Abstract.
Our study aimed at evaluating the safety and efficacy of GH treatment up to near adult height (NAH) for short children born small for gestational age (SGA). This was a multi-center, open-label, long-term extension study after a one-year, randomized, open-label, dose-response study. The primary objective was to assess safety, determined by adverse events and laboratory test parameters. Height parameters were evaluated as a secondary objective. The final data after all patients completed the study were reported. Overall, 61 patients were enrolled in the study. GH treatment was well tolerated. No notable changes in HbA1c levels, oral glucose tolerance tests and glucose metabolism were observed. No new safety concerns related to long-term treatment up to NAH were identified. Twenty patients (11 boys and 9 girls) reached NAH with a mean height of 159.1 cm and 146.9 cm, respectively. The mean change in height SDS from baseline to NAH was +1.9 in boys and +1.8 in girls. Long-term GH treatment for SGA short stature was confirmed to be safe and effective for the normalization of adult height.
Keywords: SGA, adult height, GH treatment, Clinical trial, Japan
Introduction
GH treatment is internationally recognized as the only standard therapy for short children born small for gestational age (SGA) (1,2,3). Although data regarding the efficacy and safety of long-term GH therapy are being accumulated, there is no data for Japanese SGA patients who were followed up to adult height.
This Phase III study started in 2002 and has continued through long-term follow-up. We have published several interim reports on this study, including a dose-response study (4), a follow-up study reporting outcomes of long-term GH treatment (4–8 yr) in a subpopulation of Japanese short children born SGA (5), that in those who met all criteria for GH treatment according to the Japanese guidelines (3), and an interim analysis of up to 10 yr of treatment (6). Here, we report the final data, including those on near adult height (NAH), that have been collected upon completion of the study.
Methods
Ethics
The present trial was performed in accordance with the Declaration of Helsinki and The Ministerial Ordinance on Good Clinical Practice for Drugs. Written informed consent was obtained from the patients’ parents/legal guardians and, when possible, the patients themselves. The study protocol was approved by the institutional review boards at each participating facility.
Patients
The total number of patients was 61, with 29 in the 0.033/0.067 mg group and 32 in the 0.067/0.067 mg group (see below for definitions). Inclusion and exclusion criteria were described previously (4).
Study design
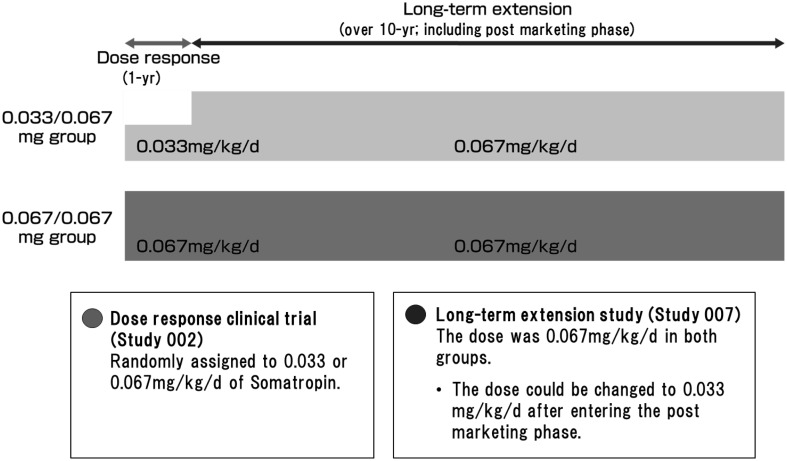
This was a multi-center, open-label, long-term extension study after a one-year, randomized, open-label, dose-response study conducted at 19 institutions listed in the Institutions Participated in the Study. This study consists of a one-year dose response study (Study 002) followed by a long-term extension study (Study 007) of over 10 yr, including the post-marketing phase (Fig. 1). In study 002, patients were randomly assigned to two groups receiving 0.033 or 0.067 mg/kg/d GH. In Study 007, the dose was increased to 0.067 mg/kg/d in the group previously receiving 0.033 mg/kg/d GH (0.033/0.067 mg group), while the dose did not change for children who had previously received 0.067 mg/kg/d (0.067/0.067 mg group). The summary of Study 007 is disclosed in Clinicaltrials.gov (NCT01859949) (9).
Fig. 1.
Study design of Studies 002 and 007 including the post-marketing phase. 0.033/0.067 mg group: Participants who were treated with somatropin 0.033 mg/kg/d in the preceding study for 12 mo received a dose of 0.067 mg/kg/d. 0.067/0.067 mg group: Participants who were treated with somatropin 0.067 mg/kg/d in the preceding study for 12 mo were maintained on the same dose. After entering the post marketing phase study, the dose could be changed to 0.033 mg/kg/d from the viewpoint of age, puberty, height velocity, and safety.
