Abstract
Whether the level of circulating eosinophils in chronic obstructive pulmonary disease (COPD) patients can predict the risk of exacerbations of COPD (ECOPD) or response to treatment is debated. Here, we evaluate the prevalence of elevated eosinophils in COPD patients and its relationship with severe ECOPD requiring hospitalisation.
We retrospectively reviewed the charts of COPD patients hospitalised in our centre between January 1, 2005 and November 30, 2015 because of ECOPD or other reasons (controls). In a second analysis, the ECOPD patients were divided into two subgroups based on having ECOPD in the next year after the index event or not. Circulating eosinophils, both during clinical stability and hospitalisation, as well as clinical and functional data and the relation to recurrent exacerbations were analysed.
We studied 992 COPD patients (318 ECOPD patients and 674 controls). Among ECOPD patients, 121 had one or more ECOPD during the year after the index event. The prevalence of eosinophils ≥2% was 72% in ECOPD patients and 71% in controls (p=0.93). Among ECOPD patients, eosinophil levels ≥2%, ≥4% or ≥300 cells·μL−1, either when clinically stable or during hospitalisation, did not show a significant association with the rate of recurrent severe exacerbations. The severity of airflow limitation was associated with recurrent exacerbations, but inhaled corticosteroid treatment was not.
The majority of COPD patients have circulating eosinophils >2% and a significant association with the risk of severe ECOPD or response to inhaled corticosteroids was not demonstrated.
Short abstract
Eosinophil levels are not associated with increased risk of future severe COPD exacerbation and treatment with ICSs does not show a significant association with risk reduction of severe exacerbation in patients with low or high circulating eosinophils http://ow.ly/Cc8W30kuPWr
Introduction
Chronic obstructive pulmonary disease (COPD) is a major health problem because of its high prevalence, increasing incidence, and associated personal, familial, social and economic costs [1, 2]. During the course of the disease, COPD patients can suffer from episodes of exacerbations of COPD (ECOPD) that are associated with increased morbidity and mortality, particularly if they require hospitalisation (severe ECOPD) [3, 4]. Preventing ECOPD is therefore a major therapeutic goal in COPD. As a result, there is currently great interest in identifying and validating reliable biomarkers to predict the risk of future ECOPD and, eventually, intervene therapeutically to prevent them with, for instance, inhaled corticosteroids (ICSs). In this context, eosinophilic airway inflammation seems a promising candidate.
Eosinophilic airway inflammation occurs in 20–40% of COPD patients [5]. Sputum eosinophilia in clinically stable patients predicts the risk of future ECOPD and is associated with a favourable response to ICSs [6–10]. However, sputum induction and analysis is cumbersome and not readily accessible in all clinical settings. As an alternative, investigators reported a good correlation between sputum eosinophilic inflammation and blood eosinophil levels. For instance, Bafadhel et al. [11] showed that a cut-off of 2% circulating blood eosinophils had a sensitivity of 90% and a specificity of 60% to identify sputum eosinophil levels >3% during ECOPD. Other studies confirmed that blood eosinophil levels may be a good biomarker of the future risk of ECOPD and the effect of ICSs on ECOPD prevention [12–17], while recent studies did not find a correlation between circulating eosinophils, at a cut-off of ≥200 or ≥300 cells·μL−1, or ≥2%, ≥3% or ≥4%, and the risk for exacerbations in COPD [18–20]. Furthermore, not only is the association between blood eosinophil levels with exacerbation risk controversial, another issue is, if it is indeed related, what is the appropriate cut-off value (2%, 4% or 300 cells·μL−1). Several studies reported that a 2% eosinophil level should be the ideal cut-off, while others focused on absolute eosinophil numbers [12–15], and a post hoc analysis by Watz et al. [16] suggested that any association between blood eosinophil counts and exacerbation rate is only seen for baseline eosinophil counts ≥4% or ≥300 cells·μL−1.
To gain further insight into the relationship between circulating eosinophils and the risk of severe (i.e. hospitalised) ECOPD, we investigated: 1) if circulating levels of eosinophils when clinically stable predict future severe ECOPD and 2) if circulating levels of eosinophils during hospitalisation because of ECOPD predict the risk of future episodes of severe ECOPD.
Methods
Study design
This study was carried out in Carmel Medical Center, a public hospital in Haifa, north Israel. The hospital serves a large population of around 800 000 individuals, together with two more public hospitals. Our study was authorised by the Ethics Committee of the hospital according to the Declaration of Helsinki.
