Abstract
For more than 20 years, Copaxone (glatiramer acetate, Teva), a non-biological complex drug, has been a safe and effective treatment option for multiple sclerosis. In 2016, a follow-on glatiramer acetate product (FOGA, Synthon) was approved in the EU. Traditional bulk-based methods and high-resolution assays were employed to evaluate the physicochemical, functional, and bio-recognition attributes, as well as the in vivo toxicity profile of the active substances in Copaxone and Synthon EU FOGA lots. These tests included quality control tests applied routinely in release of Copaxone lots, as well as additional characterization assays, gene expression studies and a rat toxicity study. Even though the Synthon FOGA was designed to copy and compete with Copaxone, the active substances were found to be similar in only 7 of the tested 14 (50%) methods (similar is defined as within approved specifications or within the inherent microheterogeneity range of tested Copaxone batches, or not showing statistically significant differences). With additional methods applied, consistent compositional differences in attributes of surface charge distribution, molecular size, and spatial arrangement were observed. These marked differences were concordantly observed with higher biological activity of some of the Synthon EU FOGA lots compared with Copaxone lots, including potency and cytotoxicity activities as well as gene expression of pathways that regulate apoptosis, IL-2, and inflammation signaling. These observations raise concerns for immunogenicity differences, particularly in (repeated) substitution settings. Another orthogonal finding demonstrated increased frequency of injection-site local toxicity observations for the Synthon EU FOGA in an in vivo daily dosing rat study, thus warranting further qualification of the link between compositional and functional differences in immunogenicity, and potential impact on long-term efficacy and safety.
Keywords: Copaxone, Glatiramer acetate, FOGA, Follow-on glatiramer acetate product, Non biological complex drug, NBCD, Substitutability
Highlights
-
•
A Synthon follow-on glatiramer acetate (FOGA; 20 mg/mL) was approved in Europe in 2016.
-
•
Molecular charge distribution and spatial arrangement differ in FOGA vs Copaxone lots.
-
•
Higher potency and cytotoxicity activities in some FOGA lots vs Copaxone lots
-
•
Increased frequency of injection-site reactions in in vivo rat study for FOGA
-
•
Gene expression differences in immunological pathways between FOGA and Copaxone lots
1. Introduction
Copaxone (glatiramer acetate), manufactured by Teva Pharmaceuticals, has provided a safe and effective treatment option for multiple sclerosis for 20+ years. The active substance of Copaxone is glatiramer acetate (GA), a non-biological complex drug (NBCD) composed of a mixture of immunogenic polypeptides of varying sequences and sizes that are impossible to characterize even with state-of-the-art analytical methods [1]. Thus, unavoidably, GA is defined by the reaction conditions utilized across multiple synthesis [2], preparatory, and final purification stages. As a result, the composition of GA, its micro-heterogeneity range, and the consequential therapeutic activity of Copaxone all strongly rely on the robustness of the manufacturing process, ensuring antigen homology, quality control, and the resultant consistent safety and efficacy profile of Copaxone.
In 2016, a 20 mg/mL follow-on glatiramer acetate product (FOGA, Synthon) was approved in the EU and Switzerland and is referred to herein as Synthon's EU FOGA.The tradename for the Synthon's EU FOGA product varies from one country to another (for instance, in Sweden the tradename is Copemyl, in Germany Clift, in Slovakia Remurel and in Switzerland Glatiramyl). The EU regulatory assessment of FOGA relied on establishing similarity of the active substances in FOGA and Copaxone, and furthermore, the complexity of the glatiramer acetate and the particular challenges it presents for demonstrating equivalence was acknowledged [3]. Thus, the marketing authorization in the EU for Synthon's FOGA was granted pursuant to Article 10(3) of Directive 2001/83/EC as a hybrid application. In accordance, the FOGA is not considered a generic product, but rather a “hybrid product.” Moreover, it was recognized [3] that, because of the complexity of the substance, the production process of the drug substance is an important factor for consideration, because the compositional reproducibility is linked to the tightly controlled manufacturing process. Thus, although Copaxone and the Synthon EU FOGA are not biological medicinal products, Synthon followed a regulatory strategy similar to the dossier requirements of biosimilar applications and, in addition to quality data, also provided nonclinical and clinical data in support of the similarity of its product [3]. Therefore, the approval was supported by the Glatiramer Acetate Clinical Trial to Assess Equivalence with Copaxone (GATE), a nine-month clinical study [4,5] with a 15-month, open-label extension [5,6].
Given the aforementioned characterization challenges, a battery of peptide/protein-appropriate evaluation methods was employed by Teva to examine the compositional characteristics of glatiramer acetate in Copaxone and FOGA lots, as well as their associated functional ramifications. This battery, which includes quality control release tests used routinely for release of Copaxone lots, high resolution physicochemical tests [[7], [8], [9], [10], [11]], biological characterization tests, as well as gene expression studies and a rat toxicity study, has been applied to other FOGA products marketed globally, and the findings of these analyses have been published [[12], [13], [14], [15], [16], [17]]. Consistently, the collected results of these analyses highlight the difficulties and challenges in manufacturing a complex peptide mixture such as GA and demonstrate that many of the FOGA lots produced by different manufacturers globally do not contain the same active substance as Copaxone, as differences and similarities are demonstrated in different assays. Furthermore, differences in physicochemical and functional attributes were detected for all FOGAs tested to-date [[12], [13], [14], [15], [16], [17]], which remain to be qualified with respect to their impact on clinical immunogenicity and long-term safety and efficacy, as well as (repeated) substitutability in real-world settings (the term “substitution” herein refers to the practice of dispensing one medicine instead of another at the pharmacy level without the consultation of a physician).
The present study sought to determine the comparability of the active ingredient in Copaxone to that in the Synthon EU FOGA, employing a battery of physicochemical and biological assays reported previously [[12], [13], [14], [15], [16], [17]]. To this end, six lots of Synthon's FOGA purchased in four different European countries were compared with Copaxone specifications or inherent variability ranges, utilizing a total of fourteen methods. Intra-product lot-to-lot variability (also termed microheterogeneity) was characterized and compared across products, alongside analysis of the inter-product lot-to-lot assessments. The compositional and biological activity attributes observed to be markedly and consistently different between the products were summarized and their functionality characterized. Furthermore, for the first time, in vivo toxicity was tested in rats, orthogonally examining the frequency of injection-site reactions elicited by each of the treatments.
2. Methods
Physicochemical characterization was pursued for six different lots of the Synthon EU FOGA drug product marketed in four countries (Germany, Clift lots 1503713B, 1601798A, 1503711E; Austria, Perscleran lot 1503711D; Switzerland, Glatiramyl lot 1601982B; and Slovakia, Remurel lot 1601434B) with expiration dates between October 2018 and May 2019, and compared with a random set of six Copaxone lots (P63250, P63256, P63260, P63265, P63266, P63275) with expiration dates between August 2017 to March 2018. Of note, the Synthon FOGAs for Germany, Austria, and Slovakia were approved via the same decentralized procedure and are considered to be the same product throughout Europe; the Swiss Synthon FOGA product also relied on the GATE clinical study [3] for its approval and therefore it is also assumed to be the same product.
Testing involving comparative low- and high-resolution physicochemical assays, in addition to biological, functional, and toxicological analyses, was conducted from March 2017 to December 2017. The battery of high-resolution methods (developed by Teva based on well-established methodologies widely used for characterization of complex polypeptide mixtures and antibody preparations) includes techniques that characterize the intact GA product as is, with its polypeptide chains unmodified, rather than following degradation procedures usually incorporated in traditional/low-resolution methods. This is the most clinically relevant approach, as the mixture is injected in MS patients in its intact form. As has been published previously [1], once artificially degraded for the purpose of such analyses, even marked differences between GA products are easily masked.
Inherent variability (microheterogeneity) exists for all biological and synthetic complex polypeptide mixtures, including Copaxone. In accordance, the testing strategy set forth defines either the test specifications or the range of the intrinsic microheterogeneity per quality attribute, as detected across multiple Copaxone lots. A head-to-head comparison can demonstrate whether a FOGA product is within the Copaxone lot-to-lot intrinsic microheterogeneity limits for the specific quality attribute being tested. For each assay and quality attribute, this microheterogeneity of Copaxone, as well as the analytical test variability, has been taken into consideration. Statistical simulations of the relevant available comparative data (peak maximum molecular weight and relative potency) were provided.