The treatment termination criteria defined in the protocol were “reaching a height SDS of 0 for chronological age”, “reaching a bone age of 17 yr in boys or 15 yr in girls”, “annual height velocity (HV) < 2 cm after achieving peak velocity at puberty” and “annual height velocity < 1.0 cm”. When patients met any one of these criteria, study participation was terminated. Onset of puberty was defined as the immediate time point after the point when secondary sexual characteristics reached Tanner Stage II. NAH was defined as height at the immediate time point after the point when annual HV became less than 2 cm after achieving peak velocity at puberty or when bone age reached 17 yr in boys or 15 yr in girls. Patients whose height SDS reached 0 and GH administration was discontinued due to the termination criteria were included in the NAH analysis when the above NAH definition was satisfied. In addition, height SDS at NAH was calculated using standard height at chronological age for Japanese boys and girls when each patient reached NAH.
Safety assessment methods, including laboratory tests, bone age evaluation, and puberty evaluation, were described previously (5). Hemoglobin A1c (HbA1c), fasting blood glucose, oral glucose tolerance tests (OGTT), immune reactive insulin (IRI) and homeostasis model assessment of insulin resistance (HOMA-IR) were evaluated as glucose metabolism parameters. OGTTs were performed at baseline and on termination of the 12-mo treatment. Children were administered 1.75 g/kg glucose (maximum: 75 g), and subsequently blood glucose and IRI levels were determined for up to 120 min. Glucose tolerance data were assessed using standards established by Kuzuya et al. (7) and individual patients were classified into three categories: normal, borderline, or diabetic. Diabetic is defined as fasting plasma glucose (FPG) levels ≥ 126 mg/dl, and/or plasma glucose levels ≥ 200 mg/dl at 2 h after glucose load (2hPG), while the normal pattern is defined as FPG levels < 110 mg/dl and plasma glucose levels < 140 mg/dl at 2hPG. The borderline pattern is defined as intermediate between the diabetic and normal patterns.
Statistics
Statistical methods were described previously (5, 6). Full analysis set (FAS) was used and it was defined as the set of patients who met the inclusion/exclusion criteria as well as excluded individuals who were never administered the study medication and had no study assessment data after the start of Study 007. In the efficacy analysis, baseline values of Study 002 were used for Study 007. In the safety analysis, treatment-related adverse events (AEs) during Study 007 were reported. AEs and abnormal changes in laboratory tests that occurred during Study 002 and were not resolved at the start of Study 007 were included in the final analysis. When AEs or abnormal changes in the laboratory testing values were resolved during Study 002, but subsequently occurred again during Study 007, they were included in the final analysis as new AEs or abnormal changes in laboratory tests.
IGF-1 SDS was calculated with Japanese reference values (8) of serum IGF-1 concentration in children classified by sex and age.
