with aCMP had decreased left ventricular mass upon completion of therapy (86.9 ± 24.5 vs. 81.1 ± 22.3 g; P = 0.02), while patients without aCMP did not show a change in left ventricular mass (81.8 ± 21.0 vs. 79.2 ± 18.1 g; P > 0.05). No patient developed new myocardial scars or compact myocardial fibrosis under chemotherapy.
Conclusions
Early decrease of T1 times 48 h after first treatment with anthracyclines can predict the development of subsequent aCMP after completion of chemotherapy.
Keywords: Anthracyclines, Cardiomyopathy, T1 mapping, Cardiac MR, Cardiovascular MR, Cardiotoxicity
Introduction
Anthracyclines are the mainstay of treatment in many malignancies. Approximately 32% of all breast cancer patients,1 60% of elderly lymphoma patients, and most soft tissue sarcoma patients receive anthracyclines during the course of their oncological treatment.2
As outcomes of most cancer patients significantly improved over the last decades, the number of cancer survivors has drastically increased.3
This fortunate development leads to a progressive importance of long‐term side effects of chemotherapy.
Anthracyclines are known to frequently have cardiotoxic side effects. Heart failure due to anthracyclines has severe prognostic implications as it can lead to mortality rates worse than that associated with many malignancies.4
The overall incidence of cardiotoxicity, defined as a reduction in left ventricular ejection fraction (LVEF) of >10%, has been reported in up to 30% of patients receiving anthracyclines.5
While the exact mechanism of cardiotoxicity has not been fully understood yet, studies have shown that oxidative tissue injury from free radicals, impaired protein synthesis, and altered calcium handling in cardiomyocytes contribute to its development.6, 7
There is empirical evidence for the existence of risk factors increasing the likelihood of cardiac dysfunction due to anthracyclines, that is age, known cardiovascular disease, dosage and bolus application.8
However, until now, there is no tool for prediction of cardiotoxicity before or early in the treatment. Hence, clinical guidelines and consensus statements of major cardiology and oncology societies on prevention and treatment of anthracycline‐induced cardiomyopathy (aCMP) concentrate on serial screening for LVEF reduction before, during, and after chemotherapy.9, 10
Cardiovascular magnetic resonance (CMR) is a non‐invasive imaging technique that allows thorough myocardial tissue differentiation. Myocardial T1 and T2 mapping are promising techniques in this regard as they enable quantitative assessment of diffuse myocardial tissue alteration through a pixel‐wise analysis approach.11, 12
Several studies with animals and human cancer survivors revealed that anthracycline therapy can induce diffuse myocardial fibrosis several years after completion of treatment, which can be assessed by T1 mapping.13, 14
While myocardial fibrosis is well known to be associated with increased risk for development of congestive heart failure and worse patient outcome, it can only be detected late into or after completion of chemotherapy and, hence, has no substantial preventive benefit for patients receiving anthracyclines.15, 16
Early preclinical detection of patients with aCMP could lead to a benefit of earlier treatment, thus enabling patients to receive full chemotherapy with lower risk for development of aCMP.17
Until today, there is no single universally accepted definition or categorization of cardiotoxicity by anthracyclines. There are several types of aCMP that are categorized according to timing of onset of cardiac dysfunction into acute (days after start of chemotherapy), early‐onset (months after start of chemotherapy), and late‐onset aCMP (years after completion of chemotherapy).18 In this study, we focused on development of early‐onset aCMP.
We hypothesized that cardiotoxicity of anthracyclines affects cardiac tissue morphology already very early into the treatment and that these alterations may be detected by CMR. Accordingly, the purpose of this study was to assess the predictive value of parametric mapping techniques for detection of acute effects of anthracyclines on cardiac tissue at the beginning of treatment, which may lead to reduction in LVEF after completion of treatment.
Methods
Study population
A total of 30 patients were prospectively recruited. All patients had histologically confirmed soft tissue sarcoma and were planned for anthracycline‐based chemotherapy with a cumulative doxorubicin‐equivalent dose of 360–400 mg/m2. Exclusion criteria were chronic renal failure (glomerular filtration rate < 30 mL/m2), cardiac metastases, previous treatment with anthracyclines, known incompatibility for gadolinium contrast media, and contraindication for magnetic resonance imaging. All enrolled individuals were approved by the local ethical review board and gave written informed consent before participation.
Cardiovascular magnetic resonance protocol
All study participants underwent three CMR scans of 45 min each on a 1.5 T Siemens AvantoFit® scanner (Siemens Healthineers, Erlangen, Germany) with a 32 channel phased array coil. The first CMR scan was performed within 48 h before start of anthracycline treatment (baseline CMR), the second scan 48 h after the first anthracycline treatment, and the third scan 4 weeks after the last anthracycline treatment (usually 5 to 6 months after begin of therapy). Participants received 0.2 mmol/kg of gadoteridol contrast agent (ProHance®, Bracco Diagnostics, Princeton, New Jersey) during each scan. All imaging sequences were performed according to previously published techniques. Left ventricular (LV) and right ventricular (RV) volumetric and functional parameters were assessed in long‐axis and short‐axis steady‐state free precession cine sequences. Cine imaging parameters included field of view (FOV) 340 mm, voxel size 1.8 × 1.8 × 7 mm3, 3 mm gap, echo time (TE) 1.2 ms, repetition time (TR) 33.4 ms, flip angle 74°, and bandwidth 930 Hz.