Gene expression profiling was applied for three lots of Synthon EU FOGA (Germany Clift lots 1503711E and 1503713B; Slovakia Remurel lot 1601434B) and three lots of Copaxone (P63256, P63265, P63275) in two complementary biological assays. These two experiments, validated previously [12,13,16], model the molecular effects of treatment on antigen presenting cells (APCs) and T cells, which represent the two cell types that, along with the antigen (GA or FOGA, respectively), form the “immunological triad” that is critical to the mechanism of action of Copaxone [18]. Furthermore, the T-cell model system was designed to identify molecular differences associated with switching, relevant for the consideration of safety and immunogenicity in the context of (repeated) substitution in real-world settings. Finally, a comparative 13-week, repeated dose, subcutaneous in vivo toxicity study in Sprague Dawley (SD) male rats was performed with Copaxone and Synthon EU FOGA under Good Laboratory Practice (GLP) regulations [19].
3. Results and discussion
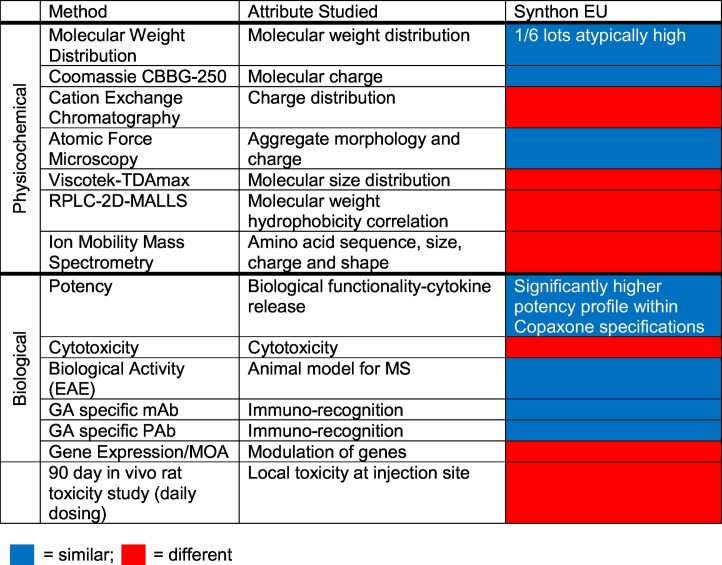
Six of the standard traditional methods and one of the high resolution methods detected no differences between GA and Synthon EU FOGAs. The remaining seven methods identified marked deviation of physicochemical and biological properties between GA and Synthon EU FOGA even after taking into account GA lot-to-lot microheterogeneity and analytical test variability. Notably, lot-to-lot variability among Synthon EU FOGA lots was substantially higher than GA's intrinsic microheterogeneity in certain parameters. Table 1 summarizes the tests, the measured attributes, and the results. The proceeding sections provide descriptions of the findings for each of the methods.
Table 1.
Summary of comparative analyses, methods, attributes and results.
3.1. Physicochemical analyses
3.1.1. Molecular weight distribution (MWD)
MWD is a basic bulk physicochemical characterization parameter reflecting the general distribution of the polypeptides in a complex mixture according to their hydrodynamic size, rather than their primary structure. The MWD in Copaxone and Synthon EU FOGA samples diluted to 4 mg/mL was evaluated by size exclusion chromatography (SEC) using a Superose 12 column (GE Healthcare Life Sciences, Pittsburgh, PA, USA) with pH 1.5 phosphate buffer mobile phase at 0.5 mL/min with UV detection at 208 nm. The test system is calibrated with a set of polypeptide MW markers containing the same amino acids as Copaxone.
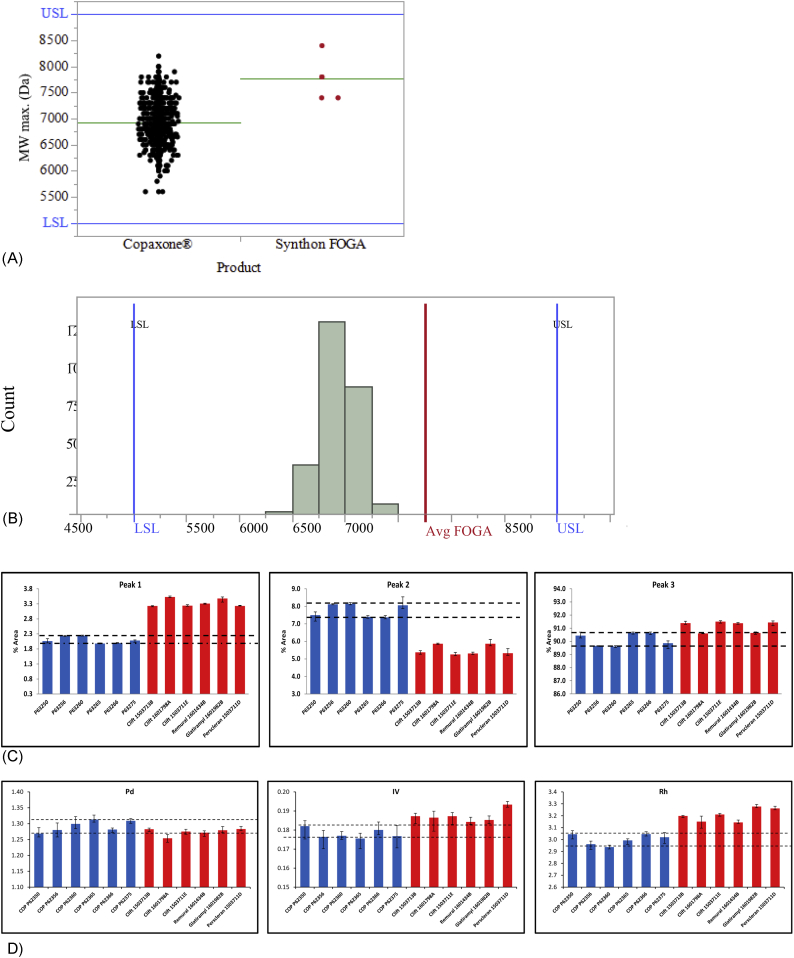
Copaxone polypeptides have an average MW ranging from 5000 to 9000 Da measured at peak max (the MW of all Copaxone components range from 2500 to 20,000 Da). In general, the Synthon EU FOGAs' MW values complied with Copaxone specifications, although one out of the six tested lots (1601434B - REMUREL) showed higher MWD within Copaxone specifications (Fig. 1A). Statistical comparison of the Copaxone and Synthon EU product datasets required simulation to address the imbalance in number of available lots for comparison (Copaxone 957 samples, Synthon EU FOGA product 6 samples). For this, six samples were randomly selected from the available 957 samples of Copaxone and then a mean value of each of the six samples was calculated. This process was repeated 2500 times, hence creating a data set of 2500 mean values of those samples. The distribution of those means was plotted and a statistical analysis was used to compare the distribution of the simulated mean MW values of Copaxone averages with the actual mean value MW of the total available six Synthon EU FOGA lots. Simulation was performed using R software, The R Foundation, version 3.4.0, and presentation of the results was performed with JMP Statistical Discovery, Ver. 13.1, SAS Institute Inc.
Fig. 1.
MWD, CEX, and Viscotek TDAmax Assays.
A. Peak maximum molecular weight for Copaxone and Synthon FOGA product – Actual data.
B. Distribution of peak maximum molecular weight (Da) for simulated Copaxone data and Synthon EU FOGA mean value.
Avg = average; FOGA = follow-on glatiramer acetate; LSL = lower specification limit; USL = upper specification limit. Grey columns represent the distribution of peak maximum MW of 2500 simulated Copaxone averages of the six samples randomly drawn from the total 957 Copaxone readings. The red line represents the observed average peak maximum MW of the six available Synthon EU lots.
C. CEX. Characterization of surface charge distribution of Copaxone lots and Synthon EU FOGA lots (Peak 1 = Negatively Charged Population, Peak 2 = Weak Positively Charged Population; Peak 3 = Strong Positively Charged Population).
D. Viscotek TDAmax. Conformational characterization of polymer molecular weight distribution for Copaxone lots and Synthon EU FOGA lots.
