Abstract
Reducing insulin/IGF‐1 signaling (IIS) extends lifespan, promotes protein homeostasis (proteostasis), and elevates stress resistance of worms, flies, and mammals. How these functions are orchestrated across the organism is only partially understood. Here, we report that in the nematode Caenorhabditis elegans, the IIS positively regulates the expression of caveolin‐1 (cav‐1), a gene which is primarily expressed in neurons of the adult worm and underlies the formation of caveolae, a subtype of lipid microdomains that serve as platforms for signaling complexes. Accordingly, IIS reduction lowers cav‐1 expression and lessens the quantity of neuronal caveolae. Reduced cav‐1 expression extends lifespan and mitigates toxic protein aggregation by modulating the expression of aging‐regulating and signaling‐promoting genes. Our findings define caveolae as aging‐governing signaling centers and underscore the potential for cav‐1 as a novel therapeutic target for the promotion of healthy aging.
Keywords: aging, caveolae, insulin/IGF‐1 signaling, lifespan, proteostasis
Subject Categories: Ageing, Membrane & Intracellular Transport, Signal Transduction
Introduction
Insulin/IGF‐1 signaling (IIS) is a highly conserved pathway that regulates aging and lifespan of worms 1, flies 2, mice 3, and probably humans 4. In the nematode Caenorhabditis elegans (C. elegans), the binding of insulin‐like ligands 5 to the worm's lone insulin/IGF‐1 receptor DAF‐2 results in phosphorylation events that negatively regulate the activity of at least three transcription factors: DAF‐16/FOXO 6, SKN‐1/NRF 7, and the heat shock factor 1 (HSF‐1) 8 through changes in their cellular localization. The IIS downstream kinases, PDK‐1 and AKT‐1, directly phosphorylate DAF‐16 9 and SKN‐1 7, preventing them from entering the nucleus and from regulating their target gene networks. Similarly, the IIS prevents the phosphorylation of the HSF‐1‐interacting protein DDL‐1, which retains HSF‐1 in the cytosol 8. Accordingly, the knockdown of daf‐2 hyper‐activates its downstream transcription factors extends lifespan 1 and promotes resistance to various environmental insults, including elevated temperatures 10 and exposure to pathogenic bacteria 11 or ultra‐violet (UV) radiation 12. IIS reduction also protects worms from toxic aggregation (proteotoxicity) of various neurodegeneration‐linked proteins, including the human Alzheimer's disease‐associated peptide Aβ 13 and the Huntington's disease‐linked, aberrantly long, polyQ stretches 14. These counter‐proteotoxic effects of IIS reduction are conserved from worms to mammals 15.
Among its many functions, IIS has also been found to play a strong role in the regulation of metabolism and is required for the expression of a significant number of lipid‐metabolizing enzymes 16, 17. For instance, daf‐2 regulates the expression of the triglyceride lipase, lipl‐4. Reduced expression of lipl‐4 suppresses the longevity phenotype of daf‐2 (e1370) mutant animals while having no effect on the lifespan of wild‐type animals 18. Similarly, the lipid gene regulator MDT‐15 cooperates with DAF‐16 to regulate gene expression 19. Collectively, these findings indicate that lipid metabolism and IIS‐promoted longevity are correlated; nonetheless, the mechanistic details of this link are only partially understood.
The plasma membrane consists of a highly heterogeneous bilayer of lipids responsible for the control of a large number of essential functions, including signaling, transport, adhesion, and protein localization. While the cell membrane is formed of large amounts of different lipids and cholesterol, embedded within it are small pockets of liquid‐ordered microdomains rich in cholesterol and sphingolipids 20. These “lipid rafts” play a significant role in signal transduction and in the immune response, in part by providing docking stations for groups of proteins in the plasma membrane 20. Lipid rafts have been also reported to be physiologically important for neurodegeneration‐associated processes. For instance, the replication of infectious prions 21 and the biogenesis of the Alzheimer's disease (AD)‐associated peptide Aβ 22 were attributed to these microdomains and altered lipid metabolism characterizes AD patients 23. Caveolae constitute a unique subtype of lipid rafts whose formation depends on the protein caveolin‐1 (CAV‐1). Among other roles, caveolae serve as scaffolds for the formation of signaling complexes 20 and functionally interact with the IGF‐1 receptor 24. Interestingly, CAV‐1 was found to regulate cell senescence and contribute to aging phenotypes of skin cells 25. Moreover, CAV‐1 was shown to be, at least partially, regulated by IGF‐1 signaling 26 and by the FOXO transcription factor 27. While in vertebrates, the caveolin gene family has three members: caveolin‐1, 2 and 3, which are expressed in distinct tissues 28, in C. elegans only two family members have been characterized: caveolin‐1 and caveolin‐2. How caveolae and the IIS interact at the organismal level, how such interactions affect lifespan and whether they modulate proteostasis across tissues, are unknowns.
Here, we utilized C. elegans to investigate whether IIS reduction modulates protein content and functionality of lipid microdomains and discovered that this manipulation changes the protein composition of lipid rafts. Lipid rafts extracted from worms that were treated with daf‐2 RNAi contain highly reduced quantities of subset of the aging‐related, IIS‐regulated proteins typically found in lipid rafts of untreated animals. We also found that in the nematode, IIS reduction lowers the expression of cav‐1 which is foremost expressed in neurons of the adult animal 29 thereby, lessening the amount of caveolae in these cells. The knockdown of cav‐1 extends lifespan, enhances proteostasis, protects the worm from pathogenic bacteria, and elevates survival after exposure to UV radiation. In contrast, the knockdown of cav‐1 has no effect on survival of worms that were exposed to heat. Interestingly, while the lifespan extension effect of knocking down cav‐1 depends on daf‐16 and skn‐1, the knockdown of cav‐1 facilitates proteostasis independently of these transcription factors. Analyzing gene expression patterns, we discovered that reduced cav‐1 expression attenuates the expression of an array of genes involved in signaling and in the determination of lifespan. Our observations designate neuronal caveolae as aging‐regulating cellular sites that are modulated by the IIS and provide novel insights into the roles of lipid microdomains in the regulation of aging.
Results
IIS reduction modifies lipid rafts’ protein content
We first asked whether the lipid rafts found in animals whose IIS was suppressed were inherently different from those found in animals that exhibit natural IIS activity. To address this we employed 1‐day‐old, conditionally sterile worms (strain CF512) that were either treated with daf‐2 RNAi of left untreated (grown on empty vector bacteria (EV)), and isolated detergent‐resistant membranes (DRMs) using a modified floatation procedure (Fig EV1A and B) 30. The sterility of these animals enabled the purification of DRMs from somatic tissues with no background from developing embryos. DRMs consist of a mixture of structures which contain several subtypes of lipid microdomains including plasma membrane rafts and caveolae (reviewed in 20). Intriguingly, we observed several protein bands in DRMs of untreated worms (EV) that were undetectable in samples of animals treated with daf‐2 RNAi (Fig 1A, arrows). Conversely, we found that a smaller number of proteins appeared in our daf‐2 RNAi‐treated animals that were absent in the EV samples. These analyses preliminarily suggested that daf‐2 RNAi promotes a dramatic remodeling of the lipid microdomains’ proteome.
Figure EV1. Caveolin‐1 floats in TX114 gradients.

- Worm homogenates were incubated with TX‐114 and subjected to floatation. The DRM‐resident proteins CAV‐1 and γ tubulin but not fumarase, float to the upper fractions (top).
- This floatation was prevented when the homogenates were pre‐incubated with the detergent saponin which dissolves DRMs.
Figure 1. The knockdown of daf‐2 by RNAi modifies the protein content of detergent‐resistant membranes (DRMs).
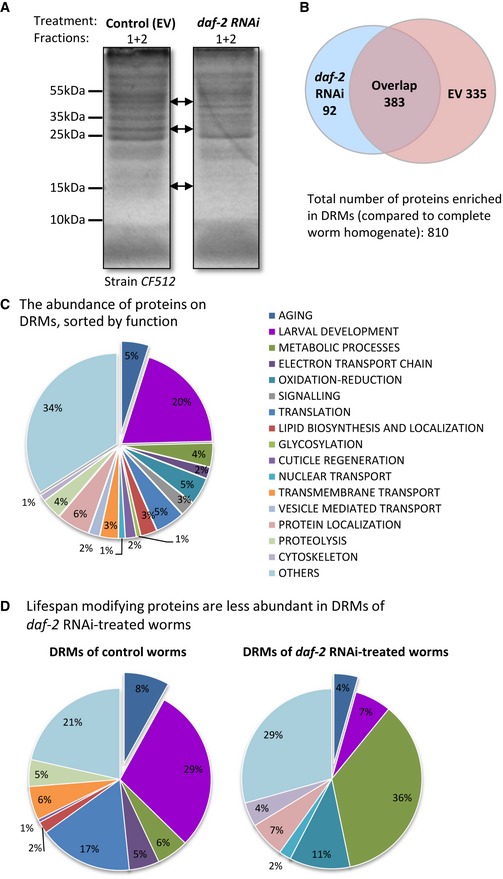
- CF512 worms were either grown on daf‐2 RNAi bacteria or left untreated (EV). At day 1 of adulthood, the worms were homogenized, DRMs were isolated by floatation, and total proteins were separated and stained. IIS reduction by daf‐2 RNAi modulated protein content of DRMs (arrows).
- Mass spectrometry‐based analysis of DRM‐resident proteins revealed that 335 proteins were detectable solely in samples of untreated (EV) worms and 92 proteins were identified exclusively in DRMs of daf‐2 RNAi‐treated worms. Three hundred and eighty‐three proteins were detected in both DRM samples.
- The DAVID bioinformatic source was used to sort the identified DRM‐resident proteins according to their biological functions. Proteins that were reported to have roles in the regulation of aging (5%) were surprisingly abundant in DRMs.
- A comparison of proteins in DRMs of control (EV) and daf‐2 RNAi‐treated worms unveiled that various DRM‐resident lifespan‐controlling proteins were present in control samples but not in DRMs of daf‐2 RNAi‐treated worms. In contrast, proteins involved in metabolic processes were notably enriched in DRMs of daf‐2 RNAi‐treated animals.
Using quantitative mass spectrometry, we next identified a total of 810 DRM‐resident proteins that met our criteria for significance; 92 were identified exclusively in DRMs of daf‐2 RNAi‐treated worms, 335 were detected solely in samples of untreated animals and 383 were present in both samples (Fig 1B) (http://www.ebi.ac.uk/pride accession number PXD009704).
Using the DAVID Bioinformatics resource 31, we sorted all identified proteins based on their functions (Fig 1C) and discovered that 5% of the lipid raft proteome had been previously characterized as lifespan regulators. In addition, all proteins were sorted by their cellular compartment (Appendix Fig S1) and we found that, as expected, membrane proteins (41%) and mitochondrial proteins (8%) were most abundant. We next compared the DRM‐resident proteins of untreated and daf‐2 RNAi‐treated worms and found that IIS reduction remarkably modifies the DRMs’ protein content (Fig 1D). It increases the abundance of proteins that are involved in metabolism while decreasing those involved in larval development. Importantly, proteins that regulate aging were much less prevalent in DRMs of daf‐2 RNAi‐treated worms; 27 aging‐controlling proteins were identified in DRMs of untreated animals while only four were detected in DRMs of their daf‐2 RNAi‐treated counterparts (Fig 1D). These observations establish a functional link between lipid microdomains and the IIS and propose that IIS activity modulates the formation of these cellular structures.
The IIS governs the expression of caveolin‐1
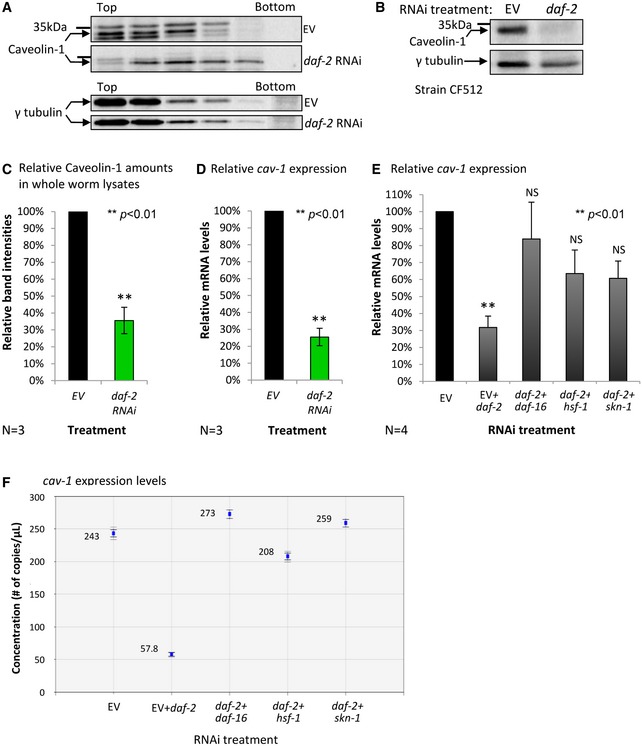
In our mass spectrometry analyses, we observed a dramatic reduction in CAV‐1 protein in the DRMs of daf‐2 RNAi‐treated worms (22%) compared to the quantity detected in DRMs of untreated animals. We verified this finding using Western blot (WB) (Fig 2A). A reduction of approximately 65% in CAV‐1 levels was also observed in whole worm homogenates (i.e., prior to lipid extraction) (Fig 2B and C). These results confirm that cav‐1 is a downstream target of daf‐2 signaling. To further test this, we used quantitative real‐time PCR (qPCR) and cav‐1‐specific primers and found that cav‐1 expression is reduced by about 75% upon IIS reduction (Fig 2D).