Summary statistics of patients who reached NAH during GH treatment, whose change in height SDS from baseline to NAH, or whose change in height and height SDS from puberty onset to NAH were calculated according to sex. Height, height SDS and age at puberty onset were also summarized by sex for patients who reached puberty. NAH SDS and height SDS at puberty onset were calculated based on Japanese reference standard values according to sex and age when patients reached the relevant milestone. Scatter plots of height to age at puberty onset were generated by sex.
Results
Patient background and disposition
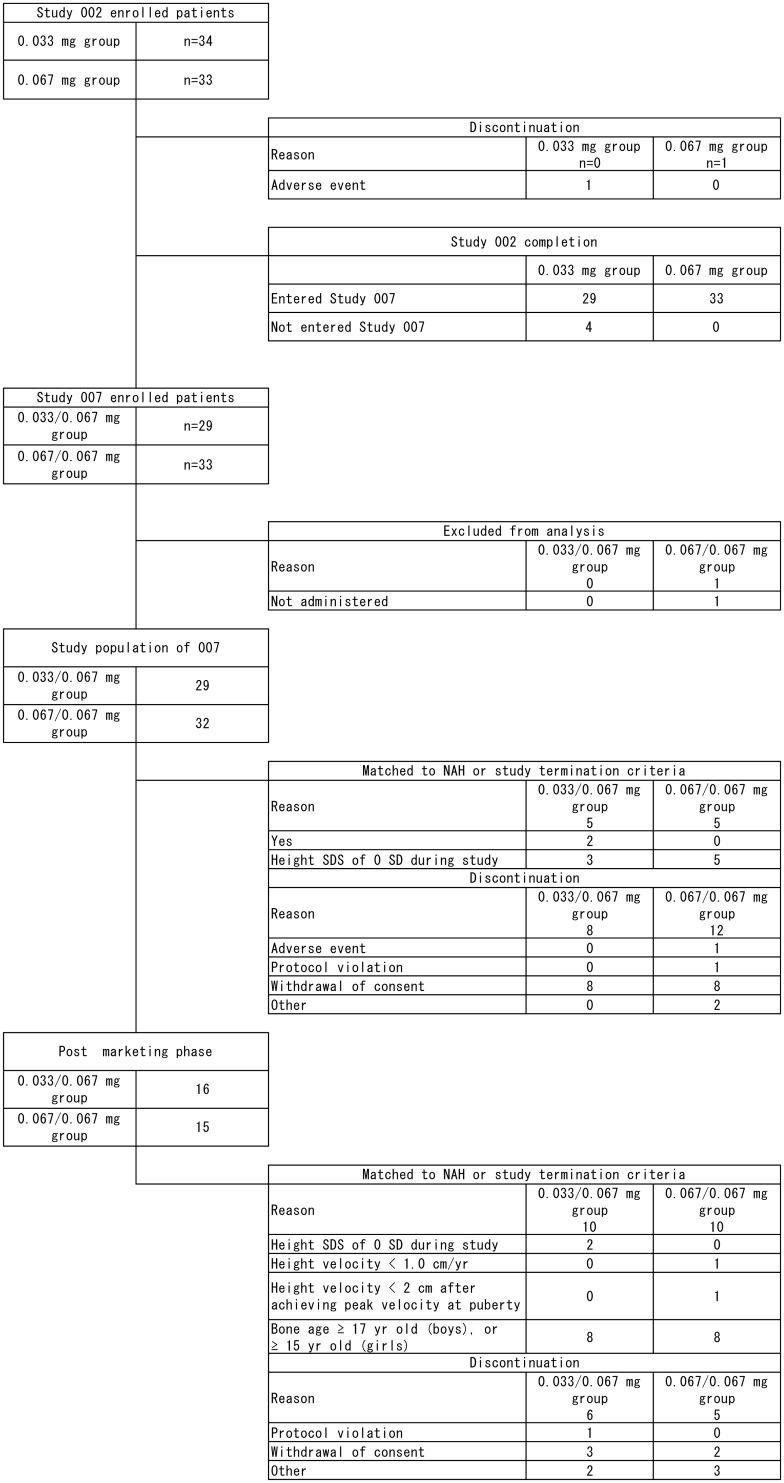
After completing the previous one-year treatment in Study 002, 61 out of 62 patients who entered Study 007 were treated with 0.067 mg/kg/d GH and continued until the pre-specified treatment termination criteria were satisfied. Three of those had comorbid Silver-Russell syndrome. Changes in the number of patients in both studies are summarized in Fig. 2. One patient was maintained at the dosage of 0.033 mg/kg/d even after completion of Study 002, as the investigator considered that 0.033 mg/kg/d is sufficiently effective for this patient. In this report this patient was included in the 0.033/0.067 mg group.
Fig. 2.
Flowchart of the selection of patients in Studies 002 and 007 including the post marketing phase. Patients were randomized into two dose groups: 34 and 33 patients received GH at 0.033 and 0.067 mg/kg/d for one yr (Study 002), respectively. After Study 002, the dose was escalated to 0.067 mg/kg/d in the group receiving 0.033 mg/kg/d of GH (hereafter abbreviated as the 0.033/0.067 mg group), while children assigned to the group that received 0.067 mg/kg/d remained on the same dose (hereafter abbreviated as the 0.067/0.067 mg group) (Study 007).
Mean duration of GH treatment during the total period of the study was 7.15 (range: 1.49–13.53) yr in the 0.033/0.067 mg group (29 patients), and 6.44 (1.80–12.24) yr in the 0.067/0.067 mg group (32 patients). During the total period of Studies 002 to 007 post-marketing phase, the mean adherence of drug administration per patient was favorable (pre-defined as more than 75%) in all patients except for one with a mean adherence of 64.7%.
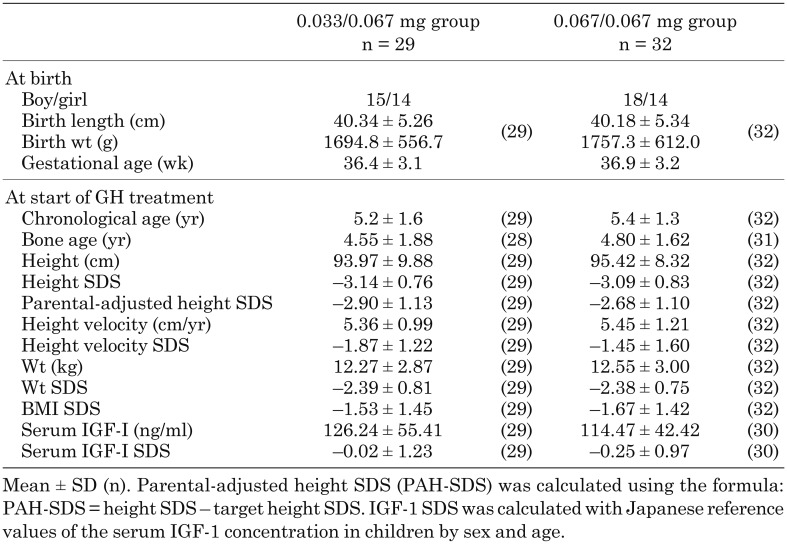
The clinical characteristics of the 61 patients at birth and the start of GH treatment are shown in Table 1. Demographic characteristics were similar between the two treatment groups. Among 44 patients in this study who met the criteria of the Japanese guidelines for GH treatment initiation (height SDS < –2.5), 20 patients werein the 0.033/0.067 mg group and 24 in the 0.067/0.067 mg group.
Table 1. Clinical characteristics.
Efficacy
A summary of patients who reached NAH during GH treatment is shown in Table 2. Twenty patients (11 boys and 9 girls) reached NAH. Among these patients, the mean height at NAH was 159.1 cm (–1.56 SDS) in boys and 146.9 cm (–1.61 SDS) in girls at the time NAH was reached; the change in height SDS from baseline to NAH was +1.87 SDS in boys and +1.82 SDS in girls. The mean pubertal height gain (from onset of puberty to NAH) was 22.2 cm in boys and 20.6 cm in girls. Age at NAH ranged from 14.6 to 16.8 yr in boys and from 12.8 to 16.5 yr in girls.
