Abstract
Recent studies support either X‐dampening or X‐inactivation as the mechanism of dosage compensation in human embryos. The data and possible underlying causes for these conflicting models are discussed here.

Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Development & Differentiation
Eutherian female mammals compensate the dosage of X‐linked gene expression between XY male and XX female, via transcriptional silencing of one of the two X‐chromosomes during embryonic development, a phenomenon known as X‐chromosome inactivation 1. Studies have shown that like murine adult female somatic cells, the human counterparts also have many genes inactivated in one of the X‐chromosomes. In mouse, there are two forms of X‐inactivation: imprinted and random (Fig 1). Humans on the other hand do not undergo imprinted X‐inactivation 2. However, X‐chromosome dynamics in human pre‐implantation embryos remains elusive, largely due to the restricted availability of human embryos and technical difficulties. Early experiments on human embryos reported conflicting results 3, 4, 5. One study showed progressive accumulation of XIST on one of the X‐chromosomes along with the inactivation of X‐linked genes in pre‐implantation female embryos 3. In contrast, another study reported XIST coating on both X‐chromosomes accompanied by partial exclusion of RNA‐Pol II in most early embryonic cells without the transcriptional silencing of X‐linked genes, indicating incipient X‐inactivation during pre‐implantation development. However, a minor population of cells showed monoallelic Xist expression, and the authors also reported XIST coating of the X‐chromosomes in male embryos 4. These differences were most likely caused by differences in the sensitivity of the techniques used and/or the low numbers of X‐linked genes studied. In addition, potential influences of different embryo sources and culture conditions may be other underlying causes of these conflicting results. Two recent studies, by Petropoulos et al 6 and De Mello et al 7, used single‐cell RNA‐Sequencing (scRNA‐Seq) to investigate dosage compensation in human pre‐implantation embryos and yielded conflicting models: X‐dampening versus X‐inactivation. In this Opinion, we provide some critical insights into these recent findings and discuss some enduring questions.
Figure 1. X‐chromosome dampening versus inactivation.
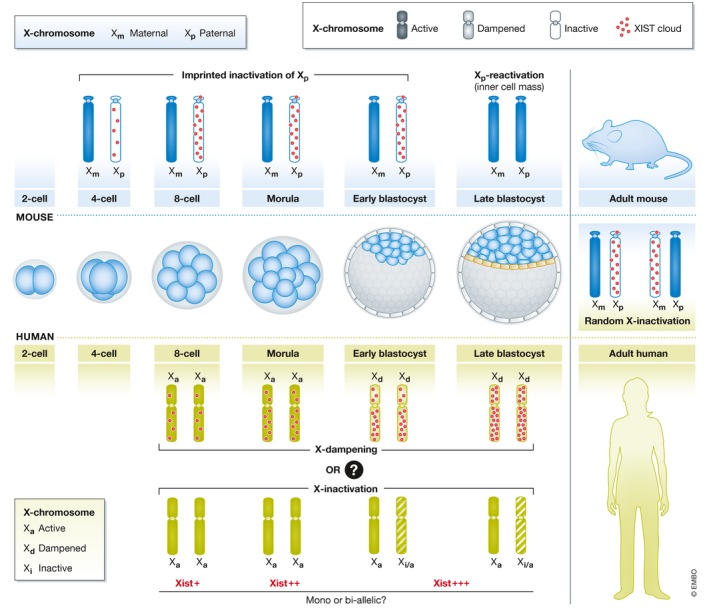
Schematic representation of X‐chromosome dynamics in early embryonic mouse and human development. In mouse, paternal‐X (Xp) (imprinted) gets inactivated initially. Later in the epiblast progenitor cells of the late blastocyst, inactive‐Xp is reactivated. The embryo proper then undergoes random X‐inactivation. However, in humans there is no imprinted X‐inactivation. Recent studies have inferred contradictory models of X‐chromosome dynamics in human pre‐implantation embryos: X‐chromosome dampening versus inactivation. According to the dampening model, following the biallelic Xist expression initiation at the 8‐cell stage, expression of X‐linked genes is downregulated while maintaining biallelic expression until the late blastocyst stage. On the contrary, another study suggests the initiation of X‐inactivation in human pre‐implantation development as they found Xist expression was followed by a majority of X‐linked genes showing monoallelic expression.
Petropoulos et al comprehensively profiled the transcriptome of early pre‐implantation human embryos from the 8‐cell to the late blastocyst stage by scRNA‐Seq, to explore the dynamics of lineage specification as well as dosage compensation during early development 6. These embryos were sourced from IVF facilities from two separate cohorts of embryonic day (E) 4 and E2 embryos. Soon after zygotic genome activation (ZGA) at the 8‐cell stage, female embryos showed biallelic expression of the X‐linked genes, indicating the presence of two active‐X chromosomes. Expression of the long non‐coding RNA (lncRNA) XIST, master regulator of X‐inactivation, was concomitant to ZGA and its level kept increasing till the late blastocyst stage. X‐chromosome expression was downregulated in female embryos from the morula stage and was minimal at the late blastocyst stage. Interestingly, X‐chromosome expression was found to be biallelic even at the late blastocyst stage. SNP analysis also showed XIST expression to be biallelic. On the other hand, male X‐chromosome expression remained constant during early development. Xist expression was higher in female compared with male embryos in all lineages at later stages of pre‐implantation development. Based on these observations, Petropoulos et al proposed X‐chromosome dampening as a likely dosage compensation mechanism during human pre‐implantation development (Fig 1). However, the underlying mechanism of X‐dampening remains unclear, and the role of XIST in dampening needs further investigation.