Figure 2. The IIS positively regulates the expression of cav‐1 .
-
A–CWB analysis shows lower CAV‐1 levels in DRMs (A) and in total homogenates (B) of daf‐2 RNAi‐treated CF512 worms compared to their untreated (EV) counterparts. Band intensity quantification of three independent experiments as in (B) confirms the significance of CAV‐1 reduction by daf‐2 RNAi (C, unpaired, error bar represent ± SEM, two‐tailed Student's t‐test, **P < 0.01).
-
DA qPCR‐based comparison of cav‐1 expression levels in control (EV) and in daf‐2 RNAi‐treated CF512 worms revealed a reduction of approximately 75% in the expression of cav‐1, N = 3 (unpaired, error bar represents ± SEM, two‐tailed Student's t‐test, **P < 0.01).
-
E, FCF512 worms were grown on the indicated mixes of RNAi bacteria, harvested at day 1 of adulthood and subjected to qPCR (E) or to digital droplet PCR (ddPCR) (F) to ascertain the expression levels of cav‐1. The knockdown of daf‐2 by RNAi (EV + daf‐2) resulted in approximately 70% reduction in cav‐1 expression. Simultaneous knockdown of daf‐2 and daf‐16, daf‐2 and hsf‐1 or of daf‐2 and skn‐1 led to a significant increase in the expression of cav‐1 ((E) Data represent the relative mRNA levels ± SEM, N = 4, unpaired, two‐tailed Student's t‐test, **P < 0.01, (F) representative ddPCR experiment, data represent the number of cav‐1 mRNA transcripts/μl ± SEM, N = 3).
We next examined whether any of the canonical DAF‐2‐regulated transcription factors, DAF‐16, HSF‐1, or SKN‐1, govern the expression of cav‐1. To test this, sterile CF512 worms were grown throughout development on either EV bacteria or on one of the following mixtures of bacterial strains: (i) EV and daf‐2 RNAi, (ii) daf‐2 + daf‐16 RNAi, (iii) daf‐2 + hsf‐1 RNAi, or (iv) daf‐2 + skn‐1 RNAi and qPCR was used to measure the expression levels of cav‐1. If a certain transcription factor serves as a negative regulator of cav‐1, it was expected that a simultaneous knockdown of daf‐2 and of the gene that encodes this factor would elevate the cav‐1 expression to the level seen in untreated worms. We found that all three transcription factors are needed to lower cav‐1 expression by IIS reduction in 1‐day‐old worms (Fig 2E). These results were further confirmed using digital droplet PCR (ddPCR) 32. While knocking down daf‐2 (EV + daf‐2 RNAi) reduced the expression of cav‐1 by approximately 75%, concurrent knockdown of daf‐2 and any transcription factor restored cav‐1 expression to nearly the natural level (Fig 2F), confirming that all three transcription factors are needed for the negative regulation of cav‐1 expression by IIS.
Reduced cav‐1 expression extends lifespan
Since in cells, CAV‐1 expression is associated with the onset of cellular senescence 33, we hypothesized that cav‐1 is involved in the regulation of lifespan. To test this hypothesis, we treated sterile CF512 worms with cav‐1 RNAi bacteria or left them untreated (EV) and measured their lifespans. We found that the knockdown of cav‐1 results in lifespan extension (Figs 3A and EV2A and Appendix Table S1), as the mean lifespan of untreated animals was 16.44 ± 0.42 days (Fig 3A, black) and of cav‐1 RNAi‐treated worms (blue) was 18.26 ± 0.42, representing a mean lifespan extension of 11.07% (***P < 0.002).
Figure 3. Reduced cav‐1 expression extends lifespan and protects from pathogenic bacteria but does not affect resistance to heat stress.
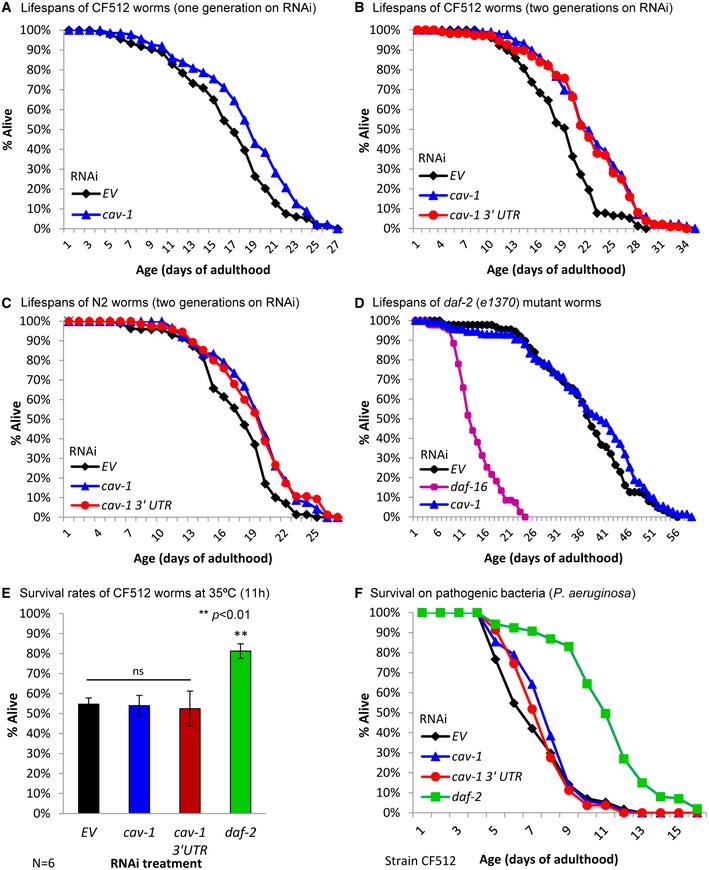
- CF512 worms were grown on cav‐1 RNAi bacteria or left untreated (EV) for one generation, and their lifespans were scored. Lifespan extension of 11.07% (unpaired, one‐tailed Student's t‐test, ***P < 0.002) was observed in worms that were treated with cav‐1 RNAi. Representative lifespan experiment of at least 95 worms per treatment, N = 4 (for details, see Appendix Table S1).
- Treating CF512 worms with cav‐1 RNAi or cav‐1 3′UTR RNAi for two generations resulted in lifespan extensions of 20.2 and 17.2%, respectively (unpaired, one‐tailed Student's t‐test, ***P < 0.001 for both RNAi treatments). Representative lifespan experiment of at least 77 worms per treatment, N = 4 (for details, see Appendix Table S1).
- Wild‐type worms (N2) that were grown for two generations on cav‐1 or with cav‐1 3′UTR RNAi bacteria lived 11.4 and 9.9% (unpaired, one‐tailed Student's t‐test, ***P < 0.001 and ***P < 0.005, respectively) longer than their untreated counterparts, respectively. Lifespan experiment of at least 70 worms per treatment (for details, see Appendix Table S1).
- Unlike daf‐16 RNAi, cav‐1 RNAi had no effect on the lifespan of daf‐2 (e1370) mutant worms. While daf‐16 RNAi‐treated worms exhibited a mean lifespan of 13.77 ± 0.5 days, untreated and cav‐1 RNAi‐treated nematodes showed similar mean lifespans (36.88 ± 1.07 and 37.05 ± 1.46 days, respectively, unpaired, one‐tailed Student's t‐test, P = 0.4). Representative lifespan experiment of at least 54 worms per treatment, N = 3 (for details, see Appendix Table S1).
- CF512 worms were grown throughout development on the indicated RNAi bacteria. At the first day of adulthood, the nematodes were exposed to 35°C for 11 h and their rates of survival were scored. While the knockdown of daf‐2 significantly increased the worms’ survival rates, no such effect was seen in cav‐1 and cav‐1 3′UTR RNAi‐treated worms. Data present mean survival ± SEM of six independent experiments (unpaired, one‐tailed Student's t‐test, **P < 0.01).
- CF512 animals were treated with RNAi as in (E) and transferred at day 1 of adulthood onto plates seeded with the pathogenic bacteria Pseudomonas aeruginosa. Treatment with both cav‐1 RNAi strains significantly increased the nematodes’ survival rates (unpaired, one‐tailed Student's t‐test, **P < 0.01 and ***P < 0.001, respectively). However, the knockdown of daf‐2 had much more prominent effect. Representative survival experiment of at least 96 worms per treatment, N = 3 (for details, see Appendix Table S2).
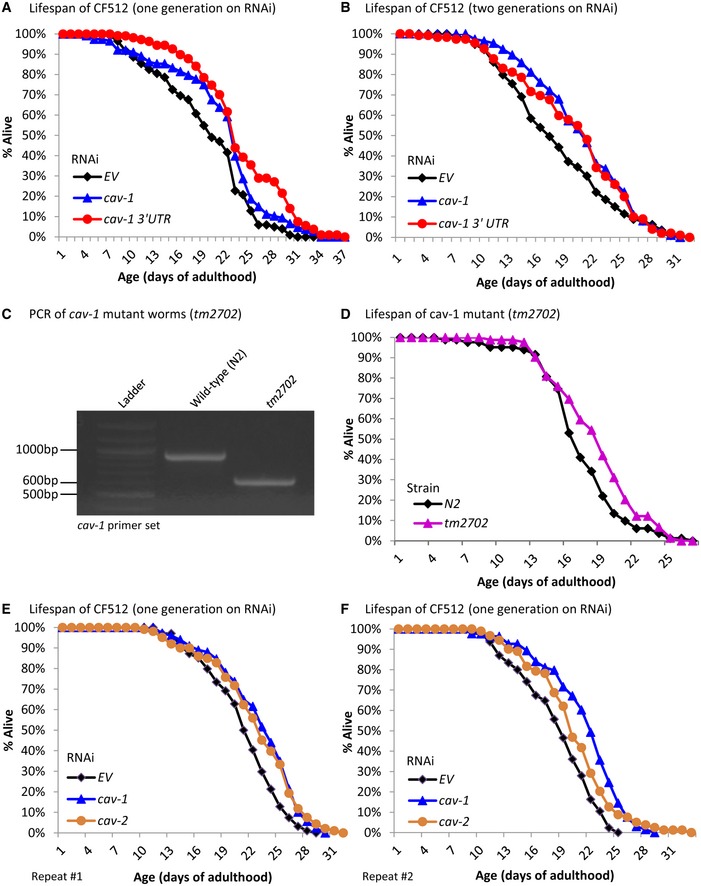
Figure EV2. The knockdown of cav‐1 and cav‐2 extends lifespan.
-
AKnocking down the expression of cav‐1 by RNAi (two RNAi bacterial strains) for one generation extends the lifespans of CF512 worms (mean lifespans of 19.45 ± 0.60 (EV), 20.93 ± 0.69 (cav‐1) and 23.59 ± 0.57 days (cav‐1 3′UTR); unpaired, one‐tailed Student's t‐test, P < 0.052 and ***P < 0.001 for cav‐1 and cav‐1 3′UTR RNAi, respectively) (“A” is a repeat of the experiment displayed as Fig 3A). Lifespan experiment of at least 101 worms per treatment (for details see Appendix Table S1).
-
BSimilarly, CF512 worms that were treated with cav‐1 or cav‐1 3′UTR RNAi for two generations lived longer than their untreated counterparts (mean lifespans of 17.97 ± 0.55 (EV), 20.38 ± 0.52 (cav‐1) and 19.8 ± 0.6 days (cav‐1 3′UTR); unpaired, one‐tailed Student's t‐test,***P < 0.0009 and **P < 0.015, for cav‐1 and cav‐1 3′UTR RNAi, respectively) (“B” is a repeat of Fig 3B) lifespan experiment of at least 99 worms per treatment (for details, see Appendix Table S1).
-
CWorm PCR using specific cav‐1 primers confirmed that tm2702 worms carry a deletion in the sequence of cav‐1.
-
Dtm2702 worms live longer than their wild‐type (strain N2) counterparts (unpaired, one‐tailed Student's t‐test, **P < 0.02). Representative lifespan experiment of at least 74 worms per strain, N = 3 (for details, see Appendix Table S1).
-
E, FThe knockdown of cav‐2 by RNAi slightly, but significantly, extends the lifespans of CF512 worms (E: mean lifespans of 21.07 ± 0.42, 22.88 ± 0.47and 22.39 ± 0.52 for EV, cav‐1 and cav‐2 RNAi‐treated worms, respectively; F: mean lifespans of 18.35 ± 0.45, 20.84 ± 0.56 and 20.1 ± 0.52 for EV, cav‐1 and cav‐2 RNAi‐treated worms, respectively) (unpaired, one‐tailed Student's t‐test, **P < 0.02 for E and ***P < 0.006 for F).