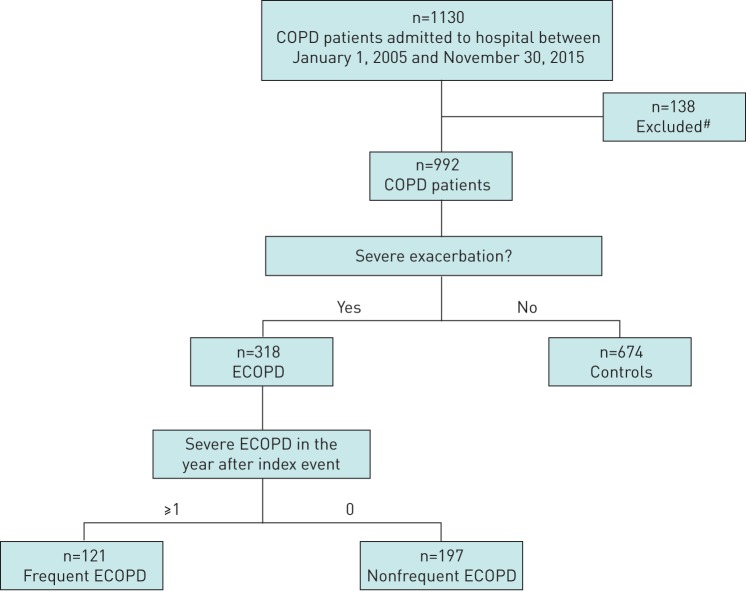
We retrospectively reviewed the charts of all COPD patients admitted to our hospital for any reason (including ECOPD) between January 1, 2005 and November 30, 2015, and identified 1130 patients with a diagnosis of COPD and a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio <0.7 (as per Global Initiative for Chronic Obstructive Lung Disease criteria [21]). We included patients with a diagnosis as defined by the International Classification of Diseases, Ninth Revision, using the codes 496 for chronic airway obstruction and 491.21 for obstructive chronic bronchitis with acute exacerbation. Severe ECOPD was defined as acute shortness of breath with aggravation in the patient's respiratory status that requires hospitalisation or treatment for >24 h in the emergency room [21]. After discharge, all these patients were followed at community pulmonary clinics, so information during clinical stability was also available. As shown in figure 1, we excluded from the current analysis 138 patients (12.2%) that had a previous diagnosis of asthma in childhood or asthma–COPD overlap in adulthood, as well as nonsmokers (current smokers or per history). Of the remaining 992 patients, ECOPD was the cause of hospitalisation in 318 patients (32.1%) during the study period, whereas the remaining 674 COPD patients (67.9%) did not suffer from any severe ECOPD during the study period and were hospitalised for reasons not related to ECOPD, and served as controls. The patients included in the control group did not experience ECOPD during the follow-up period of 1 year. Patients in the ECOPD group were further stratified in two subgroups according to events that followed their index hospitalisation: those with no other severe ECOPD during the 12 months after discharge and those with one or more similar episodes. The data were analysed per patient and the first severe ECOPD was recorded as the index event. The next severe ECOPD for each patient were referred to as future events, regardless of the hospital that they were admitted to. Patients that were hospitalised for any reason other than ECOPD in the next year after the index event were not included in the frequent exacerbators group; they were in the group having only index ECOPD.
FIGURE 1.
CONSORT flow diagram of the study. COPD: chronic obstructive pulmonary disease; ECOPD: exacerbations of COPD. #: previous diagnosis of asthma in childhood or asthma–COPD overlap in adulthood, as well as nonsmokers (current smokers or per history).
Measurements
The following data were collected for all participants in the study from the patients’ charts: demographics, comorbidities, spirometry results and regular COPD therapy. In addition, circulating eosinophil levels, both as absolute values and percentages, were determined when clinically stable (defined as at least 3 months before the index ECOPD event in the ECOPD group and at least 3 months before admission among the controls) and at ECOPD (as defined by the first evaluation in the emergency room). Blood eosinophil levels before admission were available for the entire cohort as periodic follow-up blood tests performed routinely at ambulatory clinics.
Spirometry was performed using identical spirometers in the hospital during hospitalisation and in the ambulatory follow-up, with the same method of calculation [22]. Blood eosinophils were also examined in the same laboratory that serves the hospital and the community.