T1 mapping was performed using a modified Look–Locker inversion recovery sequence in a mid‐ventricular short‐axis slice before and 15 min after contrast administration. Sequence parameters include native T1: 5s(3s)3s with FOV 360 mm, voxel size 1.6 × 1.6 × 7 mm3, TE 1 ms, TR 339.4 ms, flip angle 35°, and bandwidth 1063 Hz and post‐contrast: 4s(1s)3s(1s)2s with FOV 360 mm, voxel size 1.6 × 1.6 × 7 mm3, TE 1 ms, TR 419.4 ms, flip angle 35°, and bandwidth 1063 Hz.
Motion‐corrected T2 mapping was performed using an established T2 prepared steady‐state free precession technique (3 single‐shot images with T2 preparation times of 0/24/55 ms and voxel size of 1.6 × 1.6 × 6.0 mm).19
Late gadolinium enhancement (LGE) was used for focal fibrosis imaging and performed in the same slice positions as cine imaging using a gradient echo‐based segmented phase‐sensitive inversion recovery sequence in single‐slice, single‐breathhold fashion. LGE scan parameters: FOV 380 mm, voxel size 1.8 × 1.8 × 7 mm, no interslice gap, TE 1 ms, TR 700 ms, flip angle 65°, and bandwidth 1184 Hz.
Image analysis
Experienced readers (F. M., S. F., L. Z., and A. S.) with at least 3 years of experience in cardiac MR analysis in a centre with 3.000 scans per year were blinded to clinical patient information. All image analysis was performed using cvi42® post‐processing software version 4.2 (Circle Cardiovascular Imaging Inc., Calgary, Canada). LV and RV size and function as well as LV mass were assessed in short‐axis cine images, and atrial volumes were assessed monoplanar (right atrium) or biplanar (left atrium) in long‐axis cine four‐chamber and two‐chamber views.
Epicardial and endocardial contours in a mid‐ventricular short‐axis slice were traced for T1 and T2 mapping analyses and a 5% safety margin was applied endocardial and epicardial to minimize partial volume effects. Both T2 and T1 maps were quantified as average global values in the analysed slice as previously reported.19 Visual surveys were evaluated for artefacts before quantification, and segments with relevant artefacts were excluded from analysis (e.g. caused by susceptibility, unintended motion effects, or incorrect motion correction).
Relative and absolute extracellular volume (ECV) fraction were calculated by means of native and post‐contrast T1 values and haematocrit as previously established.12 Relative ECV was reported as per cent of myocardial volume of the corresponding short‐axis plane, and absolute ECV in gram extrapolated towards LV mass.
Visual evaluation of LGE images was performed by two independent readers and included presence, location, and transmurality of identified lesions. Differentiation of real LGE lesions from artefacts was realized during image acquisition by verification in two perpendicular slices or altered readout direction.
Interobserver and intraobserver variability analysis was performed on subsets of 10 subjects.
According to current guidelines, patients with LVEF drop of >10% points were defined as patients with aCMP. All other patients were defined non‐aCMP patients for further analysis.
Laboratory blood analysis
On the day of each CMR scan, venous blood samples were obtained and immediately sent for laboratory analysis at our central laboratory. High‐sensitivity cardiac troponin T concentrations were measured using the Elecsys® hsTNT STAT assay (Roche Diagnostics, Mannheim, Germany). The analytical limit of detection was 5 ng/L, and the 99th percentile upper reference limit was 14 ng/L.
Plasma N terminal pro brain natriuretic peptide (NT‐proBNP) concentrations were measured using the Elecsys® proBNP II assay (Roche Diagnostics). The analytical limit of detection of NT‐proBNP was 5 pg/mL.
Electrocardiography
A 12 lead electrocardiography (ECG) was obtained before each CMR scan and assessed for pathological findings. In detail, patients were ranked positive if one of the following criteria was newly present: no sinus rhythm, PQ interval of ≥200 ms, QRS duration of ≥120 ms, and atrioventricular blockage, elevation, or depression of ST interval > 0.1 mV. 30.
Statistical analysis
All measured values are shown as mean ± standard deviation. Statistical analysis was performed using SPSS Statistics 22.0.0 (IBM, Armonk, NY, USA). Using Wilcoxon signed‐rank test, significant values were accepted by P < 0.05.
Univariate analysis for prediction of LVEF drop was performed using two‐sided t‐test embedded into ANOVA analysis.
Correlation analyses were performed using the Spearman rank correlation coefficients. To test for group‐differences of categorical variables χ2‐test was applied.
For intraobserver and interobserver reproducibility, images were analysed twice by blinded readers. The results were evaluated by intraclass correlation coefficients.
Results
Patient characteristics
We initially recruited 30 patients. Seven individuals had to be excluded because of early study abort because of individual wish (n = 2) or termination of anthracycline chemotherapy during the study (n = 5). Finally, we had 23 data sets for analysis. Mean age of study cohort was 58.7 ± 13.4 years; 12 patients (52%) were female. Baseline characteristics of study cohort are summarized in Table 1. Patients received a mean cumulative dose of doxorubicin‐equivalent chemotherapy of 342 ± 23 mg/m2 within a mean treatment time of 19.1 ± 2.1 weeks.
