Abstract.
Uniparental disomy (UPD) refers to a condition in which two homologous chromosomes or chromosomal regions are inherited from one parent. Recent studies have shown that UPD is not rare among the general population, arising from trisomy rescue, gamete complementation, and other mechanisms. Although UPD is not necessarily pathogenic, it can lead to various disease phenotypes by causing imprinting disorders or by unmasking autosomal recessive mutations. Notably, known UPD-mediated autosomal recessive disorders include congenital adrenal hyperplasia due to 21-hydroxylase deficiency, 11β-hydroxylase deficiency, and 3β-hydroxysteroid dehydrogenase deficiency. In addition, UPD can occur in combination with additional cytogenetic abnormalities that may affect growth and development. Therefore, UPD represents a clinically important condition that accounts for a certain percentage of the etiology of growth failure and endocrine abnormalities. Although UPD is barely detectable by standard karyotyping or sequence analyses, it can be screened by single nucleotide polymorphism- and microsatellite-genotyping of patients and their parents, or by DNA methylation analysis of the patients. This mini-review introduces the underlying mechanisms and phenotypic consequences of UPD in association with pediatric endocrine disorders.
Keywords: DNA methylation, growth failure, imprinting, uniparental disomy, unmasked autosomal recessive mutation
Introduction
Uniparental disomy (UPD) refers to a type of chromosomal variation in which two homologous chromosomes or chromosomal regions are inherited from one parent (1). UPD includes uniparental heterodisomy and uniparental isodisomy. Patients with uniparental heterodisomy inherit two different alleles from one parent, whereas those with uniparental isodisomy carry two identical copies of one allele from either the mother or the father. Moreover, a substantial percentage of UPD patients harbor a combination of heterodisomy and isodisomy. Notably, isodisomy can unmask autosomal recessive mutations.
Currently, UPD is a hot topic in the fields of human genetics and reproductive medicine. Recent studies have shown that UPD is present in nearly 1 of 3,500 live births (2). Although UPD is not necessarily pathogenic, it can result in imprinting disorders and autosomal recessive disorders. Moreover, UPD can be accompanied by additional cytogenetic abnormalities. UPD plays certain roles in the development of various diseases including pediatric endocrine disorders. In this mini-review, we introduce the underlying mechanisms and phenotypic consequences of UPD.
Mechanisms Leading to UPD
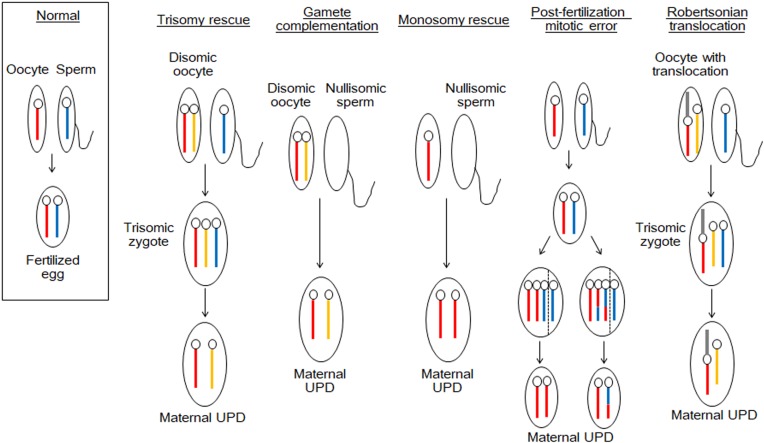
UPD is caused by various mechanisms such as trisomy rescue, gamete complementation, monosomy rescue, and post-fertilization mitotic error (3). Fig. 1 shows a schema of the development of maternal UPD. Paternal UPD also occurs through the same mechanisms. Trisomy rescue results from fertilization of a disomic oocyte and a normal sperm. Subsequently, one of the supernumerary chromosomes in the trisomic zygote is eliminated to restore the normal chromosomal number. Theoretically, this process creates maternal UPD at a rate of 33%, when the paternally derived chromosome is lost. Gamete complementation is a rare condition caused by fertilization of a disomic oocyte and a nullisomic sperm. Monosomy rescue results from fertilization of a normal oocyte and a nullisomic sperm, and subsequent amplification of the maternally derived chromosome. Post-fertilization mitotic errors are caused by chromosomal mis-segregation that occurs after the formation of a normal zygote. In addition, non-homologous Robertsonian translocation can also lead to UPD.
Fig. 1.