Statistical analysis
Results are described as mean with standard deviation, number, proportion or range as appropriate. We used the Chi-squared test, Fisher's exact test and the t-test to compare categorical and quantitative variables. The level of significance was set at p=0.05. All analyses were carried out using SPSS version 23 (IBM, Armonk, NY, USA). In our study, 318 patients with ECOPD were identified and 674 patients without ECOPD were sampled. Prior data indicate that the probability of patients with eosinophils ≥2% among patients without ECOPD ranges from 0.5 to 0.7. Assuming that the rate of eosinophils ≥2% in ECOPD patients is at least 10% higher, given α=0.05, we are able to reject the null hypothesis that the eosinophil ≥2% rates are equal between the two study groups with probability (power) >80%. Since in our study the rate of eosinophils ≥2% among the controls is 71% and the rate among patients with ECOPD is 72%, the null hypothesis of equal rates was not rejected.
Results
Patient characteristics
Table 1 presents the main clinical characteristics of the population studied. Most of the 318 patients hospitalised for ECOPD during the study period (80%) were attended in the internal medicine or intensive care department, whereas 20% were treated in the emergency room for >24 h and then discharged home. The majority of these patients were males with a high number of pack-years smoked. Background treatment included ICSs with a long-acting β2-agonist (LABA) or with a long-acting antimuscarinic (LAMA), or triple therapy (ICS, LABA and LAMA) in 252 patients (79%), while 66 patients (21%) were treated with long-acting bronchodilators (LABA, LAMA or both) without ICSs. Among the 674 patients of the control group (i.e. COPD patients who were hospitalised for reasons other than ECOPD), there was male predominance and higher pack-years smoked; however, their average FEV1 was significantly higher (p<0.0001) and the proportion of patients treated with ICSs, with or without long-acting bronchodilators, was lower (p<0.0001) (table 1). When we stratified our patient population according to ICS treatment, the only significant difference that was found was that patients who were treated with ICSs had a lower FEV1 (p<0.0001).
TABLE 1.
Main clinical characteristics of the population studied
| ECOPD patients | Control patients | p-value | ICSs | No ICSs | p-value | |
| Subjects | 318 | 674 | 576 | 416 | ||
| Age years | 71.4±11 | 70.2±13 | 0.155 | 71.9±10 | 72.8±9 | 0.145 |
| Male | 208 (65) | 425 (63) | 0.541 | 372 (64.6) | 275 (66.1) | 0.625 |
| BMI kg·m−2 | 28.5±6.6 | 28.2±7 | 0.393 | 28.6±6.5 | 28.4±7.2 | 0.648 |
| Pack-years (median) | 50 | 51 | 0.194 | 53 | 50 | 0.131 |
| FEV1 % pred | 49±18 | 64.5±19 | <0.0001 | 47.3±18 | 56.6±19 | <0.0001 |
| ICS | 253 (79) | 323 (47.9) | <0.0001 |
Data are presented as n, mean±sd or n (%), unless otherwise stated. ECOPD: exacerbations of chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; BMI: body mass index; FEV1: forced expiratory volume in 1 s.
In the control group, there were various reasons for admission. All were non-COPD-related or with clear correlation to eosinophils, and included cardiac (i.e. myocardial infraction, congestive heart failure and arrhythmias), infectious (i.e. urinary tract infection, cellulitis and pneumonia without ECOPD), vascular (i.e. deep vein thrombosis), haematological and neurological conditions.
During ECOPD, all the patients were treated with systemic corticosteroids (intravenous or oral), antibiotics and short-acting bronchodilators. Data regarding arterial blood gases, ventilation support or radiological findings were not available for all the patients.
Circulating eosinophil counts at clinical stability and risk of severe ECOPD
The level of circulating eosinophils when clinically stable was not different between the two groups of patients compared, neither as percentages nor absolute values (table 2). Likewise, the proportion of COPD patients with ≥2% or ≥4% circulating eosinophils when clinically stable was also similar, whereas that of patients with ≥300 cells·μL−1 was higher in controls (p<0.001).
TABLE 2.