Table 1.
Patient characteristics
| Patient characteristics | |
|---|---|
| Age | 58.7 ± 13.4 years |
| Gender | 11 M/12 F |
| BMI | 23.5 ± 3.4 kg/m2 |
| Hypertension | 11/23 (48%) |
| Diabetes | 3/23 (13%) |
| CAD | 1/23 (4%) |
| (Ex‐) Smoker | 5/23 (22%) |
BMI, body mass index; CAD, coronary artery disease.
Left ventricular and right ventricular function
At the end of chemotherapy, nine patients had developed LVEF reduction >10% as compared with baseline and were defined as aCMP patients for further analysis. See Figure 1 A,B for representative example. In this aCMP group mean, LVEF decreased from 63.5% ± 5.8% at baseline to 49.9% ± 5.0% after chemotherapy (P < 0.01), whereas the remaining 14 non‐aCMP patients had no difference in LVEF until completion of therapy (baseline: 59.2% ± 10.3%; after chemotherapy: 58.3 ± 7.8%; P = 0.47). Individual LVEF changes between baseline and completion of chemotherapy are displayed in Figure 2 , and detailed results of anatomical and functional parameters of all CMR studies are illustrated in Table 2 A. Notably, in aCMP patients, LV mass decreased from baseline (86.9 ± 24.5 g) until completion of therapy (81.1 ± 22.3 g; P = 0.02), while LV mass did not change in non‐aCMP patients over the course of chemotherapy (baseline: 81.8 ± 21.0 g; after therapy: 79.2 ± 18.1 g).
Figure 1.
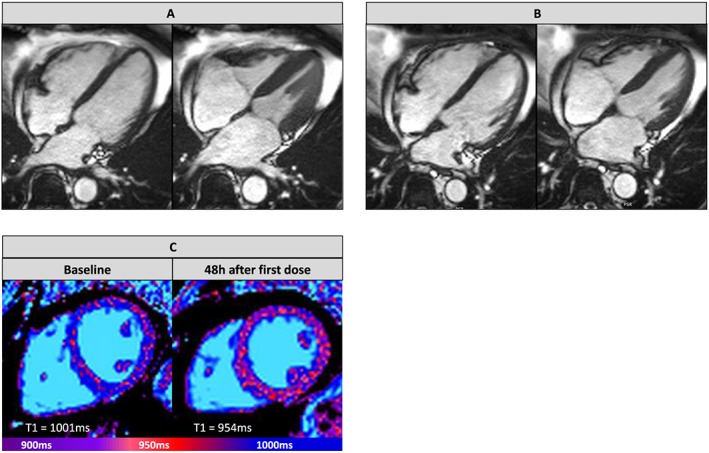
Representative anthracycline‐induced cardiomyopathy imaging data. Steady‐state free precession cine imaging captures in diastole and systole (A) before start of chemotherapy [baseline (left ventricular ejection fraction 65%)] and (B) after completion of chemotherapy (left ventricular ejection fraction 45%). (C) Native myocardial T1 maps of patient with anthracycline‐induced cardiomyopathy before chemotherapy and 48 h after first dose application. Displayed T1 times were measured as average global left ventricular times in each slice.
Figure 2.
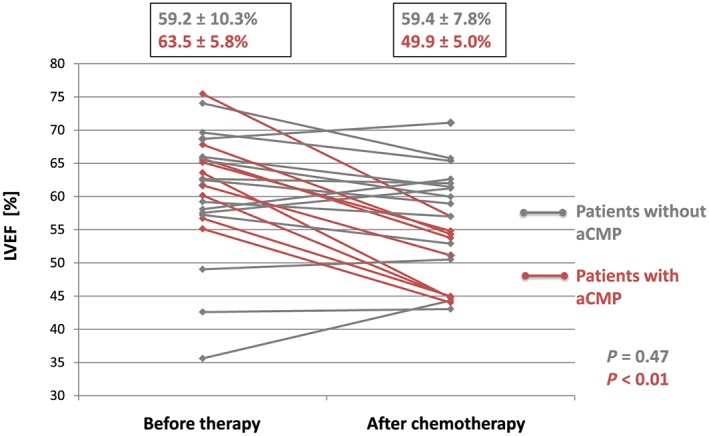
Left ventricular ejection fraction (LVEF) development before and after chemotherapy. Red data points indicate anthracycline‐induced cardiomyopathy (aCMP) patients with LVEF drop of >10% during chemotherapy. Averages for aCMP and non‐aCMP patients are displayed above.
Table 2.