As shown in Fig. 1B for the peak maximum MW, the observed mean value of Synthon EU FOGA lots (“Avg FOGA”) was significantly higher (+12%) than that of simulated mean values of Copaxone, and the probability of the observed (actual) FOGA MW mean value being part of the distribution of the simulated Copaxone data was <0.0001.
3.1.2. Coomassie Brilliant Blue Dye-250 (CBBG-250)
The Coomassie Brilliant Blue Dye-250 (CBBG-250) is known to interact with different polypeptides in solution such that distinct color changes are produced. For this identification test, Copaxone and Synthon EU FOGA samples were mixed with CBBG-250 dye solution at 1:1 ratio, followed by centrifugation at 10,000 rcf (relative centrifugal force) for 30 min. The supernatant was then diluted 6:100 and absorbance measured at 590 nm. Absorbance was calculated relative to a CBBG-250 dye control. The CBBG-250 values for Synthon EU FOGAs complied with Copaxone specifications. The relative absorption for Synthon EU FOGA were Clift 1503713B 3.0, Clift1601798A 2.8, Clift 1503711E 3.0, Remurel 1601434B 3.2, Glatiramyl 1601982B 2.9, and Perscleran 1503711D 2.8, ie the peptide-dye interaction mode was similar in both products.
3.1.3. Cation exchange chromatography (CEX)
Polypeptide surface charge distribution may be a key attribute affecting binding properties of antigens to their immunological counterparts, ie antigen presenting cells and T cells [20]. This attribute was therefore measured by cation exchange chromatography (CEX). The method is based on a nondestructive separation of the polypeptide mixture into subgroups according to their average overall charge, ie affinity to the negatively charged stationary phase of a separating column. Further details on the methods have been provided previously [13].
In a typical Copaxone chromatogram, three subpopulations are evident and defined to exhibit negative, weak positive, or strong positive charge distributions [13]. The polypeptide charge distribution in Synthon EU FOGA samples was shown to be consistently different regardless of the country of purchase. There was generally a larger negatively-charged subpopulation than seen for Copaxone, and a smaller weak positive-charged subpopulation, and larger (4 out of 6) strong positive-charged subpopulation (Fig. 1C). These findings are indicative of differences in overall polypeptide composition, including primary structure and conformation.
3.1.4. Viscotek TDAmax
Conformational characterization of polymers, including molecular size and weight, hydrodynamic radius (Rh), intrinsic viscosity (IV: inverse of molecular density) and Pd (polydispersity or uniformity of mixture with regard to MWD), was assessed using Viscotek TDAmax multi-detector size exclusion chromatography (SEC) analysis system for polymers and macromolecules (Malvern Instruments) and Superose 12 column (GE Healthcare).
Differences between Copaxone and Synthon's EU FOGAs were detected, indicating that the Synthon EU FOGA polypeptide chains are folded more loosely in their spatial arrangement compared with Copaxone (Fig. 1D), ie exhibit higher effective molecular size. This finding is indicative of differences in peptide primary sequence and structure.
3.1.5. Reverse phase liquid chromatography 2-dimensional multi-angle laser light scattering detector (RPLC 2D-MALLS)
Polypeptide molecular mass of subpopulations in a mixture reflect compositional characteristics that are not currently evaluated directly by any other conventional QC methods. The 2-dimensional analysis of the mixtures was performed by sequentially attaching the reverse phase high performance liquid chromatography (RP-HPLC) and 2D-MALLS detectors as described previously [13], to obtain comparative molecular mass elution profiles as a function of hydrophobicity for the Copaxone and Synthon EU FOGA samples.
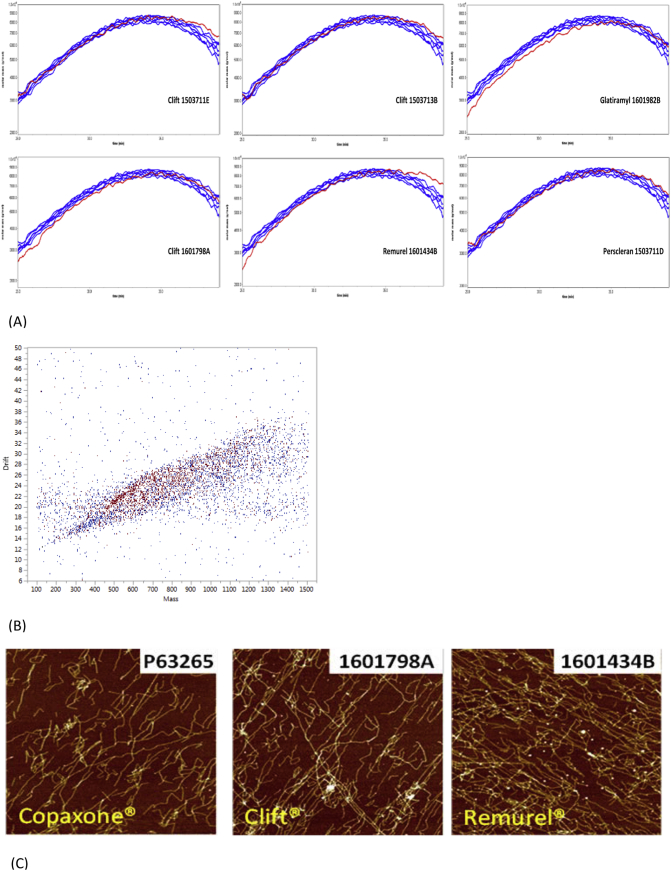
In general, Synthon EU FOGA lots showed similarity to Copaxone (Fig. 2A). However, the Remurel sample (Slovakia) contained atypical highly hydrophobic, high molecular mass constituents, ie was compositionally dissimilar to Copaxone. This suggests that a measurable difference in overall polypeptide composition can occur that cannot be revealed using conventional methods such as SEC.
Fig. 2.
2D-MALLS, IMMS, and AFM Assays.
A. 2D-MALLS. Molecular mass elution profiles as a function of hydrophobicity for Copaxone and Synthon EU FOGA lots.
2D-MALLS = 2 dimensional multi angle laser light scattering; EU = European Union; FOGA = follow-on glatiramer acetate Synthon EU FOGA samples are shown in red; 6 lots of Copaxone (overlay) are shown in blue.
B. IMMS. The pixel-by-pixel compositional comparison of Synthon lots to a randomly selected Copaxone lot identified extensive differences (as exemplifed by the red and blue dots in the scattergraph of Clift 1503711E). IMMS = ion mobility mass spectrometry; Red = above range; Blue = below range; White = within range of COPAXONE's microheterogeneity, as tested by 6 randomly selected lots of similar expiration dates.
C. AFM. Comparison of Copaxone and Synthon EU FOGAs are generally similar in morphology. EU = European Union; FOGA = follow-on glatiramer acetate.
3.1.6. Ion mobility mass spectrometry (IMMS)
IMMS is a 2-dimensional technique that allows structural analysis of heterogeneous mixtures by separation of ionized molecules based on molecular size, shape, and mass-to-charge ratio (m/z) [10]. Reverse phase liquid chromatography (RPLC) was applied to six Copaxone and six Synthon EU FOGA lots with Agilent 6560 Ion Mobility Q-TOF LC/MS. Approximately 52 million data points (m/z/drift values) were collected for each lot (injected twice), followed by a qualitative data analysis.
As shown in Fig. 2B, a scatterplot of a Synthon EU lot demonstrates the observed differences seen in IMMS analyses between Copaxone and the Synthon EU FOGA lots. For each, the range (max-min) of the combination of drift and mass of the 6 tested Copaxone lots was calculated. Then, each matching result of a Synthon EU FOGA lot was compared with the range for Copaxone, being either within range (marked as white color), below range (marked as blue color), or above range (marked as red color), ie, the colored dots observed on the white background represent the mass/drift combinations in FOGAs that deviate from those within Copaxone range.
Table 2 presents the frequencies of pixel by pixel IMMS results. The frequencies of results that were outside the range of Copaxone tested batches were about 50%, with about 30% being below that range and 20% above it. The data produced in this study demonstrate that the Synthon EU FOGA lots have peptide compositions that vary significantly from Copaxone and likely arise from a combination of differences in amino acid sequence, length, and content of various peptide constituents in the product.
