Abstract
Objective
Generalized tonic-clonic seizures are accompanied by cardiovascular and respiratory sequelae that threaten survival. The frequency of these seizures is a major risk factor for sudden unexpected death in epilepsy (SUDEP), a leading cause of untimely death in epilepsy. The circumstances accompanying such fatal events suggest a cardiovascular or respiratory failure induced by unknown neural processes rather than an inherent cardiac or lung deficiency. Certain cortical regions, especially the insular, cingulate, and orbitofrontal cortices, are key structures that integrate sensory input and influence diencephalic and brainstem regions regulating blood pressure, cardiac rhythm, and respiration; output from those cortical regions compromised by epilepsy-associated injury may lead to cardiorespiratory dysregulation. The aim here was to assess changes in cortical integrity, reflected as cortical thickness, relative to healthy controls. Cortical alterations in areas that influence cardiorespiratory action could contribute to SUDEP mechanisms.
Methods
High-resolution T1-weighted images were collected with a 3.0-Tesla MRI scanner from 53 patients with generalized tonic-clonic seizures (Mean age ± SD: 37.1 ± 12.6 years, 22 male) at Case Western Reserve University, University College London, and the University of California at Los Angeles. Control data included 530 healthy individuals (37.1 ± 12.6 years; 220 male) from UCLA and two open access databases (OASIS and IXI). Cortical thickness group differences were assessed at all non-cerebellar brain surface locations (P < 0.05 corrected).
Results
Increased cortical thickness appeared in post-central gyri, insula, and subgenual, anterior, posterior, and isthmus cingulate cortices. Post-central gyri increases were greater in females, while males showed more extensive cingulate increases. Frontal and temporal cortex, lateral orbitofrontal, frontal pole, and lateral parietal and occipital cortices showed thinning. The extents of thickness changes were sex- and hemisphere-dependent, with only males exhibiting right–sided and posterior cingulate thickening, while females showed only left lateral orbitofrontal thinning. Regional cortical thickness showed modest correlations with seizure frequency, but not epilepsy duration.
Significance
Cortical thickening and thinning occur in patients with generalized tonic-clonic seizures, in cardiovascular and somatosensory areas, with extent of changes sex- and hemisphere-dependent. The data show injury in key autonomic and respiratory cortical areas, which may contribute to dysfunctional cardiorespiratory patterns during seizures, as well as to longer-term SUDEP risk.
Keywords: Autonomic, Respiratory, Insula, Cingulate, SUDEP
Abbreviations: ACC, anterior cingulate cortex; CWRU, Case Western Reserve University; GTCS, generalized tonic-clonic seizures; OASIS, Open Access Series of Imaging Studies; PCC, posterior cingulate cortex; ROI, region of interest; SUDEP, sudden unexpected death in epilepsy; UCL, University College London; UCLA, University of California Los Angeles.
Highlights
-
•
Cortical thickness differs in multiple cardiorespiratory and sensory areas in GTCS.
-
•
GTCS patients show increased insular, cingulate, and postcentral gyrus thickening.
-
•
Extensive neocortical thinning, particularly in frontal cortex, occurs in GTCS.
-
•
Extent of cortical thickening or thinning differs by sex and brain hemisphere.
1. Introduction
The mechanisms underlying sudden unexpected death in epilepsy (SUDEP) remain obscure, despite recognition that SUDEP is among the most common causes of death in people with epilepsy (Devinsky, 2011). Patients who experience frequent generalized tonic-clonic seizures (GTCS) are at even higher risk for SUDEP (Harden et al., 2017). Autonomic and respiratory disturbances typically accompany GTCS, with extreme transient elevations and profound declines in blood pressure and heart rate, together with induction of dangerous cardiac arrhythmia. In addition, the severe extensor and flexor somatomotor efforts accompanying tonic and clonic phases of the seizures often result in sustained apnea or apneusis (Fisher et al., 2014; Goldman, 2012; Lederman, 2012). The potential for unrecoverable loss of perfusion from prolonged hypotension or cardiac arrhythmia, or sustained hypoxia from extended cessation of airflow is significant during a GTCS (Bateman et al., 2008).
The brain mechanisms responsible for maintaining blood pressure and preserving left and right balance of sympathetic and parasympathetic output to avoid fatal arrhythmia from asymmetric outflow do not solely rest in the brainstem. Insular, cingulate, and frontal cortices exert significant influences on other limbic and hypothalamic structures that, in turn, project to brainstem structures comprising the final common output pathways for autonomic outflow (Craig, 2003; Hurley et al., 1991; Loewy, 1982; Terreberry and Neafsey, 1987; Verberne and Owens, 1998; Westerhaus and Loewy, 2001). These cortical structures also influence respiratory patterning, either through blood pressure and breathing interactions (James et al., 2013; Ohtake and Jennings, 1992; Trelease et al., 1985), or through direct projections to brainstem respiratory nuclei (Moga et al., 1990; Terreberry and Neafsey, 1987). If the cortical and diencephalic structures mediating autonomic and breathing control are injured, as they are in other diseases such as heart failure and obstructive sleep apnea (Fatouleh et al., 2014; Harper et al., 2012; Kumar et al., 2011; Kumar et al., 2015; Macey et al., 2008; Ogren et al., 2014; Park et al., 2016; Woo et al., 2009), the consequences for regulation of sympathetic outflow and respiratory patterning can be severe.
Significant alterations occur in functional connectivity between brain structures in patients with GTCS (Blumenfeld et al., 2009; Elshahabi et al., 2015; Ji et al., 2014; Song et al., 2011), presumably arising from loss of volume in neural structures (Huang et al., 2011), or fiber injury (Kori et al., 2013). The findings suggest a potential for involvement of insular and cingulate cortex regions, both of which mediate autonomic, and especially cardiovascular patterning (Kimmerly et al., 2005; Oppenheimer, 2001; Oppenheimer et al., 1991). The insular cortices exert lateralized autonomic influences (principally sympathetic influences on the right side, parasympathetic on the left) (Oppenheimer et al., 1992), and the cingulate cortex serves major blood pressure and respiratory regulatory roles (Devinsky et al., 1995; Frysinger and Harper, 1986). In addition, frontal cortex regions which typically play inhibitory roles for motor regulation (Chase and McGinty, 1969, Chase and McGinty, 1970; Knauss et al., 1968; Sauerland et al., 1967) are affected in patients with GTCS (Huang et al., 2011), a concern considering the potential effects on the respiratory somatic musculature.
The concern for autonomic and respiratory dysregulation in GTCS is enhanced by findings that risk for SUDEP, a common cause of death in people with epilepsy, accounting for 7.5–17% of deaths in individuals with the condition (Terra et al., 2013), and over half of deaths in patients with intractable epilepsy (Devinsky, 2011; Tolstykh and Cavazos, 2013), is especially increased in patients who experience frequent GTCS (Hesdorffer et al., 2011).
The objective of this study was to determine whether cortical regions serving autonomic regulation of cardiovascular action and somatomotor influences on breathing are altered in patients with GTCS, with the long-term goal of relating the changes to pathological autonomic and breathing patterns that may lead to a compromised status, including SUDEP. A number of analytic techniques are available to assess different aspects of cortical injury; we chose quantification of cortical thickness, an objective measure that can be systematically evaluated by a standard software package, FreeSurfer, which has been well-validated in assessments of injury in other disease conditions (Dale et al., 1999; Fischl et al., 1999; Fischl et al., 2004; Macey et al., 2012b), and has shown good reliability across various scanners (Han et al., 2006). Because females exhibit distinct patterns of functional changes and structural injury in other conditions of hypoxia and extreme blood pressure changes (Macey et al., 2012a), and because males and females normally show different cortical thicknesses in disparate regions, we also considered GTCS changes by sex. In addition, since selected cortical areas, e.g., insula, show lateralized influences on autonomic action (Oppenheimer et al., 1992; Oppenheimer et al., 1996), partitioning of cortical thickness changes by left or right hemisphere was also required.
2. Materials and methods
2.1. Participants
We assessed cortical thickness changes in patients with GTCS using high-resolution T1-weighted MRI scans, collected from 53 GTCS patients (mean age ± SD:37.1 ± 12.6 years, 22 male) scanned at UCLA, CWRU, and UCL. The studies were approved by institutional review boards at each institution, and those documents are preserved at CWRU and at the individual institutions. Written, informed consent was obtained from each subject. Clinical variables, such as type of epilepsy and GTCS frequency, were obtained using the Center for SUDEP Research Multi-Modality Epilepsy Data Capture and Integration System (MEDCIS) database (Zhang et al., 2014; Zhang et al., 2015). We compared the patient group with a population set of 530 control subjects (37.1 ± 12.6 years, 220 male). The controls were selected to have 10 control subjects matched to each GTCS subject on the basis of age and gender. Data from 36 control subjects were collected at UCLA under similar informed consent procedures, and these local control scans were supplemented by data from Hammersmith Hospital of London's open-access IXI dataset http://www.brain-development.org (n = 322) (Ericsson et al., 2008; Heckemann et al., 2003), and the OASIS patient database http://www.oasis-brains.org/ (n = 172) (Herrick et al., 2016; Marcus et al., 2007), which has approval for public sharing of the images (Marcus et al., 2007).