Schema of the mechanisms underlying maternal uniparental disomy (UPD). The blue bars denote the chromosomes of the father, and the red and yellow bars indicate the homologous chromosomes of the mother. The gray bars depict the translocated chromosomes of the mother. These mechanisms are also applicable to paternal UPD.
Notably, disomic oocytes that serve as the source of trisomy rescue and gamete complementation are created through meiotic errors during oogenesis. Specifically, nondisjunction of two homologous chromosomes or the premature separation of sister chromatids underlies disomy in oocytes. Since the frequency of such meiotic errors is known to increase with age (4), advanced maternal age appears to be a risk factor of UPD in the offspring (5, 6).
UPD and Diseases
Patients with UPD usually retain the normal genomic structures of all chromosomes; therefore, they have a normal gene dosage. Consistent with this, recent studies have identified UPD in several phenotypically normal individuals (summarized in the UPD source, http://upd-tl.com/upd.html). However, UPD can exert negative effects on human health in the following situations: 1) when an imprinted gene(s) of functional importance is located in the UPD region(s), 2) when a heterozygous autosomal recessive mutation is unmasked by uniparental isodisomy, and 3) when the UPD is accompanied by other cytogenetic abnormalities, such as mosaicism with an abnormal cell lineage or small supernumerary marker chromosomes.
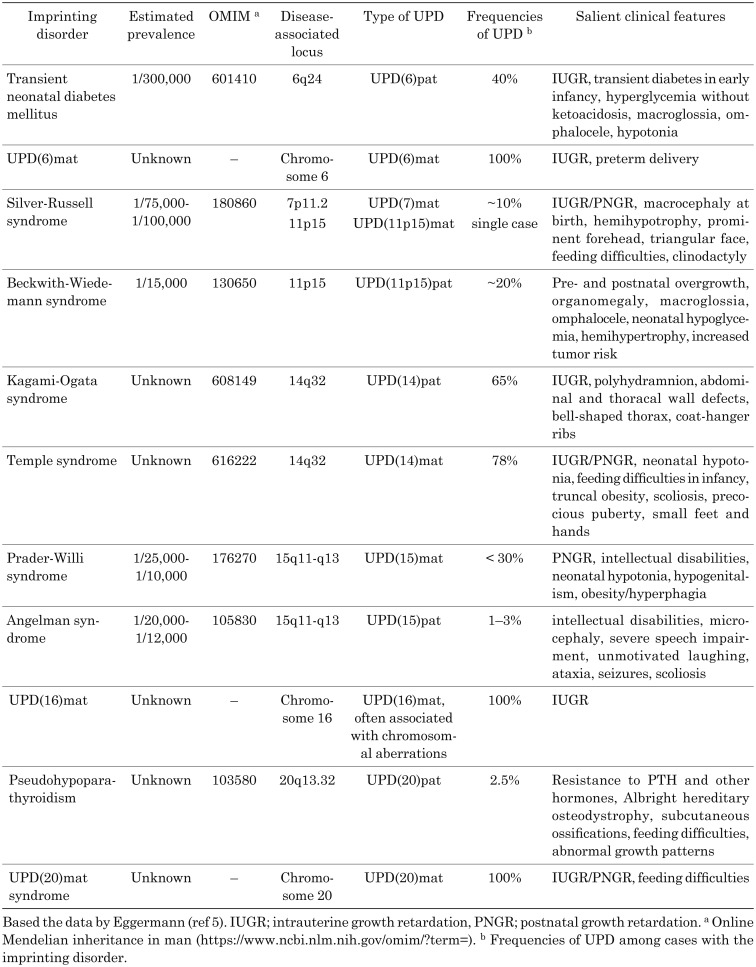
To date, UPD has been documented for almost all human chromosomes. The information of previously reported cases is shown in the UPD database (http://upd-tl.com/upd.html). Table 1 shows an overview of the phenotypic data of various UPDs based on this database. The phenotypes of affected individuals varied from an apparently normal phenotype to specific malformation syndromes.
Table 1. Overview of uniparental disomy (UPD) cases in the UPD database.