Since the efficiency of cav‐1 RNAi‐mediated knockdown could be reduced due to high stability of the CAV‐1 protein or due to its expression in neurons, which are less amenable to gene knockdown by RNAi 34, we tested whether the knockdown of cav‐1 for two generations results in a more prominent lifespan‐extending effect. To control for RNAi off‐targeting effect, we created a new RNAi construct that hybridizes with the gene's 3′ untranslated region (cav‐1 3′UTR RNAi). CF512 worms were grown from hatching until day 1 of adulthood on cav‐1 or cav‐1 3′UTR RNAi bacteria or left untreated (EV). Eggs were extracted from each worm group and placed on plates seeded with the same RNAi bacterial strain. Lifespans of worms of the second generation were scored. Our results (Figs 3B and EV2B) showed that knocking down cav‐1 for two generations more prominently extend lifespan compared to knockdown for one generation (Appendix Table S1). While the mean lifespan of untreated worms was 18.58 ± 0.54 days, dual generation cav‐1 RNAi treatment extended the worms’ mean lifespan to 22.34 ± 0.57 days and with cav‐1 3′UTR RNAi to 21.77 ± 0.57 days, extensions of 20.2 and 17.2%, respectively (***P < 0.001). A similar effect was seen when the experiment was performed using wild‐type reproductive worms (strain N2, Fig 3C, mean lifespans of 17.39 ± 0.46 (EV), 19.37 ± 0.39 (cav‐1 RNAi) ***P < 0.001, and 19.11 ± 0.48 days (cav‐1 3′UTR RNAi) ***P < 0.005). Finally, we compared the lifespans of wild‐type animals with these of mutant worms harboring a partial deletion of 287 base pairs in cav‐1 (Fig EV2C, strain tm2702) and found that these mutant animals live longer than N2 worms (Fig EV2D, mean lifespans of 17.12 ± 0.40 (N2) and 18.41 ± 0.44 days (tm2702) worms, **P < 0.02). Collectively, these results indicate that cav‐1 is a negative regulator of lifespan.
It remains possible that cav‐1 extends the lifespan of animals via a mechanism that is distinct from the IIS. If so, we would predict that cav‐1 RNAi treatment further extends the lifespan of daf‐2 (e1370) mutant worms. We treated these mutant animals throughout life with daf‐16 or cav‐1 RNAi or cultured them on control bacteria (EV), and followed lifespans (Fig 3D). While the knockdown of daf‐16 shortened lifespan (mean lifespans of 13.77 ± 0.50 (daf‐16 RNAi) and 36.88 ± 1.07 (EV) days, ***P < 0.001), the knockdown of cav‐1 had no significant effect on lifespans of these worms (mean lifespan of 37.05 ± 1.46 days, P = 0.4 against untreated worms). Thus, our observations support the notion that cav‐1 expression is an important component of the lifespan‐regulating mechanism downstream of the IIS cascade.
We also examined whether the knockdown of cav‐2, the additional member of the caveolin family of C. elegans, affects lifespan. CF512 worms were treated from hatching with RNAi toward cav‐1, cav‐2 or left untreated (EV) and lifespans were followed. Two independent experiments (Fig EV2E and F) indicated that the knockdown of cav‐2 extends the worms’ lifespans (mean lifespans of 22.39 ± 0.52 and 20.1 ± 0.52 for repeats 1 and 2, respectively) compared to these of the control animals (mean lifespans of 21.07 ± 0.42 and 18.35 ± 0.45 days, **P < 0.02 and ***P < 0.006) albeit, not to the same extent as cav‐1 RNAi treatment (mean lifespans of 22.88 ± 0.47 and 20.84 ± 0.56 days, ***P < 0.001). Since CAV‐2 was reported to be involved in apical lipid trafficking 35, our results suggest that this protein also plays roles in membrane remodeling and signaling.
The knockdown of cav‐1 provides partial protection from pathogenic bacteria and UV radiation but does not affect resistance to heat
The discovery that cav‐1 RNAi extends lifespan raised the prospect that this gene is also involved in other IIS‐regulated functions. Therefore, we sought to determine whether survivorship of animals that are exposed to environmental stressors was affected by cav‐1 expression. First, we tested whether the level of cav‐1 affects heat stress resistance. CF512 worms were treated throughout development with cav‐1, cav‐1 3′UTR, daf‐2 RNAi or grown on EV bacteria. At day 1 of adulthood, the animals were exposed to 35°C for 11 h and rates of survival were recorded. While the knockdown of daf‐2 elevates survival in 47% compared to the rate seen in untreated worms (mean survival of 55% (EV) and 81% (daf‐2 RNAi), **P < 0.01) the knockdown of cav‐1 (54%) or cav‐1 3′UTR (52%) RNAi had no such effect (Fig 3E).
We further tested the possible roles of cav‐1 in heat stress resistance by repeating the experiment using wild‐type (N2) worms that were grown on the indicated RNAi bacteria for two generations. Our results (Appendix Fig S2A) showed no significant effect of cav‐1 RNAi on heat stress resistance as on average of 53% of the EV, 45% of cav‐1 RNAi‐treated and 46% of the cav‐1 3′UTR RNAi‐treated worms survived the heat stress. In contrast, 82% of the daf‐2 RNAi‐treated animals were alive at the end of the experiment. Finally, we examined whether cav‐1 mutant worms (strain tm2702) exhibit an elevated heat stress resistance compared to N2 worms. Day 1 old adult worms were exposed to 35°C for 12 h and survival rates were recorded. No difference in survival was observed as 46% of the N2 and 49% of the tm2702 nematodes were alive at the end of the experiment (Appendix Fig S2B). Collectively, these results indicate that cav‐1 is dispensable for heat stress resistance.
Next, we examined whether the knockdown of cav‐1 modulates resistance to pathogenic bacteria. In this experiment, CF512 worms were treated from hatching with cav‐1, cav‐1 3′UTR, daf‐2 RNAi or left untreated (EV). At day 1 of adulthood, the worms were transferred onto plates seeded with the toxic bacteria Pseudomonas aeruginosa 11, and survival rates were recorded. As previously reported, IIS reduction by daf‐2 RNAi extended survival compared to untreated worms (mean survival of 8.08 ± 0.26 and 5.57 ± 0.15 days, respectively, ***P < 0.001). Similarly, the knockdown of cav‐1 elevated resistance to these pathogenic bacteria, albeit to a much lesser extent than daf‐2 RNAi (Fig 3F, mean survival of 6.12 ± 0.18 (cav‐1 RNAi) and 6.36 ± 0.18 days (cav‐1 3′UTR RNAi), **P < 0.01, see also Appendix Fig S2C and Appendix Table S2).
We also tested whether knocking down cav‐1 confers protection from UV radiation. CF512 worms were grown from hatching to day 1 of adulthood on RNAi bacteria as described above, exposed to a sub‐lethal dose of UV and rates of survival were followed. While treatment with daf‐2 RNAi elevated resistance to this insult, UV‐radiated worms that were treated with cav‐1 or cav‐1 3′UTR RNAi, and untreated animals (EV) exhibited indistinguishable rates of survival (Appendix Fig S2D and Appendix Table S3). Similar results were obtained when we repeated this experiment using wild‐type (N2) worms (Appendix Fig S2E and Appendix Table S3). We also asked whether cav‐1 mutant animals (strain tm2702) are more resistant than wild‐type worms to UV. Animals of both strains were exposed to UV radiation at day 1 of adulthood and survival rates were followed. We found no significant difference in survival rates among N2 and tm2702 worms (Appendix Fig S2F and Appendix Table S3).
Since tm2702 worms carry a partial deletion in cav‐1 (Fig EV2C) and the usage of cav‐1 RNAi for one generation may not efficiently suppress the number of caveolae, we tested whether the knockdown of cav‐1 for two generation protects the animals from UV radiation. N2 worms were treated with cav‐1, cav‐1 3′UTR or daf‐2 RNAi or grown on EV bacteria and eggs were extracted. Eggs of each worm group were placed on plates that were seeded with the same RNAi bacteria as their parents. At day 1 of adulthood, the worms of the second generation were exposed to UV radiation and survival rates were followed. Surprisingly, the survival rates of worms whose cav‐1 was knocked down were elevated compared to these of untreated worms (Appendix Fig S2G). While untreated animals exhibited mean survival of 10.57 ± 0.43 days, the average survival of cav‐1 RNAi‐treated worms was 12.77 ± 0.31 and of animals that were treated with cav‐1 3′UTR RNAi was 12.65 ± 0.65 days (***P < 0.001). Yet, the mean survival rates of UV‐radiated daf‐2 RNAi‐treated worms were 20.38 ± 1.47 days, significantly (***P < 0.001) longer than these of worms whose cav‐1 expression was knocked down.
Taken together, our results imply that cav‐1 has a limited role in the promotion of resistance to UV radiation and pathogenic bacteria but plays no role in resistance to heat stress by IIS reduction. These observations support the notion that the ability to resist heat stress is separable from lifespan 36, 37.
Reduced cav‐1 expression promotes proteostasis by inducing the disaggregation of proteotoxic species
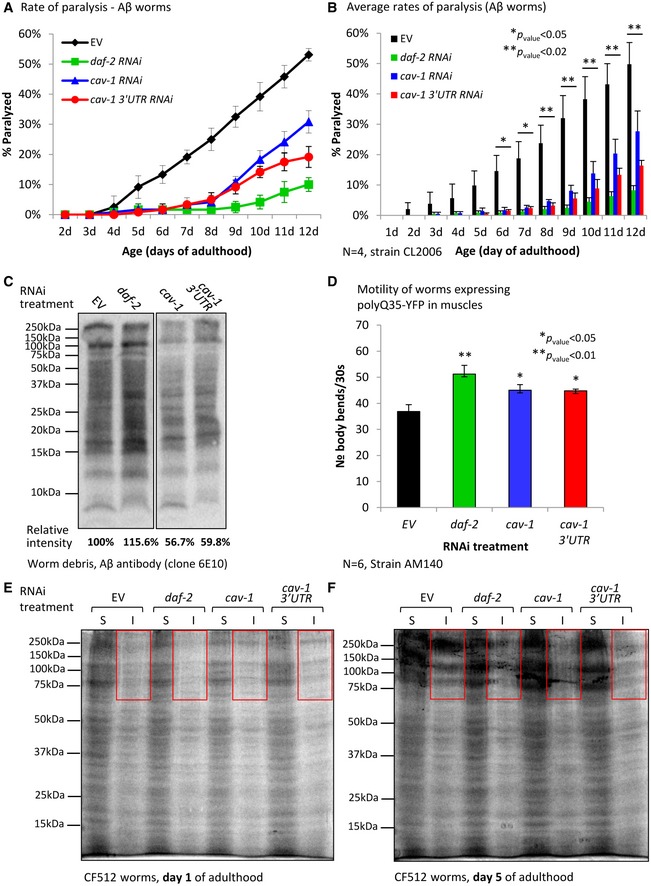
The critical roles of IIS in the regulation of proteostasis 13, 14 have prompted us to test whether reducing cav‐1 expression attenuates proteotoxicity. Using worms that express the Alzheimer's disease‐linked peptide, human Aβ3–42 38 in their body wall muscles (strain CL2006 39—Aβ worms), we tested whether the knockdown of cav‐1 affects proteostasis. The expression of Aβ results in progressive paralysis within the worm population, a phenotype that can be mitigated by IIS reduction 13. We treated Aβ worms with RNAi as above, followed the rates of paralysis and found that knocking down cav‐1 partially, but significantly, protects the worms from this toxic phenotype (Fig 4A and B). While approximately 50% of the untreated animals were paralyzed at day 12 of adulthood, merely 28% of the cav‐1 RNAi‐treated and 16% of the cav‐1 3′UTR RNAi‐treated worms showed this phenotype.
Figure 4. Reduced cav‐1 levels provide partial protection from proteotoxicity.
-
ACL2006 worms (Aβ worms) were treated with daf‐2, cav‐1 or cav‐1 3′UTR RNAi or left untreated (EV) and rates of paralysis were followed daily. The knockdown of cav‐1 lowered the rate of paralysis compared to the control group (EV), although not as efficiently as daf‐2 RNAi. A representative experiment, error bars represent the average rates of paralysis within the worm populations ± SD.
-
BThe counter‐proteotoxic effects of cav‐1 and cav‐1 3′UTR RNAi treatments were confirmed by four independent paralysis assays. Bars represent the average daily rates of paralysis within the worm populations ± SEM (unpaired, one‐tailed Student's t‐test, *P < 0.05 and **P < 0.02).
-
CWB analysis using an Aβ antibody (clone 6E10) indicates that the knockdown of cav‐1 by two different RNAi constructs reduces the rate of Aβ aggregation.
-
DWorms that express polyQ35‐YFP in their muscles that were treated with cav‐1 or cav‐1 3′UTR RNAi exhibited significantly higher rate of motility compared to untreated animals. Bars show average rates of combined six independent experiments ± SEM (unpaired, one‐tailed Student's t‐test, *P < 0.05 and **P < 0.01).
-
E, FCF512 were grown on the control bacteria (EV) or were treated with the indicated RNAi. At days 1 and 5 of adulthood, worm groups were homogenized and subjected to high‐speed centrifugation to separate insoluble (I) from soluble (S) proteins. Colloidal blue stain of total proteins indicated that treating the worms with daf‐2, cav‐1 or with cav‐1 3′UTR reduced general protein aggregation in day 5 (F) but not in young (day 1 old) animals (red rectangles).
Since HSF‐1 and DAF‐16 promote opposing activities, disaggregation and hyper‐aggregation, respectively 13, we examined how the knockdown of cav‐1 affects Aβ aggregation. To this end, Aβ worms were treated with RNAi as described above, homogenized, and spun to separate soluble fractions from worm debris. Analyzing Aβ by WB analysis unveiled that while the knockdown of daf‐2 increased Aβ aggregation in the worm debris 13, the knockdown of cav‐1 reduces the quantities of aggregated Aβ (Fig 4C). This suggests that CAV‐1 inhibits disaggregation.