2.2. Magnetic resonance imaging
High-resolution three-dimensional anatomical brain scans were obtained from GTCS subjects and controls at UCLA, CWRU, and UCL with 3.0-Tesla MRI scanners. In addition, OASIS and IXI control images, collected with both 1.5- and 3.0-Tesla scanners, were used (Ericsson et al., 2008; Herrick et al., 2016; Marcus et al., 2007); no systematic differences in FreeSurfer measures between images collected at different magnetic field strengths have been found (Han et al., 2006; McCarthy et al., 2015; Reuter et al., 2012).
2.3. Data processing
Prior to importing to FreeSurfer 5.3, all T1-weighted images were manually rigid-body shifted to overlap the Montreal Neurological Institute template (as included with SPM12). All images were resliced into a common space and voxel size (0.9 × 0.9 × 0.9 mm) to ensure consistent processing within FreeSurfer. These resliced images were imported into FreeSurfer and processed with the standard protocol (Dale et al., 1999; Fischl et al., 2004). This protocol includes skull stripping and segmentation of gray and white matter tissue types; these steps were manually checked and corrections performed if needed, as per standard FreeSurfer procedures. Images were normalized to common space and smoothed (10 mm kernel) in preparation for statistical analysis. First, a regional analysis was performed. Second, regions-of-interest were selected based on examination of the regional analysis. Using the standard FreeSurfer atlas (Desikan et al., 2006), the average cortical thickness within each ROI was extracted for each subject.
2.4. Statistical analysis
FreeSurfer includes an implementation of a general linear model that allows for a variety of statistical tests. Group characteristics of cortical thickness, laterality of changes, and age- and sex- thickness correlations were performed (p < 0.05, false discovery rate correction for multiple comparisons). Whole brain values were calculated for each subject, and then averaged to obtain mean global cortical volume and overall cortical thickness. In the regional analysis, group differences were assessed using the “qdec” graphical user interface tool. The mean thickness for each selected ROI was compared between groups. ROI mean thickness values were analyzed using two-sample, two-tailed t-tests assuming unequal variances. Additionally, correlations between ROI mean thickness, scaled for total intracranial volume (TIV), and GTCS frequency or epilepsy duration were assessed.
3. Results
3.1. Demographics & global cortical changes
Subject characteristics are shown in Table 1. Mean ages did not differ significantly between GTCS and Control groups, and the proportion of males vs females in each group was the same (GTCS: 22/53 male, Control: 220/530 male). For each hemisphere, total cortical volume and average cortical thickness were calculated. Left and right total cortical volumes were similar in GTCS patients and controls, while mean cortical thickness in the right hemisphere was 1.6% thinner in the GTCS group (Right GTCS thickness mean ± SE: 2.42 ± 0.016, Control: 2.46 ± 0.006, two-sample t-test p = 0.03).
Table 1.
Subject characteristics.
| GTCS (N = 53) |
Mean ± SD | Control (N = 530) |
|||
|---|---|---|---|---|---|
| Mean ± SD | Range | Range | P value | ||
| Age (years) | 37.1 ± 12.6 | 16.5–63.5 | 37.1 ± 12.6 | 18.5–63.9 | <0.98 |
| Sex | 22 Male, 31 Female | 220 Male, 310 Female | = 1.00 | ||
| Cortical volume (cm3) | |||||
| Left | 219.9 ± 25.4 | 165.4–274.2 | 226.6 ± 27.7 | 149.3–31.4 | <0.077 |
| Right | 224.0 ± 25.1 | 170.6–27.1 | 227.7 ± 27.4 | 152.6–31.8 | <0.319 |
| Mean ± SE | Range | Mean ± SE | Range | P value | |
| Thickness (mm) | |||||
| Left | 2.42 ± 0.017 | 2.13–2.72 | 2.45 ± 0.005 | 2.14–2.75 | <0.071 |
| Right | 2.42 ± 0.016 | 2.16–2.70 | 2.46 ± 0.006 | 2.14–2.77 | <0.031* |
Two-sample, two-tailed t-tests assuming unequal variances. (*) indicates significant p-value.
3.2. Characteristics of epilepsy
The majority of patients (34/53) had more than three GTCS in the past year, while eight had between one and three GTCS, and nine had no GTCS in the past year. For patients with well-documented seizure frequency data, the mean number of GTCS per year was similar in female and male patient groups (15 and 17 per year, respectively), although GTCS frequency in females of this study was less likely to be well-documented. Twenty-five patients had epilepsy durations of <15 years, 18 had durations of 15–29 years, and 10 of the 53 GTCS patients had epilepsy durations of 30 or more years. Females and males had similar mean durations of epilepsy (mean ± std. dev; females: 15.9 ± 12.3 years, males: 17.7 ± 11.5 years, p < 0.62). Approximately half of patients had presumed temporal lobe onset with secondary generalization (Table 2).
Table 2.
Characteristics of Epilepsy.
| Region of Seizure Onset | Females | Males | Mean #GTCS/yr |
|---|---|---|---|
| Temporal Lobe | |||
| Left | 6 | 8 | 5.8 |
| Right | 3 | 2 | 26 |
| Bitemporal | 2 | 2 | 18.5 |
| Unspecified | 1 | 0 | <1 |
| Focal | 0 | 4 | 16.5 |
| Multifocal | 1 | 1 | 40 |
| Frontal | |||
| Left | 1 | 1 | 9.5 |
| Right | 3 | 1 | 36.4 |
| Frontotemporal | |||
| Left | 0 | 1 | 4.5 |
| Right | 1 | 0 | >100 |
| Temporo-occipital | |||
| Right | 0 | 1 | 12 |
| Generalized | 8 | 1 | 14.7 |
| Unknown | 5 | 0 | 9.4 |
3.3. Regional cortical thickness differences
3.3.1. Combined sexes
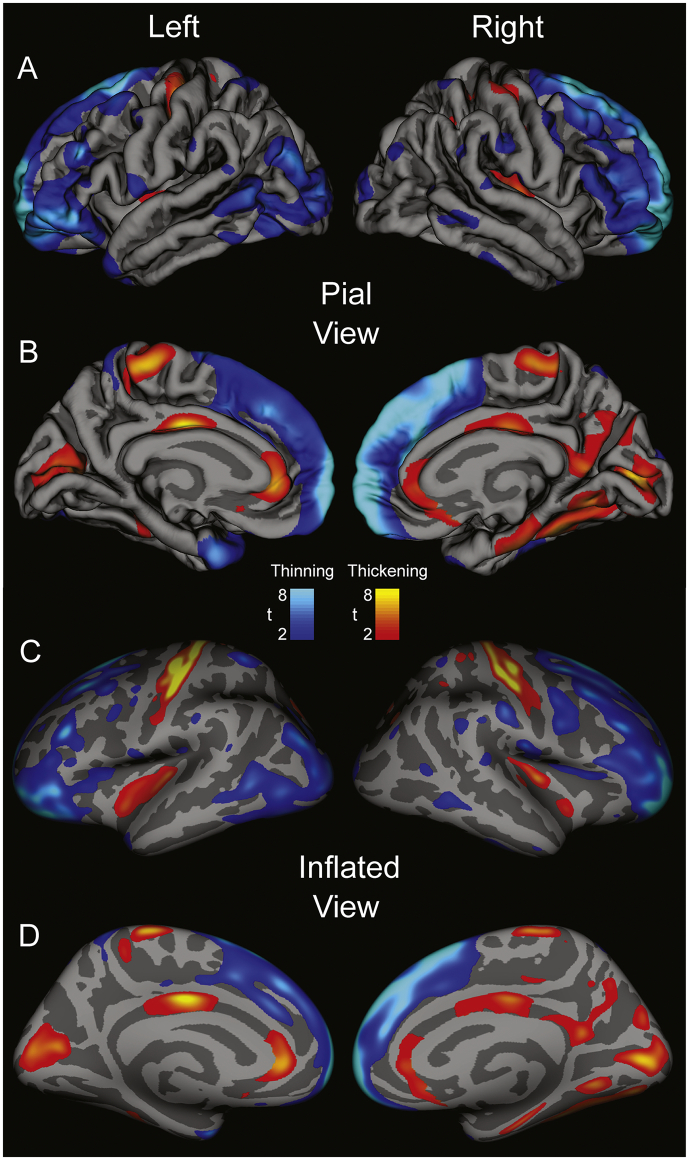
The frontal cortex, temporal pole, and lateral parietal and occipital cortices showed cortical thinning (Fig. 1). T values are expressed in a colored scale, with thinning represented in cool (blue) colors, and thickening in warm (red-yellow) colors.