Quantitative magnetic resonance parameters. (A) Volumetric and functional assessment. (B) Myocardial tissue differentiation parameters.
| 48 h after first dose | After therapy | ||||||
|---|---|---|---|---|---|---|---|
| Before therapy | P value | P value | |||||
| A | |||||||
| Non‐aCMP | LVEDV | mL | 165.1 ± 39.6 | 177.5 ± 36.2 | 0.03 | 164.7 ± 34.8 | 0.67 |
| LVEF | % | 59.2 ± 10.2 | 63.9 ± 7.9 | 0.02 | 58.3 ± 7.8 | 0.47 | |
| RVEDV | mL | 185.7 ± 41.8 | 196.0 ± 43.0 | 0.06 | 184.6 ± 40.7 | 0.52 | |
| RVEF | % | 47.3 ± 5.9 | 51.1 ± 6.2 | <0.01 | 47.2 ± 5.8 | 0.98 | |
| LVM | g | 81.8 ± 21.0 | 81.8 ± 20.1 | 0.92 | 79.2 ± 18.1 | 0.33 | |
| aCMP | LVEDV | mL | 154.1 ± 32.2 | 164.3 ± 30.4 | 0.17 | 155.8 ± 25.0 | 0.86 |
| LVEF | % | 63.5 ± 5.8 | 65.1 ± 4.9 | 0.34 | 49.9 ± 5.0 | <0.01 | |
| RVEDV | mL | 171.2 ± 36.0 | 181.6 ± 40.7 | 0.11 | 156.9 ± 33.1 | <0.01 | |
| RVEF | % | 51.7 ± 3.5 | 53.3 ± 3.7 | 0.34 | 44.2 ± 5.1 | <0.01 | |
| LVM | g | 86.9 ± 24.5 | 85.5 ± 24.6 | 0.52 | 81.1 ± 22.3 | 0.02 | |
| B | |||||||
| Non‐aCMP | T2 | ms | 52.0 ± 3.5 | 52.9 ± 2.7 | 0.07 | 54.6 ± 3.2 | 0.08 |
| Relative ECV | % | 26.4 ± 2.0 | 28.1 ± 2.7 | 0.12 | 29.4 ± 1.6 | 0.06 | |
| Absolute ECV | g | 21.0 ± 5.4 | 23.4 ± 5.8 | 0.20 | 23.7 ± 3.9 | 0.08 | |
| aCMP | T2 | ms | 54.3 ± 2.8 | 55.3 ± 1.5 | 0.37 | 54.8 ± 2.9 | 0.73 |
| Relative ECV | % | 27.5 ± 2.7 | 29.3 ± 2.7 | 0.16 | 29.8 ± 1.7 | 0.04 | |
| Absolute ECV | g | 23.4 ± 9.2 | 25.4 ± 8.6 | 0.33 | 26.8 ± 4.5 | 0.15 | |
aCMP, anthracycline‐induced cardiomyopathy; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end‐diastolic volume; LVM, left ventricular mass; RVEF, left ventricular ejection fraction; RVEDV, right ventricular end‐diastolic volume.
P values indicate statistical significance towards baseline data (before therapy).
As illustrated in Table 2 A, at 48 h after the first treatment with anthracyclines, non‐aCMP patients showed an increase in LVEF (59.2% ± 10.2% vs. 63.9% ± 7.9%, P = 0.02), LV end‐diastolic volume (165.1 ± 39.6 vs. 177.5 ± 36.2 mL, P = 0.03), and RV ejection fraction (47.3% ± 59.% vs. 51.1% ± 6.2%, P < 0.01) as compared with baseline, while patients with subsequent aCMP did not. RV end‐diastolic volume and LV mass did not change in either group after the first dose of anthracyclines.
Myocardial tissue differentiation
In patients who developed aCMP, we observed a significant decrease of native T1 times from 1002.0 ± 37.9 ms at baseline to 956.5 ± 29.2 ms at 48 h after the first dose of anthracyclines (P < 0.01). Patients without development of aCMP until completion of chemotherapy did not show a significant change in native T1 at 48 h (990.9 ± 56.4 ms at baseline; 978.4 ± 57.4 ms at 48 h; P = 0.08). After completion of therapy, native T1 times were not significantly different from baseline values in either group. For details, see Figure 3 , a representative imaging example is displayed in Figure 1 C. Reliability was excellent for both interobserver and intraobserver evaluations (Spearman rank correlation for native T1 times r s = 0.90 with P = 0.01 and for T2 times r s = 0.91 with P = 0.01, intraclass correlation coefficient 0.96 for native T1 and 0.98 for T2).
Figure 3.
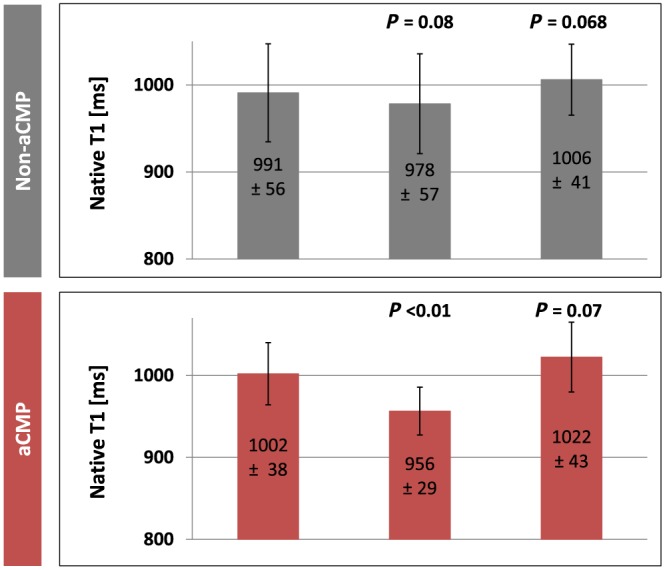
Native T1 mapping. Grey columns represent patients without development of anthracycline‐induced cardiomyopathy (aCMP), and red columns represent aCMP patients. P values indicate statistical significance towards baseline data (before therapy).