Table 2. Summary of patients who reached NAH during GH treatment.
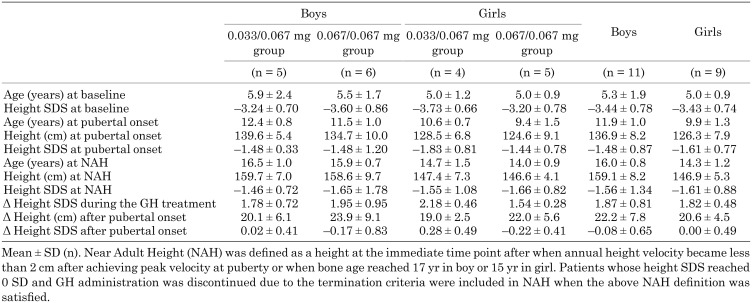
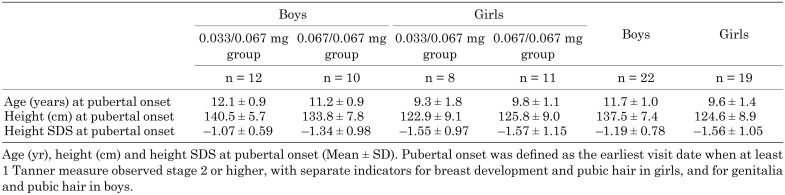
Among the 41 patients (22 boys and 19 girls) who reached pubertal onset, the mean age at pubertal onset was 11.7 yr in boys and 9.6 yr in girls. The mean height at pubertal onset was 137.5 cm (–1.19 SDS) in boys and 124.6 cm (–1.56 SDS) in girls (Table 3).
Table 3. Summary of height, height SDS and age at pubertal onset.
The number of patients whose height SDS of chronological age exceeded –2.0 at the completion or discontinuation of the study was 26 out of 33 (78.8%) in boys and 19 out of 28 (67.9%) in girls. However, the number of patients whose NAH SDS exceeded –2.0 was 9 out of 11 and 6 out of 9 in boys and girls, respectively.
Exploratory analysis
Since we started this study before the establishment of GH treatment guidelines for SGA in Japan, height SDS ≤ –2 was applied as the criterion for initiating GH treatment, consistent with other growth-related studies. Japanese guidelines published later stipulated that height SDS < –2.5 consists a treatment initiation criterion (3). Due to the difference in criteria between our study and the Japanese guidelines, we analyzed NAH endpoints in a subgroup of patients with height SDS < –2.5 at baseline. Out of 20 patients who reached NAH, 17 were < –2.5 SDS in height SDS at baseline. Among them, mean height at NAH was 158.7 cm (–1.60 SDS) in boys and 146.6 cm (–1.77 SDS) in girls, which was above the academically defined short stature of –2 SDS. Mean change in height SDS from baseline to NAH was +1.97 SDS in boys, and +1.94 SDS in girls. Height gain in this subpopulation was comparable to the overall population.
Safety
AEs were observed in 58 out of 61 patients (95.1%) during Study 007. There were no differences between the treatment groups regarding the incidence and types of adverse events. Since the last interim report (6), there was no additional information altering the safety profile. The summary of the AE is disclosed in Clinicaltrials.gov (9).
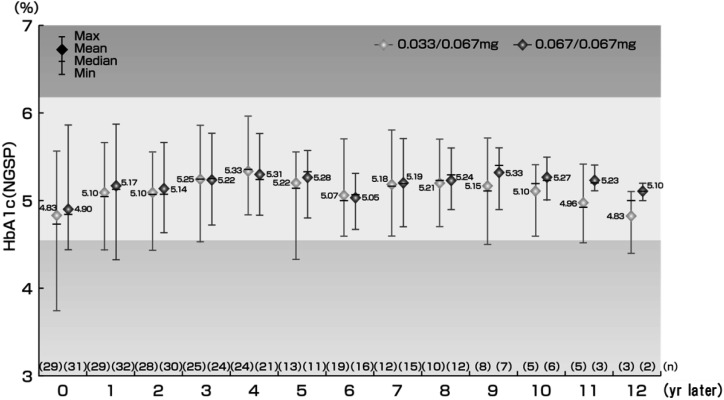
HbA1c (NGSP) levels of the patients were stable and within the normal range (4.6–6.2%) in both groups without any significant changes (Fig. 3). One patient in the 0.033/0.067 mg group had high HbA1c (5.9%) at 63 mo, which recovered to normal range at 66 mo without decreasing the GH dose or administering additional medication.
Fig. 3.
Change in HbA1c. The white background color is the HbA1c reference range (4.6 to 6.2).