Table 2.
Summary of IMMS pixel by pixel frequencies of FOGA lots within or beyond Copaxone range.
| Lot ID | Level | Count | Frequency |
|---|---|---|---|
| CLIFT_1503711E | Below range | 1,247,040 | 0.301 |
| Within range | 2,081,769 | 0.502 | |
| Above range | 814,088 | 0.197 | |
| CLIFT_1503713B | Below range | 1,229,792 | 0.299 |
| Within range | 2,096,431 | 0.509 | |
| Above range | 791,927 | 0.192 | |
| CLIFT_1601798A | Below range | 1,219,945 | 0.295 |
| Within range | 2,094,527 | 0.507 | |
| Above range | 818,715 | 0.198 | |
| Remurel_1601434B | Below range | 1,237,396 | 0.299 |
| Within range | 2,075,940 | 0.501 | |
| Above range | 831,627 | 0.201 | |
| Glatiramyl_1601982B | Below range | 1,254,863 | 0.300 |
| Within range | 2,136,073 | 0.511 | |
| Above range | 790,539 | 0.189 | |
| Perscleran_1503711D | Below range | 1,232,418 | 0.299 |
| Within range | 2,092,241 | 0.507 | |
| Above range | 798,299 | 0.194 |
3.1.7. Atomic Force Microscopy (AFM)
AFM is a standard technique to determine sample topography, such as aggregation forms [8]. The polypeptide mixtures were placed on negatively charged plates and washed as described previously [13,21]. The morphology of Synthon EU FOGA sample aggregates was similar to that of Copaxone, as shown in Fig. 2C.
3.2. Biological activity and gene expression assays
Copaxone is a complex mixture of polypeptides, each containing multiple amino-acid sequences that are immunogenic antigens. As such, important antigenic attributes are assessed for the purpose of ensuring consistent efficacy and safety, including potency, cytotoxicity, biorecognition, local toxicity, and gene expression modulation.
3.2.1. Potency ex vivo cell-based assay
The potency ex vivo cell-based assay reflects specific potency results by measuring the levels of a single cytokine (interleukin-2 [IL-2]) secreted by GA-primed T cells following response to recall antigen (GA). Mice were immunized with glatiramer acetate reference standard (GA RS); after 4 to 5 days, they were sacrificed, and a primary culture of pooled spleen (SPL) cells was prepared. The SPL cells were activated in vitro with serial concentrations of either GA RS or tested drug product lot. After 24 h incubation, the cell supernatants were collected for Interleukin-2 (IL-2) measurement. The quantification of IL-2 secretion as a marker for cellular response was performed using a commercial Enzyme Linked Immunosorbent Assay (ELISA) kit. The reported result for each tested lot was calculated as relative to the reference product (relative potency).
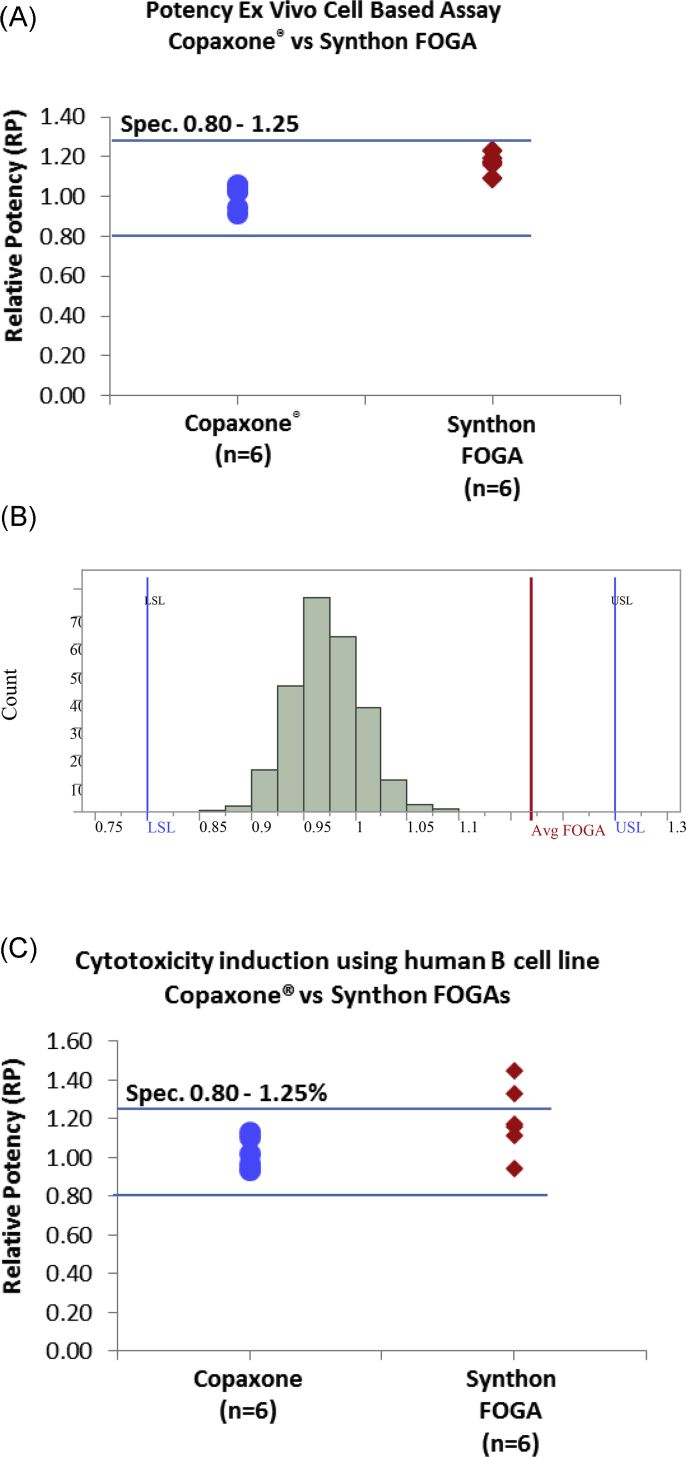
The activity for the Synthon EU FOGAs was higher, although within, the Copaxone specifications for this method (Fig. 3A). Statistical comparison of the Copaxone and Synthon EU FOGA datasets required simulation to address the imbalance in number of available lots for comparison. To this end, six samples were randomly selected from the available 231 Copaxone samples and then a mean value of each of these samples was calculated. This process was repeated 2500 times, thereby creating a data set of 2500 mean values of those samples. The distribution of those means was then plotted and compared with the actual mean value of the available six Synthon EU FOGA lots. Simulation was performed using R software, The R Foundation, version 3.4.0, and presentation of the results was performed with JMP Statistical Discovery Ver. 13.1, SAS Institute Inc. As shown in Fig. 3B for the relative potency, the observed mean value of Synthon EU FOGA lots (“Avg FOGA”) was significantly higher (+20%) than the distribution of simulated mean values of Copaxone, and the probability of the observed (actual) FOGA mean value being part of the distribution of the simulated Copaxone data was <0.0001.
Fig. 3.
Potency ex vivo cell-based assay.
A. Relative potency of Copaxone and Synthon EU FOGA - actual data.
B. Distribution of average relative potency (%) simulated data for Copaxone vs Synthon EU FOGA product - actual data.
Avg = average; LSL = lower specification limit; USL = upper specification limit.
Grey columns represent the distribution of averages of relative potency of 2500 simulated Copaxone mean values, drawn out of the total observed data from 231 lots tested. The red line represents the observed average relative potency of the 6 available Synthon EU FOGA lots.
Note: Specification limits relate to the individual values not averages since the variation of mean values - standard error (SE) is smaller than the variation of individual readings - standard deviation (SD). Therefore, specification limits on chart are given for display purposes only.
C. Relative potency of cytotoxicity induction Copaxone and Synthon EU FOGA product - actual data.
3.2.2. Cytotoxicity - induction of in vitro cytotoxicity using human B cell lines
This cell-based in vitro assay determines the dose-dependent cytotoxic effect of tested product lots in serial concentrations by using an established human B cell-line. The cytotoxic effect of tested drug product lots (Copaxone or Synthon EU FOGA samples) on Epstein-Barr virus (EBV)-transformed B-cell line was tested following 2 h incubation with serial concentrations of either the reference standard (RS) or tested drug product lot. The reported result for each tested lot was calculated as relative cytotoxicity values to the reference product.