Extracellular volume analysis showed that relative and absolute ECV values did not change significantly between baseline and 48 h after first dose of anthracyclines in aCMP and non‐aCMP patients (Table 2 B). Because of the loss in LV mass over the course of chemotherapy, we observed an increase of relative ECV after completion of chemotherapy in aCMP patients against baseline from 27.5% ± 2.7% to 29.8% ± 1.7% (P = 0.04). However, this change was not verifiable in absolute ECV values (23.4 ± 9.2 g at baseline vs. 26.8 ± 4.5 g after therapy, P = 0.15; for details, see Table 2 B).
On evaluation of T2 maps, we did not find focal lesions in any patient at any time point. At baseline, average T2 times were not different between patients with development of subsequent aCMP (54.3 ± 2.8 ms) and non‐aCMP patients (52.0 ± 3.5 ms) (P = 0.11). Also, on follow‐up at 48 h and after completion of therapy, average T2 times did not significantly change in either patient group. For details, see Table 2 B.
LGE analysis revealed that three patients had myocardial fibrosis at baseline CMR. Two of these three subjects were in the non‐aCMP group and had minor subendocardial scars as well as known coronary artery disease. One individual was in the aCMP group and had a small intramyocardial fibrosis inferolateral. None of the detected LGE lesions changed on both follow‐up CMR scans. No patient developed new LGE lesions over the course of this study.
Laboratory results
High‐sensitive troponin T and NT‐proBNP blood analysis are displayed in Table 3. There was no significant difference in troponin T levels at baseline between aCMP (11.4 ± 11.2 ng/L) and non‐aCMP patients (8.1 ± 6.5 ng/L) and no significant change at 48 h after first anthracycline treatment (aCMP: 11.4 ± 7.2 ng/L; non‐aCMP: 8.6 ± 7.0 ng/L). However, upon completion of study, we observed an increase of troponin T levels against baseline in both patient groups (aCMP: 17.7 ± 8.5 ng/L – P = 0.04; non‐aCMP: 23.3 ± 25.3 ng/L – P < 0.01). NT‐proBNP was neither different between aCMP and non‐aCMP patients at any time point nor did we see a statistically significant increase at 48 h or upon completion of chemotherapy (Table 3). Glomerular filtration rates did not significantly change in aCMP (baseline: 87.8 ± 14.1 mL/min/1.73 m2; at 48 h: 91.1 ± 16.5 mL/min/1.73 m2; after chemotherapy: 90.8 ± 13.6 mL/min/1.73 m2, P > 0.05) and non‐aCMP patients (baseline: 88.1 ± 18.0 mL/min/1.73 m2; at 48 h: 91.8 ± 19.1 mL/min/1.73 m2; after chemotherapy: 93.9 ± 20.3 mL/min/1.73 m2, P > 0.05) over the course of the study.
Table 3.
Laboratory blood test assessment
| Before therapy | 48 hr after first dose | After therapy | |||||
|---|---|---|---|---|---|---|---|
| P value | P value | ||||||
| Non‐aCMP | High‐sensitive troponin T | ng/L | 8.1 ± 6.5 | 8.6 ± 7.0 | 0.71 | 23.3 ± 25.3 | <0.01 |
| NT‐proBNP | pg/mL | 169.9 ± 160.8 | 175.0 ± 163.5 | 0.33 | 203.0 ± 208.4 | 0.19 | |
| aCMP | High‐sensitive troponin T | ng/L | 11.4 ± 11.2 | 11.4 ± 7.2 | 0.65 | 17.7 ± 8.5 | 0.02 |
| NT‐proBNP | pg/mL | 160.7 ± 209.0 | 169.4 ± 181.2 | 0.55 | 265.0 ± 304.7 | 0.30 | |
aCMP, anthracycline‐induced cardiomyopathy; NT‐proBNP, N terminal pro brain natriuretic peptide.
P values indicate statistical significance towards baseline data (before therapy).
Conduction abnormalities
We found ECG abnormalities only in three patients, two of which were in the aCMP group. One aCMP patient had first degree atrioventricular blockage after completion of therapy, which was not present at baseline. In the other aCMP patient, we detected atrial fibrillation after chemotherapy but normal sinus rhythm at baseline and at 48 h after therapy start. One non‐aCMP patient had complete LV branch block at baseline, which was persistent on follow‐up ECGs.
Discussion
In this study, we were able to detect several myocardial tissue changes predicting an early‐onset cardiomyopathy because of anthracyclines. Firstly, 30% of recruited patients showed a decrease in LVEF of more than 10% over the course of an anthracycline‐based chemotherapy, which was defined as development of aCMP. Secondly, all patients who develop aCMP (30% of recruited patients) had decreased LV mass upon completion of therapy, while patients without aCMP did not show a change in LV mass. Thirdly, a decrease of native myocardial T1 time within 48 h after the first dose of anthracyclines was associated with subsequent development of aCMP. This acute decrease in native T1 time resolved in all aCMP patients until completion of therapy. Finally, myocardial T2 mapping, absolute ECV values, and blood biomarkers did not qualify to discriminate between patients with and without development of aCMP in this study.