As a secondary approach to test for the effects of cav‐1 on proteotoxicity, we employed worms that express a chimera of a poly glutamine 35 stretch fused to the yellow fluorescent protein in their muscles (polyQ35‐YFP, strain AM140). The animals were treated with RNAi as above, and the thrashing assay 40 was used to follow muscle dystrophy. We observed that the knockdown of cav‐1 protects 4‐day‐old worms from motility impairment (Fig 4D). This indicates that the counter‐proteotoxic effect of cav‐1 knockdown is not restricted to the toxicity of Aβ.
To further investigate the roles of caveolae in the maintenance of proteostasis, we asked whether the knockdown of cav‐1 mitigates aging‐associated protein aggregation 41. To test this, CF512 worms were grown on EV, daf‐2, cav‐1, or cav‐1 3′UTR RNAi bacteria, homogenized, and subjected to ultra‐centrifugation to separate soluble from insoluble proteins. Proteins of the different fractions were separated on gels and stained by colloidal blue reagent. In these analyses, we found no difference in protein aggregation in homogenates of young (day 1) worms across all treatments (Fig 4E). In contrast, knocking down cav‐1 slows aging‐associated protein aggregation of older animals (day 5) as the ratios of insoluble (I) to soluble (S) proteins were lower in 5‐day‐old worms that were treated with either daf‐2, cav‐1, or cav‐1 3′UTR RNAi compared to the ratio seen in fractions of untreated animals of the same age (Fig 4F). Similar results were obtained when we conducted an identical experiment labeling the proteins by silver staining (Appendix Fig S3A–D).
These observations indicate that the knockdown of cav‐1 protects from both acute proteotoxicity and chronic aging‐associated protein aggregation and that caveolae play crucial roles in proteostasis maintenance.
The knockdown of cav‐1 extends lifespan in daf‐16‐ and skn‐1‐dependent manners but promotes proteostasis independently of these factors
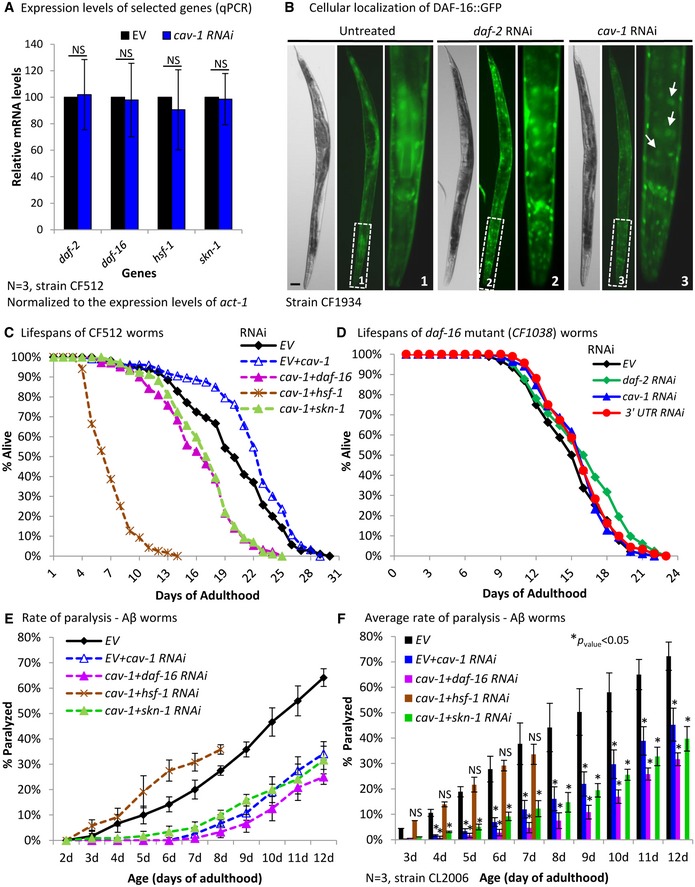
Are IIS‐regulated transcription factors needed for the cav‐1 knockdown‐mediated longevity and proteostasis phenotypes? To address this question, we took several measures. First, we asked whether the knockdown of cav‐1 affects the expression levels of daf‐16, skn‐1, hsf‐1 and of daf‐2. CF512 animals were either left untreated or treated from hatching with RNAi toward one of the aforementioned genes. qPCR analysis indicated no difference in the expression levels of all four genes in control (EV) and cav‐1 RNAi‐treated worms populations (Fig 5A). These results show that although all three transcription factors regulate the expression of cav‐1 (Fig 2E and F), cav‐1 does not control the expression of these factors nor of daf‐2.
Figure 5. The IIS‐regulated transcription factors DAF‐16 and SKN‐1 are required for lifespan extension but not for protection from proteotoxicity by the knockdown of cav‐1 .
-
AqPCR analysis indicates that the knockdown of cav‐1 by RNAi does not affect the expression levels of daf‐2, daf‐16, hsf‐1, and skn‐1 (N = 3, data represent the relative mRNA levels ± SEM, unpaired, two‐tailed Student's t‐test).
-
BUsing worms that express DAF‐16 fused to GFP (strain CF1934), we found that the RNAi‐mediated knockdown of cav‐1 (arrows) or of daf‐2 induces the entry of DAF‐16::GFP into the nucleus (scale bar, 30 μm).
-
CWhile worms that were grown on a mixture of cav‐1 RNAi and EV bacteria were long‐lived compared to control worms (EV) (unpaired, one‐tailed Student's t‐test, ***P < 0.002), the concurrent knockdown of cav‐1 and daf‐16, cav‐1, and hsf‐1 or of cav‐1 and skn‐1 shortens lifespan compared to these of untreated worms (EV) (unpaired, one‐tailed Student's t‐test, ***P < 0.001). Representative lifespan experiment of at least 58 worms per treatment, N = 3 (for details, see Appendix Table S1).
-
DNo lifespan extension was seen in daf‐2, cav‐1, or cav‐1 3′UTR RNAi‐treated worms that lack functional daf‐16 (strain CF1038). Lifespan experiment of at least 82 worms per treatment (for details see Appendix Table S1).
-
E, FSimultaneous knockdown of cav‐1 and daf‐16 or of cav‐1 and skn‐1 by RNAi protected Aβ worms from proteotoxicity. In contrast, Aβ worms that were treated concomitantly with cav‐1 and hsf‐1 RNAi exhibited higher rate of paralysis than their untreated counterparts (E). Data represent the average rate of paralysis within the worm populations ± SD. This effect was reproducible and significant (F). Bars represent the average daily rates of paralysis within the worm populations ± SEM, N = 3 (unpaired, one‐tailed Student's t‐test, *P < 0.05).
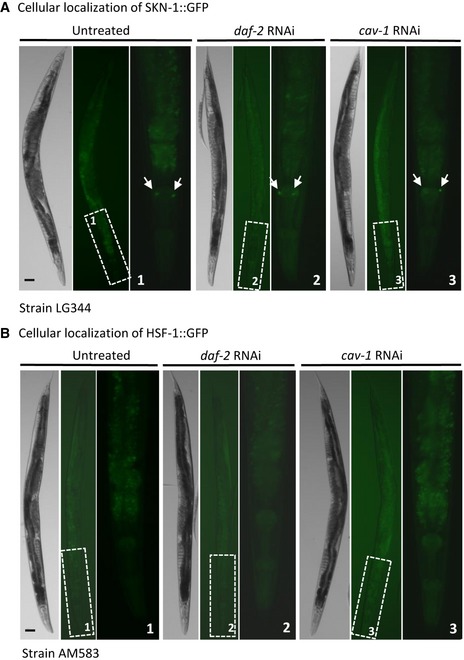
We next tested whether the knockdown of cav‐1 leads to the migration of the three transcription factors from the cytosol to the nucleus, a prerequisite for their activities. To examine this we used worms strains that express either DAF‐16::GFP (CF1934), SKN‐1::GFP (LG344), or HSF‐1::GFP (AM583). All worm strains were grown from hatching on either EV, cav‐1, or daf‐2 RNAi bacteria. At day 1 of adulthood, we visualized the worms using a fluorescence microscope and found that the knockdown of cav‐1 and of daf‐2 induced the migration of DAF‐16::GFP into the nucleus (Fig 5B, arrows). Similarly, these two RNAi treatments resulted in a slight increase in the SKN‐1::GFP signals in two neurons (Fig EV3A, arrows) that were previously reported to be ASI neurons 42. In contrast, we observed no difference in the fluorescence patterns of HSF‐1::GFP in all RNAi treatments (Fig EV3B).
Figure EV3. The knockdown of cav‐1 slightly enhances the accumulation of SKN‐1 in neurons but has no effect on truncated HSF‐1.
-
A, BWorms that express a GFP‐tagged SKN‐1 (strain LG344) or HSF‐1 (strain AM583) were either treated from hatching with daf‐2 or cav‐1 RNAi or left untreated. The worms were visualized by a fluorescent microscope at day 1 of adulthood. While a slight, but not significant, increase in SKN‐1::GFP signal was seen in neurons, no difference in HSF‐1::GFP distribution was observed (scale bar, 30 μm).
These results suggest roles for DAF‐16 and SKN‐1 in the cav‐1 RNAi‐promoted phenotypes. To scrutinize this notion, we employed CF512 worms and RNAi mixtures to examine whether these three transcription factors are needed for the lifespan extension that emanates from the knockdown of cav‐1 (all three transcription factors were reported to be critically needed for lifespan extension by IIS reduction 1, 7, 43). The worms were fed from hatching with cav‐1 RNAi bacteria mixed (1:1) with bacterial strains that express either one of the following RNAi: EV, daf‐16, skn‐1, or hsf‐1. An identical worm group was fed from hatching with EV bacteria (control). Lifespans of all worm groups were recorded. Our results (Fig 5C) indicated that while the mixture of EV+cav‐1 RNAi extended lifespan by approximately 11% (21.9 ± 0.5 days) compared to untreated worms (19.8 ± 0.5 days), concurrent knockdown of cav‐1 and of either daf‐16 or skn‐1 resulted in lifespan shortening of 18 and 15% compared to the lifespan of untreated animals, respectively. The concomitant knockdown of cav‐1 and hsf‐1 by RNAi resulted in a remarkable lifespan shortening, as the average lifespan of these worms was only 7.04 ± 0.2 days.
To further characterize the roles of the transcription factors in the mediation of longevity by the knockdown of cav‐1, we asked whether cav‐1 RNAi treatment is capable of extending the lifespan of worms that lack functional DAF‐16 (strain CF1038) or SKN‐1 (strain EU31). We also tested whether reducing the expression of cav‐1 affects the lifespans of worms that express HSF‐1 which harbors a stop codon at residue 585 and thus lacks its C‐terminal trans‐activation domain (strain PS3551) 44. Worms of all strains were treated from hatching with either cav‐1, cav‐1 3′UTR, or daf‐2 RNAi or left untreated (EV). No significant difference was observed in lifespans of daf‐16 mutant worms that were treated with cav‐1 or cav‐1 3′UTR RNAi for one (Fig 5D) or two generations (Fig EV4A, Appendix Table S1). Similarly, the knockdown of cav‐1 had no effect on the lifespans of skn‐1 mutant animals (Fig EV4B, Appendix Table S1). Interestingly, the knockdown of daf‐2 extended the lifespans of skn‐1 mutant worms (mean lifespans of 16.03 ± 0.38 and 23.81 ± 2.55 days for EV and daf‐2 RNAi‐treated worms, respectively). These observations further support the conclusion that daf‐16 and skn‐1 are required for the longevity phenotype of cav‐1 RNAi‐treated worms. In contrast, cav‐1 RNAi extended the lifespan of PS3551 worms by approximately 12% (Fig EV4C, Appendix Table S1), showing that although the knockdown of hsf‐1 drastically shortens the animals’ lifespans (Fig 5C), the lifespan extension mechanism that is activated upon the knockdown of cav‐1 is independent on the C‐terminal trans‐activation domain of HSF‐1.
Figure EV4. IIS‐regulated transcription factros and cav‐1‐mediated lifespan extension.
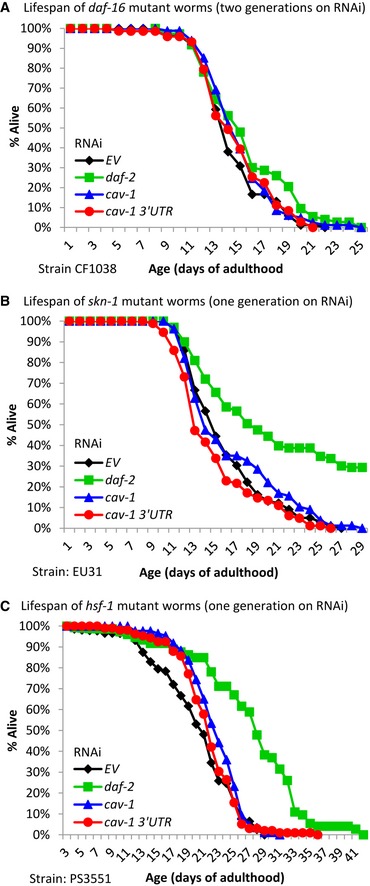
- Worms that lack functional DAF‐16 (strain CF1038) were treated with the indicated RNAi bacterial strains. No significant differences in lifespans were observed between untreated worms and their counterparts that were treated with RNAi toward daf‐2 or cav‐1, N = 3 (unpaired, one‐tailed Student's t‐test, P = 0.08).
- Worms that lack functional SKN‐1 (strain EU31) were treated from hatching with RNAi as above. While the knockdown of daf‐2 extended the animals’ lifespans, no such effect was seen when cav‐1 was knocked down, N = 2 (unpaired, one‐tailed Student's t‐test, **P = 0.027).