Two lots of the Clift FOGA were out of range for the Copaxone specifications using this method, demonstrating higher in vitro cytotoxicity (Fig. 3C). Two other Synthon EU FOGA lots also had cytotoxic activity that was higher, although within the Copaxone specifications. The lot-to-lot variability noted for Synthon EU FOGA lots was higher than for Copaxone.
3.2.3. Anti-Glatiramer acetate antibodies biorecognition assays
The two bio-recognition assays were based on Enzyme Linked Immunosorbent Assay (ELISA) for the specific bio-recognition of Glatiramer Acetate (GA) using 2 anti-GA monoclonal antibodies in one assay and rabbit IgG polyclonal antibodies (PAbs) in the second assay. A microplate was coated with GA reference standard (GA RS) and tested drug product (DP) lot (Copaxone or Synthon EU FOGA). Following coating and washing steps, the detection antibodies are added and incubated for 30 min at 37 °C. Then TMB was added and the optical density measured.
The results were expressed as percent binding of the GA-specific monoclonal and polyclonal antibodies to drug product lots, relative to GA RS lot. A drug product lot passes the test when its binding is between 85% and 115% for each of the antibodies. The Synthon EU FOGA samples were within the Copaxone specifications for the mAbs-based release method, with percentage relative binding ranging from 86% to 100% for mAb 6B3/57 and from 87% to 108% for mAb 1C4/220, and for the polyclonal release method, with percentage relative binding ranging from 96% to 99%. As an identification release method, both have failed previously to be sensitive enough to detect differences between Copaxone and other FOGAs known to differ from Copaxone when analyzed using different methods [[12], [13], [14], [15], [16], [17]].
3.2.4. Inhibition of tumor necrosis factor-alpha secretion using human monocyte cell-based assay
This cell-based in vitro assay determines the effect of tested lots in different concentrations to reduce tumor necrosis factor-alpha (TNFα) secretion using a human monocyte cell line (THP-1). The culture of THP-1 monocyte cell line was nonspecifically stimulated with lipopolysaccharide together and separately with serial concentrations of GA RS and tested lots. Following 24-h incubation, the cell supernatants were collected for TNFα determination using ELISA. The relative potency of a tested lot was calculated relative to the GA RS using the PLA software. The inhibition of TNFα secretion results for the Synthon EU FOGAs were within the Copaxone specifications for this method, ranging between 0.958 and 1.027.
3.2.5. Gene expression profiling and analyses in human THP-1 cell line and mouse splenocytes
To understand the gene expression and pathway modulation by Copaxone compared with Synthon EU FOGA, two complementary methods were applied. In the APC model, the human APC monocyte cell line THP-1 was treated for 6 h with Copaxone, Synthon EU FOGA, or mannitol control, with 6 replicates per treatment, and then subjected to RNA extraction and gene expression profiling. In the T-cell model, mouse splenocytes (T-cell rich) were used to assess the impact of substitution: (1) a patient previously treated with Copaxone and possessing Copaxone-reactive T cells, who was then switched to Synthon EU FOGA, and (2) the reverse situation of a patient treated with Synthon EU FOGA who was then switched to Copaxone. Mice were immunized with either Copaxone (lot P62356) or Synthon EU FOGA (Clift 1503711E). After 3 days, the splenocytes were removed and treated with each drug ex vivo for 24 h, with 6 replicates per treatment. Finally, RNA was extracted and gene expression profiling performed. Correction for batch variation was performed using ComBat [22,23], as implemented in the SVA R package sva: Surrogate Variable Analysis, available at http://www.bioconductor.org/packages/release/bioc/html/sva.html.
Differentially expressed probesets were identified across conditions using linear models for microarray data (LIMMA) [24], a standard R Bioconductor package. To compare Copaxone and Synthon EU FOGA, differential expression corrected for mannitol was used for each treatment (eg, [Copaxone vs mannitol] was compared via LIMMA to [Synthon EU FOGA vs mannitol]). Probesets were filtered by MAS5 calls of presence on the chip (to be considered present, a probeset was required to have on average a call of present or marginal across samples). Probesets were mapped to genes using the annotation available for the HG U133 Plus 2 chip (THP-1 study) and the Mouse 430 2 chip (splenocyte study) from Affymetrix. Benjamini Hochberg method was used for FDR, as implemented in the LIMMA R package.
3.2.5.1. Analysis of probeset expression differences
Overall comparisons of Copaxone vs. Synthon EU FOGA with mannitol correction showed 1802 probesets to differ at adj. p < 0.05 in the THP-1 model, and 510 (Copaxone immunization) and 460 (Synthon EU FOGA immunization) probesets in the splenocyte model. In both models, the results confirmed expected modulation of GA-related mechanism of action genes related to inflammation. For example, in the THP-1 model, IL10RA expression, important to IL10 signaling, was significantly upregulated, and in the splenocyte model, anti-inflammatory cytokines Il10 and Il4 were up-regulated by GA treatment and pro-inflammatory cytokine IL-12a was down-regulated, as observed previously [12]. Lot-to-lot comparisons showed a consistent differential profile when Synthon EU FOGA lots were compared with Copaxone lots, regardless of model system tested, but no differences were observed when lots were compared within product.
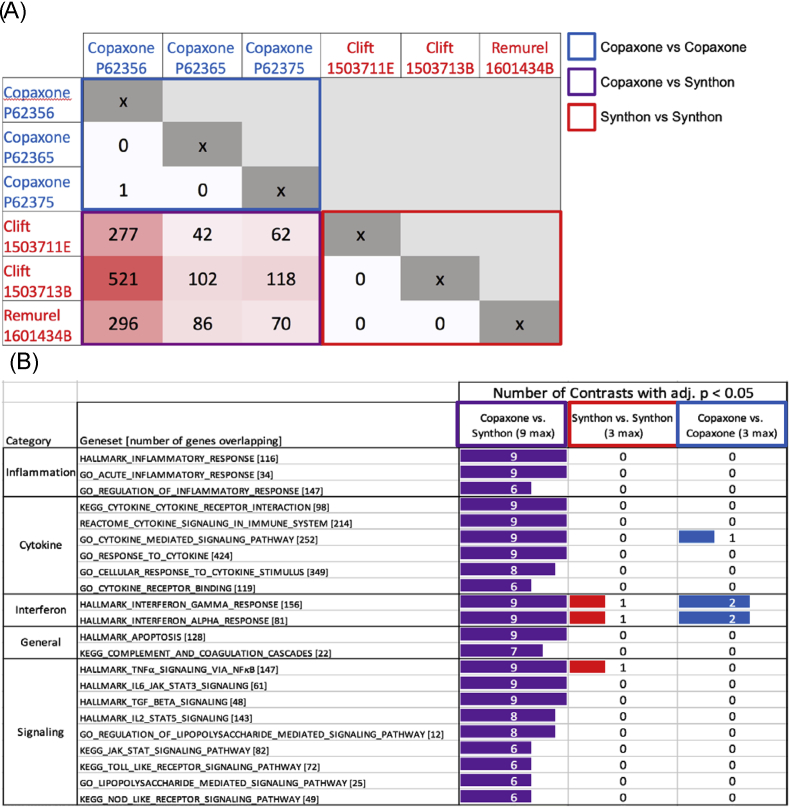
Lot vs. lot contrasts in THP-1 model are indicated by the number of differentially expressed probesets (adj. p < 0.05), after removal of 216 suspect probes previously identified [12], in Fig. 4A. The values in the cells are encoded by the intensity of red. All 9 Copaxone vs. Synthon EU FOGA lot comparisons had a significantly larger number of differentially expressed probesets (bottom-left quadrant, purple) than within-Copaxone lot comparisons (upper left, blue) or within-Synthon EU FOGA lot comparisons (bottom right, red). This pattern highlights the differences between Copaxone and Synthon EU FOGA at the lot level, and also indicates that those differences significantly exceed the lot-to-lot variability, or microheterogeneity, observed within Copaxone or FOGA.
Fig. 4.
Gene expression in THP-1 experiments.
A. Number of differentially expressed probesets in each lot vs. lot contrast. Virtually no differences were found between lots of Copaxone (upper left quadrant) and lots of Synthon (lower right quadrant); however, each Copaxone vs. Synthon lot comparison exhibits differentially expressed probesets.