Fig. 1.
Regions of significant group difference overlaid on “pial” views (A & B: showing topical view of sulci and gyri) and “inflated” views (C & D: sulci expanded and gyri flattened) for combined male and female cortical thickness data of left and right lateral (A & C) and medial (B & D) brain views of cortical thickness differences in 53 GTCS patients vs 530 age- and gender-matched controls, using sex as a covariate. t-statistic thresholds set using FDR (Rate: p = 0.05) Blue scale represents thinning, red-yellow scales indicate cortical thickening in GTCS patients over controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The combined-sex values for thinning frontal cortex in the GTCS group included the left and right superior frontal, rostral and caudal middle frontal, frontal pole, temporal pole, and medial orbitofrontal areas. The lateral orbitofrontal significantly thinned, but only on the left side (Fig. 1A & C, blue areas, Table 3). Increased cortical thickness appeared bilaterally in the left and right paracentral lobule, and the left postcentral gyrus. Bilateral thickening along the medial aspect (Fig. 1B) was observed in the right and left rostral anterior cingulate and cuneus, and right-sided thickening emerged in the mid-posterior cingulate, as well as in the parahippocampal, fusiform, and lingual areas (Fig. 1, Table 4). Both left and right insulae thickened, as did the adjacent transverse temporal areas.
Table 3.
Regions of Cortical Thinning.
| Areas of cortical thinning: cortical thickness (mm) | |||
|---|---|---|---|
| GTCS (N = 53) |
Control (N = 530) |
||
| Mean ± SD | Mean ± SD | P value | |
| Lateral orbitofrontal | |||
| Left | 2.53 ± 0.157 | 2.65 ± 0.164 | <0.001* |
| Right | 2.55 ± 0.168 | 2.58 ± 0.210 | <0.22^ |
| Medial orbitofrontal | |||
| Left | 2.33 ± 0.156 | 2.40 ± 0.183 | <0.002*^ |
| Right | 2.32 ± 0.181 | 2.38 ± 0.212 | <0.03* |
| Frontal pole | |||
| Left | 2.46 ± 0.410 | 2.81 ± 0.295 | <0.001* |
| Right | 2.44 ± 0.418 | 2.80 ± 0.278 | <0.001* |
| Superior frontal | |||
| Left | 2.62 ± 0.176 | 2.75 ± 0.155 | <0.001* |
| Right | 2.57 ± 0.179 | 2.77 ± 0.169 | <0.001* |
| Rostral Middle frontal | |||
| Left | 2.25 ± 0.139 | 2.35 ± 0.141 | <0.001* |
| Right | 2.19 ± 0.145 | 2.34 ± 0.151 | <0.001* |
| Caudal middle frontal | |||
| Left | 2.45 ± 0.161 | 2.54 ± 0.161 | <0.001* |
| Right | 2.44 ± 0.154 | 2.54 ± 0.180 | <0.001* |
| Precentral gyrus | |||
| Left | 2.50 ± 0.162 | 2.48 ± 0.186 | <0.48 |
| Right | 2.46 ± 0.149 | 2.47 ± 0.194 | <0.84 |
Mean cortical thickness values of frontal lobe ROIs in combined male and female group from 53 GTCS patients vs 530 age- and gender-matched controls. Two-sample, two-tailed t-tests assuming unequal variances. (*) indicates significant p-value. (^) indicates significant GTCS sex-related differences relative to controls; see Table 5 for details.
Table 4.
Regions of cortical thickening.
| Areas of cortical thickening: cortical thickness (mm) | |||
|---|---|---|---|
| GTCS (N = 53) |
Control (N = 530) |
||
| Mean ± SD | Mean ± SD | P value | |
| Insula | |||
| Left | 3.03 ± 0.186 | 2.98 ± 0.185 | <0.0506 |
| Right | 3.05 ± 0.178 | 2.97 ± 0.170 | <0.003* |
| Cingulate (mean) | |||
| Left | 2.64 ± 0.182 | 2.58 ± 0.151 | <0.04* |
| Right | 2.61 ± 0.194 | 2.54 ± 0.148 | <0.011*^ |
| Rostral anterior cingulate | |||
| Left | 2.93 ± 0.201 | 2.79 ± 0.254 | <0.001* |
| Right | 2.89 ± 0.265 | 2.74 ± 0.287 | <0.001* |
| Caudal anterior cingulate | |||
| Left | 2.65 ± 0.267 | 2.59 ± 0.226 | <0.15 |
| Right | 2.56 ± 0.257 | 2.52 ± 0.222 | <0.37 |
| Posterior cingulate | |||
| Left | 2.51 ± 0.198 | 2.49 ± 0.172 | <0.43 |
| Right | 2.54 ± 0.202 | 2.48 ± 0.173 | <0.04*^ |
| Isthmus cingulate | |||
| Left | 2.46 ± 0.286 | 2.46 ± 0.213 | <0.98 |
| Right | 2.45 ± 0.250 | 2.41 ± 0.212 | <0.18 |
| Paracentral lobule | |||
| Left | 2.34 ± 0.174 | 2.28 ± 0.196 | <0.013*^ |
| Right | 2.36 ± 0.167 | 2.31 ± 0.212 | <0.027* |
| Postcentral gyrus | |||
| Left | 2.06 ± 0.191 | 1.99 ± 0.137 | <0.0097*^ |
| Right | 2.02 ± 0.160 | 1.99 ± 0.151 | <0.11^ |
| Cuneus | |||
| Left | 1.81 ± 0.186 | 1.77 ± 0.192 | <0.08 |
| Right | 1.82 ± 0.143 | 1.76 ± 0.181 | <0.0084*^ |
| Pericalcarine | |||
| Left | 1.62 ± 0.159 | 1.49 ± 0.198 | <0.001* |
| Right | 1.59 ± 0.151 | 1.48 ± 0.185 | <0.001* |
Mean cortical thickness values of insula, cingulate, parietal, and occipital ROIs in combined male and female group from 53 GTCS patients vs 530 age- and gender-matched controls. Two-sample, two-tailed t-tests, assuming unequal variances. (*) indicates significant p-value. (^) indicates significant GTCS sex-related differences relative to controls; see Table 5 for details.
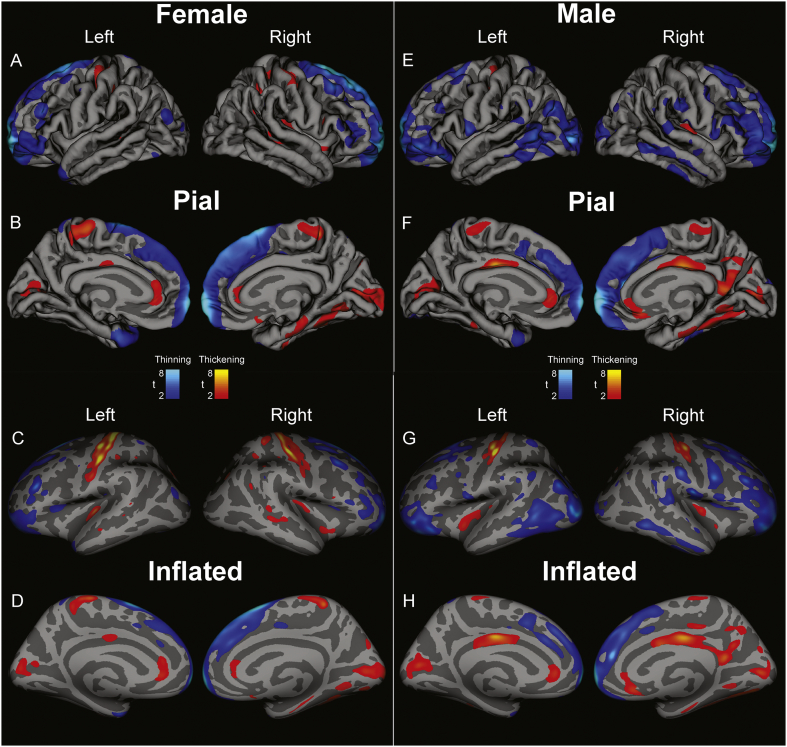
3.3.2. Separated by sex
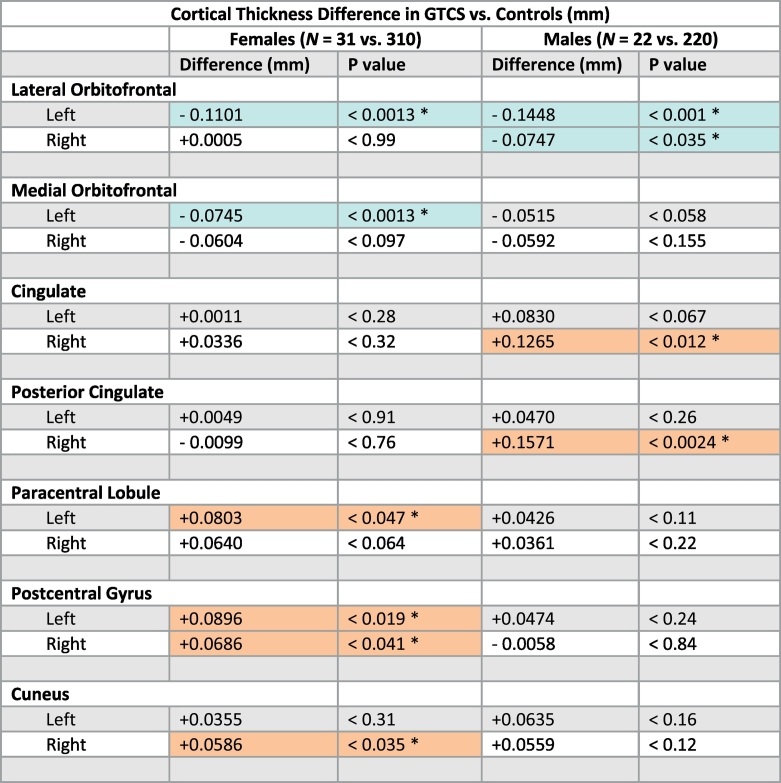
The magnitude of increase in cortical thickness was lateralized, i.e., more marked in one hemisphere over the other, with the extent of change dependent on sex (Fig. 2, Table 4). Males showed bilateral thinning of the lateral orbitofrontal cortex, but females had significant thinning only on the left (Table 5). In the postcentral gyrus, females showed bilateral thickening (Fig. 2A & C, Table 5), but males showed only insignificant trends of thickening on the left, and no difference on the right. Males showed significant thickening in the posterior cingulate (bilaterally) and right isthmus (Fig. 2F & H, Table 5), but females showed no differences in thickness on the right or left. No differences appeared in the caudal anterior (i.e., mid) cingulate with either sex or combined sexes. Taken separately by sex, both males and females had significant thickening on the right-side insula, but insignificant changes on the left; however, combining the sexes, significant thickening also appeared on the left. A similar pattern was found in the cuneus. Both sexes showed bilateral thickening of the rostral anterior cortex. These findings are displayed in Fig. 2, with statistical values in Table 5.