Circulating eosinophil counts (percentages and absolute values) in the study population
| ECOPD patients | Control patients | p-value | |
| Subjects | 318 | 674 | |
| Eosinophils % | 2.8±1.5 | 2.9±1.7 | 0.284 |
| Eosinophils cells·μL−1 | 235.6±143 | 233.2±139 | 0.809 |
| Eosinophils ≥2% | 226 (72) | 478 (70.9) | 0.929 |
| Eosinophils ≥4% | 47 (14.8) | 135 (20) | 0.053 |
| Eosinophils ≥300 cells·μL−1 | 47 (14.8) | 229 (33.9) | <0.001 |
Data are presented as n, mean±sd or n (%), unless otherwise stated. ECOPD: exacerbations of chronic obstructive pulmonary disease.
Circulating eosinophil counts during ECOPD and risk of recurrent severe ECOPD after discharge
Out of the 318 patients who were hospitalised because of ECOPD, 121 (38%) were re-hospitalised one or more times because of an ECOPD in the year that followed the index event (mean±sd number of severe ECOPD 1.7±1.1). Table 3 shows the main characteristics of the ECOPD subgroups, with or without recurrent ECOPD. The patients in both groups had similar demographic characteristics. The proportion of patients with dominant chronic bronchitis was similar between the groups, while chest computed tomography showed a nonsignificant higher rate of bronchiectasis and significantly more predominance of emphysema in the group with frequent ECOPD. As shown in table 4, there were no significant differences between these two groups whether the eosinophil cut-off was 2%, 4% or 300 cells·μL−1 when clinically stable. When we looked at treatment with ICSs on top of long-acting bronchodilators, as opposed to treatment with dual bronchodilators during clinical stability, no significant protective effect of ICSs was found at any eosinophil cut-off value (table 5). Similar results were obtained when patients were stratified within each group by different eosinophil cut-off values determined during the index ECOPD (tables 4 and 5). Finally, we observed (figure 2) a significant relationship between the severity of airflow limitation and the risk of incident severe ECOPD (p<0.0001).
TABLE 3.
Main clinical characteristics of the exacerbations of chronic obstructive pulmonary disease (ECOPD) subgroups divided according to frequency of ECOPD in the year after the index event
| Nonfrequent ECOPD | Frequent ECOPD | p-value | |
| Subjects | 197 | 121 | |
| Age (mean) | 72.2 | 71.9 | 0.81 |
| Males | 64.5 (127) | 66.1 (80) | 0.74 |
| BMI kg·m−2 (mean) | 28.9 | 27.8 | 0.18 |
| Pack-years (median) | 50 | 55 | 0.082 |
| COPD pattern | |||
| Chronic bronchitis | 60.1 (119) | 62.8 (76) | 0.658 |
| Emphysema | 40.9 (81) | 57 (69) | 0.007 |
| Bronchiectasis | 18.7 (37) | 22.3 (27) | 0.342 |
Data are presented as n or % (n), unless otherwise stated. BMI: body mass index.
TABLE 4.
Relation between circulating blood eosinophil level and number of severe exacerbations of chronic obstructive pulmonary disease (ECOPD) in the year after the index event
| ECOPD 0 | ECOPD ≥1 | p-value | |
| Subjects | 197 | 121 | |
| Eosinophils: clinically stable | |||
| <2% | 54 (27.4) | 37 (30.6) | 0.610 |
| ≥2% | 143 (72.6) | 84 (69.1) | |
| <4% | 163 (82.7) | 94 (77.7) | 0.305 |
| ≥4% | 34 (17.3) | 27 (22.3) | |
| <300 cells·μL−1 | 124 (62.9) | 75 (62) | 0.905 |
| ≥300 cells·μL−1 | 73 (37.1) | 46 (38) | |
| Eosinophils: first ECOPD | |||
| <2% | 122 (61.9) | 83 (68.6) | 0.278 |
| ≥2% | 75 (38.1) | 38 (31.4) | |
| <4% | 179 (90.9) | 106 (87.6) | 0.352 |
| ≥4% | 18 (9.1) | 15 (12.4) | |
| <300 cells·μL−1 | 167 (84.8) | 100 (82.6) | 0.639 |
| ≥300 cells·μL−1 | 30 (15.2) | 21 (17.4) |
Data are presented as n or n (%), unless otherwise stated.
TABLE 5.