The prevalence of aCMP has been shown to be 3% to 48% depending on several variables.5, 20, 21 Besides the cumulative dose of anthracyclines administered being one major risk factor for aCMP, there is evidence that the type of imaging modality for LVEF evaluation impacts detection rates for aCMP. CMR is widely accepted as the reference method for measurement of LV volumes and LVEF because of best reproducibility among non‐invasive imaging techniques.22, 23
In several studies, CMR was shown to be superior to echocardiography in detection of LVEF decline under anthracycline therapy, that is revealing an LVEF drop to <50% in 26% of all patients within 6 months of therapy using CMR, which is in line with our results.23, 24
Our finding that patients with aCMP show a reduction of LV mass over the course of chemotherapy is in line with previous limited data on anthracyclines as well as trastuzumab therapy.25
Lipshultz et al. performed serial echocardiography on children receiving anthracyclines. They found that LV decreased over time under anthracycline therapy and that the reduction in LV mass was inversely related to cumulative dose of anthracyclines.26
Because patients in this study received similar cumulative dose of anthracyclines independent of development of aCMP, the dosing factor should play a minor role in our cohort.
Myocardial atrophy and cachexia are known to be independent predictors for mortality in cancer patients.27 The decrease of LV mass in patients with aCMP in this study shows that myocardial atrophy may also serve as phenotypic criterion for aCMP. However, it is difficult to say if LV atrophy is cause or consequence of aCMP.
Myocardial T1 and T2 mapping, including the derived ECV, are recognized as potential biomarkers of chemotherapy cardiotoxicity that may have the ability to detect myocardial tissue damage earlier than conventional functional metrics. Several CMR studies with cancer survivors have investigated changes in native myocardial T1 and T2 times and found that anthracyclines can cause an increase of native T1 times and ECV because of development of diffuse myocardial fibrosis years after completion of treatment.14, 28
Hence, there clearly is a long‐term myocardial remodelling effect because of anthracyclines, which increases risk for cardiovascular disease.
In this study, we demonstrated evidence that an early decrease of native myocardial T1 time after the first administration of anthracyclines is linked to subsequent development of aCMP, allowing for very early identification of patients at high risk for aCMP.
The pathophysiologic mechanism behind this decrease in native T1, however, remains unclear. There is a lack of previous histologic or imaging data at this early timing of myocardial tissue assessment after drug administration.
In an earlier study from our group, we reported that changes in early myocardial enhancement after gadolinium administration can predict LVEF drop after 28 days in patients treated with anthracyclines.29 However, there was no longer follow‐up of patients.
One hypothesis includes the involvement of radical oxygen species, which are known to play a key role in anthracycline‐mediated cardiotoxicity and which affect mitochondrial function and increase lipid peroxidation.30
Native T1 time is increased in case of myocardial oedema or inflammation and decreased by iron overload such as in haemochromatosis or in case of lipid deposition as in Fabry disease.31
While it is unlikely that a temporary myocardial iron overload is triggered by anthracyclines, they may lead to an increase of intracellular lipid contents affecting native myocardial T1 times.
Other acute anthracycline‐meditated biochemical abnormalities in cardiomyocytes may also play a role in the observed decrease of native T1 times.
Our observation that T1 times normalize again upon completion of therapy further strengthen the possibility that acute toxic effects are meditating the early native T1 decrease rather than permanent structural changes such as interstitial fibrosis.
This is supported by our finding that absolute ECV values did not change in our study population—neither acutely after the first administration of anthracyclines nor upon completion of therapy. Other groups have reported that there is increase in relative ECV because of anthracyclines, which may occur even years after therapy.14
In our opinion, there certainly is a degree of increased fibrosis attributing to increased relative ECV in the long term. However, the reduction of LV mass during and after cancer therapy due to weight loss and systemic atrophy may also affect relative ECV values, while absolute ECV values remain largely unaffected as reported in this study.
In our study, we did not find significant changes in myocardial T2 time under and after chemotherapy, which other groups have reported. Farhad et al. found increased myocardial T2 times in mice treated with anthracyclines at 5 weeks after beginning of anthracycline treatment, which resolves upon a 20 week follow‐up.32
In our opinion, timing of imaging is crucial for interpretation of results. Cardiotoxicity of anthracyclines may not translate into a single long‐term pathophysiolic mechanism but may rather consist of several phases. Acute toxic effects may lead to early native T1 changes as observed in this study, while chronic myocardial remodelling due to anthracyclines may lead to increased native T1 times and elevated ECV because of diffuse interstitial fibrosis in cancer survivors.
Besides imaging parameters, blood biomarkers have been shown to be altered in patients with aCMP.33, 34
In our study, we observed significant elevations of high‐sensitive troponin T. However, these changes occurred independent of development of aCMP. Troponin T is a sensitive biomarker for myocardial cell death and may therefore be elevated even in patients with minor myocardial damage, which does not lead to systolic heart failure.
