Abstract
Cardiac amyloidosis is an under-appreciated cause of heart failure. Establishing a diagnosis is important because traditional heart failure treatment regimens can worsen left ventricular failure in this disease. Endomyocardial biopsy is the gold standard for diagnosis; however, scintigraphy with radiolabeled phosphate derivatives and cardiac magnetic resonance imaging have been shown to have high sensitivity and specificity in diagnosing cardiac amyloidosis. Furthermore, cardiac scintigraphy can reliably differentiate amyloid subtypes. We present a case of transthyretin-related cardiac amyloidosis with a negative endomyocardial biopsy but positive 99m-technetium pyrophosphate single photon emission computed tomography scan and cardiac magnetic resonance imaging. We discuss the utility of 99m-technetium pyrophosphate imaging in cardiac amyloidosis and the role of single photon emission computed tomography. Finally, we review the several forms of cardiac amyloidosis and how they pertain to cardiac scintigraphy.
Keywords: Cardiac amyloidosis, Transthyretin, Tc-99m pyrophosphate
Case report
A 72-year-old man with a history of chronic systolic heart failure presented with progressive dyspnea and lower extremity swelling. Echocardiogram demonstrated a hyperechoic myocardium, enlarged left atrium, mild right ventricular dilatation with preserved systolic function, and an ejection fraction of 20%-25%. Cardiac amyloidosis was suspected and a serum protein electrophoresis was obtained, which showed an increase in polyclonal gamma globulins suggestive of a chronic inflammatory reaction; however, no suspicious monoclonal bands were present. Free kappa light chains were 45.61 mg/L (normal < 19.4 mg/L) and the kappa/lambda ratio was 2.01 (normal < 1.65).
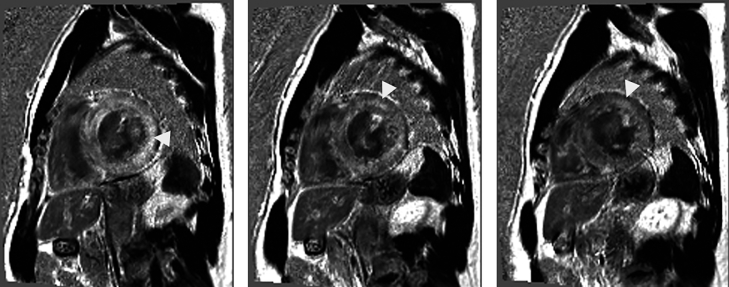
The patient underwent cardiac magnetic resonance imaging (MRI), which showed both ventricles to be moderately enlarged and have globally depressed function. The left ventricle demonstrated moderate concentric hypertrophy. Left ventricular ejection fraction was 35% with an absolute cardiac output of 5.4 L/min. Delayed enhancement imaging showed abnormal gadolinium kinetics, as well as patchy diffuse enhancement in a pattern typical for amyloid cardiomyopathy (Fig. 1).
Fig. 1.
Serial short-axis phase-sensitive inversion-recovery post-contrast magnetic resonance images through the left ventricle demonstrate diffuse, patchy linear and transmural areas of enhancement (arrowheads) predominantly involving the left ventricular myocardium.
The patient subsequently underwent a right heart catheterization with 3 endomyocardial biopsies of the right ventricular septum. Pathology showed myocardial tissue with focal fibrosis; however, no amyloid deposition was present by Congo red stain under polarized light. Immunohistochemistry or mass spectroscopy was not performed.
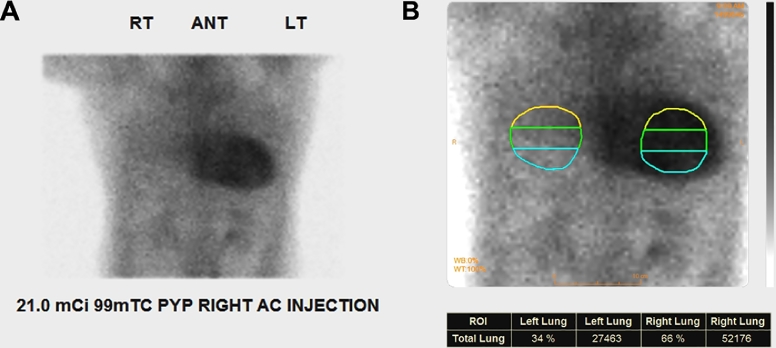
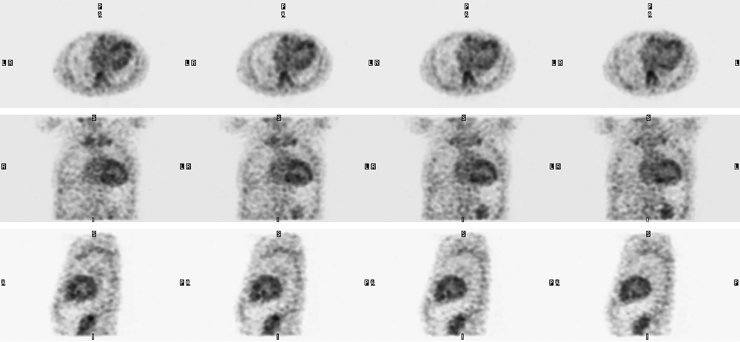
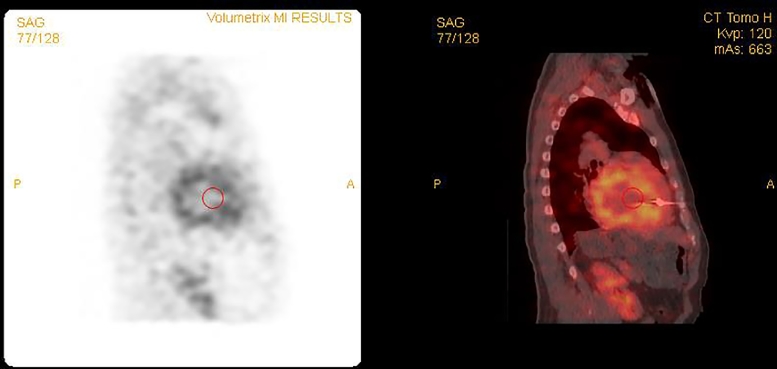