Fig. 2.
Regions of significant group difference overlaid on “pial” views (showing topical view of sulci and gyri) and “inflated” views (i.e. sulci expanded and gyri flattened) for data from 31 female GTCS patients vs 310 age-matched female controls (Female, A-D) and from 22 male GTCS vs. 220 age-matched male controls (Male, E-H). Lateral views (A, C, E, F) and medial views (B, D, F, H) in left and right brain views show areas of cortical thinning (blue scale) and thickening (red-yellow scales) in GTCS patients over controls, partitioned by sex. t-statistic thresholds (FDR Rate: p = 0.05) set in the combined male-female analysis (Fig. 1) were maintained here for consistency. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 5.
Differences in mean cortical thickness values (GTCS vs Controls).
Differences in mean cortical thickness values (GTCS vs Controls) in ROIs where change in cortical thickness differed between females and males. Females: 31 GTCS patients vs 310 age- and gender-matched controls, Males: 22 GTCS patients vs 220 age- and gender-matched controls. Two-sample, two-tailed t-tests, assuming unequal variances. (*) indicates significant p-value. Blue-shaded cells indicate significant (asterisks) thinning in GTCS over controls, orange-shaded cells indicate significant (asterisks) thickening in GTCS over controls.
3.3.3. Regional cortical thickness correlations
ROIs exhibiting significant thinning or thickening (Tables 3 & 4), were subjected to further correlation analysis. There were no significant correlations between mean ROI thickness (TIV-scaled) and duration of epilepsy (n = 53). The strongest correlation was observed in the left medial orbitofrontal cortex, where r = 0.17. For all other ROIs, │r│ < 0.14. Initial analyses showed only weak correlations between GTCS frequency and mean cortical thickness. However, when patients were divided into moderate-high (3–15 GTCS/yr, n = 16, 7F, mean age ± std. dev:32.6 ± 13.0 years, mean duration: 18.9 ± 14.8 years) and very high (18–78 GTCS/yr, n = 14, 6F, mean age ± std. dev:38.1 ± 11.9 years, mean duration: 16.0 ± 9.7 years) frequency groups, strong correlations were found. In the moderate-high group, all ROIs examined showed positive correlations with GTCS frequency (r = 0.18–0.71, mean r = 0.50). The strongest correlation (r = 0.72) was found in the left insula, but strong positive correlations were also observed in regions of cortical thinning (right caudal midfrontal cortex: r = 0.67). The very high frequency group showed opposite trends, with all but the frontal pole showing negative correlations with frequency (r = −0.03--0.52, mean r = −0.22). The right caudal midfrontal cortex, which had one of the strongest positive correlations in the moderate-high group, had one of the strongest negative correlations (r = −0.51) in the very high frequency group.
4. Discussion
4.1. Overview
Cortical thickness differs in multiple areas in GTCS patients relative to a large number of age- and gender-matched controls. Moreover, the extent of thickening or thinning of cortical sites varies by sex and by brain hemisphere. Changes in thickness of the cingulate and insular cortices were of special interest for their established roles in autonomic and respiratory regulation and the concern of those regulatory issues in SUDEP. Other cortical regions, especially the frontal and temporal cortices, showed tissue thickness declines, and additional sensorimotor areas, particularly the pre- and post- central gyri, showed increased thickness, as did posterior areas, including the medial occipital cortex. Cortical thinning was greater in males in frontal cortex; cortical thickening was more marked in post-central gyri of females. The right anterior cingulate thickening was more widespread than the left, and affected subgenual portions of the cingulate.
4.2. Combined sexes
4.2.1. Cortical thinning
Changes in cortical thickness can reflect multiple intrinsic and pathologic processes. Decreased regional thickness appears in numerous pathological conditions, including epilepsy (Bernhardt et al., 2009; Burge et al., 2016; Geisseler et al., 2016; Lin et al., 2007; McEvoy et al., 2009; van Haren et al., 2011). Here, we report extensive neocortical thinning in selected areas in patients with GTCS relative to controls, particularly in frontal cortex. Frontal neocortex thinning is common in frontal or idiopathic generalized epilepsies (Bernhardt et al., 2009; Hong et al., 2016), but also occurs in temporal lobe epilepsy (Bernhardt et al., 2008; Lin et al., 2007), where such thinning is associated with poor surgical outcome (Kamson et al., 2016), and tends to correlate with duration of epilepsy and seizure frequency (Bernhardt et al., 2009; Lin et al., 2007). Neocortical thinning, particularly of the frontal cortex, may stem from remodeling of thalamocortical networks, brought on by repeated GTCS (Bernhardt et al., 2009), a possibility consistent with reported thalamic gray matter reductions in SUDEP and high SUDEP-risk patients (Wandschneider et al., 2015). Here, we show that in patients with very high GTCS frequency, there is a weak-to-moderate correlation between neocortical thickness and GTCS frequency across numerous brain regions, even those that may show thinning in patients with lower GTCS frequency. Decreased cerebral blood flow is common in specific brain regions during secondarily-generalized GTCS, and may also contribute to thinning (Blumenfeld et al., 2009). Profound hypoperfusion occurs in the orbitofrontal, lateral frontal, and anterior and posterior cingulate cortices during seizure generalization and into the postictal period (Blumenfeld et al., 2009), thus establishing conditions for loss of tissue.
Frontal cortical thinning has significant implications for seizure characteristics and physiological expression. Decreased inhibition, caused by loss or restructuring of frontal inhibitory networks (Chase and McGinty, 1970; Sauerland et al., 1967), can lead to increased seizure frequency, duration, or severity. Frontal cortex thinning thus has the potential to worsen the seizure condition. Further concerns rest with the roles of frontal cortex regions in cardiovascular and somatic muscle action. Autonomic areas, such as the orbitofrontal cortex, exert an integral role in cardiovascular regulation, especially in blood pressure control (Kimmerly et al., 2005), and in suppression of somatic musculature, including respiratory musculature, and particularly upper airway musculature (Chase and McGinty, 1970; Marks et al., 1987). Loss of neurons in the orbitofrontal region will diminish its normal regulatory influences on both somatic respiratory musculature and autonomic control, thus hindering the potential to recover from disruptive respiratory muscle action or extreme hypotension.