- Worms that carry a mutated hsf‐1 which carries a stop codon at residue 585 and thus lack the C‐terminal trans‐activation domain (strain PS3551) were treated with RNAi as above. The knockdown of either daf‐2 or cav‐1 extended the animals’ lifespans indicating that the HSF‐1 C‐terminal domain is dispensable for lifespan extension by the knockdown of these genes (unpaired, one‐tailed Student's t‐test, ***P < 0.001).
Next, we asked whether these transcription factors are also needed for the promotion of proteostasis by reducing the cav‐1 expression levels. Importantly, all three transcription factors were shown to promote proteostasis in previous studies 13, 45 and inhere (Appendix Fig S4). Aβ worms were treated with mixtures of RNAi toward cav‐1 and each transcription factor as described above, and the rates of paralysis within the worm populations were recorded. Unexpectedly, we discovered that simultaneous knockdown of cav‐1 and of daf‐16 or cav‐1 and of skn‐1 protects the animals from proteotoxicity, implying that these two transcription factors are dispensable for the promotion of proteostasis by the knockdown of cav‐1 (Fig 5E). The reproducibility and significance of this phenotype was demonstrated by three independent repeats (Fig 5F). In contrast, the knockdown of hsf‐1 completely abolished the cav‐1 RNAi‐conferred protection from proteotoxicity.
cav‐1 is foremost expressed in neurons of the adult worm
The roles of neurons in the regulation of proteostasis 36, 37, 46 and the prominent lifespan extension seen in worms that were treated with cav‐1 RNAi for two generations (Fig 3B) raised the prospect that cav‐1 is expressed in neurons of the adult nematode. It was previously reported that cav‐1 is strongly expressed in the developing embryo, most prominently in the nervous system of larvae 30. Furthermore, cav‐1 expression was observed in both neurons and body wall muscles of adult worms; however, in muscle cells, CAV‐1 was found to be localized adjacent to neurons, probably at the neuromuscular junction 29. A single‐cell transcriptional profiling indicated that in the L2 larval stage, the expression of cav‐1 is about 70 times higher in neurons compared to body wall muscles 47.
To further explore the localization patterns of cav‐1 in the adult worm, we created transgenic animals that express the red fluorescent protein tdTomato 48 under the regulation of the cav‐1 promoter (strain EHC123). As previously reported, we observed strong cav‐1 promoter activity in developing embryos as well as in all larval stages. During larval development, the signal was most prominent in and around the pharynx (including the nerve ring), along the worm's ventral cord, and in the tail (Fig EV5).
Figure EV5. Expression patterns of cav‐1 in eggs and larval stages.
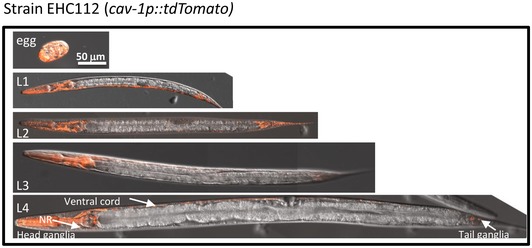
Visualization of worms that express the fluorescent protein tdTomato under the cav‐1 promoter region indicates that cav‐1 is expressed during embryonic development and during all stages of larval development. cav‐1 is mainly expressed in neurons of the larvae (scale bar, 50 μm).
To determine the expression pattern of cav‐1 in the adult worm, we crossed EHC123 worms with animals that express YFP under the pan‐neuronal promoter unc‐119 (strain TU3335) and observed nearly complete overlap of the yellow and red signals, indicating that cav‐1 is foremost expressed in neurons (Fig 6A). Higher magnification showed prominent cav‐1 expression in the nerve ring (Fig 6B, NR), implying the existence of caveolae in neurons of this cluster. To visualize and quantify caveolae‐like invaginations in neurons of the nerve ring, we employed transmission electron microscopy (TEM). CF512 worms were grown to day 1 of adulthood on daf‐2 RNAi bacteria or left untreated (EV), prepared for TEM visualization, sectioned along the body axis, and structures were observed (Fig 6C and D). The average number of caveolae‐like invaginations in worms grown on EV bacteria was 3.5 ± 0.2/section compared with 1.25 ± 0.24/section in worms in which the IIS was reduced (Fig 6E), suggesting that IIS reduction lessens the number of neuronal caveolae. This important result provides the first direct microscopic evidence of membrane remodeling following daf‐2 RNAi treatment.
Figure 6. cav‐1 is foremost expressed in neurons of adult nematodes.
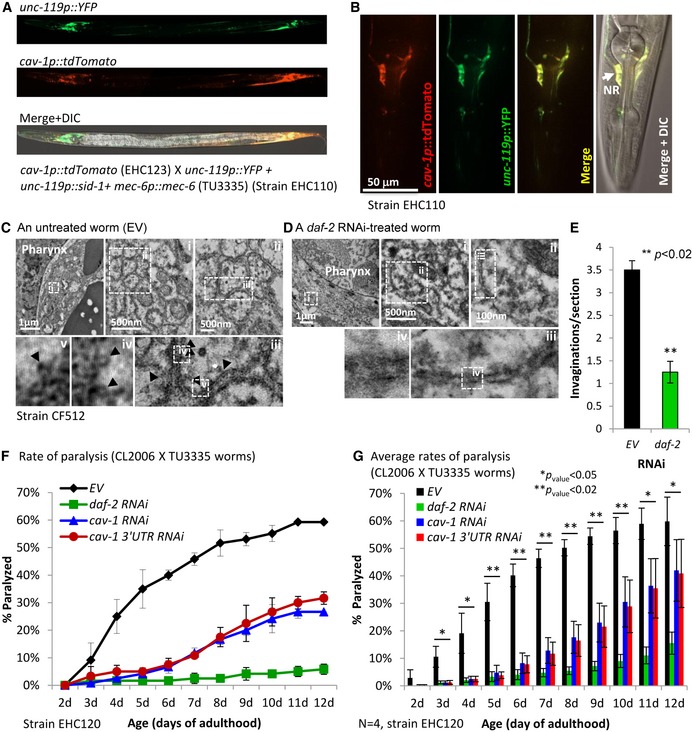
-
ANeuronal YFP (driven by the unc‐119 promoter) and tdTomato that is expressed under the regulation of the cav‐1 promoter show similar expression patterns in worms that co‐express these fluorescent proteins.
-
BHigher magnification of the worm's head shows high level of red fluorescence adjacent to the pharynx, indicating high cav‐1 expression in the nerve ring (NR) (scale bar, 50 μm).
-
C–ETransmission electron microscopy (TEM) of thin worm sections, unveils lower number of caveolae‐like invaginations in neurons of the nerve ring of a daf‐2 RNAi‐treated worm (D) compared to their untreated counterpart (EV) (C, arrowheads), scale bars are marked on the panels. Quantification of these invaginations indicated that this phenomenon is significant; data represent the average of invaginations per section ± SEM (E), (unpaired, one‐tailed Student's t‐test, **P < 0.02).
-
F, GThe knockdown of cav‐1 by either one of the two different RNAi constructs protected Aβ worms that over‐express sid‐1 in their neurons (and thus, exhibit hypersensitivity to RNAi in these cells) from proteotoxicity (F); data represent the average rate of paralysis within the worms population ± SD. The significance of this phenotype was shown by four independent repeats (G), and bars represent the average daily rates of paralysis within the worm populations ± SEM (unpaired, one‐tailed Student's t‐test, *P < 0.05 and **P < 0.02).
It will be important to further investigate whether the knockdown of cav‐1 or of either one of the IIS‐regulated transcription factors, daf‐16, skn‐1, and hsf‐1 plays any roles in membrane remodeling and caveolae formation.
To further examine whether neuronal caveolae regulate proteostasis in remote tissues, we crossed Aβ and TU3335 worms to obtain worms that exhibit progressive Aβ‐mediated paralysis and are foremost sensitive to RNAi in their neurons 49 (strain EHC120). The worms were treated from hatching with daf‐2, cav‐1, or cav‐1 3′UTR RNAi or left untreated and the rates of paralysis were followed daily. Our results (Fig 6F) indicated that an efficient knockdown of cav‐1 in the worms’ neurons provides protection from proteotoxicity. The reproducibility and significance of this protection was demonstrated by four independent experiments (Fig 6G). These observations support the notion that neuronal caveolae are involved in the regulation of proteostasis across the organism.
The knockdown of cav‐1 modulates the expression of genes that mediate signaling and regulate lifespan
The functional outcomes of cav‐1 knockdown and the prominent expression of cav‐1 in neurons have prompted us to ask which genes’ expression is affected by this manipulation. To address this, CF512 worms were treated for two generations with daf‐2 or cav‐1 RNAi and gene expression profiles were compared to these of untreated worms (EV) using RNA sequencing (RNA‐Seq). The knockdown of daf‐2 modulated the expression of 1900 genes of which the expression levels of 980 genes were reduced and those of 920 genes were elevated compared to the levels observed in untreated animals (Fig 7A–C, accession GSE100045). IIS reduction significantly reduced the expression level of cav‐1 (log2 fold change −2.326), confirming our previous observations (Fig 2A–D). As a control, we verified that daf‐2 RNAi treatment also modulated the expression of known targets of this pathway, including sod‐3 (log2 fold change, 1.901), dod‐22 (−4.119), and dod‐24 (−3.825) 16.
Figure 7. The knockdown of cav‐1 modulates gene expression.
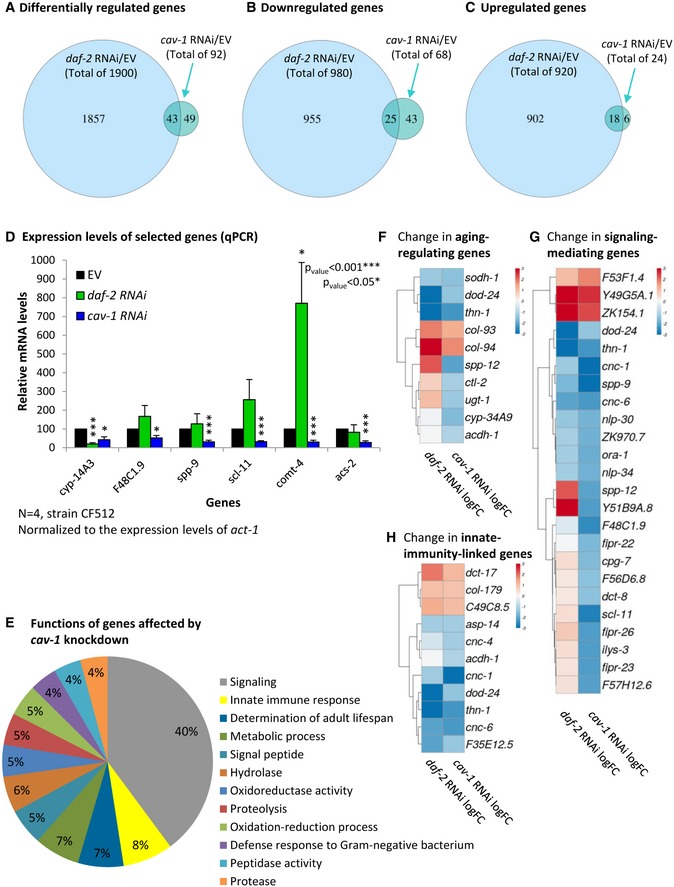
-
A–CCF512 worms were grown for two generations on either daf‐2 or cav‐1 RNAi and their gene expression profiles were analyzed by RNA‐Seq. While the knockdown of daf‐2 modulates the expression of 1,900 genes, the knockdown of cav‐1 only affects the expression levels of 92 genes (A). The expression levels of 68 of these genes were downregulated (B), and those of 24 were upregulated (C).
-
DAn analysis of the expression levels of six selected genes in worms that were treated with the indicated RNAi confirmed the results of the RNA‐Seq experiment. Data represent the relative mRNA levels ± SEM, N = 4 (unpaired, two‐tailed Student's t‐test, *P < 0.05 and ***P < 0.001).
-
EUsing the DAVID bioinformatic source, we found that 40% of the genes, that were found to be affected by the knockdown of cav‐1, are involved in signaling, 8% are known to mediate innate immunity, 7% to be involved in the determination of lifespan, and 7% in metabolic processes.
-
F–HHeat maps displaying changes in the expression levels of aging‐regulating (F), signaling‐mediating (G), and innate immunity‐promoting (H) genes upon the knockdown of daf‐2 or cav‐1.
In contrast to the large network of genes that are modulated by the knockdown of daf‐2 expression, cav‐1 RNAi‐treated worms exhibited modulated expression of only 92 genes; 68 genes of which were downregulated and 24 of which were upregulated (Fig 7A–C, see lists at Appendix Table S4). Using qPCR to measure the expression levels of six of these targets, we validated the fact that our selected genes did show significantly lower expression upon the knockdown of cav‐1 (Fig 7D and Appendix Fig S5) (the reduction in the expression of F48C1.9 was apparent but not significant when normalized to cdc‐42). We next compared the lists of genes that were affected by the knockdown of daf‐2 to those which show differential expression levels upon treatment with cav‐1 RNAi and found only a partial overlap, as the expression levels of 43 genes were affected by both daf‐2 and cav‐1 RNAi treatments and those of 49 genes were modulated by the knockdown of cav‐1 but not of daf‐2 (Fig 7A). This partial overlap implies that the knockdown of cav‐1 has also an IIS‐independent effect on gene expression.