Imprinting Disorders and UPD
The human genome encompasses more than 100 imprinted genes. These genes show monoallelic expression depending on the parental origin and are classified into maternally expressed genes (MEGs) and paternally expressed genes (PEGs). When an imprinted gene is located on a chromosome or chromosomal region of UPD, the expression level of the gene is altered, reflecting the parental origin of the chromosome. For example, maternal UPD results in overexpression and downregulation of MEGs and PEGs on the chromosome, respectively. The aberrant expression or function of some imprinted genes has been implicated in the development of imprinting disorders (for a review, see http://www.geneimprint.com/site/genes-by-species). UPD is known as the major cause of imprinting disorders, although such disorders can also be caused by epimutations, genomic rearrangements, and mutations in the imprinted genes. The contribution of each molecular abnormality to the etiology varies among imprinting disorders.
Known imprinting disorders include Silver-Russell syndrome, Temple syndrome, Prader-Willi syndrome, transient neonatal diabetes mellitus, Beckwith-Wiedemann syndrome Kagami-Ogata syndrome, Angelman syndrome, and pseudohypoparathyroidism (Table 2). In addition, the concept of imprinting disorders is still expanding (Table 2) (7). For example, the maternal UPD of chromosome 6 [UPD(6)mat], UPD(16)mat, and UPD(20)mat have been detected in patients with growth failure (8,9,10,11). Furthermore, paternally derived mutations in MKRN3, a PEG on chromosome 15q, have recently been reported as a genetic cause of precocious puberty (12). Likewise, mutations in CDKN1C, a MEG on 11p, were shown to underlie IMAGE syndrome characterized by skeletal and adrenal abnormalities, as well as Silver-Russell syndrome characterized by growth failure (13, 14). Moreover, some imprinting disorders may remain unrecognized. In fact, several imprinted genes could be hidden in the human genome, although the apparently normal phenotypes of individuals with UPD for chromosomes 13 and 22 indicate the absence of clinically important imprinted genes on these chromosomes.
Table 2. Imprinting disorders associated with uniparental disomy (UPD).
It is worth mentioning that growth failure is a common feature of most imprinting disorders. Patients with imprinting disorders often present with intrauterine growth retardation, short stature, and/or poor weight gain. Recent studies have shown that UPD can also lead to prenatal and/or postnatal growth failure without additional clinical features (15). On the other hand, overgrowth has also been reported as a clinical manifestation of some imprinting disorders (4, 16). These phenotypes are consistent with the critical roles of imprinted genes in the regulation of cell proliferation and differentiation. Furthermore, imprinting disorders frequently involve endocrinological manifestations. For example, transient neonatal diabetes mellitus due to aberrant expression of PLAGL1 at 6q24, and pseudohypoparathyroidism due to aberrant DNA methylation of the GNAS loci at 20q13 represent unique endocrine disorders (17, 18). Moreover, pubertal disorders are frequently seen in patients with Temple syndrome or Prader-Willi syndrome (19, 20). Collectively, imprinting disorders are clinically important conditions, particularly in pediatric endocrinology clinics.
Autosomal Recessive Disorders and UPD
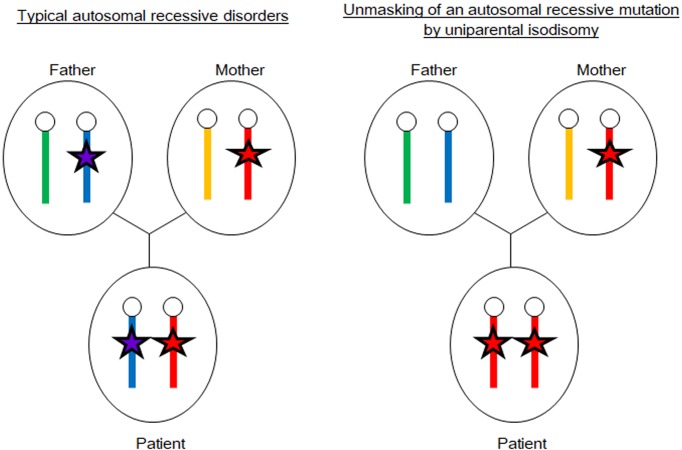
Usually, patients with autosomal recessive disorders inherit pathogenic mutations from both parents. Thus, the patients are homozygous or compound heterozygous for the mutation, and both parents are heterozygous. However, uniparental isodisomy can lead to autosomal recessive disorders in the presence of one non-carrier parent. As shown in Fig. 2, uniparental isodisomy can unmask heterozygous pathogenic mutations on the disomic chromosomes. The UPD review site (http://upd-tl.com/upd.html) shows several examples of monogenic disorders, including pediatric endocrine disorders, caused by unmasked autosomal recessive mutations. Moreover, recent studies have revealed that some patients with 21-hydroxylase deficiency (21OHD), the most common form of congenital adrenal hyperplasia (CAH), were born to families in which one of the parents is a carrier of a disease-causing mutation and the other is a non-carrier (21). In these cases, the disorder was caused by uniparental isodisomy of chromosome 6 or de novo mutations in CYP21A2. Finkielstain et al. examined a large cohort of 21OHD patients and identified UPD(6) and de novo mutations in 0.9% and 1.9% of the subjects, respectively (22). Similarly, other forms of CAH, namely, 11β-hydroxylase deficiency or 3β-hydroxysteroid dehydrogenase deficiency, were also shown to be caused by pathogenic mutations unmasked by uniparental isodisomy (23, 24).