4.2.2. Cortical thickening
While thinning typically reflects loss or impaired function, interpretation of neocortical thickening is not straightforward. In dysplasia-related frontal lobe epilepsy, thickening may reflect delayed pruning (Hong et al., 2016), while increased cortical thickness in patients with macular degeneration likely reflects compensatory “gain of function” in peripherally-responsive primary visual cortex reflecting spared peripheral vision (Burge et al., 2016). Cortical thickening, including prefrontal and anterior insular cortex, may reflect experience-dependent increased neural volume (Lazar et al., 2005; Luders et al., 2009), perhaps analogous to that of enhanced hippocampal volume after spatial navigation practice (Maguire et al., 2006), and reorganization in visual cortex following vision loss (Burge et al., 2016). The bilaterally increased insular thickness in GTCS may develop from specialized experiences related to the condition. Repeated insular overactivation from GTC seizure processes, or as compensatory mechanisms to overcome the resulting enhanced sympathetic outflow could initiate cortical thickening. In patients with moderate-high GTCS frequency, thickness of the left insula strongly correlated with GTCS frequency. The left insula, with more involvement in parasympathetic responses vs sympathetic action on the right (Oppenheimer et al., 1992), also shows more extensive thickening in GTCS and poses a particular concern for SUDEP, since exaggerated parasympathetic action can lead to profound hypotension, with loss of perfusion.
The circumstances surrounding SUDEP, which include, by deduction in unobserved cases, silence and occurrence at times when the patient would be asleep, with little evidence of trauma, suggest a cardiovascular event or respiratory failure during the fatal sequence. Such physiological patterns have been confirmed in isolated observed instances (Kloster and Engelskjon, 1999; Ryvlin et al., 2013). A range of potential cardiovascular failure eventualities may occur. One scenario involves a profound loss of blood pressure, which may result from severe arrhythmia, reduced cardiac output, or marked loss of vascular tone. However, major neural reflex mechanisms are normally in place to rescue blood pressure from such scenarios. These mechanisms include recruitment of action of the cerebellar deep nuclei, which serve to dampen profound hypotension or marked hypertension, with somatic responses to normalize pressure. Hypotension can be eased with exaggerated tidal volumes or tachypneic respiratory efforts, or with axial muscle extension to increase blood pressure (Harper et al., 1999), while transient hypertension can be normalized with cessation of respiratory efforts (Trelease et al., 1985). If an extreme loss of blood pressure takes place during a GTCS, those recovery mechanisms that depend on the integrity of neural areas mediating blood pressure and breathing for vital support may not respond appropriately. Similarly, if the fatal event results from sustained apnea or apneusis, normal oxygen and carbon dioxide reflex recovery mechanisms may not adequately function. A need exists to determine the nature of potential neural injury that may contribute to dangerous cardiovascular sequelae in patients with GTCS, or that would interfere with recovery from such events; cortical structures exert critical roles in protection of that recovery.
Cortical thickness changes may also reflect long-term functional reorganization accompanying chronic epilepsy (Elger et al., 2004; McDonald et al., 2008). Thickening of the ACC and cuneus, for example, may result from the marked increase in interhemispheric functional connectivity between these two regions in patients with GTCS (Ji et al., 2014), while thinning of superior frontal cortex is consistent with demonstrated declines in functional connectivity within the default mode network (Song et al., 2011). The findings here of increased thickening in the paracentral lobules and precentral gyrus are supported by a recent, smaller study of patients with secondarily-generalized seizures, which showed greater gray matter volumes in the motor pathway (paracentral regions) (Hsin et al., 2017). Such changes may underlie reduced seizure threshold, or a decreased latency to seizure generalization. The ACC helps mediate bradycardia via influences on blood pressure and heart rate (Critchley et al., 2003). Although the ACC is spared from acute neuronal/axonal swelling in GTCS patients, long-term cortical thickening found here suggests that impaired respiratory and cardiovascular regulation may result from damage to that area. The ACC thickening may relate to decreased thalamic-ACC functional connectivity in patients at risk for SUDEP (Allen et al., 2017).
The neuronal architectural changes common in the hippocampus in temporal lobe epilepsy (Mathern et al., 1995; Scheibel et al., 1974) can also take place in neocortex (Salin et al., 1995), and include significant increases in length and number of axon collaterals and swellings. Thickening may also reflect progressive architectural changes, stemming from glial, neuronal, or synaptic reorganization, such as those resulting from repeated episodes of severe seizure activity or status epilepticus (Colciaghi et al., 2014). In patients with moderate-high GTCS frequency, seizure frequency correlated strongly with thickness of the cingulate cortex. Increased cortical thickness in the posterior and anterior cingulate is also consistent with glial activation, which is accompanied by cell swelling. Inflammatory processes resulting from repeated GTCS may contribute to such swelling. Previously, we found increased tissue homogeneity in the PCC (presumably from inflammation); however, the ACC was spared these changes in homogeneity (Ogren et al., 2016a; Ogren et al., 2016b). Altered glial function in the PCC would suggest that GTCS could induce long-term injury to both sites. The PCC plays a significant role in “intrinsic” homeostatic control, and altered functional connectivity of the PCC to other brain regions has been associated with seizures (Bharath et al., 2015; Song et al., 2011). Increased glial activation and corresponding neuronal swelling in the PCC may be associated with dysregulation of homeostatic control, perhaps contributing to the cardiovascular sequelae in patients with GTCS.
Understanding the source of the changes in cingulate volume in GTCS patients could elucidate the physiological mechanisms underlying changes in autonomic and breathing control in epilepsy. While cingulate cortex structures were evaluated here, those structures project to frontal and temporal areas which also showed significant changes, and an understanding of the operating mechanisms must include interactions between these other cortical areas and the cingulate cortex.
4.3. Sex-specific effects
A remarkable aspect of the GTCS-induced changes in cortical thickness was the different extent of changes by sex, particularly since we observed no major differences in duration of epilepsy or frequency of GTCS between these groups. A significant factor in determining neural influences on breathing and cardiovascular control in disease conditions is sex; such conditions, including obstructive sleep apnea, heart failure, and congenital central hypoventilation syndrome can show significant injury in cardiovascular and respiratory regulatory brain sites (Kumar et al., 2012; Kumar et al., 2015; Macey et al., 2008; Macey et al., 2012b; Ogren et al., 2014), but that injury frequently differs substantially between males and females, especially in limbic areas (Macey et al., 2012a). Moreover, responsiveness of affected brain sites to evoked pain or blood pressure challenges significantly differs between sexes (Henderson et al., 2008; Macey et al., 2016; Macey et al., 2017). The mechanisms underlying these pattern differences between males and females are unclear, but there is a potential for sex-related protective mechanisms and consequent injury differences to exist in GTCS, where analogous exposure to hypoxic and extreme blood pressure changes are common in the condition.
Differences in cortical thickness exist between healthy males and females (Luders et al., 2006; Sowell et al., 2007). To account for any inherent sex differences contributing to the findings, we partitioned by sex, comparing male and female GTCS patients to male and female controls, respectively. The sex differences we identified were not confined to autonomic regions, but also included somatosensory areas such as the postcentral gyri, which showed bilateral increases in females but only left-sided increases in males. Males showed bilateral thinning of the orbitofrontal cortex, but female thinning appeared only on the left. Cortical areas with prominent autonomic regulatory roles also showed sex-related thickness changes; the posterior cingulate and isthmus were significantly thicker in males, but not so in females, and the anterior cingulate, while thicker in GTCS over controls, showed no such gender separation. The right insula was thicker for both sexes, but thickening in males was less on the left. Some sex-related processes modify the extent and localization of cortical thickening. Those processes may be related to perfusion-related differences between the sexes (Gur et al., 1982; Satterthwaite et al., 2014), the presence of neuroprotective factors in females, such as progesterone (Herzog et al., 1997) or estrogen (Miller et al., 2005; Singer et al., 1999; Whitehead and McNiel, 1935), differences in the origin or duration of epilepsy, or other contribution. Differences in the extent of injury between sexes in other conditions with remarkable cortical damage, such as obstructive sleep apnea are well-known (Macey et al., 2012a), and have been attributed to altered perfusion or susceptibility to hypoxic injury between males and females (Gur et al., 1982). Such disparities in laterality and extent of injury have direct implications for specific severities of symptoms accompanying GTCS. Equally important is the knowledge of sites of such injury, and the need to tailor specific interventions for males versus females to counteract the neural pathology.
4.4. Hemispheric lateralization
The lateralization of cortical thickness changes, whether sex-dependent or not, deserves attention, since cortical regions within the left or right hemisphere exert such substantially different influences on cardiovascular and somatic output. The most obvious of these lateralization issues as a concern rest with the insular cortices, with the right side exerting much more influence on sympathetic action, and the left, parasympathetic influences (Oppenheimer et al., 1992). Both sides showed thickening, but only barely on the left with combined sex data. Presumably, this finding suggests a more-robust sympathetic influence during seizures. Compromised function of the right insula may contribute directly to increased cardiovascular risk. Lesions of the right, but not left, insula, along with right frontal and parietal lesions, are associated with cardiac arrhythmias following stroke (Seifert et al., 2015). The unequal sex-related thinning of the orbitofrontal cortex, with males showing more thinning suggests that more cortex may remain in females to suppress respiratory motor and autonomic actions than in males. There is evidence to suggest that GTCS alter SUDEP risk differently in males from females. While male gender is associated with an increased overall risk of SUDEP (Hesdorffer et al., 2011), the association between high seizure frequency and increased SUDEP occurrence is much stronger in females (Walczak et al., 2001).