B. Top GSEA immune pathways that significantly differ in lot vs. lot comparisons, broken down by number of contrasts with adj. p < 0.05 in the 9 Copaxone vs. Synthon contrasts (purple), 3 Synthon vs. Synthon contrasts (red), and 3 Copaxone vs. Copaxone contrasts (blue). The pathways differ by 2/3 or more of the Copaxone vs. Synthon contrasts, but rarely differ in the Synthon vs. Synthon or Copaxone vs. Copaxone contrasts.
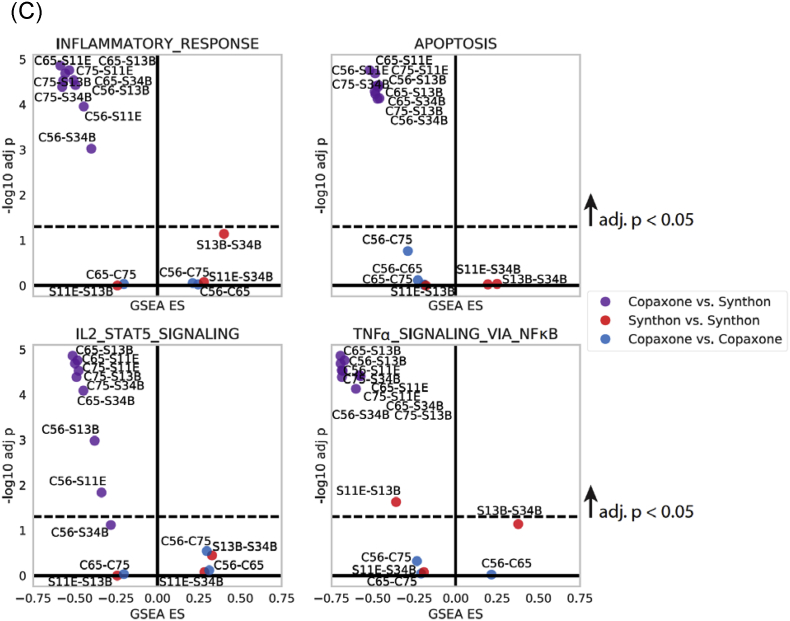
C. The volcano plots, which highlight Copaxone vs. Synthon differences in 4 hallmark pathways particularly relevant to the mechanism of action of Copaxone, show GSEAresults, with y-axis indicating significance (-log10 adj p) and x-axis indicating the GSEA enrichment score. In each plot, the number of datapoints within each category corresponds to the lot vs. lot contrasts indicated in Fig. 4A (9 Copaxone vs. Synthon, and 3 each of Synthon vs. Synthon and Copaxone vs. Copaxone).
In each lot vs lot contrast, the GSEA analysis is performed on the differential expression data of the first lot versus the second one (lot1–lot2). Each dot is labeled with the specific lot contrast, abbreviated to drug (C=Copaxone, S=Synthon), and the last two (Copaxone) or three (Synthon) characters (for example Copaxone P62356 is abbreviated to C56). The abundance of significant and negative ES values (upper left corner of plot) for Copaxone vs. Synthon lot comparisons reflects lower expression of pathway genes in Copaxone relative to Synthon in each of the 4 pathways.
In the splenocyte model, Copaxone-immunized arm, differences were observed in 5 of 9 contrasts between Copaxone vs. Synthon lots, whereas inter-lot similarity was observed within Copaxone (3/3 contrasts) and within Synthon (2/3 contrasts). Notably, Remurel 1601434B differed the most from all other lots in the splenocyte model, whereas Clift 1503713B differed the most in the THP-1 model. These results show that both splenocyte and THP-1 models highlight differences in Copaxone vs. Synthon EU FOGA lots in their overall patterns, but that individual models may be sensitive to different aspects of the biology, resulting in different FOGA lot-level observations.
3.2.5.2. General and immune-specific pathway analyses
Pathway enrichment was calculated using two methods: Gene Set Enrichment Analysis (GSEA) [25], and hypergeometric test for increased or decreased genes subset by adj p < 0.05 and fold change cut-off on filtered probesets as previously [12], to identify pathways enriched in the differential expression results between Synthon EU FOGA and Copaxone (mannitol-corrected). Probesets were filtered to use present probesets only and ranked by fold change for GSEA pre-ranked analysis. Probesets were collapsed to genes using the chip annotations available in the GSEA tool with the tool default.
Pathway analyses were initially applied against msigdb v6.1 Hallmark, C2 KEGG, and C5 pathway sets. Overall, the most consistently differentially expressed pathways were TNFα signaling via NFkB and MTORC1 signaling. The THP-1 results yielded a substantial number of pathways differentially expressed between the two products and consistently observed across all Copaxone vs. Synthon EU FOGA lot comparisons. In contrast, the more complex splenocyte model revealed fewer pathway enrichments with sparser and more variable results (Supplementary Fig. 1). Subsequent pathway analyses are focused on the THP-1 results.
As it is known that the primary effect of Copaxone is believed to result from its function as an antigen modulating the immune system [18], pathway analyses were subsequently applied to immune-related pathways. A focused 121-pathway list that specifically consisted of immune and cytokine-signaling- related gene sets was curated from the msigdb v6.1 universe based on keyword analyses of pathway names to extract those that contained immune-related terms such as “inflammation,” “chemokine,” and “lymphocyte.”
The focused enrichment analyses of immunological and cytokine-related pathway enrichment analyses, grouped by category (inflammation, cytokine, etc.), are shown in Fig. 4B. It is apparent from the table that the number of contrasts passing adjusted p-value significance are consistently differentially expressed in all 9 Copaxone vs. Synthon EU lot contrasts, and only a few are also differentially expressed within lots of Synthon EU FOGA or Copaxone. All pathways shown in Fig. 4B also showed significant differences in the comparison of Copaxone lots combined vs. Synthon EU FOGA lots combined.
In Fig. 4C, volcano plots are shown for 4 pathways from Fig. 4B to highlight the –log10 adj. p (y-axis) and GSEA Enrichment Score (x-axis). In each of the 4 plots, a dotted line demarcates the 0.05 adj. p cut-off in –log10 space. The lot-vs-lot contrasts are color-coded by comparison type in the same manner as in Fig. 4A and B: Copaxone vs. Synthon EU (purple), Synthon EU vs. Synthon EU (red), Copaxone vs. Copaxone (blue). The majority of the Copaxone vs. Synthon EU contrasts separate dramatically from within-Copaxone and within-Synthon EU contrasts, further highlighting the significant differences in pathway enrichment of Copaxone vs. Synthon EU. Although overall THP-1 TNFα secretion experiments described in Section 3.2.6 did not show differences in Copaxone relative to Synthon EU FOGA, the volcano plot on the bottom right shows that TNFα signaling was likely impacted in other ways.
These findings highlight at a pathway level the enrichment of differences when comparing Copaxone with Synthon EU FOGA, as Fig. 4A shows at the probeset level. Many of the immunological pathways that differ between Synthon EU FOGA and Copaxone are clearly relevant to the mechanism of action of Copaxone. Several of the pathways observed in these studies as differing between Copaxone and Synthon EU FOGA (including cytokine-cytokine receptor interaction and regulation of adaptive immune response) also differed in prior studies examining other FOGAs, including Synthon's Argentinian product, Polimunol [12], and Probioglat [16].
3.2.6. In vivo assays
3.2.6.1. EAE blocking test
The experimental autoimmune encephalomyelitis (EAE) blocking test infers the biological activity of a tested sample by the ability to block the induction of EAE in mice. The results show that Synthon EU FoGA samples are within the Copaxone specifications for the EAE release method, with activity ranging between 90% and 100% and mean maximal score ratio of 0.0 for all lots. As an in vivo release method, it failed to be sensitive enough to detect differences between Copaxone and other FoGAs, especially as this assay does not detect higher activity (ie, 100% activity is the maximum).