NT‐proBNP is well established as a biomarker for heart failure, thus maybe elevated in patients developing aCMP. However, in contrast to troponin T, we did not see significant changes of NT‐proBNP levels in patients with or without aCMP. This is in line with findings from other groups.35, 36
In conclusion, native myocardial T1 mapping may represent a suitable tool for aCMP risk stratification very early into the treatment. In contrast to other imaging parameters, it can help to predict aCMP development before functional cardiac impairment occurs. Larger, interventional studies are needed to investigate if preventive measures—such as primary aCMP prevention with beta‐blockers or angiotensin‐converting enzyme inhibitors—inhibit the early decrease of native T1 times after anthracycline treatment.
Limitations
In this study, we observed patients only until completion of therapy; however, long‐term effects of anthracyclines can also develop years after completion of treatment. Studies with longer follow‐up may help to discriminate between patients with early‐onset and late‐onset aCMP.
Conflict of interest
The authors have declared the following potential conflicts of interest (sorted by initials). As members of the cardiac MR working group, F.M., S.F., L.Z., F.V.K.B., E.B., A.S., and J.S.M. received direct or indirect grant support and non‐financial support from HELIOS Kliniken GmbH, SIEMENS Healthineers, Bayer Healthcare, and Circle Cardiovascular Imaging. P.R. received personal fees from Bayer Healthcare, Roche, Novartis, Pfizer, Pharma Mar, Clinigen Healthcare, Lilly Deutschland GmbH, Deciphera Pharmaceuticals, Merck, and ARIAD Pharmaceuticals. A.R. and S.G. have no conflicts of interest to declare.
Funding
This work was funded partially by HELIOS Kliniken through HELIOS Research Center grant no. HRC‐ID 058429.
Acknowledgements
We want to thank or study nurses and MR technicians for their continuous support with data acquisition and recruitment. Special thanks to the entire team of the Department of Interdisciplinary Oncology and Sarcoma Center for their help with patient recruitment.
Muehlberg, F. , Funk, S. , Zange, L. , von Knobelsdorff‐Brenkenhoff, F. , Blaszczyk, E. , Schulz, A. , Ghani, S. , Reichardt, A. , Reichardt, P. , and Schulz‐Menger, J. (2018) Native myocardial T1 time can predict development of subsequent anthracycline‐induced cardiomyopathy. ESC Heart Failure, 5: 620–629. 10.1002/ehf2.12277.
References
- 1. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol 2012; 30: 2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nabhan C, Byrtek M, Rai A, Dawson K, Zhou X, Link BK, Friedberg JW, Zelenetz AD, Maurer MJ, Cerhan JR, Flowers CR. Disease characteristics, treatment patterns, prognosis, outcomes and lymphoma‐related mortality in elderly follicular lymphoma in the United States. Br J Haematol 2015; 170: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, Marrett L, Gjerstorff ML, Johannesen TB, Adolfsson J, Lambe M, Lawrence G, Meechan D, Morris EJ, Middleton R, Steward J, Richards MA, ICBP Module 1 Working Group . Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995‐2007 (the International Cancer Benchmarking Partnership): an analysis of population‐based cancer registry data. Lancet 2011; 377: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alvarez JA, Russell RR. Cardio‐oncology: the Nuclear Option. Curr Cardiol Rep 2017; 19: 31. [DOI] [PubMed] [Google Scholar]
- 5. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003; 97: 2869–2879. [DOI] [PubMed] [Google Scholar]
- 6. Singal PK, Iliskovic N. Doxorubicin‐induced cardiomyopathy. N Engl J Med 1998; 339: 900–905. [DOI] [PubMed] [Google Scholar]
- 7. Zhang S, Liu X, Bawa‐Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med 2012; 18: 1639–1642. [DOI] [PubMed] [Google Scholar]
- 8. Cueva JF, Antolin S, Calvo L, Fernandez I, Ramos M, de Paz L, Mata JG, Lopez R, Constenla M, Perez E, González A, Pellón ML, Varela S, López T. Galician consensus on management of cardiotoxicity in breast cancer: risk factors, prevention, and early intervention. Clin Transl Oncol 2017; 19: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tarantini L, Massimo Gulizia M, Di Lenarda A, Maurea N, Giuseppe Abrignani M, Bisceglia I, Bovelli D, De Gennaro L, Del Sindaco D, Macera F, Parrini I, Radini D, Russo G, Beatrice Scardovi A, Inno A. ANMCO/AIOM/AICO Consensus Document on clinical and management pathways of cardio‐oncology: executive summary. Eur Heart J Suppl 2017; 19: D370–D379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014; 15: 1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim PK, Hong YJ, Im DJ, Suh YJ, Park CH, Kim JY, Chang S, Lee HJ, Hur J, Kim YJ, Chang S, Lee HJ, Hur J, Kim YJ, Choi BW. Myocardial T1 and T2 mapping: techniques and clinical applications. Korean J Radiol 2017; 18: 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piechnik SK, Jerosch‐Herold M. Myocardial T1 mapping and extracellular volume quantification: an overview of technical and biological confounders. Int J Cardiovasc Imaging 2018; 34: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jordan JH, Vasu S, Morgan TM, D'Agostino RB Jr, Melendez GC, Hamilton CA, Arai AE, Liu S, Liu CY, Lima JA, Bluemke DA, Burke GL, Hundley WG. Anthracycline‐associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging 2016; 9: pii: e004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neilan TG, Coelho‐Filho OR, Shah RV, Feng JH, Pena‐Herrera D, Mandry D, Pierre‐Mongeon F, Heydari B, Francis SA, Moslehi J, Kwong RY, Jerosch‐Herold M. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline‐based chemotherapy. Am J Cardiol 2013; 111: 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM, Gheorghiade M. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol 2017; 2: 995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yingchoncharoen T, Jellis C, Popovic ZB, Wang L, Gai N, Levy WC, Tang WH, Flamm S, Kwon DH. Focal fibrosis and diffuse fibrosis are predictors of reversed left ventricular remodeling in patients with non‐ischemic cardiomyopathy. Int J Cardiol 2016; 221: 498–504. [DOI] [PubMed] [Google Scholar]
- 17. Heck SL, Gulati G, Hoffmann P, von Knobelsdorff‐Brenkenhoff F, Storas TH, Ree AH, Gravdehaug B, Rosjo H, Steine K, Geisler J, Schulz–Menger J, Omland T. Effect of candesartan and metoprolol on myocardial tissue composition during anthracycline treatment: the PRADA trial. Eur Heart J Cardiovasc Imaging 2017. 10.1093/ehjci/jex159. [DOI] [PubMed] [Google Scholar]
- 18. Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline‐based therapy: what is the evidence and what are the potential harms? Lancet Oncol 2017; 18: e445‐e456. [DOI] [PubMed] [Google Scholar]
- 19. Schmacht L, Traber J, Grieben U, Utz W, Dieringer MA, Kellman P, Blaszczyk E, von Knobelsdorff‐Brenkenhoff F, Spuler S, Schulz‐Menger J. Cardiac involvement in myotonic dystrophy type 2 patients with preserved ejection fraction: detection by cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2016; 9: e004615. [DOI] [PubMed] [Google Scholar]
- 20. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin 2016; 66: 309–325. [DOI] [PubMed] [Google Scholar]
- 21. Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol Off J Am Soc Clin Oncol 1997; 15: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 22. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90: 29–34. [DOI] [PubMed] [Google Scholar]
- 23. Ylanen K, Eerola A, Vettenranta K, Poutanen T. Three‐dimensional echocardiography and cardiac magnetic resonance imaging in the screening of long‐term survivors of childhood cancer after cardiotoxic therapy. Am J Cardiol 2014; 113: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 24. Drafts BC, Twomley KM, D'Agostino R Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline‐based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013; 6: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neilan TG, Coelho‐Filho OR, Pena‐Herrera D, Shah RV, Jerosch‐Herold M, Francis SA, Moslehi J, Kwong RY. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol 2012; 110: 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005; 23: 2629–2636. [DOI] [PubMed] [Google Scholar]
- 27. Williams GR, Muss HB, Shachar SS. Cachexia in patients with cancer. Lancet Oncol 2016; 17: e220. [DOI] [PubMed] [Google Scholar]
- 28. Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1‐mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson 2013; 15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wassmuth R, Lentzsch S, Erdbruegger U, Schulz‐Menger J, Doerken B, Dietz R, Friedrich MG. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging‐a pilot study. Am Heart J 2001; 141: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 30. Asensio‐Lopez MC, Soler F, Pascual‐Figal D, Fernandez‐Belda F, Lax A. Doxorubicin‐induced oxidative stress: The protective effect of nicorandil on HL‐1 cardiomyocytes. PLoS One 2017; 12: e0172803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu JM, Liu A, Leal J, McMillan F, Francis J, Greiser A, Rider OJ, Myerson S, Neubauer S, Ferreira VM, Piechnik SK. Measurement of myocardial native T1 in cardiovascular diseases and norm in 1291 subjects. J Cardiovasc Magn Reson 2017; 19: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farhad H, Staziaki PV, Addison D, Coelho‐Filho OR, Shah RV, Mitchell RN, Szilveszter B, Abbasi SA, Kwong RY, Scherrer‐Crosbie M, Hoffmann U, Jerosch–Herold M, Neilan TG. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 2016; 9: e003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitayama H, Kondo T, Sugiyama J, Kurimoto K, Nishino Y, Kawada M, Hirayama M, Tsuji Y. High‐sensitive troponin T assay can predict anthracycline‐ and trastuzumab‐induced cardiotoxicity in breast cancer patients. Breast Cancer 2017; 24: 774–782. [DOI] [PubMed] [Google Scholar]
- 34. Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, Cinieri S, Martinelli G, Fiorentini C, Cipolla CM. Myocardial injury revealed by plasma troponin I in breast cancer treated with high‐dose chemotherapy. Ann Oncol 2002; 13: 710–715. [DOI] [PubMed] [Google Scholar]
- 35. Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, Volkers C, Haaksma J, de Vries EG, Sleijfer DT, van der Graaf WT. Prospective evaluation of early cardiac damage induced by epirubicin‐containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 2001; 19: 2746–2753. [DOI] [PubMed] [Google Scholar]
- 36. Daugaard G, Lassen U, Bie P, Pedersen EB, Jensen KT, Abildgaard U, Hesse B, Kjaer A. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail 2005; 7: 87–93. [DOI] [PubMed] [Google Scholar]