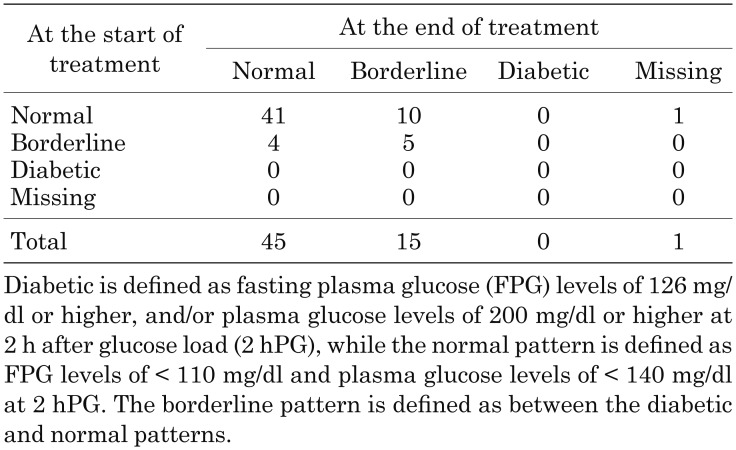
OGTT patterns at baseline and the end of GH treatment are shown in Table 4. The shift in OGTT patterns from baseline to the end of GH treatment were “Normal to Normal” in 41 patients, “Normal to Borderline” in 10, “Borderline to Normal” in 4 and “Borderline to Borderline” in 5 patients. One patient shifted from normal to a diabetic pattern at 36 months, but recovered to the normal pattern at 48 months with the same dose of study drug. At the last observation (72 mo), the patient’s status was borderline pattern.
Table 4. Cross table for OGTT patterns at the start and the end of GH treatment.
Elevated levels of fasting blood glucose and fasting IRI were observed after the start of GH treatment, and these levels were maintained during the treatment (data not shown). No significant changes were observed in the insulinogenic index or the HOMA-IR.
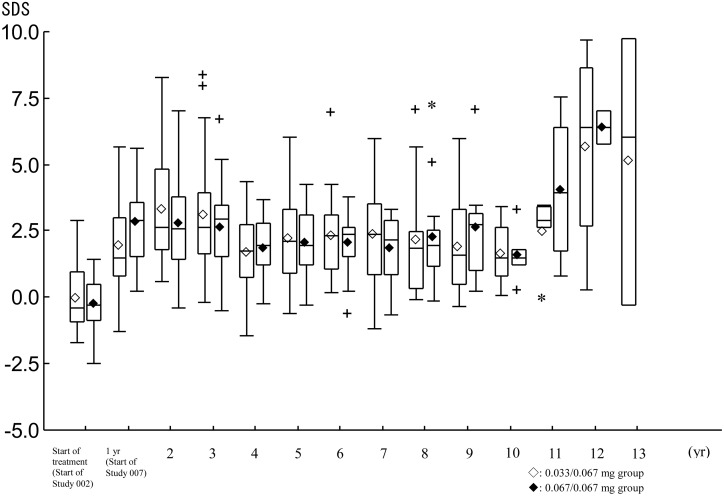
Mean IGF-1 levels increased during the study period in both groups. Mean IGF-1 SDS was high at 24 mo and showed 3.329 SDS in the 0.033/0.067 mg group and 2.830 SDS in the 0.067/0.067 mg group, and remained mostly close to 2.0 SDS (Fig. 4).
Fig. 4.
Changes in IGF-I SDS. ◊♦: mean, Horizontal line in the box: median, Box: from the 25th percentile to the 75th percentile, Vertical line (whisker): the most remote point from median, which is within 1.5 IQR from the box. +: 1.5 IQR~3 IQR from the box. *: remote point over 3 IQR from the box.
Consistent with the previous report (5), there was no new concerns regarding excessive bone maturation.
Discussion
We evaluated safety and efficacy of GH treatment for over 10 yr in 61 Japanese SGA children, including 20 children who reached their NAH. In Study 007, the most common all-causality AEs were due to infection or allergy, which were mostly mild or moderate in severity. These findings were consistent with the previous reports from Study 002 (4) and the generally known safety profile of GH treatment. No new safety concerns related to long-term treatment up to NAH were reported in our study. In this study, the patients were administered a GH dose of 0.067 mg/kg/d for a maximum of approximately 15 yr. IGF-1 SDS increased early during GH treatment, and then remained constantly close to 2 SDS. We observed no increase in mortality risks suggested by the SAGhE study (10); however, follow-up monitoring of patients who have completed treatment is necessary.
An influence on glucose metabolism is a concern of GH treatment for children with SGA (11). Mild increase in blood glucose and insulin levels was observed during GH treatment; an increase in insulin secretion, determined with OGTT, was also observed. An increase in insulin resistance can be considered as a result of administration of GH. This trend observed is similar to that reported by Sas et al. (11), and Horikawa et al. (12). In this study, HbA1c levels in most patients were maintained within normal range and no concerns regarding glucose metabolism were raised related to long-term GH treatment. Considering that insulin and GH compete with each other during glucose metabolism, changes in glucose tolerance related parameters were considered as a physiological response (13).