Some of the alterations, such as thickening of the ACC and cuneus, likely reflect increased interhemispheric functional connectivity (Ji et al., 2014), possibly leading to facilitated seizure generalization. We speculate that thinning in frontal areas, particularly in the orbitofrontal cortex, may interfere with important inhibitory processes on respiratory somatic musculature action and on blood pressure control, prolonging activation of inspiratory musculature to recover from an apnea, or diminishing influences on other brain structures needed to restore blood pressure. The bilateral increased thickness in the insular cortices are a particular concern, since the right insula exerts major influences on extent of sympathetic outflow, with the possibility of exaggerated asymmetric outflow levels increasing the possibility of dangerous cardiac arrhythmia (Seifert et al., 2015), while the left (parasympathetic influences) could lead to significant postictal bradycardia/asystole, a finding associated with GTCS and with SUDEP or near-SUDEP (Ryvlin et al., 2013).
5. Limitations
Numerous processes have the potential to influence changes in brain structure in GTCS patients, with some of these changes possibly altering cardiovascular and respiratory regulation. Those factors include long-standing use of antiepileptic medications, which, although not well described, may influence cortical thickness (Pardoe et al., 2013). In other conditions, such as schizophrenia, typical antipsychotic medications lead to cortical thinning, while other “atypical” antipsychotics appear to be associated with cortical thickening in the same regions (van Haren et al., 2011). Sampling and other issues precluded distinguishing many of these factors in this study. Future large-scale studies could factor antiepileptic drug use into the analysis, and might also group patients by etiology, duration of epilepsy, and seizure frequency. Here, we combined patients with generalized and initially focal temporal lobe onsets. Prospective analyses should separate these groups, and would also benefit from the addition of a separate group, comprised of patients who experience only focal seizures.
The processes underlying semi-automated FreeSurfer analysis pose potential issues with respect to the validity of “cortical thickness” as a measure. Statistical analysis of discrete brain regions relies upon a degree of independence from adjacent regions. These assumptions of independence may not be met, due to interdependence of adjacent brain structures. In addition, statistical thresholds would ideally be calculated across individual regions; however, the present cortical thickness analysis methodology uses only a single whole-brain threshold. Such statistical issues would lead to overly-conservative findings (i.e., increased risk of false negatives). Although the GTCS patients and the UCLA control patient values were all measured with a 3 T scanner, the OASIS control subjects were assessed with 1.5 T devices, leading to a concern of signal precision with lower-field scanners. However, FreeSurfer values are relatively immune to such field strength variations, likely because the processing algorithms down-sample the scans to a common resolution (Han et al., 2006; Reuter et al., 2012).
6. Conclusions
Widespread cortical thickness changes occur in patients with GTCS relative to controls, with alterations appearing in cortical areas with significant influences on cardiovascular and breathing patterns. Occurrence of GTCS is the major risk factor for SUDEP (Harden et al., 2017). For patients with as few as three GTCS per year, SUDEP risk is increased 15-fold over GTCS-free patients (Harden et al., 2017), but the mechanisms by which GTCS increase this risk are unknown. The morphologic changes in cortical areas regulating sympathetic outflow, together with the alterations that can modify breathing patterns and recovery from apnea suggest the development of injurious circumstances that enhance conditions for SUDEP. The extent, localization and lateralization of changes in cortical thickness varied by sex. Whether these sex differences result from neuroprotective effects, male-female differences in cerebral blood flow, or other unknown factors is unclear. However, the differences emphasize that consideration of sex is essential in assessing brain changes with GTCS. Cardiovascular and other autonomic control, as well as certain somatomotor regulation is typically lateralized; thus, the preferential cortical thickness changes by hemisphere must be a major consideration in determining MRI-measurable biomarkers of increased cardiovascular and respiratory pathology. The correlation data emphasize again the importance of reducing the number of GTCS, a factor that exerts a significant influence on extent of cortical changes. The current practice guidelines urge management of epilepsy therapies and counseling GTCS patients on the importance of medication in reducing SUDEP risk. Future guidelines might include tailored recommendations based on MRI findings.
Acknowledgments
Acknowledgements
The authors thank all patients who participated in this study. Data were provided, in part, by Hammersmith Hospital of London (IXI database) and the Open Access Series of Imaging Studies (OASIS patient database).
Funding
This work was supported by the National Institute of Neurologic Disorders and Stroke [U01 NS090407] and the Amgen Foundation. The OASIS database for control images was supported by the National Institutes of Health [P50 AG05681, P01 AG03991, R01 AG021910, P20 MH071616, and U24 RR021382]. The UCL group is grateful to the Wolfson Foundation and the Epilepsy Society for supporting the Epilepsy Society MRI scanner. The UCL contributions were also supported by the National Institute for Health Research, University College London Hospitals Biomedical Research Centre.
References
- Allen L.A., Harper R.M., Kumar R., Guye M., Ogren J.A., Lhatoo S.D., Lemieux L., Scott C.A., Vos S.B., Rani S., Diehl B. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front. Neurol. 2017;8(544) doi: 10.3389/fneur.2017.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman L.M., Li C.S., Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. Pt 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B.C., Worsley K.J., Besson P., Concha L., Lerch J.P., Evans A.C., Bernasconi N. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage. 2008;42(2):515–524. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Rozen D.A., Worsley K.J., Evans A.C., Bernasconi N., Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. NeuroImage. 2009;46(2):373–381. doi: 10.1016/j.neuroimage.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Bharath R.D., Sinha S., Panda R., Raghavendra K., George L., Chaitanya G., Gupta A., Satishchandra P. Seizure frequency can alter brain connectivity: evidence from resting-state fMRI. Am. J. Neuroradiol. 2015;36(10):1890–1898. doi: 10.3174/ajnr.A4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H., Varghese G.I., Purcaro M.J., Motelow J.E., Enev M., McNally K.A., Levin A.R., Hirsch L.J., Tikofsky R., Zubal I.G., Paige A.L., Spencer S.S. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. Pt 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge W.K., Griffis J.C., Nenert R., Elkhetali A., DeCarlo D.K., ver Hoef L.W., Ross L.A., Visscher K.M. Cortical thickness in human V1 associated with central vision loss. Sci. Rep. 2016;6(23268) doi: 10.1038/srep23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M.H., McGinty D.J. Orbital cortical originating somato-motor inhibition and excitation during sleep and wakefulness. Electroencephalogr. Clin. Neurophysiol. 1969;26(3):337. [PubMed] [Google Scholar]
- Chase M.H., McGinty D.J. Somatomotor inhibition and excitation by forebrain stimulation during sleep and wakefulness: orbital cortex. Brain Res. 1970;19(1):127–136. doi: 10.1016/0006-8993(70)90242-8. [DOI] [PubMed] [Google Scholar]
- Colciaghi F., Finardi A., Nobili P., Locatelli D., Spigolon G., Battaglia G.S. Progressive brain damage, synaptic reorganization and NMDA activation in a model of epileptogenic cortical dysplasia. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Josephs O., O'Doherty J., Zanini S., Dewar B.K., Cipolotti L., Shallice T., Dolan R.J. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. Pt 10. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Sudden, unexpected death in epilepsy. N. Engl. J. Med. 2011;365(19):1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. Pt 1. [DOI] [PubMed] [Google Scholar]
- Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3(11):663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Elshahabi A., Klamer S., Sahib A.K., Lerche H., Braun C., Focke N.K. Magnetoencephalography reveals a widespread increase in network connectivity in idiopathic/genetic generalized epilepsy. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A., Aljabar P., Rueckert D. 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 2008. Construction of a patient-specific atlas of the brain: Application to normal aging; pp. 480–483. [Google Scholar]
- Fatouleh R.H., Hammam E., Lundblad L.C., Macey P.M., McKenzie D.K., Henderson L.A., Macefield V.G. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin. 2014;6:275–283. doi: 10.1016/j.nicl.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., Engel J., Jr., Forsgren L., French J.A., Glynn M., Hesdorffer D.C., Lee B.I., Mathern G.W., Moshe S.L., Perucca E., Scheffer I.E., Tomson T., Watanabe M., Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Frysinger R.C., Harper R.M. Cardiac and respiratory relationships with neural discharge in the anterior cingulate cortex during sleep-walking states. Exp. Neurol. 1986;94(2):247–263. doi: 10.1016/0014-4886(86)90100-7. [DOI] [PubMed] [Google Scholar]
- Geisseler O., Pflugshaupt T., Bezzola L., Reuter K., Weller D., Schuknecht B., Brugger P., Linnebank M. Cortical thinning in the anterior cingulate cortex predicts multiple sclerosis patients' fluency performance in a lateralised manner. Neuroimage Clin. 2016;10:89–95. doi: 10.1016/j.nicl.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. W.B. Saunders; Philadelphia: 2012. 50 – Approach to the Patient with Possible Cardiovascular Disease. Goldman's Cecil Medicine (Twenty-Fourth Edition) pp. 246–255. [Google Scholar]