The discordance between the positive MRI and negative endomyocardial biopsy results prompted further investigation with 99m-technetium pyrophosphate (99mTc-PYP) scintigraphy. Images of the thorax were acquired in planar projections, as well as with single photon emission computed tomography (SPECT), to improve anatomic localization of radiotracer. Cardiac retention of radiotracer was assessed quantitatively by drawing a region of interest over the heart followed by the contralateral right lung and calculating a heart-to-contralateral ratio (H/CL). The patient was injected with 21.0 mCi of 99mTc-PYP and planar images were acquired 1 hour later, which showed significantly increased radiotracer activity in the heart (Fig. 2A) with a calculated H/CL of 1.9 (normal < 1.5; Fig. 2B). SPECT/computed tomography images showed abnormally increased radiotracer activity throughout the myocardium, greatest in the left ventricle (Figs. 3 and 4), consistent with a transthyretin amyloidosis.
Fig. 2.
Thoracic scan in the anterior view performed 1 hour after administration of 99m-technetium pyrophosphate. (A) There is intense radiotracer accumulation in the myocardium. (B) Region of interest markers placed over the myocardium and contralateral right lung for quantification of radiotracer activity. The total counts within the myocardial region of interest divided by the contralateral counts were used to calculate the heart-to-contralateral ratio.
Fig. 3.
99m-technetium pyrophosphate cardiac single photon emission computed tomography (top: axial plane, middle: coronal plane, bottom: sagittal plane) shows intense, left greater than right ventricular myocardium radiotracer accumulation.
Fig. 4.
99m-technetium pyrophosphate cardiac single photon emission computed tomography (left) and fusion single photon emission computed tomography/computed tomography (right) images in the sagittal plane through the left ventricle confirm intense and diffuse radiotracer accumulation in the myocardium.
Discussion
Cardiac amyloidosis, often considered as a single clinical entity, is better characterized as a group of diseases caused by the extracellular deposition of fibrillary proteins in the heart [1]. There are three main types defined by different pathological substrates and clinical courses. Cardiac amyloid light-chain (AL) is due to clonal proliferation of immunoglobulin light-chains in the bone marrow leading to protein aggregation and fibrillary deposits. Transthyretin-related cardiac amyloidosis is due to misfolding of the transthyretin protein (TTR) with resulting aggregation into fibrillary deposits. It can be due to mutations in TTR, in which case it is called the ATTRm form of cardiac amyloidosis, or due to wild-type, non-mutant TTR, in which case it is called ATTRwt and mainly affects older men [2]. Differentiating between AL and ATTR variants is important because AL portends a worse prognosis and is typically treated with chemotherapy targeting plasma cells. Treatment of ATTR subtypes of cardiac amyloidosis involves liver and/or heart transplantation in select individuals, and conventional heart failure management, with numerous potentially disease-modifying strategies emerging over the past several years [3].
The gold standard for diagnosing cardiac amyloidosis is endomyocardial biopsy. However, non-invasive imaging methods are being increasingly used to allow safer and earlier detection [4]. In a study of 33 patients with endomyocardial biopsy proven cardiac amyloidosis, MRI was shown to have a sensitivity of 94% and specificity of 80% in diagnosing cardiac amyloidosis [5]. Late gadolinium enhancement has also been shown to precede left ventricular morphologic alterations in many cases [6]. However, cardiac MRI is unable to reliably differentiate between AL and ATTR subtypes of cardiac amyloidosis, an important distinction that dictates treatment and prognosis [7].