Fig. 2.
Unmasking of an autosomal recessive mutation by uniparental isodisomy. The left panel shows a typical autosomal recessive disorder caused by homozygous or compound heterozygous mutations (red and purple stars) transmitted from both parents. The right panel shows an example of autosomal recessive disorders caused by maternal isodisomy encompassing a heterozygous mutation (red stars).
These findings suggest that UPD should be considered a possible cause of autosomal recessive disorders, particularly when a patient harbors a rare homozygous mutation despite being born to non-consanguineous parents. This notion is important for genetic counseling because the recurrence risk of autosomal recessive disorders is 25% in most cases and negligible when the disease is associated with UPD. Therefore, the carrier status of parents should be examined before genetic counseling of families with autosomal recessive disorders.
UPD Combined with Other Cytogenetic Abnormalities
UPD occurs either alone or in combination with other cytogenetic abnormalities such as mosaicism with abnormal cell lineages or small supernumerary marker chromosomes (25,26,27). For example, UPD due to trisomy rescue can be associated with trisomy mosaicism depending on the timing of trisomy rescue during embryogenesis. In addition, small supernumerary marker chromosomes may be formed during the trisomy rescue process. Indeed, there have been several reports on mosaicism of UPD cells and trisomy cells and/or supernumerary marker chromosomes. The presence of abnormal cell lineages or supernumerary marker chromosomes may affect clinical manifestations of UPD patients, even when the UPD itself has no deleterious effects on the phenotype. On the other hand, it has been reported that additional cytogenetic abnormalities can occasionally rescue the severity of phenotypes of UPD patients (28).
UPD in Clinical Practice
In most cases, UPD occurs by chance. The frequency of UPD was estimated to be approximately 1 in 3,500 live births (2), while in cases with nonhomologous Robertsonian translocation it is as high as ~0.6–0.8% (29). Since advanced maternal age is associated with the risk of UPD (6, 7), and the average age of child bearing is continuously increasing in many countries (data from Japan, USA, and EU are available at http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei16/index.html, https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01_tables.pdf and http://ec.europa.eu/eurostat/web/population-demography-migration-projections/births-fertitily-data/database, respectively), the frequency of UPD may increase in the future.
Notably, UPD is barely detectable by karyotyping or copy-number analyses unless it is combined with other cytogenetic abnormalities. Standard sequencing is also inadequate for identifying UPD. Thus, specific techniques are required for the molecular diagnosis of UPD (30, 31). Single nucleotide polymorphism (SNP)-genotyping or microsatellite analyses using the DNA samples of the patients and their parents are useful tools to detect UPD. Furthermore, comparative genomic hybridization (CGH) using SNP plus CGH arrays is a powerful method to screen uniparental isodisomy in the entire genome. Various methods, such as pyrosequencing, methylation-specific PCR, and combined bisulfite restriction analysis, are utilized to detect DNA methylation abnormalities.
Conclusions
Accumulating evidence suggests that UPD occurs in approximately 1 in 3,500 live births (2). UPD plays certain roles in the development of growth failure and endocrine abnormalities; therefore, it is a clinically important condition in the field of pediatric endocrinology. SNP- or microsatellite-genotyping or DNA methylation analyses are useful to diagnose patients with UPD.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Grant-in-aid for Scientific Research on Innovative Areas (17H06428) from JSPS, and grants from AMED (18ek0109204h0002, 17ek0109266h0001 and 17ek0109278h0001), National Center for Child Health and Development (26-11), and Takeda Foundation.