Relation between circulating blood eosinophil level and number of severe exacerbations of chronic obstructive pulmonary disease (ECOPD) in the year after the index event, stratified by treatment with inhaled corticosteroids (ICSs)
| Without ICSs | With ICSs | p-value | |||
| ECOPD 0 | ECOPD ≥1 | ECOPD 0 | ECOPD ≥1 | ||
| Subjects | 74 | 244 | |||
| Eosinophils: clinically stable | |||||
| <2% | 14 | 9 | 40 | 28 | 0.769 |
| ≥2% | 28 | 23 | 115 | 61 | |
| <4% | 36 | 23 | 127 | 71 | 0.395 |
| ≥4% | 6 | 9 | 28 | 18 | |
| <300 cells·μL−1 | 28 | 16 | 96 | 59 | 0.319 |
| ≥300 cells·μL−1 | 14 | 16 | 59 | 30 | |
| Eosinophils: first ECOPD | |||||
| <2% | 24 | 18 | 98 | 65 | 0.569 |
| ≥2% | 18 | 14 | 57 | 24 | |
| <4% | 39 | 25 | 140 | 81 | 0.270 |
| ≥4% | 3 | 7 | 15 | 8 | |
| <300 cells·μL−1 | 36 | 22 | 131 | 78 | 0.187 |
| ≥300 cells·μL−1 | 6 | 10 | 24 | 11 | |
Data are presented as n, unless otherwise stated.
FIGURE 2.
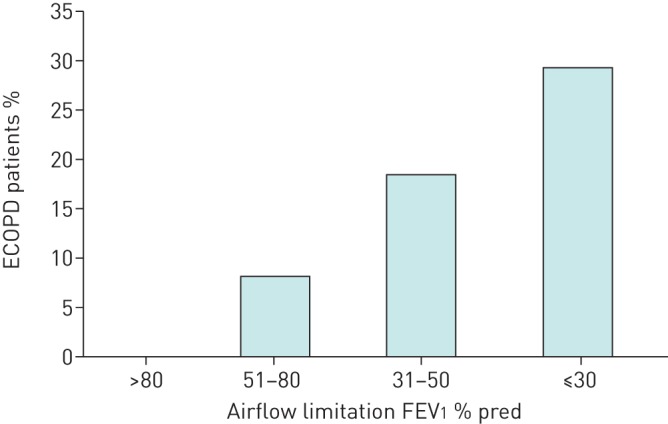
Proportion of patients with hospitalisations for recurrent exacerbations of chronic obstructive pulmonary disease (ECOPD) in the year after the index exacerbation, stratified by severity of airflow limitation (forced expiratory volume in 1 s (FEV1) % pred).
Discussion
This retrospective analysis of medical records of COPD patients hospitalised in our institution during the past decade shows that, in those with severe ECOPD: 1) circulating eosinophils ≥2%, a cut-off value often suggested to predict future exacerbations [12–15], was common during clinical stability (>70%), and 2) association between circulating eosinophil levels when clinical stable, expressed as an absolute value or as a percentage, and future severe ECOPD was not demonstrated. The only parameter related with incident severe ECOPD was the severity of airflow limitation (p<0.0001).
Previous studies
There has been considerable recent interest and debate about the role of blood eosinophil counts in COPD. Post hoc analyses have suggested that higher sputum and blood eosinophil counts appear to be associated with increased risk of future ECOPD as well as with corticosteroid responsiveness [12–17]. These analyses used a cut-off level of 2% circulating eosinophils because this value has high sensitivity in predicting sputum eosinophilia, albeit it is entirely within the normal range of eosinophil levels [11–13].
Furthermore, the percentage of blood eosinophils in the general population is variable and changes over time. For instance, in the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) study, 49% of the COPD patients had variable blood eosinophil levels above and below the 2% cut-off [10]. On the contrary, in the Copenhagen General Population Study, Vedel-Krogh et al. [15] reported that blood eosinophil counts >340 cells·μL−1 were associated with a 1.76-fold increased risk of severe ECOPD, but a post hoc analysis of the WISDOM trial, which included patients with severe COPD on maintenance treatment with a LABA and a LAMA (not ICSs), suggested that the association between blood eosinophil counts and ECOPD rate is seen only when the baseline eosinophil count is ≥4% or ≥300 cells·μL−1 [16]. Likewise, a recent study demonstrated that blood eosinophil counts ≥2% or ≥200 cells·mL−1 at admission for severe ECOPD, when assessed in a corticosteroid-free timeframe, were associated with a >3-fold increase in 12-month readmission for COPD [23].