Over the recent years, there is increasing interest in the use of cardiac scintigraphy for the early detection and differentiation of cardiac amyloidosis [8]. Radiolabeled bisphosphonates were first shown to detect cardiac amyloidosis and distinguish AL from ATTR subtypes with the use of 99m-technetium-3,3-diphosphonate-1,2-propanodicarboxylic acid [9]; however, this isotope is not Food and Drug Administration-approved and not available in the United States. More recently, the widely available isotope 99mTc-PYP has been shown to be able to distinguish cardiac amyloid subtypes. Using the quantitative measure H/CL with a cutoff value >1.5, ATTR subtype amyloidosis can be detected with a 97% sensitivity and 100% specificity [2]. Other quantitative methods have also been proposed, including the “pyrophosphate score,” which is the ratio of the myocardial mean counts to ventricular cavity mean counts. This method has a reported sensitivity of 85% and specificity of 95% for diagnosing cardiac amyloidosis without differentiating amyloid subtypes [10]. The use of SPECT has also been investigated to improve anatomic radiotracer localization [11]. However, it is currently difficult to quantify radiotracer activity on SPECT, which makes it difficult to assess disease burden and response to therapy [12]. Interestingly, once a diagnosis of ATTR is made, follow-up 99mTc-PYP scintigraphy does not show significant changes despite obvious clinical disease progression [13].
Negative endomyocardial biopsy in the setting of highly suspected cardiac amyloidosis is rare. Biopsies positive for cardiac amyloidosis will demonstrate Congo red staining with apple-green birefringence under polarized light microscopy. In false-negative cases, electron microscopy has been shown to demonstrate the presence of rigid, non-branching fibrils [14]. Under-sampling has also been shown to decrease the diagnostic yield from endomyocardial biopsy with a 45% accurate pathologic diagnosis with 5 or more samples compared to only 20% with 1-3 samples [15]. In our case, electron microscopy was not performed and only three samples were obtained from the right ventricle.
Our case illustrates the value of 99mTc-PYP cardiac scintigraphy in diagnosing cardiac amyloidosis and specifically its use in subtyping the disease in the setting of a negative endomyocardial biopsy. The non-invasive nature coupled with high sensitivity and specificity and ability to distinguish cardiac amyloid subtypes make 99mTc-PYP cardiac scintigraphy a very useful test in patients with suspected cardiac amyloidosis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.06.012.
Appendix. Supplementary materials
References
- 1.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 2.Bokhari S, Castaño A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castaño A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20:163–178. doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeyda-Pommersheim F, Hwang M, Chen SS, Strollo D, Fuhrman C, Bhalla S. Amyloidosis: modern cross-sectional imaging. RadioGraphics. 2015;35:1381–1392. doi: 10.1148/rg.2015140179. [DOI] [PubMed] [Google Scholar]
- 5.Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 6.Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–1579. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristen AV, aus dem Siepen F, Scherer K, Kammerer R, Andre F, Buss SJ. Comparison of different types of cardiac amyloidosis by cardiac magnetic resonance imaging. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloid. 2015;22:132–141. doi: 10.3109/13506129.2015.1020153. [DOI] [PubMed] [Google Scholar]
- 8.Bokhari S, Shahzad R, Castaño A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2014;21:175–184. doi: 10.1007/s12350-013-9803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perugini E, Guidalotti PL, Salvi F, Cooke RMT, Pettinato C, Riva L. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Onoguchi M, Haramoto M, Kodani N, Komatsu A, Kitagaki H. Novel method for quantitative evaluation of cardiac amyloidosis using (201)TlCl and (99m)Tc-PYP SPECT. Ann Nucl Med. 2012;26:634–643. doi: 10.1007/s12149-012-0627-y. [DOI] [PubMed] [Google Scholar]
- 11.Casset-Senon D, Secchi V, Arbeille P, Cosnay P. Localization of myocardial amyloid deposits in cardiac amyloidosis by Tc-99m pyrophosphate myocardial SPECT: implication for medical treatment. Clin Nucl Med. 2005;30:496–497. doi: 10.1097/01.rlu.0000167664.47995.d9. [DOI] [PubMed] [Google Scholar]
- 12.Andrikopoulou E, Bhambhvani P. Nuclear imaging of cardiac amyloidosis. J Nucl Cardiol 2017:1–4. doi: 10.1007/s12350-017-1028-3. [DOI] [PubMed]
- 13.Castaño A, DeLuca A, Weinberg R, Pozniakoff T, Blaner WS, Pirmohamed A. Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2016;23:1355–1363. doi: 10.1007/s12350-015-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Z, Cui Q, Tian Z, Zhao D, Zhu K, Fang Q. Electron microscopy in patients with clinically suspected of cardiac amyloidosis who underwent endomyocardial biopsy and negative Congo red staining. Int J Cardiol. 2013;168:3013–3015. doi: 10.1016/j.ijcard.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 15.Khan T, Selvakumar D, Trivedi S, Rao K, Harapoz M, Thiagalingam A. The value of endomyocardial biopsy in diagnosis and guiding therapy. Pathology (Phila) 2017;49:750–756. doi: 10.1016/j.pathol.2017.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.