Next, we sorted the genes that are affected by the knockdown of cav‐1 according to biological functions using the DAVID bioinformatic resource and found that 40% of the genes that show modulated expression upon the knockdown of cav‐1 are involved in signaling, 8% in resistance to pathogenic bacteria, 7% in the determination of lifespan, and 7% in metabolism (Fig 7E). These results are consistent with the extended lifespan (Fig 3A–C) and elevated resistance to Pseudomonas aeruginosa (Fig 3F) observed in cav‐1 RNAi‐treated worms. They also support the theme that caveolae serve as assembly centers for aging and proteostasis‐regulating complexes.
A detailed analysis indicated that in many cases, daf‐2 and cav‐1 RNAi treatments similarly affect gene expression levels. For instance, the expression of dod‐24, a regulator of lifespan and of the innate immune response (Fig 7F–H) that is controlled by DAF‐16 16, was reduced upon the knockdown of both daf‐2 and cav‐1. Similarly, the DAF‐16 target dct‐17, which is critical for innate immunity, was upregulated by both RNAi treatments (Fig 7H). In contrast, the level of spp‐12, an antibacterial gene, exhibited opposing effects (Fig 7F–G). These results further indicate that while caveolae are influenced by the IIS, the disruption of these cellular structures also has IIS‐independent consequences.
Discussion
Lipid rafts in general and caveolae in particular have important roles in cell organization and functionality. The regulation of genes that encode for lipid‐metabolizing enzymes by the IIS 16 and the roles of such genes in the determination of lifespan 18 led us to ask whether IIS reduction affects the protein content of lipid rafts. Using biochemical techniques and mass spectrometry, we found that IIS reduction by daf‐2 RNAi modifies the protein content of DRMs. Interestingly, proteins which are known as lifespan regulators appear to be enriched in DRMs but their quantities were modulated by IIS reduction. These results suggest the intriguing idea that IIS may modulate global signaling pathways, in part, through the physical remodeling of the plasma membrane surfaces in which they are otherwise embedded. One of the DRM proteins that are significantly affected by IIS reduction is CAV‐1. The regulation of cav‐1 by daf‐2 is dependent on all three IIS‐regulated transcription factors, DAF‐16, SKN‐1, and HSF‐1 (Fig 8‐I). Reduced amount of CAV‐1 leads to the presence of less caveolae‐like invaginations in neurons (Fig 8‐II) and possibly to the disintegration of existing caveolae (Fig 8‐III). This is associated with lifespan extension, protection from pathogenic bacteria, elevated survival after UV radiation, and enhanced proteostasis (Fig 8‐IV). Unexpectedly, the knockdown of cav‐1 showed no effect on survival of heat‐stressed worms, further supporting the theme that lifespan and stress resistance are separable 36, 37, 50. The observations that knocking down cav‐1 modulates the expression of genes that are involved in signaling, determination of lifespan, and innate immunity (Fig 8‐V) also support the notion that neuronal caveolae are signaling centers that enable the orchestration of organismal functions. Yet, the less prominent effects of reduced cav‐1 expression on longevity and proteostasis compared to the influences of daf‐2 RNAi treatment clearly indicate that additional, cav‐1‐independent lifespan and proteostasis‐governing mechanisms are controlled by the IIS (Fig 8‐VI).
Figure 8. A model.

Upon the binding of its ligand, DAF‐2 activates its downstream signaling cascade that negatively regulates the transcription factors SKN‐1, HSF‐1 and DAF‐16, suppression that enhances the expression level of cav‐1 (I). Accordingly, IIS reduction by daf‐2 RNAi significantly reduces the levels of CAV‐1 in DRMs of neurons (II). Reduced amount of CAV‐1 leads to the reduction in caveolae formation and possibly to the disintegration of existing caveolae (III). This probably impairs the organization of caveolae‐resident protein complexes, extends lifespan in a DAF‐16‐ and SKN‐1‐dependent manner and provides protection from proteotoxicity in a DAF‐16‐ and SKN‐1‐independent fashion (IV) by modulating gene expression (V). Additional, cav‐1‐independent, lifespan and proteostasis‐governing mechanisms are controlled by the IIS (VI).
What roles are played by caveolin‐1 in neurons?
Our results, as well as previous reports 30, show that cav‐1 is primarily expressed in neurons of the adult nematode. This raises the question of what roles CAV‐1 and caveolae play as lifespan and proteostasis regulators in these cells. It is possible that caveolae serve as scaffolds for the assembly of protein complexes that sense hormonal and perhaps environmental cues and respond by promoting signaling to other tissues, thereby orchestrating the organismal pace of aging. This notion is supported by our finding that knocking down cav‐1 protects muscle cells from Aβ‐and polyQ35‐YFP‐mediated toxicity (Fig 4). This effect was also apparent when cav‐1 was knocked down in worms that are foremost sensitive to RNAi in neurons (Fig 6F and G). It is also reinforced by the observations that neuronal components are needed for the activation of stress responses and for the maintenance of proteostasis in the soma 36, 37, 46, 51. But the strongest support for this theme is provided by our RNA‐Seq results showing that 40% of the genes whose expression is affected by the knockdown of cav‐1 are involved in signaling (Fig 7E). It will be interesting to identify caveolae‐resident signaling complexes, examine whether caveolae are needed for the release of neuro‐transmitters or neuro‐peptides and identify these molecules.
Studies that were performed in mammalian systems showed that caveolae are also involved in oxidative stress and mitochondrial function 52 as well as in the etiology of human diseases 53. Thus, the knockdown of cav‐1 and reducing the amount of caveolae in neurons may involve reduction of oxidative stress and as a result, lifespan extension.
The role of the cellular membrane in maintaining homeostasis is undisputable: As the sole barrier between the cell and its surroundings, it literally defines the cell. Our results suggest the intriguing idea that lipid rafts might be important sites in which environmental changes are sensed by the cell, enabling its adaptation to the new conditions. Moreover, the report that CAV‐1 interacts with the IGF‐1 receptor 54 raises the possibility that caveolae‐resident protein complexes regulate the activity of the IIS. However, although DAF‐2 was not detected in our mass spectrometry analysis, further research is needed to elucidate the mechanistic links between caveolae and the IIS and to better characterize the mechanisms by which caveolae control lifespan and proteostasis.
Differential regulation of lifespan, stress resistance, and proteostasis by caveolae
Interestingly, we discovered that the knockdown of cav‐1 extends lifespan and promotes proteostasis but has no effect on resistance to heat. The uncoupling of IIS‐governed functions has been already shown. For instance, abolishing the worms’ ability to induce the heat shock response does not affect lifespan but protects from proteotoxicity 36, 37. The mechanistic basis of this uncoupling is poorly understood; however, a possible explanation suggests that different daf‐2 splicing isoforms are responsible for the different functions downstream of the IIS. This idea may be supported by the report that the translocation of a specific DAF‐2 isoform to the synapses of chemosensory neurons is essential for nematode's learning and that diverse DAF‐2 splicing isoforms differentially affect biological functions such as dauer formation and chemotaxis 55. Thus, it will be interesting to test whether DAF‐2 resides in neuronal caveolae and if so, to characterize the caveolae‐specific isoforms and dissect their functional relevance.
An alternative explanation suggests that the IIS mediates its distinct functions by modulating signaling activities of factors that reside in different sub‐cellular structures. Accordingly, signaling complexes that regulate lifespan, coordinate proteostasis, and govern resistance to pathogens assemble and function on caveolae, while signaling apparatuses that control other IIS‐regulated functions are located elsewhere. The much smaller longevity effect of cav‐1 knockdown compared to the remarkable lifespan extension that results from the knockdown of daf‐2, implies that IIS reduction concurrently modulates the activity of different signaling complexes that reside in various cellular locations to achieve a cumulative longevity effect. In contrast, treating Aβ‐ and polyQ35‐YFP‐expressing worms with cav‐1 RNAi resulted in a notable counter‐proteotoxic effect, suggesting that caveolae play pivotal roles in the coordination of proteostasis and raises the prospect that the knockdown of cav‐1 could slow the progression of neurodegenerative disorders with minimal effect on lifespan. To assess the therapeutic potential of this approach, the effects of cav‐1 knockdown in mammalian cells should be tested to evaluate whether they modulate proteotoxicity and disease. It will also be necessary to examine whether a late life application of such treatment can efficiently mitigate disease‐associated symptoms. If conserved in mammals, an anti‐CAV‐1 intervention could be combined with IIS inhibitors 56 and perhaps additional compounds to achieve synergistic therapeutic efficiency.
Materials and Methods
Worm and RNAi strains
N2 (wild‐type, Bristol), daf‐2(e1370), TU3335 (unc‐119p::YFP + unc‐119p::sid‐1 + mec‐6p::mec‐6), CL2006 (unc‐54p::human Aβ3–42), EU31 (zu135), and LG344 (gpa‐4p::skn‐1b::GFP) worms were obtained from the Caenorhabditis Genetics Center (CGC, Minneapolis, MN), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). tm2702 worms (carrying deletion of 287 base pairs in the sequence of cav‐1) were provided by the Mitani laboratory through the National Bio‐Resource Project of the MEXT, Japan. CF1934 (daf‐16p::daf‐16::GFP) and CF1038 (mu86) worms were obtained from Dr. Andrew Dillin (Berkeley, CA USA). AM583 (hsf‐1p::hsf‐1::GFP) and PS3551 (hsf‐1p::hsf‐1 585 stop mutant) were obtained from Dr. Anat Ben‐Zvi (Ben‐Gurion University of the Negev, Israel). The worms were grown at 20°C. CF512 (fer‐15(b26)II; fem‐1(hc17)IV) animals are heat‐sensitive sterile that were routinely grown at 15°C. To avoid progeny, CF512 worms were hatched at 20°C and L1 larvae transferred to 25°C for 48 h and back to 20°C until harvested. To reduce gene expression, we used bacterial strains expressing dsRNA: empty vector (pAD12), daf‐2 (pAD48), daf‐16 (pAD43). cav‐1, hsf‐1, and skn‐1 dsRNA expressing bacteria were from cDNA RNAi library (M. Vidal). Each RNAi bacteria colony was grown at 37°C in LB with 100 μg/ml ampicillin and then seeded onto NG‐ampicillin plates supplemented with 100 mM isopropyl β‐D‐1‐thiogalactopyranoside (IPTG 1 mM final concentration).
The creation of worm strains and RNAi constructs
Strain EHC123—The following primers were used to amplify the promoter region of cav‐1 (including restriction sites): forward—CGACCCAAGCTTATGACTGAG; reverse—TATCCCGGGCGGTGGAGATGATGAAATGAG. The PCR reaction was performed using Phusion High‐Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA; #M0530S) on genomic DNA from N2 worms. The PCR product was cloned between the BamHI and XmaI restriction sites upstream of tdTomato. The final construct was sequenced and microinjected into the gonad of N2 (WT) L4 worms to create the transgenic line together with rol‐6 marker. Selection was made by picking rollers and visualization by a fluorescence microscope.
Strain EHC110‐TU3335 (unc‐119p::YFP + unc‐119p::sid‐1 + mec‐6p::mec‐6) males were crossed with EHC123 hermaphrodites. To create the cav‐1 3′UTR RNAi, the following gBlock consisting of a dsDNA of cav‐1 3′UTR was synthesized (IDT—Integrated DNA Technologies, Inc): AAGCTTatttatttaatttatctcaaattcattttactttttttgttgtatattctcatccctcaaagagtggtaataacgatccggagtgcaaaaaataaaatttgaaagcaaaaCTCGAG. The gBlock was digested with HindIII and XhoI restriction enzymes and cloned into the corresponding sites in the pL4440 plasmid.
Isolation of detergent‐resistant membranes (DRMs) and protein identification
One hundred thousand CF512 worms were treated with RNAi bacteria as indicated and were washed with 1×PBS. The worms were then washed with 1% Triton X‐114 in PBS containing Protease Inhibitor (Millipore, Billerica MA; #539134) and homogenized using a Dounce homogenizer. The worm homogenates were incubated on ice for 1 h and then were spun for 3 min at 850 g in a desktop Qiagen centrifuge to sediment debris. The postdebris supernatants were collected and diluted with 2.4 M sucrose to a final sucrose concentration of 1.2 M. This was placed in the bottom of a centrifuge tube and overlaid carefully with 1 ml of 1.1 M sucrose followed by 0.25 ml of 0.2 M sucrose. The tubes were centrifuged for 18 h at 166,180 g in a TLS‐55 Beckman rotor. Ten fractions were collected from top to bottom.
Protein identification by mass spectrometry
Two independent experiments were normalized according to protein concentration of total sample before placing at the bottom of the sucrose gradient. The top two fractions (1+2) from the experiments were combined. These fractions were frozen in liquid nitrogen and lyophilized for ~20 h. They were then separated on SDS–PAGE to get rid of lipids and detergents. The gel was stained using Colloidal Blue stain (Invitrogen, Carlsbad, CA; # LC6025). Each lane was cut into four slices. The samples were digested by trypsin, analyzed by LC‐MS/MS on LTQ‐OrbitrapXL (Thermo), and identified by MaxQuant versus the C. elegans section of the NCBI‐NR database and quantified by the same software using label free quantification. The data of all four gel slices of the same lane were combined. Criteria for a protein to be classified as differently expressed:
When a protein is identified in both samples, there needs to be at least 4.5 times difference between the intensities of the peptides for the difference to be considered as significant.
If a protein was identified only in one sample, the identification of the protein has to be with at least four peptides and with an intensity of at least 1E+06.