Recently, De Mello and colleagues used a novel pipeline to investigate the same scRNA‐Seq dataset of human pre‐implantation embryos reported by Petropoulos et al 6 and provided evidence for initiation of X‐inactivation 7 (Fig 1). In their analysis, genes in the pseudo autosomal regions (PAR) of the X‐chromosome were excluded as they are known to escape X‐inactivation. Moreover, only informative genes, i.e., genes with either biallelic expression in at least one cell or different monoallelic expression in at least two cells, were considered for analysis. Contrary to earlier reports, their analysis showed that the proportion of biallelically expressed genes decreased with time, with a concurrent increase in monoallelic expression during pre‐implantation development, suggesting that X‐inactivation was initiated during pre‐implantation development. Interestingly, the median X‐chromosome expression was found to be similar throughout pre‐implantation development, indicating X‐inactivation 7. Notably, the analysis of a different RNA‐Seq dataset revealed monoallelic expression of X‐linked genes by the late blastocyst stage. The ratio of biallelically expressed female X‐linked genes to male X‐linked expression (XX/XY) also confirmed ongoing X‐inactivation. Altogether, these data by De Mello et al provide evidence in support of X‐inactivation as the dosage compensation mechanism during pre‐implantation development. In accordance with the Petropoulos study, Xist was upregulated in female embryos post‐ZGA and was significantly higher than in male embryos. However, the expression pattern of Xist, whether monoallelic or biallelic, was not confirmed due to lack of informative SNPs in Xist. Another interesting result of this study, which supports Ohno's hypothesis, was that the active‐X chromosome was transcriptionally upregulated in both male and female embryos, as seen in mice to compensate the X to autosome dosage. Separately, another recent study by Vallot et al 8 has also shown a decrease in biallelic expression of X‐chromosomes with development using a different pipeline.
In summary, the dynamics of the X‐chromosome state during pre‐implantation development of human embryos remains elusive. Although the X‐chromosome dampening model proposed by Petropoulos et al is well accepted by the scientific community, there are several technical issues which raise questions about this model. The most important one is that they used a minimum of 3 SNPs to identify allelic expression in their analysis of scRNA‐Seq data, which is a very low number for confirming allelic identity. Therefore, their reports of biallelic expression of the X‐linked genes during pre‐implantation development are doubtful. In contrast, De Mello et al analyzed the same dataset using 20 SNPs as the minimum requirement to identify allelic expression and found monoallelic expression of X‐linked genes during pre‐implantation development. Moreover, Petropoulos et al denoted relative expression ratios ranging from 0.1 to 0.9 as biallelic, whereas De Mello et al used relative expression ratios from 0.2 to 0.8 to denote biallelic expression. Since both of these studies focus primarily on a single database from Petropoulos et al, the difference in their inferences arises from the analytical parameters used. Altogether, these issues question the X‐chromosome dampening model as the method of dosage compensation during early pre‐implantation development. Further studies are required to properly understand human dosage compensation during pre‐implantation development. On the other hand, despite recent reports of the generation of naïve human embryonic stem cell lines, studying dosage compensation in these systems has been reported as not being satisfactory 9, 10. However, it will be worthwhile to invest more effort into perfecting isolation of naïve human stem cell lines, which could be an important tool to resolve the present conflict.
EMBO Reports (2018) 19: e46294
References
- 1. Lyon MF (1961) Nature 190: 372–373 [DOI] [PubMed] [Google Scholar]
- 2. van den Berg IM, Galjaard RJ, Laven JSE et al (2010) Nature 35: 443–451 [Google Scholar]
- 3. van den Berg IM, Laven JSE, Stevens M et al (2009) Am J Hum Genet 84: 771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okamoto I, Patrat C, Thépot D et al (2011) Nature 472: 370–374 [DOI] [PubMed] [Google Scholar]
- 5. Briggs SF, Dominguez AA, Chavez SL et al (2015) Stem Cells 33: 1771–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petropoulos S, Edsgärd D, Reinius B et al (2016) Cell 165: 1012–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moreira de Mello JC, Fernandes GR, Vibranovski MD et al (2017) Sci Rep 7: 10794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vallot C, Patrat C, Collier AJ et al (2017) Cell Stem Cell 20: 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahakyan A, Kim R, Chronis C et al (2017) Cell Stem Cell 20: 87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahakyan A, Plath K, Rougeulle C (2017) Philos Trans R Soc Lond B Biol Sci 372: 20160363 [DOI] [PMC free article] [PubMed] [Google Scholar]