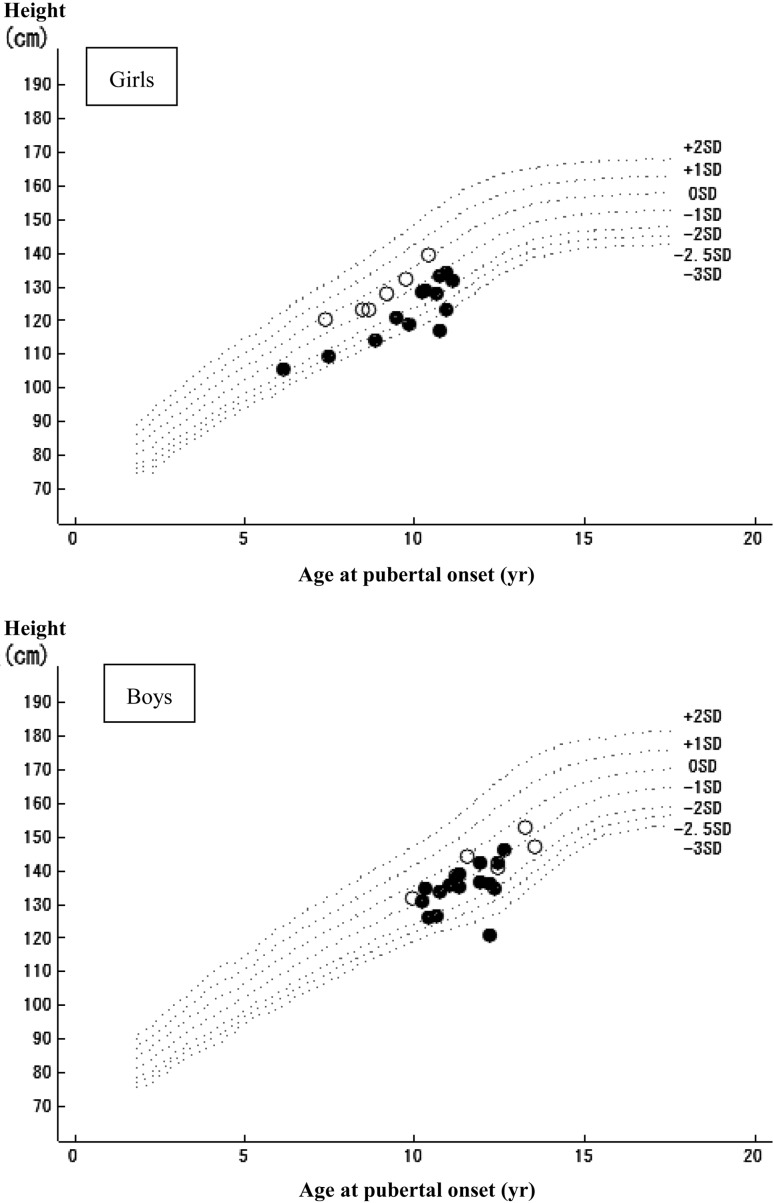
All patients developed puberty within the normal age range for Japanese children except for one girl who developed early puberty at the age of 6 yr and 2 mo, and who was therefore diagnosed with precocious puberty 36 mo after the initial dose (Fig. 5). The median age at pubertal onset was 11.4 yr in boys and 9.9 yr in girls (Table 3), which is similar to the reference population (14, 15) and consistent with the findings of Boonstra et al. (16).
Fig. 5.
Relationship between age and height at pubertal onset. Scattering chart of age and height at the onset of puberty among the subjects (boys: 22 subjects, girls: 19 subjects) who reached to puberty during GH treatment. Pubertal onset was evaluated every 3 months before approval and every 6 months during post marketing phase. -------: ± 1, ± 2, –2.5, –3 SD of standard growth curve for Japanese children, ●: baseline height SDS < –2.5 SD, ○: –2.0 SD > baseline height SDS ≥ –2.5 SD.
The mean height SDS at NAH was –1.6 in boys and –1.6 in girls in the present study. The adult height reported in previous controlled trials for the SGA population varied. van Pareren et al. (17) reported better results (–0.9 ± 0.8 SDS), while Carel et al. (18) reported lower values (–2.7 ± 0.9 SDS). Dahlgren et al. (19) and van Dijk et al. (20) showed –1.2 ± 0.7 SDS (among patients with > 2-yr treatment) and –1.4 ± 1.0 SDS, respectively. Due to the lack of control group in our study, it is difficult to directly compare our data with previous studies. However, our results showed intermediate values compared to four controlled trials published so far and thus our data showed a similar trend with previous reports.
While our study has shown that long-term GH treatment normalized adult height, height SDS at the onset of puberty was still less than –2 SDS in some patients implying that there is individual variability in treatment response, as reported previously (21). The mean height gain during puberty in healthy short children (22) was 27.1 cm in boys, whereas it was 22.2 cm in our NAH population. In girls, we observed higher height gain (20.6 cm in NAH population and 17.7 cm in healthy short children) than boys although we should consider that one girl developed early puberty and showed remarkable height gain (30.0 cm). High-dose GH treatment is reported to accelerate bone age in patients with idiopathic short stature (23) and the same tendency was observed in our study (data not shown). This may result in small pubertal height gain and unsatisfactory adult height observed in some patients.