The database for annotation, visualization, and integrated discovery (DAVID) analysis was used for functional clustering and annotation of the Mass Spec list (http://david.abcc.ncifcrf.gov/). Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources 57. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists 58. This suit consists of an integrated biological knowledgebase and analytic tools aimed at systematically extracting biological meaning from large gene/protein lists. The lists were sorted according to cellular compartments or biological processes.
Expression analysis by quantitative real‐time PCR and digital droplet PCR
Synchronized eggs were placed on NGM plates seeded with the indicated bacteria. The worms were grown from hatching until day 1 of adulthood, collected with M9 buffer, and washed in RNase‐free water. The worm pellet was resuspended in 2 volumes of QIAzol (Qiagen, Hilden, Germany; #79306;) and frozen overnight. Chloroform was used to separate RNA from protein and other materials. The RNeasy Lipid Tissue Mini Kit (Qiagen; #74804;) was used according to the manufacturer's instructions to extract RNA. cDNA was generated by reverse transcription of the total RNA samples with iScriptRT Advanced cDNA Synthesis Kit for RT–PCR (Bio‐Rad, Hercules, CA; #170–8891;). qPCR was performed in triplicate using the EvaGreen SuperMix (Bio‐Rad; #172–5204;) and normalized to the levels of act‐1 and cdc‐42 cDNA.
Primers used for PCR:
| Gene | F primer (5′–3′) | R primer (5′–3′) |
|---|---|---|
| cyp‐14A3 | TTTGTTACTCAAGGTGACG | CTCCACTAGAACCTAATACG |
| F48C1.9 | AGCCCAATTAAGTACAGC | TGTAATAGTAGTTATTTGCCG |
| spp‐9 | TTGAAGATAAATTCCTTGCC | CAGTTCCAGATTCGAGC |
| scl‐11 | CCTCGATACATTTGGACC | CAGGCCATTTGAGTAGC |
| comt‐4 | AGTCTAGACAAACTAATCGC | ACAAAAATGACACCTCCC |
| acs‐2 | ATCTACCGAGACATCCC | CCAAATTCATCGACAATGG |
| daf‐2 | TTGATGAATCATAGTGGAGG | TATTTCGTCGCGTTGG |
| daf‐16 | CTTCAAGCCAATGCCACTACC | GGAGATGAGTTGGATGTTGATAGC |
| hsf‐1 | TTGACGACGACAAGCTTCCAGT | AAAGCTTGCACCAGAATCATCCC |
| skn‐1 | CCATATCACTCACAGAGACAC | CCAACTTTCCGTAGAAACG |
| cav‐1 primers | ||
| Used for qPCR | TGGAGCACCGGGAGATGAGC | AGTGCTGTGAGTCGGCTTCTCC |
| Used for deletion analysis | TGTCCACCGAGCAAGATATC | CGGATCGTTATTACCACTCT |
PCR using worms as a template
Six worms were added to worm lysis buffer (30 mM Tris pH 8.0, 8 mM EDTA, 100 mM NaCl, 0.7% NP‐40, and 0.7% Tween‐20) supplemented with proteinase K (PK, Sigma, St. Louis, MO #P6556). The tubes were incubated for 60 min at 60°C to enable PK‐mediated protein digestion and heat‐inactivated for 15 min at 95°C to inactivate the PK.
PCR for deletion analysis was performed using REDTaq ReadyMixPCR reaction Mix with MgCl2 (Sigma; #R2648). Each tube contained: 25 μl mix, 1 μl of each primer (final concentration 2 μM), 5 μl template DNA and DDW to a volume of 50 μl. The cycling parameters were: (i) 94°C for 1 min (ii) 94°C for 1 min, (iii) 58°C for 2 min, (iv) 72°C for 3 min, (v) repeat 30 cycles from step 2, (vi) 72°C for 5 min; 25 μl from each reaction was run on 1% agarose gel.
SDS–PAGE, Western blot analysis, and protein staining
Twelve thousand worms were treated with RNAi bacteria as indicated and homogenized using a Dounce homogenizer. The worm homogenates were spun for 3 min at 850 g in a desktop centrifuge to sediment debris. The postdebris supernatants (PDS) were collected, protein amounts were measured by a Bradford kit (Bio‐Rad; #500‐0006), supplemented with loading buffer (10% glycerol, 125 mM Tris base, 1% SDS), boiled for 10 min, and 10 μg total protein was loaded into each well. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), transferred onto PVDF membranes and probed with CAV‐1 antibody (BD Transduction Laboratories NJ; #610059) or γ‐tubulin antibody (clone GTU‐88 Sigma; #T‐6557). To detect Aβ, the worms were treated and prepared as described above, protein samples were loaded on 16.5% Tris‐Tricine gels (Bio‐Rad; # 4563064) separated, transferred onto a nitrocellulose membrane (Pall Corporation, Pensacola, FL; #66485), and blotted using an Aβ antibody (clone 6E10, Covance, Emeryville CA; # SIG‐39320). HRP‐conjugated secondary antibody, chemiluminescence system, and a luminescent image analyzer (Chemidoc XRS+, Bio‐Rad) were used to detect protein signals.
Lifespan assay
Synchronized eggs were placed on master NG‐ampicillin plates seeded with the indicated RNAi bacterial strain and supplemented with 100 mM IPTG (~1 mM final). CF512 worms were hatched at 20°C transferred to 25°C for 48 h to avoid progeny and back to 20°C. PS3551 worms were grown and maintained at 15°C. daf‐2 (e1370) mutant animals as well as N2, tm2702 worms and all other strains were developed and maintained at 20°C. At day 1 of adulthood, 120 animals per treatment were transferred onto small NG‐ampicillin plates (12 animals per plate). Worms that failed to move their tips when tapped twice with a platinum wire were scored as dead. Survival rates were recorded daily.
Paralysis assay
Synchronized CL2006 worms were grown on NG plates containing 100 μg/ml ampicillin, spotted with E. coli cultures expressing dsRNA as indicated. On the first day of adulthood, 120 worms were placed on 10 plates (12 animals per plate). The plates were divided randomly into five sets (two plates, 24 worms per set). The worms were tested daily for paralysis by tapping their noses with a platinum wire. Worms that moved their noses but failed to move their bodies were scored as “paralyzed” and removed from the plates. To avoid scoring old animals as paralyzed, paralysis assays were terminated at day 12 of adulthood.
Heat, UV, and innate immunity stress assays
Synchronized CF512 or N2 eggs were placed on NG plates seeded with the RNAi bacteria (as indicated) and supplemented with 100 mM IPTG (1 mM final). For heat stress assays, 120 day 1 old nematodes were transferred onto fresh plates (12 animals per plate) spotted with RNAi bacteria, exposed to 35°C for 11 (CF512) or 12 (N2) h and survival rates were recorded. To assess resistance to ultra‐violet (UV) radiation, day 1 adult worms that were developed from hatching on the indicated RNAi bacteria were exposed to a sub‐lethal UV dose (800 J/cm2). Survival rates were scored daily. To evaluate resistance to pathogenic bacteria (innate immunity), eggs of CF512 worms were placed on plates seeded with the indicated RNAi bacteria, grown to day 1 of adulthood and transferred onto plates seeded with Pseudomonas aeruginosa. Survival rates were recorded daily.
Separation of soluble from aggregated proteins
Eggs of CF512 worms were placed on plates seeded with the indicated RNAi bacteria (18,000 eggs per treatment) and incubated at 25°C for 48 h (to achieve sterility). At the indicated age, the worms were washed twice with M9 buffer (RT), incubated for 30 min with mild agitation to reduce the amount of bacteria in their guts, and washed twice with ice‐cold PBS. The worms were then resuspended in 300 μl PBS, supplemented with a protease inhibitor cocktail (Millipore, Billerica MA #539134), homogenized using a Dounce homogenizer and cleared by low speed centrifugation (5 min, 1,000 g). Supernatants were transferred onto new tubes, supplemented with TX‐100 and deoxycholic acid (final concentration of 1% of each detergent) followed by incubation on ice for 30 min.
The samples were subsequently transferred twice through thin needles (0.4 mm) and spun at 10,000 g (30 min, 4°C). Supernatants were transferred onto new tubes, supplemented with 1% sodium lauroyl sarcosinate (Sarkosyl) and incubated on ice for 30 min followed by ultra‐centrifugation (1 h, 200,000 g, 4°C). Supernatants (soluble fractions) were transferred onto new tubes, and pellets (insoluble fraction) were resuspended in 200 μl PBS. All samples were supplemented with loading buffer (4% SDS, 40% glycerol, 0.25 M Tris, 0.5% Bromo Phenol Blue, 10% β‐mercaptoethanol), heated to 95°C for 10 min, and separated on 10% PAA gel. Proteins were stained using a silver stain kit (Pierce, Waltham, MA; #24612) or a colloidal blue staining kit (Invitrogen; # LC6025).
Expression analysis by next‐generation sequencing
mRNA library preparation
RNA ScreenTape kit (Agilent Technologies, Santa Clara, CA; #5067‐5576), D1000 ScreenTape kit (Agilent Technologies; #5067‐5582), Qubit® RNA HS Assay kit (Invitrogen; #Q32852) and Qubit® DNA HS Assay kit (Invitrogen; #32854;) were used for each specific step purpose for quality control of RNA. For mRNA library preparation: KAPA Stranded mRNA‐Seq Kit with mRNA Capture Beads (kapabiosystems, KK8421, https://www.kapabiosystems.com/) was used. In brief, 1ug was used for the library construction; library was eluted in 20 μl of elution buffer. Libraries were adjusted to 10 mM, and then, 10 μl (50%) from each sample was collected and pooled in one tube.
Multiplex samples Pool (1.5 pM including PhiX 1.5%) was loaded in NextSeq 500/550 High Output v2 kit (75 cycles) cartridge (Illumina, San Diego, CA; #FC‐4041005) and loaded on NextSeq 500 System (Illumina), with 75 cycles and single‐read sequencing conditions.
Trimming and filtering of raw reads
The NextSeq basecalls files were converted to fastq files using the bcl2fastq (v2.15.0.4) program with default parameters. The provided SampleSheet.csv file contained samples’ names and barcodes only, so no trimming or filtering was done at this stage and a fastq file was created for each sample separately.
Raw reads (fastq files) were inspected for quality issues with Fast QC (v0.11.2, http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). According to the FastQC report, reads were quality‐trimmed at both ends, using in‐house Perl scripts, with a quality threshold of 32. In short, the scripts use a sliding window of five bases from the read's end and trim one base at a time until the average quality of the window passes the given threshold. Following quality‐trimming, the SL1 and SL2 sequences were removed from the 5′ end by “cut adapt” (version 1.7.1, http://cutadapt.readthedocs.org/en/stable/), with a minimal overlap of 1 (‐O parameter) and allowing for read wildcards. The adapter sequence was then similarly trimmed from the 3′ end. Reads that became shorter than 15 nt were filtered out (‐m parameter). The remaining reads were further filtered to remove very low quality reads, using the fastq_quality_filter program of the FASTX package (version 0.0.14, http://hannonlab.cshl.edu/fastx_toolkit/), with a quality threshold of 20 at 90 percent or more of the read's positions.
Mapping and differential expression analysis
The processed fastq files were mapped to the worm transcriptome and genome using TopHat (v2.0.13). The genome version was WBcel235, with annotations from Ensembl release 84. Mapping allowed up to 5 mismatches per read, a maximum gap of five bases, and a total edit distance of 10 (full command: tophat ‐G genes.gtf ‐N 5 –read‐gap‐length 5 –read‐edit‐dist 10 –segment‐length 20 –read‐realign‐edit‐dist 4 ‐i 20 –microexon‐search –min‐segment‐intron 20 –coverage‐search –min‐coverage‐intron 20 –max‐coverage‐intron 10,000 –library‐type fr‐firststrand genome processed.fastq).
For the statistics file, quantification was done using htseq‐count (version 0.6.0, http://htseq.readthedocs.io/en/release_0.10.0/). Strand information was set to “reverse”, and an annotation file that lacked information for genes of type rRNA, tRNA, miRNA, snRNA, snoRNA, and piRNA, was used.
For further analysis, quantification was done with the Cufflinks package (v2.2.1), using the cuffquant program with the genome bias correction (‐b parameter), multi‐mapped reads assignment algorithm (‐u parameter) and masking for genes of the same types as above (rRNA, tRNA, etc.) (‐M parameter). Maximum number of fragments per locus was increased to 10,000,000, and the program was informed of the reads directionality by the –library‐type fr‐firststrand parameter. Raw counts were obtained by running cuffnorm on the cuffquant output.
Normalization and differential expression were done with the DESeq2 package (version 1.10.1). Genes with a sum of counts < 2 over all samples were filtered out prior to normalization, and then, size factors and dispersion were calculated. Differential expression was calculated with default parameters, including the significance threshold of padj< 0.1. R version was 3.2.1, and plotting was done with packages RColorBrewer_1.1‐2, pheatmap_1.0.8 and VennDiagram_1.6.17.
Visualization of worms by fluorescence microscopy
Expression patterns were tested using fluorescence microscopy system. The worms were washed twice with M9, anesthetized using 18 mM sodium azide (#S‐2002; Sigma), and placed on an agar pad for visualization. Images were obtained using a Zeiss Observer Z1 microscope equipped with an ApoTome 2 or with a Nikon AZ100 system.