References
- 1.Shaffer LG, Agan N, Goldberg JD, Ledbetter DH, Longshore JW, Cassidy SB. American College of Medical Genetics statement of diagnostic testing for uniparental disomy. Genet Med 2001;3: 206–11. doi: 10.1097/00125817-200105000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamazawa K, Ogata T, Ferguson-Smith AC. Uniparental disomy and human disease: an overview. Am J Med Genet C Semin Med Genet 2010;154C: 329–34. doi: 10.1002/ajmg.c.30270 [DOI] [PubMed] [Google Scholar]
- 3.Robinson WP. Mechanisms leading to uniparental disomy and their clinical consequences. BioEssays 2000;22: 452–9. doi: [DOI] [PubMed] [Google Scholar]
- 4.Herbert M, Kalleas D, Cooney D, Lamb M, Lister L. Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births. Cold Spring Harb Perspect Biol 2015;7: a017970. doi: 10.1101/cshperspect.a017970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggermann T, Perez de Nanclares G, Maher ER, Temple IK, Tümer Z, Monk D, et al. Imprinting disorders: a group of congenital disorders with overlapping patterns of molecular changes affecting imprinted loci. Clin Epigenetics 2015;7: 123. doi: 10.1186/s13148-015-0143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsubara K, Murakami N, Nagai T, Ogata T. Maternal age effect on the development of Prader-Willi syndrome resulting from upd(15)mat through meiosis 1 errors. J Hum Genet 2011;56: 566–71. doi: 10.1038/jhg.2011.59 [DOI] [PubMed] [Google Scholar]
- 7.Kagami M, Kato F, Matsubara K, Sato T, Nishimura G, Ogata T. Relative frequency of underlying genetic causes for the development of UPD(14)pat-like phenotype. Eur J Hum Genet 2012;20: 928–32. doi: 10.1038/ejhg.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermann T, Oehl-Jaschkowitz B, Dicks S, Thomas W, Kanber D, Albrecht B, et al. The maternal uniparental disomy of chromosome 6 (upd(6)mat) phenotype: result of placental trisomy 6 mosaicism? Mol Genet Genomic Med 2017;5: 668–77. doi: 10.1002/mgg3.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yingjun X, Zhiyang H, Linhua L, Fangming S, Linhuan H, Jinfeng T, et al. Chromosomal uniparental disomy 16 and fetal intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol 2017;211: 1–7. doi: 10.1016/j.ejogrb.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 10.Mulchandani S, Bhoj EJ, Luo M, Powell-Hamilton N, Jenny K, Gripp KW, et al. Maternal uniparental disomy of chromosome 20: a novel imprinting disorder of growth failure. Genet Med 2016;18: 309–15. doi: 10.1038/gim.2015.103 [DOI] [PubMed] [Google Scholar]
- 11.Chudoba I, Franke Y, Senger G, Sauerbrei G, Demuth S, Beensen V, et al. Maternal UPD 20 in a hyperactive child with severe growth retardation. Eur J Hum Genet 1999;7: 533–40. doi: 10.1038/sj.ejhg.5200287 [DOI] [PubMed] [Google Scholar]
- 12.Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med 2013;368: 2467–75. doi: 10.1056/NEJMoa1302160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arboleda VA, Lee H, Parnaik R, Fleming A, Banerjee A, Ferraz-de-Souza B, et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat Genet 2012;44: 788–92. doi: 10.1038/ng.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brioude F, Oliver-Petit I, Blaise A, Praz F, Rossignol S, Le Jule M, et al. CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome. J Med Genet 2013;50: 823–30. doi: 10.1136/jmedgenet-2013-101691 [DOI] [PubMed] [Google Scholar]
- 15.Bens S, Haake A, Richter J, Leohold J, Kolarova J, Vater I, et al. Frequency and characterization of DNA methylation defects in children born SGA. Eur J Hum Genet 2013;21: 838–43. doi: 10.1038/ejhg.2012.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura A, Muroya K, Ogata–Kawata H, Nakabayashi K, Matsubara K, Ogata T, et al. A case of paternal uniparental isodisomy for chromosome 7 associated with overgrowth. J Med Genet 2018. pii: jmedgenet-2017-104986. doi: . [Epub ahead of print]. [DOI] [PubMed]