There is a possible bias that could be considered as a limitation in the NAH SDS evaluation. Ten patients, who reached a height SDS of 0, terminated the study before reaching NAH. The 10 patients were not included in the NAH population in the present study. In addition, we should carefully interpret NAH SDS as adult height SDS because NAH SDS was calculated using standard height at chronological age when each patient reached NAH, was not used the standard height at adult age and thus we may have overestimated the NAH SDS. If adult height SDS is estimated from the observed NAH with the standard height at the age of 17.5 yr for Japanese boys and girls (24), assuming the observed NAH equals adult height, mean adult height SDS in boys and in girls is –2.01 SDS and –2.19 SDS respectively, and the mean increases in height SDS from the baseline to adult height are 1.42 SDS and 1.24 SDS, respectively. Based on these estimates, these increases in height SDS are smaller than those of severe GH deficiency (GHD) (2.13 SDS and 1.66 SDS in boys and girls, respectively) children, but greater than those of mild GHD (1.12 SDS and 1.04 SDS) and moderate GHD (1.22 SDS and 0.94 SDS) children in the Kabi International Growth Study (KIGS) Japan database (25). Thus, the present results are comparable to the existing reports on Japanese GHD patients.
Conclusion
Long-term GH treatment with the dose of 0.067 mg/kg/d was well tolerated in SGA patients without new safety concerns and efficacy in normalizing adult height were observed.
Conflict of Interests
This study was sponsored by Pfizer Japan Inc. Yuko Hoshino and Shintaro Hiro are employees of Pfizer Japan Inc. Nobuhiko Ohki was a Pfizer employee at the time this manuscript was drafted. Toshiaki Tanaka and Susumu Yokoya have no conflicts of interest to declare. Editorial assistance was provided by Yutaka Takahashi at WDB ICO Co. Ltd. and was funded by Pfizer Japan Inc.
Institutions participated in the study
Asahikawa Medical University Hospital (Pediatrics), Hokkaido University Hospital (Pediatrics), Obihiro Kyokai Hospital (Pediatrics), Iwate Medical University Hospital (Pediatrics), Tohoku University Hospital (Pediatrics), Gunma University Hospital (Pediatrics), Toranomon Hospital (Pediatrics), National Center for Child Health and Development (Department of Medical Subspecialties), Kitasato University Hospital (Pediatrics), University of Yamanashi Hospital (Pediatrics), Seirei Hamamatsu General Hospital (Pediatrics), Kyoto University Hospital (Pediatrics), JCHO Osaka Hospital (Pediatrics), Osaka Medical Center and Research Institute for Maternal and Child Health (Department of Pediatric Gastroenterology, Nutrition and Endocrinology), Okayama University Hospital (Pediatrics), Hiroshima City Hiroshima Citizens Hospital (Pediatrics), Tottori University Hospital (Pediatrics), University of Occupational and Environmental Health Hospital (Pediatrics) and Kumamoto University Hospital (Pediatrics, Child Development).
Acknowledgments
The authors would like to thank all participating patients and their families, as well as the institutions above, investigators, study coordinators and other staff members who participated in this clinical study.
We are grateful to Prof. Kenji Fujieda, Prof. Yoshiki Seino, Prof. Hiroshi Tada and Dr. Jun Mishina for their contributions as Coordinating Investigators and for their cooperation in the progress of this study. We would like to thank Dr. Mari Sato for centralized evaluation of bone age.
References
- 1.Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P, International Small for Gestational Age Advisory Board. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics 2003;111: 1253–61. doi: 10.1542/peds.111.6.1253 [DOI] [PubMed] [Google Scholar]
- 2.Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 2007;92: 804–10. doi: 10.1210/jc.2006-2017 [DOI] [PubMed] [Google Scholar]
- 3.The Japanese Society for Pediatric Endocrinology, Japan Society for Premature and Newborn Medicine. Guideline for GH treatment in SGA short children. Nippon Shonika Gakkai Zasshi 2007;111: 641–6(in Japanese). [Google Scholar]
- 4.Tanaka T, Fujieda K, Yokoya S, Seino Y, Tada H, Mishina J. Efficacy and safety of growth hormone treatment in children born small for gestational age in Japan. J Pediatr Endocrinol Metab 2008;21: 423–31. doi: 10.1515/JPEM.2008.21.5.423 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Yokoya S, Fujieda K, Seino Y, Tada H, Mishina J, et al. Efficacy and safety of up to 8 years of long-term growth hormone treatment in short children born small for gestational age in Japan: analysis of the subpopulation according to the Japanese guideline. Clin Pediatr Endocrinol 2012;21: 57–68. doi: 10.1297/cpe.21.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, Yokoya S, Seino Y, Tada H, Mishina J, Sato T, et al. Onset of puberty and near adult height in short children born small for gestational age and treated with GH: Interim analysis of up to 10 years of treatment in Japan. Clin Pediatr Endocrinol 2015;24: 15–25. doi: 10.1297/cpe.24.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diab Soc 1999;42: 385–404(in Japanese). [DOI] [PubMed] [Google Scholar]
- 8.Shimatsu A, Fujieda K, Haniu K, Tanaka T, Yokoya S, Miyachi Y, et al. Clinical evaluation of serum IGF-I, IGF-II and IGFBP-3 measured by IRMA kits in childhood. Horumon To Rinsho 1996;44: 1129–38(in Japanese). [Google Scholar]
- 9.ClinicalTrials.gov. Long Term Study of Genotropin (Somatropin) for Short Children Born Small for Gestational Age (SGA). https://www.clinicaltrials.gov/ct2/show/results/NCT01859949?term=A6281225&cntry1=ES%3AJP&rank=1 (Accessed on 7 Aug 2017).