- Gur R.C., Gur R.E., Obrist W.D., Hungerbuhler J.P., Younkin D., Rosen A.D., Skolnick B.E., Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217(4560):659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harden C., Tomson T., Gloss D., Buchhalter J., Cross J.H., Donner E., French J.A., Gil-Nagel A., Hesdorffer D.C., Smithson W.H., Spitz M.C., Walczak T.S., Sander J.W., Ryvlin P. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674–1680. doi: 10.1212/WNL.0000000000003685. [DOI] [PubMed] [Google Scholar]
- Harper R.M., Richard C.A., Rector D.M. Physiological and ventral medullary surface activity during hypovolemia. Neuroscience. 1999;94(2):579–586. doi: 10.1016/s0306-4522(99)00347-4. [DOI] [PubMed] [Google Scholar]
- Harper R.M., Kumar R., Macey P.M., Ogren J.A., Richardson H.L. Functional neuroanatomy and sleep-disordered breathing: implications for autonomic regulation. Anat. Rec. (Hoboken) 2012;295(9):1385–1395. doi: 10.1002/ar.22514. [DOI] [PubMed] [Google Scholar]
- Heckemann R.A., Hartkens T., Leung K.K., Zheng Y., Hill D.L.G., Hajnal J.V., Rueckert D., editors. Information Extraction from Medical Images (IXI): Developing an e-Science Application Based on the Globus Toolkit. Nottingham; UK: 2003. [Google Scholar]
- Henderson L.A., Gandevia S.C., Macefield V.G. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. NeuroImage. 2008;39(4):1867–1876. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Herrick R., Horton W., Olsen T., McKay M., Archie K.A., Marcus D.S. XNAT central: open sourcing imaging research data. NeuroImage. 2016;124:1093–1096. doi: 10.1016/j.neuroimage.2015.06.076. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog A.G., Klein P., Ransil B.J. Three patterns of catamenial epilepsy. Epilepsia. 1997;38(10):1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hesdorffer D.C., Tomson T., Benn E., Sander J.W., Nilsson L., Langan Y., Walczak T.S., Beghi E., Brodie M.J., Hauser A., Epidemiology I.C.o., Subcommission on, M Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52(6):1150–1159. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Bernhardt B.C., Schrader D.S., Bernasconi N., Bernasconi A. Whole-brain MRI phenotyping in dysplasia-related frontal lobe epilepsy. Neurology. 2016;86(7):643–650. doi: 10.1212/WNL.0000000000002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin Y.L., Harnod T., Chang C.S., Peng S.J. Increase in gray matter volume and white matter fractional anisotropy in the motor pathways of patients with secondarily generalized neocortical seizures. Epilepsy Res. 2017;137:61–68. doi: 10.1016/j.eplepsyres.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Huang W., Lu G., Zhang Z., Zhong Y., Wang Z., Yuan C., Jiao Q., Qian Z., Tan Q., Chen G., Zhang Z., Liu Y. Gray-matter volume reduction in the thalamus and frontal lobe in epileptic patients with generalized tonic-clonic seizures. J. Neuroradiol. 2011;38(5):298–303. doi: 10.1016/j.neurad.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Hurley K.M., Herbert H., Moga M.M., Saper C.B. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 1991;308(2):249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- James C., Macefield V.G., Henderson L.A. Real-time imaging of cortical and subcortical control of muscle sympathetic nerve activity in awake human subjects. NeuroImage. 2013;70:59–65. doi: 10.1016/j.neuroimage.2012.12.047. [DOI] [PubMed] [Google Scholar]
- Ji G.J., Zhang Z., Xu Q., Zang Y.F., Liao W., Lu G. Generalized tonic-clonic seizures: aberrant interhemispheric functional and anatomical connectivity. Radiology. 2014;271(3):839–847. doi: 10.1148/radiol.13131638. [DOI] [PubMed] [Google Scholar]
- Kamson D.O., Pilli V.K., Asano E., Jeong J.W., Sood S., Juhasz C., Chugani H.T. Cortical thickness asymmetries and surgical outcome in neocortical epilepsy. J. Neurol. Sci. 2016;368:97–103. doi: 10.1016/j.jns.2016.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly D.S., O'Leary D.D., Menon R.S., Gati J.S., Shoemaker J.K. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J. Physiol. 2005;569(Pt 1):331–345. doi: 10.1113/jphysiol.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloster R., Engelskjon T. Sudden unexpected death in epilepsy (SUDEP): a clinical perspective and a search for risk factors. J. Neurol. Neurosurg. Psychiatry. 1999;67(4):439–444. doi: 10.1136/jnnp.67.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauss T., Sauerland E.K., Clemente C.D. Cortically induced reflex inhibition following ablation of sensorimotor cortex in the cat. Brain Res. 1968;8(2):373–375. doi: 10.1016/0006-8993(68)90056-5. [DOI] [PubMed] [Google Scholar]
- Kori P., Garg R.K., Malhotra H.S., Gupta R.K., Verma R., Singh M.K., Rathore R.K., Gupta P.K. Evaluation of cerebral white-matter micro-structural alterations in patients with medically refractory epilepsy using diffusion tensor tractography. Epilepsy Res. 2013;107(1–2):82–90. doi: 10.1016/j.eplepsyres.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Kumar R., Woo M.A., Macey P.M., Fonarow G.C., Hamilton M.A., Harper R.M. Brain axonal and myelin evaluation in heart failure. J. Neurol. Sci. 2011;307(1–2):106–113. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Woo M.S., Macey P.M., Woo M.A., Harper R.M. Progressive gray matter changes in patients with congenital central hypoventilation syndrome. Pediatr. Res. 2012;71(6):701–706. doi: 10.1038/pr.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Yadav S.K., Palomares J.A., Park B., Joshi S.H., Ogren J.A., Macey P.M., Fonarow G.C., Harper R.M., Woo M.A. Reduced regional brain cortical thickness in patients with heart failure. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar S.W., Kerr C.E., Wasserman R.H., Gray J.R., Greve D.N., Treadway M.T., McGarvey M., Quinn B.T., Dusek J.A., Benson H., Rauch S.L., Moore C.I., Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman R.J. Bradley's neurology in clinical practice. JAMA. 2012;308(16):1694. [Google Scholar]
- Lin J.J., Salamon N., Lee A.D., Dutton R.A., Geaga J.A., Hayashi K.M., Luders E., Toga A.W., Engel J., Jr., Thompson P.M. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb. Cortex. 2007;17(9):2007–2018. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Loewy A.D. Descending pathways to the sympathetic preganglionic neurons. Prog. Brain Res. 1982;57:267–277. doi: 10.1016/S0079-6123(08)64133-3. [DOI] [PubMed] [Google Scholar]
- Luders E., Narr K.L., Thompson P.M., Rex D.E., Woods R.P., Deluca H., Jancke L., Toga A.W. Gender effects on cortical thickness and the influence of scaling. Hum. Brain Mapp. 2006;27(4):314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Toga A.W., Lepore N., Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. NeuroImage. 2009;45(3):672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Kumar R., Woo M.A., Valladares E.M., Yan-Go F.L., Harper R.M. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Kumar R., Yan-Go F.L., Woo M.A., Harper R.M. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep. 2012;35(12):1603–1613. doi: 10.5665/sleep.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Moiyadi A.S., Kumar R., Woo M.A., Harper R.M. Decreased cortical thickness in central hypoventilation syndrome. Cereb. Cortex. 2012;22(8):1728–1737. doi: 10.1093/cercor/bhr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Rieken N.S., Kumar R., Ogren J.A., Middlekauff H.R., Wu P., Woo M.A., Harper R.M. Sex differences in insular cortex gyri responses to the Valsalva maneuver. Front. Neurol. 2016;7(87) doi: 10.3389/fneur.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey P.M., Rieken N.S., Ogren J.A., Macey K.E., Kumar R., Harper R.M. Sex differences in insular cortex gyri responses to a brief static handgrip challenge. Biol. Sex Differ. 2017;8(13) doi: 10.1186/s13293-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Woollett K., Spiers H.J. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Marcus D.S., Wang T.H., Parker J., Csernansky J.G., Morris J.C., Buckner R.L. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J. Cogn. Neurosci. 2007;19(9):1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- Marks J.D., Frysinger R.C., Harper R.M. State-dependent respiratory depression elicited by stimulation of the orbital frontal cortex. Exp. Neurol. 1987;95(3):714–729. doi: 10.1016/0014-4886(87)90311-6. [DOI] [PubMed] [Google Scholar]
- Mathern G.W., Pretorius J.K., Babb T.L. Quantified patterns of mossy fiber sprouting and neuron densities in hippocampal and lesional seizures. J. Neurosurg. 1995;82(2):211–219. doi: 10.3171/jns.1995.82.2.0211. [DOI] [PubMed] [Google Scholar]
- McCarthy C.S., Ramprashad A., Thompson C., Botti J.A., Coman I.L., Kates W.R. A comparison of FreeSurfer-generated data with and without manual intervention. Front. Neurosci. 2015;9(379):379. doi: 10.3389/fnins.2015.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C.R., Hagler D.J., Jr., Ahmadi M.E., Tecoma E., Iragui V., Gharapetian L., Dale A.M., Halgren E. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49(5):794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- McEvoy L.K., Fennema-Notestine C., Roddey J.C., Hagler D.J., Jr., Holland D., Karow D.S., Pung C.J., Brewer J.B., Dale A.M., Alzheimer's Disease Neuroimaging I. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251(1):195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.R., Jover T., Cohen H.W., Zukin R.S., Etgen A.M. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146(7):3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Moga M.M., Herbert H., Hurley K.M., Yasui Y., Gray T.S., Saper C.B. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J. Comp. Neurol. 1990;295(4):624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Ogren J.A., Fonarow G.C., Woo M.A. Cerebral impairment in heart failure. Curr. Heart. Fail. Rep. 2014;11(3):321–329. doi: 10.1007/s11897-014-0211-y. [DOI] [PubMed] [Google Scholar]
- Ogren J.A., Kumar R., Eliashiv D.S., Stern J.M., Keselman I., Engel J., Jr., Macey P.M., Diehl B., Lhatoo S.D., Harper R.M. 70th Annual Meeting of the American Epilepsy Society. 2016. Insular, cingulate, and autonomic structure texture alterations in patients with generalized tonic-clonic seizures. (Houston, TX) [Google Scholar]
- Ogren, J.A., Kumar, R., Stern, J.M., Eliashiv, D.S., Keselman, I., Engel, J., Jr., Diehl, B., Lhatoo, S.D., Harper, R.M., 2016b. Reduced entropy in patients with generalized tonic-clonic seizures. 46th Annual Meeting of the Society for Neuroscience, San Diego, CA.