Furthermore, a study of Kerkhof et al. [24] showed that overall, patients with clearly elevated blood eosinophil counts (i.e. ≥450 cells·μL−1) at stable disease had a 13% higher exacerbation rate during the following year than patients in the reference group. Interestingly, ex-smokers with elevated blood eosinophil counts had the higher exacerbation rate, no interaction effect between sex or ICSs and eosinophil count on the exacerbation rate was found, and patients with elevated eosinophil counts who were currently smoking showed the lowest exacerbation rate [23]. However, Zysman et al. [20] showed that specific characteristic of COPD patients, e.g. symptoms, lung function, exacerbation rate and prognosis, were not related to circulating eosinophil levels at cut-offs of 2%, 3% or 4%.
Interpretation of results
Several observations in our study deserve comment. First, we found that the prevalence of circulating eosinophils ≥2% during clinical stability was >70% (table 2). This is similar to that reported in the ECLIPSE study, where 86% of patients had persistent or intermittently circulating eosinophils ≥2% [10]. Second, we did not find differences between ECOPD and controls with respect to circulating eosinophil levels, neither expressed as an absolute value nor as a percentage, determined during clinical stability or ECOPD (tables 3 and 4). These observations are compatible with the results of recent trials [18, 19] and cast doubt on the predictive value of eosinophil levels in “real-life” COPD [23]. Third, in our study, 79% of patients were on ICS therapy. However, ICS treatment was not associated with a reduced number of recurrent severe ECOPD in any eosinophil bin and was not superior to dual long-acting bronchodilators in preventing future exacerbations (table 5). Interestingly, we observed that treatment with ICSs among controls was significantly lower than in those with recurrent ECOPD, probably in relation to their less severe airflow limitation. Finally, as reported previously [4, 25], we found a significant relationship between the severity of airflow limitation and the rate of ECOPD, which was significantly higher in patients with severe and very severe airflow obstruction.
Potential limitations
Our study has several limitations that deserve comment. First, it is a retrospective analysis, so its results must be interpreted carefully. Second, the population studied may not reflect that of COPD at large because all participants were hospitalised (because of ECOPD or other reasons). Third, some patients started steroid treatment before referral to hospital, which may have reduced the number of patients with elevated eosinophil levels at exacerbation. Finally, we measured blood eosinophils at stable disease only once and we know that eosinophil levels change over time.
Conclusions
Our analysis shows that eosinophil levels (both when clinically stable or during hospitalisation) were not associated with increased risk of future severe ECOPD. Furthermore, treatment with ICSs did not show a significant association with risk reduction of severe ECOPD in patients with low or high circulating eosinophils. These results question the value of circulating eosinophils as a useful biomarker in COPD to predict exacerbation risk or treatment response.
Footnotes
Conflict of interest: A. Agusti reports receiving grants from GSK and AstraZeneca, lecture fees from Novartis and Chiesi, and lecture fees and personal fees for serving on advisory boards from AstraZeneca and Boehringer Ingelheim, outside the submitted work.
References
- 1.Toy EL, Gallagher KF, Stanley EL, et al. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010; 7: 214–228. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015; 386: 2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1418–1422. [DOI] [PubMed] [Google Scholar]
- 4.Hurst J, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 5.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis 2006; 1: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005; 60: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2000; 356: 1480–1485. [DOI] [PubMed] [Google Scholar]
- 8.Leigh R, Pizzichini MM, Morris MM, et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J 2006; 27: 964–971. [DOI] [PubMed] [Google Scholar]
- 9.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007; 29: 906–913. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014; 44: 1697–1700. [DOI] [PubMed] [Google Scholar]
- 11.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biological clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184: 662–671. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 14.Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax 2016; 71: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease: the Copenhagen General Population Study. Am J Respir Crit Care Med 2016; 193: 965–974. [DOI] [PubMed] [Google Scholar]
- 16.Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med 2016; 4: 390–398. [DOI] [PubMed] [Google Scholar]
- 17.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N Engl J Med 2016; 374: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 18.Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J 2017; 50: 1701162. [DOI] [PubMed] [Google Scholar]
- 20.Zysman M, Deslee G, Caillaud D, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017 Available from: http://goldcopd.org/
- 22.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6: Suppl. 16, 5–40. [DOI] [PubMed] [Google Scholar]
- 23.Couillard S, Larivée P, Courteau J, et al. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest 2017; 151: 366–373. [DOI] [PubMed] [Google Scholar]
- 24.Kerkhof M, Sonnappa S, Postma DS, et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J 2017; 50: 1700761. [DOI] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Respirology 2017; 22: 575–601. [DOI] [PubMed] [Google Scholar]