- 10.Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab 2012;97: 416–25. doi: 10.1210/jc.2011-1995 [DOI] [PubMed] [Google Scholar]
- 11.Sas T, Mulder P, Aanstoot HJ, Houdijk M, Jansen M, Reeser M, et al. Carbohydrate metabolism during long-term growth hormone treatment in children with short stature born small for gestational age. Clin Endocrinol (Oxf) 2001;54: 243–51. doi: 10.1046/j.1365-2265.2001.01178.x [DOI] [PubMed] [Google Scholar]
- 12.Horikawa R, Tanaka T, Nishinaga H, Ogawa Y, Yokoya S. The influence of a long-term growth hormone treatment on lipid and glucose metabolism: a randomized trial in short Japanese children born small for gestational age. Int J Pediatr Endocrinol 2016; 2016: 19. [DOI] [PMC free article] [PubMed]
- 13.van der Steen M, Lem AJ, van der Kaay DC, Hokken-Koelega AC. Insulin sensitivity and β-cell function in SGA children treated with GH and GnRHa: results of a long-term trial. J Clin Endocrinol Metab 2016;101: 705–13. doi: 10.1210/jc.2015-3435 [DOI] [PubMed] [Google Scholar]
- 14.Fujieda K, Matsuura N. Growth and maturation in the male genitalia from birth to adolescence. I. Change of testicular volume. Acta Paediatr Jpn 1987;29: 214–9. doi: 10.1111/j.1442-200X.1987.tb00035.x [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Imai T. The standard of breast development in normal girls: Diagnostic criteria for precocious puberty. J Child Health 2005;64: 33–8(in Japanese). [Google Scholar]
- 16.Boonstra V, van Pareren Y, Mulder P, Hokken-Koelega A. Puberty in growth hormone-treated children born small for gestational age (SGA). J Clin Endocrinol Metab 2003;88: 5753–8. doi: 10.1210/jc.2003-030512 [DOI] [PubMed] [Google Scholar]
- 17.Van Pareren Y, Mulder P, Houdijk M, Jansen M, Reeser M, Hokken-Koelega A. Adult height after long-term, continuous growth hormone (GH) treatment in short children born small for gestational age: results of a randomized, double-blind, dose-response GH trial. J Clin Endocrinol Metab 2003;88: 3584–90. doi: 10.1210/jc.2002-021172 [DOI] [PubMed] [Google Scholar]
- 18.Carel JC, Chatelain P, Rochiccioli P, Chaussain JL. Improvement in adult height after growth hormone treatment in adolescents with short stature born small for gestational age: results of a randomized controlled study. J Clin Endocrinol Metab 2003;88: 1587–93. doi: 10.1210/jc.2002-021123 [DOI] [PubMed] [Google Scholar]
- 19.Dahlgren J, Wikland KA, Swedish Study Group for Growth Hormone Treatment. Final height in short children born small for gestational age treated with growth hormone. Pediatr Res 2005;57: 216–22. doi: 10.1203/01.PDR.0000148716.71231.81 [DOI] [PubMed] [Google Scholar]
- 20.van Dijk M, Bannink EM, van Pareren YK, Mulder PG, Hokken-Koelega AC. Risk factors for diabetes mellitus type 2 and metabolic syndrome are comparable for previously growth hormone-treated young adults born small for gestational age (sga) and untreated short SGA controls. J Clin Endocrinol Metab 2007;92: 160–5. doi: 10.1210/jc.2006-1073 [DOI] [PubMed] [Google Scholar]
- 21.Loche S, Carta L, Ibba A, Guzzetti C. Growth hormone treatment in non-growth hormone-deficient children. Ann Pediatr Endocrinol Metab 2014;19: 1–7. doi: 10.6065/apem.2014.19.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka T. Natural growth in short healthy children: clinical factors related to the improvement of adult height. J Jpn Ass Hum Auxo 2010;16: 15–21(in Japanese). [Google Scholar]
- 23.Kamp GA, Waelkens JJ, de Muinck Keizer-Schrama SM, Delemarre-Van de Waal HA, Verhoeven-Wind L, Zwinderman AH, et al. High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch Dis Child 2002;87: 215–20. doi: 10.1136/adc.87.3.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suwa S, Tachibana K. Standard growth charts for height and weight of Japanese children from birth to 17 years based on a cross-sectional survey of national data. Clin Pediatr Endocrinol 1993;2: 87–97. doi: 10.1297/cpe.2.87 [DOI] [Google Scholar]
- 25.Fujieda K, Tanaka T, Takano K, Chihara K, Seino Y, Irie M, KIGS Japan Scientific Committee. Adult height after growth hormone treatment in Japanese children with idiopathic growth hormone deficiency: analysis from the KIGS Japan database. J Pediatr Endocrinol Metab 2011;24: 457–62. doi: 10.1515/jpem.2011.212 [DOI] [PubMed] [Google Scholar]