3.2.6.2. Comparative 13-week repeated dose in vivo toxicity study in Sprague Dawley (SD) male rats
A comparative 13-week, repeated-dose, subcutaneous toxicity study in male Sprague-Dawley (SD) rats was performed with Copaxone and Synthon EU FOGA following Good Laboratory Practice (GLP) regulations [19]. Groups 1, 2, and 3 (n = 15 male rats/group) received vehicle control, Copaxone 40 mg/kg, or Synthon EU FOGA 40 mg/kg, respectively, at a concentration of 20 mg/mL. Animals were administered a daily subcutaneous injection at 1 of 4 alternating sites of the lateral dorsum and were observed daily for signs of behavioral changes, reaction to treatment, or illness. Daily evaluation of any injection site reaction was conducted for all treatment groups. Local tolerance assessment throughout the study indicated that all animals (100%) receiving Copaxone or Synthon EU FOGA had at least 1 occurrence of injection site minor swelling (Table 3). However, the total number of episodes recorded for the animals in the two test article treated groups was different; the number of recorded episodes of swelling following administration of Synthon EU FOGA reached 201, while for Copaxone the number of episodes was slightly more than half of this incidence (122) (Fisher's Exact Test, p < 0.0001).
Table 3.
In vivo toxicity rat study.
| Group 1 |
Group 2 |
Group 3 |
|
|---|---|---|---|
| Placebo |
Copaxone 40 mg/kg |
Synthon EU 40 mg/kg |
|
| (n = 15) | (b = 15) | (n = 15) | |
| Injection site swelling (minimal-sight severity) | |||
| % (n) of affected animals | 0 (0) | 100 (15) | 100 (15) |
| First to last study day in which finding was observed | n/a | 23–91 | 24–91 |
| Number of incidences | 0 | 122 | 201 |
| Rate compared to reference (Copaxone) | n/a | Reference | 165%a |
| Injection site induration (miminal-slight severity) | |||
| % (n) of affected animals | 0 (0) | 87 (13) | 87 (13) |
| First to last study day in which finding was observed | n/a | 23–91 | 23–90 |
| Number of incidences | 0 | 68 | 95 |
| Rate compared to reference (Copaxone) | n/a | Reference | 140%a |
Fisher Exact test,p < 0.0001 for number of swelling in the Synthon EU group compared to Copaxone 40 mg/kg group and p = 0.0177 for induration incidences.
Likewise, 87% of the animals receiving Copaxone or Synthon EU FOGA also had at least 1 occurrence of slight injection site induration. The number of induration episodes recorded following administration of Synthon EU FOGA was higher than that recorded for Copaxone (95 vs 68, respectively, p = 0.0177, Table 3). No control animals experienced injection site swelling or induration during the 90 day treatment period. No differences were noted with respect to location of the injection site.
Noteworthy for local tolerance of subcutaneous injections, it is recommended not to calculate safety margins based on mg/m2, but rather safety margins should be normalized to concentration (eg mg/area of application) or amount of drug (mg) at application site. Therefore the dose concentration (20 mg/mL) and amount of drug (10 mg/rat vs 20 mg/patient) used in this study are relevant to human treatment [26].
4. Conclusion
Herein we evaluate and report on comprehensive characterization of the active ingredient in the Synthon FOGA product marketed in European countries in comparison to the reference product – Copaxone. Results demonstrate that methodologies appropriate for analysis of peptide mixtures illuminate consistent and marked differences in the compositional and biological characteristics of the two products. These findings are concordant with results from both functional assays (ie, genome-wide, unbiased expression profiling) and subcutaneous injection-site reactions observed in treated rats.
The high-resolution methods found differences in key compositional attributes such as the molecular density parameters, including size and spatial arrangement of the polypeptide chains as measured by Viscotek-TDAmax and the surface charge distribution parameters as measured by CEX. These may stem from differences in primary structure, molecular conformation, and overall peptide-composition between Copaxone and the Synthon EU FOGA. In addition, these qualitative and quantitative differences in polypeptide composition were orthogonally confirmed by results from IMMS. In totality, these findings indicate compositional differences between the products.
Based on published literature in other model systems, altered surface charge distribution of antigens may be associated with altered immune processes and cytotoxicity [20,[27], [28], [29]].The out-of-specification results for a third (2 out of 6) of the Synthon EU FOGA lots tested in the cytotoxicity assay (characterization assay) may be due to differences in quality attributes, that physicochemical testing is not sensitive enough to detect. Indeed, simulated statistical analyses have shown that, although still within specification range, for both peak maximum MW and relative potency there was a marked difference between the actual mean values for Synthon EU FOGA lots and the distribution of simulated mean values of Copaxone, such that the mean values for both the peak maximum MW and potency were higher for the Synthon EU FOGA lots relative to Copaxone.
Comparison of gene expression-based results to physicochemical or other biological tests yields orthogonal concordance supporting the hypothesis that functionally consistent differences between the two products exist. First, Remurel 1601434B differed from the other Synthon EU FOGA lots with respect to MWD as well as 2D MALLS. Concomitantly, in the splenocyte gene expression data, Remurel 1601434B was the consistent outlier, suggesting that it is both an outlier by physiochemical metrics as well as by gene expression in the T-cell rich splenocyte model. Secondly, both Clift lots show elevated cell-based cytotoxicity measurements, as well as strong genomic enrichments in related pathways (apoptosis, inflammatory response, interferon responses). Finally, in general, the Synthon EU FOGA potency distribution was higher than the simulated distribution of Copaxone as measured by IL2 levels in the supernatant after ex vivo activation. Correspondingly, IL2 STAT5 signaling differed in Synthon EU FOGA-treated THP-1 cells relative to Copaxone. These three examples show consistent patterns of correlations between multiple tests across the samples that may reflect underlying biological processes affected by differences between the active substances in Synthon EU FOGA and Copaxone.
Notably, there have been a few glatiramoids that exhibit out-of-specification results for cytotoxicity activity. A higher molecular weight analogue of GA, TV-5010 (produced by a slightly modified although similar manufacturing process at Teva) was found to be associated with both high potency and cytotoxic activity, and subsequently also caused in vivo lethality, hepatotoxicity, nephropathy, and other safety concerns in animals [30,31]. The clinical development program of the product TV-5010 was terminated. Furthermore, out-of-specification results of the cytotoxicity assay were also noted for a FOGA marketed in Mexico, Probioglat, manufactured by Probiomed. Probioglat has been on the market in Mexico for a few years, and its introduction was followed by an increase in both reported number of adverse events and relapses [32]. Although the underlying mechanism of the higher cytotoxicity result in some of the Synthon EU FOGA lots cannot be definitively established, this finding does raise concern regarding potential differences in biological activity that warrants further investigation.
The lot-to-lot inconsistency of the Synthon EU FOGA lots is also noteworthy. One of the 6 lots had high molecular weight distribution, as well as high MW, high hydrophobicity, and atypical constituents in the same lot as measured by RPLC -2D-MALLS. Also there were differences in the results of the cytotoxicity in vitro assay, with 2 of the 6 lots of FOGAs being out of range for the Copaxone specifications.
The differences noted in the frequency of injection site reactions in a nonclinical rat toxicity model for the Synthon EU FOGA may be concordant with the results of the physicochemical, biological and gene expression studies, all showing that the active ingredients in Copaxone and Synthon EU FOGA have different in vivo properties. These data are of concern, given the potential underreporting of injection site reactions (ISRs) in the GATE study sponsored by Synthon [4] and its open label extension [6] compared with the findings from other Copaxone clinical studies. Specifically, adverse events due to ISRs reported for Copaxone ranged from 56.4% (GLACIER) [33], 90% and 66% in the original Johnson studies [34,35] while in the double-blind phase of GATE, adverse events due to ISR were reported in 22.9% of Synthon EU FOGA subjects and 23.2% of Copaxone subjects [4]. A similar trend was noted in the open-label switch phase of the GATE study for Synthon EU FOGA/Synthon EU FOGA group at 1.2%, Copaxone/Synthon EU FOGA 0.9%, and placebo/Synthon EU FOGA 9.9% [6]. Considering that ISRs are among the most commonly reported adverse events associated with Copaxone use, any underreporting that may have occurred in the GATE study may suggest less sensitivity to detect differences between Copaxone and the Synthon EU FOGA.