- 17.Docherty LE, Kabwama S, Lehmann A, Hawke E, Harrison L, Flanagan SE, et al. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia 2013;56: 758–62. doi: 10.1007/s00125-013-2832-1 [DOI] [PubMed] [Google Scholar]
- 18.Mantovani G, Spada A, Elli FM. Pseudohypoparathyroidism and Gsα-cAMP-linked disorders: current view and open issues. Nat Rev Endocrinol 2016;12: 347–56. doi: 10.1038/nrendo.2016.52 [DOI] [PubMed] [Google Scholar]
- 19.Kagami M, Nagasaki K, Kosaki R, Horikawa R, Naiki Y, Saitoh S, et al. Temple syndrome: comprehensive molecular and clinical findings in 32 Japanese patients. Genet Med 2017;19: 1356–66. doi: 10.1038/gim.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med 2012;14: 10–26. doi: 10.1038/gim.0b013e31822bead0 [DOI] [PubMed] [Google Scholar]
- 21.Spiro RP, Christian SL, Ledbetter DH, New MI, Wilson RC, Roizen N, et al. Intrauterine growth retardation associated with maternal uniparental disomy for chromosome 6 unmasked by congenital adrenal hyperplasia. Pediatr Res 1999;46: 510–3. doi: 10.1203/00006450-199911000-00004 [DOI] [PubMed] [Google Scholar]
- 22.Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2011;96: E161–72. doi: 10.1210/jc.2010-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsubara K, Kataoka N, Ogita S, Sano S, Ogata T, Fukami M, et al. Uniparental disomy of chromosome 8 leading to homozygosity of a CYP11B1 mutation in a patient with congenital adrenal hyperplasia: implication for a rare etiology of an autosomal recessive disorder. Endocr J 2014;61: 629–33. doi: 10.1507/endocrj.EJ13-0509 [DOI] [PubMed] [Google Scholar]
- 24.Panzer K, Ekhaguere OA, Darbro B, Cook J, Shchelochkov OA. Uniparental isodisomy of chromosome 1 unmasking an autosomal recessive 3-beta hydroxysteroid dehydrogenase type II-related congenital adrenal hyperplasia. J Clin Res Pediatr Endocrinol 2017;9: 70–3. doi: 10.4274/jcrpe.3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartels I, Schlueter G, Liehr T, von Eggeling F, Starke H, Glaubitz R, et al. Supernumerary small marker chromosome (SMC) and uniparental disomy 22 in a child with confined placental mosaicism of trisomy 22: trisomy rescue due to marker chromosome formation. Cytogenet Genome Res 2003;101: 103–5. doi: 10.1159/000074163 [DOI] [PubMed] [Google Scholar]
- 26.Chen CP, Chen M, Wang LK, Chern SR, Wu PS, Chen SW, et al. Detection of paternal uniparental disomy 9 in a neonate with prenatally detected mosaicism for a small supernumerary marker chromosome 9 and a supernumerary ring chromosome 9. Taiwan J Obstet Gynecol 2017;56: 527–33. doi: 10.1016/j.tjog.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 27.Eggermann T, Krause-Plonka I, Wollmann HA, Zerres K, Dai G, Meyer E, et al. Supernumerary marker chromosome 7 and maternal uniparental disomy 7 in a boy with growth retardation and triangular face. Clin Dysmorphol 2006;15: 9–12. doi: 10.1097/01.mcd.0000181605.55382.9a [DOI] [PubMed] [Google Scholar]
- 28.Saitoh S, Hosoki K, Takano K, Tonoki H. Mosaic paternally derived inv dup(15) may partially rescue the Prader-Willi syndrome phenotype with uniparental disomy. Clin Genet 2007;72: 378–80. doi: 10.1111/j.1399-0004.2007.00860.x [DOI] [PubMed] [Google Scholar]
- 29.Shaffer LG. Risk estimates for uniparental disomy following prenatal detection of a nonhomologous Robertsonian translocation. Prenat Diagn 2006;26: 303–7. doi: 10.1002/pd.1384 [DOI] [PubMed] [Google Scholar]
- 30.Dawson AJ, Chernos J, McGowan-Jordan J, Lavoie J, Shetty S, Steinraths M, et al. Canadian College of Medical Geneticists committees. CCMG guidelines: prenatal and postnatal diagnostic testing for uniparental disomy. Clin Genet 2011;79: 118–24. doi: 10.1111/j.1399-0004.2010.01547.x [DOI] [PubMed] [Google Scholar]
- 31.Joshi RS, Garg P, Zaitlen N, Lappalainen T, Watson CT, Azam N, et al. DNA methylation profiling of uniparental disomy subjects provides a map of parental epigenetic bias in the human genome. Am J Hum Genet 2016;99: 555–66. doi: 10.1016/j.ajhg.2016.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]