- Ohtake P.J., Jennings D.B. Ventilation is stimulated by small reductions in arterial pressure in the awake dog. J. Appl. Physiol. 1992;73(4):1549–1557. doi: 10.1152/jappl.1992.73.4.1549. (1985) [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. Forebrain lateralization and the cardiovascular correlates of epilepsy. Brain. 2001;124(Pt 12):2345–2346. doi: 10.1093/brain/124.12.2345. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.M., Wilson J.X., Guiraudon C., Cechetto D.F. Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Res. 1991;550(1):115–121. doi: 10.1016/0006-8993(91)90412-o. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.M., Gelb A., Girvin J.P., Hachinski V.C. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.M., Kedem G., Martin W.M. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin. Auton. Res. 1996;6(3):131–140. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- Pardoe H.R., Berg A.T., Jackson G.D. Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology. 2013;80(20):1895–1900. doi: 10.1212/WNL.0b013e318292a2e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B., Palomares J.A., Woo M.A., Kang D.W., Macey P.M., Yan-Go F.L., Harper R.M., Kumar R. Aberrant insular functional network integrity in patients with obstructive sleep apnea. Sleep. 2016;39(5):989–1000. doi: 10.5665/sleep.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P., Nashef L., Lhatoo S.D., Bateman L.M., Bird J., Bleasel A., Boon P., Crespel A., Dworetzky B.A., Hogenhaven H., Lerche H., Maillard L., Malter M.P., Marchal C., Murthy J.M., Nitsche M., Pataraia E., Rabben T., Rheims S., Sadzot B., Schulze-Bonhage A., Seyal M., So E.L., Spitz M., Szucs A., Tan M., Tao J.X., Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- Salin P., Tseng G.F., Hoffman S., Parada I., Prince D.A. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J. Neurosci. 1995;15(12):8234–8245. doi: 10.1523/JNEUROSCI.15-12-08234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Shinohara R.T., Wolf D.H., Hopson R.D., Elliott M.A., Vandekar S.N., Ruparel K., Calkins M.E., Roalf D.R., Gennatas E.D., Jackson C., Erus G., Prabhakaran K., Davatzikos C., Detre J.A., Hakonarson H., Gur R.C., Gur R.E. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc. Natl. Acad. Sci. U. S. A. 2014;111(23):8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland E.K., Knauss T., Nakamura Y., Clemente C.D. Inhibition of monosynaptic and polysynaptic reflexes and muscle tone by electrical stimulation of the cerebral cortex. Exp. Neurol. 1967;17(2):159–171. doi: 10.1016/0014-4886(67)90142-2. [DOI] [PubMed] [Google Scholar]
- Scheibel M.E., Crandall P.H., Scheibel A.B. The hippocampal-dentate complex in temporal lobe epilepsy. A Golgi study. Epilepsia. 1974;15(1):55–80. doi: 10.1111/j.1528-1157.1974.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Seifert F., Kallmunzer B., Gutjahr I., Breuer L., Winder K., Kaschka I., Kloska S., Doerfler A., Hilz M.J., Schwab S., Kohrmann M. Neuroanatomical correlates of severe cardiac arrhythmias in acute ischemic stroke. J. Neurol. 2015;262(5):1182–1190. doi: 10.1007/s00415-015-7684-9. [DOI] [PubMed] [Google Scholar]
- Singer C.A., Figueroa-Masot X.A., Batchelor R.H., Dorsa D.M. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 1999;19(7):2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Du H., Wu N., Hou B., Wu G., Wang J., Feng H., Jiang T. Impaired resting-state functional integrations within default mode network of generalized tonic-clonic seizures epilepsy. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0017294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R., Xu D., Zhu H., Thompson P.M., Toga A.W. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra V.C., Cysneiros R., Cavalheiro E.A., Scorza F.A. Sudden unexpected death in epilepsy: from the lab to the clinic setting. Epilepsy Behav. 2013;26(3):415–420. doi: 10.1016/j.yebeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Terreberry R.R., Neafsey E.J. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res. Bull. 1987;19(6):639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Tolstykh G.P., Cavazos J.E. Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav. 2013;26(3):410–414. doi: 10.1016/j.yebeh.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Trelease R.B., Sieck G.C., Marks J.D., Harper R.M. Respiratory inhibition induced by transient hypertension during sleep in unrestrained cats. Exp. Neurol. 1985;90(1):173–186. doi: 10.1016/0014-4886(85)90050-0. [DOI] [PubMed] [Google Scholar]
- van Haren N.E., Schnack H.G., Cahn W., van den Heuvel M.P., Lepage C., Collins L., Evans A.C., Hulshoff Pol H.E., Kahn R.S. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry. 2011;68(9):871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- Verberne A.J., Owens N.C. Cortical modulation of the cardiovascular system. Prog. Neurobiol. 1998;54(2):149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Walczak T.S., Leppik I.E., D'Amelio M., Rarick J., So E., Ahman P., Ruggles K., Cascino G.D., Annegers J.F., Hauser W.A. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56(4):519–525. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- Wandschneider B., Koepp M., Scott C., Micallef C., Balestrini S., Sisodiya S.M., Thom M., Harper R.M., Sander J.W., Vos S.B., Duncan J.S., Lhatoo S., Diehl B. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain. 2015 doi: 10.1093/brain/awv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhaus M.J., Loewy A.D. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903(1–2):117–127. doi: 10.1016/s0006-8993(01)02453-2. [DOI] [PubMed] [Google Scholar]
- Whitehead R.W., McNiel E.E. The therapeutic effects of estrogenic hormone preparations in certain cases of idiopathic epilepsy and in migraine. Am. J. Psychiatr. 1935;91(6):1275–1288. [Google Scholar]
- Woo M.A., Kumar R., Macey P.M., Fonarow G.C., Harper R.M. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J. Card. Fail. 2009;15(3):214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.Q., Cui L., Lhatoo S., Schuele S.U., Sahoo S.S. MEDCIS: multi-modality epilepsy data capture and integration system. AMIA Annu. Symp. Proc. 2014;(2014):1248–1257. [PMC free article] [PubMed] [Google Scholar]
- Zhang G.Q., Tao S., Xing G., Mozes J., Zonjy B., Lhatoo S.D., Cui L. NHash: randomized N-gram hashing for distributed generation of validatable unique study identifiers in multicenter research. JMIR Med. Inform. 2015;3(4) doi: 10.2196/medinform.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]