Transmission electron microscopy
Visualization of worm neurons by TEM was performed as described previously 59. Briefly, CF512 worms were grown from hatching to adulthood on EV or on daf‐2 RNAi bacteria. At day 1 of adulthood, the worms were washed three times with M9, fixed with a fixative (1% freshly made formaldehyde, 2.5% EM grade glutaraldehyde, 0.1 M HEPES, pH 7.0), and subjected to a microwave cycle of 20 s heating, 10 s on ice and additional 20 s of heating. Then, the worms were cooled on ice for 5 min, subjected to an additional microwave cycle (as above), and incubated for 1 h at room temperature. Excess of fixative was removed, and the worms were washed three times for 10 min with 0.2 M HEPES (pH 5.6). The worms were incubated for 1 h in 1 ml of 1% OsO4, 0.5% reduced K4Fe(CN)6, 0.1 M HEPES (pH 5.6, RT, darkness), washed twice for 10 min with DDW, and subjected to dehydration with 50, 70, 90, and 100% ethanol (10‐min incubation in 50, 70, and 90% followed by 3 × 30 min in 100% ethanol). Next, the worms were incubated in sequential concentrations of Epon/ethanol (ratios of 1:2, 1:1 and 2:1 for 2 h in each solution RT) followed by an overnight incubation in 100% Epon. Next the worms were incubated three times in 100% Epon (2 h each incubation) and placed in a dry oven (60°C) for 48 h. Sections of approximately 80 nm were prepared using a Diatome diamond knife, placed on coper grids, and visualized by a JEM‐1400 JEOL TEM.
Statistical analyses
Statistical significance of the results was performed using the Student's t‐test using two‐tailed distribution and two‐sample equal variance. For lifespan and paralysis experiments unpaired, one‐tailed Student's t‐test was used. The analyses were done using at least three independent biological repeats of each experiment as indicated. Statistical information of lifespan experiments, UV, and pathogenic survival are presented at the Appendix tables.
Data availability
Mass spectrometry data are available at PRIDE archive under accession PXD009704.
NGS data are available from the GEO database under accession GSE100045.
Author contributions
EC, NR, and MB‐S initiated and designed this project. MB‐S conducted all cloning procedures. MB‐S and NR performed most of the experimental work including stress, proteotoxicity and lifespan assays. Samples for mass spectrometry were prepared by MB‐S and for NGS by NR. NR performed ddPCR. FCM performed quantitative real‐time PCR. LM, LG, EC, HB, and TE conducted paralysis and lifespan assays. YN performed computational analyses. EC, MB‐S and NR wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
This study was generously supported by the Israel Science Foundation (ISF) (EC# 981/16), the Fritz‐Thyssen Foundation (EC) (0305171), and the European Research Council (ERC) (EC# 281010). We thank Drs. Idit Shiff and Abed Nasereddin for expert assistance with next‐generation sequencing, Dr. Tamar Ziv, from the Smoler Proteomics Center at the Technion (Haifa, Israel), for mass spectrometry analyses for expert assistance with protein identification by mass spectrometry and Mrs. Hava Glickstein for preparing samples for visualization by electron microscopy.
EMBO Reports (2018) 19: e45673
References
- 1. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464 [DOI] [PubMed] [Google Scholar]
- 2. Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L (2007) Dynamics of the action of dFOXO on adult mortality in Drosophila . Aging Cell 6: 429–438 [DOI] [PubMed] [Google Scholar]
- 3. Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y (2003) IGF‐1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421: 182–187 [DOI] [PubMed] [Google Scholar]
- 4. Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P (2008) Functionally significant insulin‐like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105: 3438–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li W, Kennedy SG, Ruvkun G (2003) daf‐28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF‐2 signaling pathway. Genes Dev 17: 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henderson ST, Johnson TE (2001) daf‐16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans . Curr Biol 11: 1975–1980 [DOI] [PubMed] [Google Scholar]
- 7. Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK (2008) Direct inhibition of the longevity‐promoting factor SKN‐1 by insulin‐like signaling in C. elegans . Cell 132: 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL (2012) HSF‐1 regulators DDL‐1/2 link insulin‐like signaling to heat‐shock responses and modulation of longevity. Cell 148: 322–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee RY, Hench J, Ruvkun G (2001) Regulation of C. elegans DAF‐16 and its human ortholog FKHRL1 by the daf‐2 insulin‐like signaling pathway. Curr Biol 11: 1950–1957 [DOI] [PubMed] [Google Scholar]
- 10. Lithgow GJ, White TM, Melov S, Johnson TE (1995) Thermotolerance and extended life‐span conferred by single‐gene mutations and induced by thermal stress. Proc Natl Acad Sci USA 92: 7540–7544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans EA, Kawli T, Tan MW (2008) Pseudomonas aeruginosa suppresses host immunity by activating the DAF‐2 insulin‐like signaling pathway in Caenorhabditis elegans . PLoS Pathog 4: e1000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakami S, Johnson TE (1996) A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans . Genetics 143: 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006) Opposing activities protect against age‐onset proteotoxicity. Science 313: 1604–1610 [DOI] [PubMed] [Google Scholar]
- 14. Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine‐expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans . Proc Natl Acad Sci USA 99: 10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen E, Paulsson JF, Blinder P, Burstyn‐Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW et al (2009) Reduced IGF‐1 signaling delays age‐associated proteotoxicity in mice. Cell 139: 1157–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C (2003) Genes that act downstream of DAF‐16 to influence the lifespan of Caenorhabditis elegans . Nature 424: 277–283 [DOI] [PubMed] [Google Scholar]
- 17. Lee SS, Kennedy S, Tolonen AC, Ruvkun G (2003) DAF‐16 target genes that control C. elegans life‐span and metabolism. Science 300: 644–647 [DOI] [PubMed] [Google Scholar]
- 18. Wang MC, O'Rourke EJ, Ruvkun G (2008) Fat metabolism links germline stem cells and longevity in C. elegans . Science 322: 957–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang P, Judy M, Lee SJ, Kenyon C (2013) Direct and indirect gene regulation by a life‐extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab 17: 85–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11: 688–699 [DOI] [PubMed] [Google Scholar]
- 21. Naslavsky N, Shmeeda H, Friedlander G, Yanai A, Futerman AH, Barenholz Y, Taraboulos A (1999) Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. J Biol Chem 274: 20763–20771 [DOI] [PubMed] [Google Scholar]
- 22. Tun H, Marlow L, Pinnix I, Kinsey R, Sambamurti K (2002) Lipid rafts play an important role in A beta biogenesis by regulating the beta‐secretase pathway. J Mol Neurosci 19: 31–35 [DOI] [PubMed] [Google Scholar]
- 23. Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ (2001) Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem Res 26: 771–782 [DOI] [PubMed] [Google Scholar]
- 24. Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K (2003) Lipid rafts/caveolae are essential for insulin‐like growth factor‐1 receptor signaling during 3T3‐L1 preadipocyte differentiation induction. J Biol Chem 278: 11561–11569 [DOI] [PubMed] [Google Scholar]
- 25. Lee JA, Choi DI, Choi JY, Kim SO, Cho KA, Lee JB, Yun SJ, Lee SC (2015) Methyl‐beta‐cyclodextrin up‐regulates collagen I expression in chronologically‐aged skin via its anti‐caveolin‐1 activity. Oncotarget 6: 1942–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panetta D, Biedi C, Repetto S, Cordera R, Maggi D (2004) IGF‐I regulates caveolin 1 and IRS1 interaction in caveolae. Biochem Biophys Res Commun 316: 240–243 [DOI] [PubMed] [Google Scholar]
- 27. van den Heuvel AP, Schulze A, Burgering BM (2005) Direct control of caveolin‐1 expression by FOXO transcription factors. Biochem J 385: 795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5: 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parker S, Peterkin HS, Baylis HA (2007) Muscular dystrophy associated mutations in caveolin‐1 induce neurotransmission and locomotion defects in Caenorhabditis elegans . Invert Neurosci 7: 157–164 [DOI] [PubMed] [Google Scholar]
- 30. Scheel J, Srinivasan J, Honnert U, Henske A, Kurzchalia TV (1999) Involvement of caveolin‐1 in meiotic cell‐cycle progression in Caenorhabditis elegans . Nat Cell Biol 1: 127–129 [DOI] [PubMed] [Google Scholar]
- 31. Huang da W, Sherman BT, Zheng X, Yang J, Imamichi T, Stephens R, Lempicki RA (2009) Extracting biological meaning from large gene lists with DAVID. Curr Protoc Bioinformatics Chapter 13: Unit 13 11 [DOI] [PubMed] [Google Scholar]
- 32. Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC et al (2011) High‐throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83: 8604–8610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Volonte D, Liu Z, Musille PM, Stoppani E, Wakabayashi N, Di YP, Lisanti MP, Kensler TW, Galbiati F (2013) Inhibition of nuclear factor‐erythroid 2‐related factor (Nrf2) by caveolin‐1 promotes stress‐induced premature senescence. Mol Biol Cell 24: 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M et al (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237 [DOI] [PubMed] [Google Scholar]
- 35. Parker S, Walker DS, Ly S, Baylis HA (2009) Caveolin‐2 is required for apical lipid trafficking and suppresses basolateral recycling defects in the intestine of Caenorhabditis elegans . Mol Biol Cell 20: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volovik Y, Moll L, Marques FC, Maman M, Bejerano‐Sagie M, Cohen E (2014) Differential regulation of the heat shock factor 1 and DAF‐16 by neuronal nhl‐1 in the nematode C. elegans . Cell Rep 9: 2192–2205 [DOI] [PubMed] [Google Scholar]
- 37. Maman M, Carvalhal Marques F, Volovik Y, Dubnikov T, Bejerano‐Sagie M, Cohen E (2013) A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans . J Neurosci 33: 6102–6111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McColl G, Roberts BR, Gunn AP, Perez KA, Tew DJ, Masters CL, Barnham KJ, Cherny RA, Bush AI (2009) The Caenorhabditis elegans A beta 1‐42 model of Alzheimer disease predominantly expresses A beta 3‐42. J Biol Chem 284: 22697–22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Link C (1995) Expression of human beta‐amyloid peptide in transgenic Caenorhabditis elegans . Proc Natl Acad Sci USA 92: 9368–9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Volovik Y, Marques FC, Cohen E (2014) The nematode Caenorhabditis elegans: a versatile model for the study of proteotoxicity and aging. Methods 68: 458–464 [DOI] [PubMed] [Google Scholar]
- 41. David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C (2010) Widespread protein aggregation as an inherent part of aging in C. elegans . PLoS Biol 8: e1000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bishop NA, Guarente L (2007) Two neurons mediate diet‐restriction‐induced longevity in C. elegans . Nature 447: 545–549 [DOI] [PubMed] [Google Scholar]
- 43. Hsu AL, Murphy CT, Kenyon C (2003) Regulation of aging and age‐related disease by DAF‐16 and heat‐shock factor. Science 300: 1142–1145 [DOI] [PubMed] [Google Scholar]
- 44. Morton EA, Lamitina T (2013) Caenorhabditis elegans HSF‐1 is an essential nuclear protein that forms stress granule‐like structures following heat shock. Aging Cell 12: 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ravichandran M, Priebe S, Grigolon G, Rozanov L, Groth M, Laube B, Guthke R, Platzer M, Zarse K, Ristow M (2018) Impairing L‐threonine catabolism promotes healthspan through methylglyoxal‐mediated proteohormesis. Cell Metab 27: 914–925 e915 [DOI] [PubMed] [Google Scholar]
- 46. Prahlad V, Morimoto RI (2011) Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc Natl Acad Sci USA 108: 14204–14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ et al (2017) Comprehensive single‐cell transcriptional profiling of a multicellular organism. Science 357: 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572 [DOI] [PubMed] [Google Scholar]
- 49. Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M (2010) Enhanced neuronal RNAi in C. elegans using SID‐1. Nat Methods 7: 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Raamsdonk JM, Hekimi S (2012) Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci USA 109: 5785–5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prahlad V, Cornelius T, Morimoto RI (2008) Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320: 811–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caravia L, Dudau M, Gherghiceanu M, Tanase C, Enciu AM (2015) Could caveolae be acting as warnings of mitochondrial ageing? Mech Ageing Dev 146–148: 81–87 [DOI] [PubMed] [Google Scholar]
- 53. Parton RG, del Pozo MA (2013) Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14: 98–112 [DOI] [PubMed] [Google Scholar]
- 54. Maggi D, Biedi C, Segat D, Barbero D, Panetta D, Cordera R (2002) IGF‐I induces caveolin 1 tyrosine phosphorylation and translocation in the lipid rafts. Biochem Biophys Res Commun 295: 1085–1089 [DOI] [PubMed] [Google Scholar]
- 55. Ohno H, Kato S, Naito Y, Kunitomo H, Tomioka M, Iino Y (2014) Role of synaptic phosphatidylinositol 3‐kinase in a behavioral learning response in C. elegans . Science 345: 313–317 [DOI] [PubMed] [Google Scholar]
- 56. El‐Ami T, Moll L, Carvalhal Marques F, Volovik Y, Reuveni H, Cohen E (2014) A novel inhibitor of the insulin/IGF signaling pathway protects from age‐onset, neurodegeneration‐linked proteotoxicity. Aging Cell 13: 165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- 58. Huang da W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cohen M, Tzur YB, Neufeld E, Feinstein N, Delannoy MR, Wilson KL, Gruenbaum Y (2002) Transmission electron microscope studies of the nuclear envelope in Caenorhabditis elegans embryos. J Struct Biol 140: 232–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Data Availability Statement
Mass spectrometry data are available at PRIDE archive under accession PXD009704.
NGS data are available from the GEO database under accession GSE100045.