In conclusion, the use of high-resolution, peptide-appropriate methodologies illuminated marked differences between the Synthon EU FOGA and Copaxone products. These consistent compositional differences indicate that the amino acid antigenic sequences, length, and amount of peptides are not the same between the active substances in Synthon EU FOGAs and Copaxone. The higher biological activity of the Synthon EU FOGAs, as demonstrated in potency and cytotoxicity assays, raises specific concerns regarding possible immunogenicity differences. Further supporting evidence was found in the gene expression differences, which independently indicated enrichment for pathways including inflammatory response, apoptosis, IL2 STAT5 signaling, and TNFα signaling, as well as in the differences noted in the frequency of swelling and induration at injection sites in the rat toxicity assessment. The totality of evidence indicates that further methodological experience is necessary to ascertain the long-term medical relevance of these findings in a chronic condition such as multiple sclerosis, including considerations for (repeated) substitution in real-world settings.
The following are the supplementary data related to this article.
Declaration of interest
All authors are employees of Teva Pharmaceuticals Ltd. or Immuneering. Teva is the manufacturer of Copaxone and provided financial support for the studies described in this article and was involved in study design, collection, analysis, and interpretation of data, manuscript writing and the decision to submit the manuscript for publication.
References
- 1.Weinstein V., Schwartz R., Grossman I. Non-Biological Complex Drugs. The Science and the Regulatory Landscape. In: Crommelin D.J.A., de Vlieger J.S.B., editors. Glatiramoids. Springer; Switzerland: 2015. pp. 107–148. [Google Scholar]
- 2.Campos-García V.R., López-Morales C.A., Benites-Zaragoza E. Design of a strong cation exchange methodology for the evaluation of charge heterogeneity in glatiramer acetate. J. Pharm. Biomed. Anal. 2017;132:133–140. doi: 10.1016/j.jpba.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Public Assessment Report Public Assessment Report. Scientific Discussion. Glatirameeracetaat Mylan 20 mg/ml, solution for injection, pre-filled syringe (glatiramer acetate) 2016. http://db.cbg-meb.nl/Pars/h115993.pdf (NL/H/3213/001/DC. Date: 6 June 2016. Available at)
- 4.Cohen J., Belova A., Selmaj K. Equivalence of Generic glatiramer Acetate in Multiple Sclerosis: a randomized clinical trial. JAMA Neurol. 2015;72:1433–1441. doi: 10.1001/jamaneurol.2015.2154. [DOI] [PubMed] [Google Scholar]
- 5.Annovazzi P., Bertolott A., Morra V.B. et al., A comprehensive review on Copemyl. Neurol Ther. DOI 10.1007/540120-017-0079.3
- 6.Selmaj K., Barkhof F., Belova A.N. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult. Scler. 2017;23:1909–1917. doi: 10.1177/1352458516688956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fekete S., Beck A., Fekete J. Method development for the separation of monoclonal antibody charge variants in cation exchange chromatography, part I: salt gradient approach. J. Pharm. Biomed. Anal. 2015;102:33–44. doi: 10.1016/j.jpba.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri F.S., Habchi J., Cerreta A. AFM-based single molecule techniques: unraveling the amyloid pathogenic species. Curr. Pharm. Des. 2016;22:3950–3970. doi: 10.2174/1381612822666160518141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Gatta A., De Rosa M. A complete hyaluronan hydrodynamic characterization using a size exclusion chromatography-triple detector array system during in vitro enzymatic degradation. Anal. Biochem. 2010;404:21–29. doi: 10.1016/j.ab.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Uetrecht C., RJ Rose E. van Duijn. Ion mobility mass spectrometry of proteins and protein assemblies. Chem. Soc. Rev. 2010;39:1633–1655. doi: 10.1039/b914002f. [DOI] [PubMed] [Google Scholar]
- 11.Shvartsburg A.A., Bush M.F. 2014 ASMS fall workshop: ion mobility mass spectrometry. J. Am. Soc. Mass Spectrom. 2015;26:1051–1054. doi: 10.1007/s13361-015-1155-5. [DOI] [PubMed] [Google Scholar]
- 12.Hasson T., Kolitz S., Towfic F. Functional effects of the antigen glatiramer acetate are complex and tightly associated with its composition. J. Neuroimmunol. 2016;290:84–95. doi: 10.1016/j.jneuroim.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Grossman I., Kolitz S., Komlosh A. Compositional differences between Copaxone and Glatopa are reflected in altered immunomodulation ex vivo in a mouse model. Ann. N. Y. Acad. Sci. 2017 doi: 10.1111/nyas.13547. [DOI] [PubMed] [Google Scholar]
- 14.Komlosh A., Hasson T., Wells-Knecht K. Similarities and differences in properties of glatiramer acetate (Copaxone®, Teva) versus Polimunol (Synthon) using standard and emerging technologies. Eur. J. Neurol. 2016;23:205. [Google Scholar]
- 15.Komlosh A., Hasson T., Wells-Knecht K. The 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS. 2015. Comparison of physicochemical, biological, and genomic characteristics of differently manufactured glatiramoids to ensure MS patient safety.https://onlinelibrary.ectrims-congress.eu/ectrims/2015/31st/115149 [Google Scholar]
- 16.Kolitz S., Hasson T., Towfic F. Gene expression studies of a human monocyte cell line identify dissimilarities between differently manufactured glatiramoids. Sci. Rep. 2015;5 doi: 10.1038/srep10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towfic F., Funt J.M., Fowler K.D. Comparing the biological impact of glatiramer acetate with the biological impact of a generic. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aharoni R. Immunomodulation neuroprotection and remyelination - the fundamental therapeutic effects of glatiramer acetate: a critical review. J. Autoimmun. 2014;54:81–92. doi: 10.1016/j.jaut.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Federal Register Good Laboratory Practice for Nonclinical Laboratory Studies. 2016. https://www.federalregister.gov/documents/2016/08/24/2016-19875/good-laboratory-practice-for-nonclinical-laboratory [PubMed]
- 20.Fromen C.A., Rahhal T.B., Robbins G.R. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomedicine. 2016;12:677–687. doi: 10.1016/j.nano.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molotsky T., Krispin R., Hasson T. 18th Annual Meeting of the Israel Analytical Chemistry Society. 2015. Characterization of Copaxone by atomic force microscopy (AFM) and dynamic light scattering (DLS)http://bioforumconf.com/analytica-abs/outofhtml/isranalytica_2015/characterizatio_Tatiana_Molotsky.html (abstract) [Google Scholar]
- 22.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 23.Leek J.T., Johnson W.E., Parker H.S. vol. 3. 2013. SVA: Surrogate Variable Analysis. R Package Version. [Google Scholar]
- 24.Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. (Accessed June 15, 2017) [DOI] [PubMed] [Google Scholar]
- 25.Subramanian A., Tamayo P., Mootha V.K. Gene set enrichment analysis: a knowledgebased approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Pharmacology and Toxicology Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. July, 2005. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf
- 27.Bhattacharjee S., de Haan L.H.J. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol. 2010;7:25. doi: 10.1186/1743-8977-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foged C., Brodin B., Frokjaer S. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramot Y., Rosenstock M., Klinger E. Comparative long-term preclinical safety evaluation of two glatiramoid compounds (glatiramer acetate, COPAXONE(R), and TV-5010, protiramer) in rats and monkeys. Toxicol. Pathol. 2012;40:40–54. doi: 10.1177/0192623311424169. [DOI] [PubMed] [Google Scholar]
- 31.Varkony H., Weinstein V., Klinger E. The glatiramoid class of immunomodulator drugs. Expert. Opin. Pharmacother. 2009;10:657–668. doi: 10.1517/14656560902802877. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez D., Flores J.D., Verdi D. Rates of adverse events and multiple sclerosis relapses before and after introduction of a purported generic glatiramer acetate in Mexico: results from a large patient support program in Mexico. Value Health. 2015;18:A877. [Google Scholar]
- 33.Wolinsky J.S., Borresen T., Dietrich D. GLACIER: an open-label, randomized, multicenter study to assess the safety and tolerability of glatiramer acetate 40 mg three-times weekly versus 20 mg daily in patients with relapsing-remitting multiple sclerosis. Mult. Scler. Rel. Disord. 2015;4:370–376. doi: 10.1016/j.msard.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Johnson K.P., Brooks B.R., Cohen J.A. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 35.Johnson K.P., Brooks B.R., Cohen J.A. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Neurology. 1998;50:701–708. doi: 10.1212/wnl.50.3.701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.