Abstract
Breast Cancer (BC) encompasses numerous entities with different biological and behavioral characteristics, favored by tumor molecular complexity. Azadirachta indica (neem) presents phenolic compounds, indicating its potential as an antineoplastic compound. The present study aimed to evaluate the cellular response of MCF10, MCF7, and MDA-MB-231 breast cell lines to ethanolic extracts of neem leaves (EENL) obtained by dichloromethane (DCM) and ethyl acetate (EA) solvent. Extracts’ antiproliferative activities were evaluated against MCF 10A, MCF7, and MDA-MB-231 for 24 and 48 h using MTT assay. ESR1, ESR2, AR, AR-V1, AR-V4, and AR-V7 transcripts were quantified through qPCR for 0.03125 μg/mL of DCM and 1.0 μg/mL for EA for 48 h. The EENL was tested on Drosophila melanogaster as a sole treatment and then also together with doxorubicin. Antiproliferative effect on tumor cell lines without affecting MCF 10A were 1.0 µg/mL (P < 0.001) for EA, and 0.03125 µg/mL (P < 0.0001) for DCM, both after 48 h. Transcriptional levels of AR-V7 increased after treatment. In vivo assays demonstrated that EENL induced fewer tumors at a higher concentration with doxorubicin (DXR). The behavior of AR-V7 in the MDA-MB-231 tumor lineage indicates new pathways involved in tumor biology and this may have therapeutic value for cancer.
Keywords: breast cancer, Azadirachta indica, neem, ethanolic extracts, androgen receptor variant-7
1. Introduction
According to the World Health Organization (WHO) [1], Breast Cancer (BC) is the most common malignant tumor among women. An increase from 14 million in 2012 to 22 million new cases is estimated by 2022. By 2035, the number of deaths is expected to grow by 70% [2]. BC is a disease that encompasses numerous entities, with peculiar biological and behavioral characteristics favored by a complex molecular microenvironment [3].
Malignancy results from regulatory imbalances involving pathways closely related to growth and proliferation [4,5,6,7]. In this context, receptors for estrogen and androgenic hormones are important proteins in cancer progression and play key roles in deciding the appropriate treatments [8].
Estrogen hormone binds to receptors on the nucleus membrane and regulates the expression of genes associated with survival, proliferation, and differentiation of mammary cells [9]. The estrogen receptor alpha (ESR1) is the main receptor responsible for these events, thus hormone therapy is considered when tumors display this receptor. However, therapies using the ESR1 as a target may cause some patients to become more resistant [8,10]. The ESR1 protein forms a homodimer or a heterodimer with the protein ESR2 (estrogen receptor beta) and is responsible for regulating gene function [11,12,13].
The AR gene encodes for the androgen receptor, which is a transcription factor activated by a steroid hormone [14,15]. Structurally related to ESR1, this protein is expressed in 80% of breast tumors, of which 55% are ESR1-positive and 35% are classified as triple-negative tumors (negative for ESR, progesterone receptor, and HER2 receptor). In recent years, 18 AR variants (AR-V1 to AR-V18) have been described and characterized, especially focusing on their role in disease progression [16,17]. In prostate cancer, AR-V7 is involved in cancer cell growth in the absence of androgens, which represents a highly advanced form of the disease [14,15,18,19]. From a clinical perspective, AR can be a favorable prognostic indicator [20], but its role in BC needs a deeper understanding [21,22,23,24].
In light of the regulatory role of ESR and AR, agents able to modulate these receptors’ gene expression emerges as a fundamental strategy for tumor aggressiveness control, and could potentially be used as new therapies.
Brazil has approximately 25% of the world’s biodiversity, providing great opportunities for the development of cancer drugs and therapies [25]. Different natural products present antitumor properties, endorsing the importance of scientific studies that elucidate their mode of action [26,27,28,29].
Among these diverse plants, the extracts from the species Azadirachta indica A. Juss., commonly known as “neem”, have been used for the treatment of inflammation, viral infections, hypertension, and displays insecticidal, nematicide, and fungicidal properties [30,31,32,33]. Although the bioactive compounds present in neem are found in different tissues of this plant, those from their seeds and leaves are more concentrated, accessible, and easily obtained by water or organic solvents extraction methods, such as those that use hydrocarbons, alcohols, ketones, or ethers [34,35].
Considering that natural phytochemicals contain phenolic compounds with antimetastatic activity [36,37,38,39], A. indica should be investigated in cancer research, since phenolic compounds were found in this species [40]. Balasenthil et al. (1999) [41] demonstrated that neem leaves extract administered to hamsters with oral carcinoma promoted tumor suppression by modulating lipid peroxidation, antioxidant action, and detoxification. Leaves of this species are also capable of activating an immune response [42]. It has also been reported that flavones isolated from neem flowers have antimutagenic effects by inhibition of the enzymatic activation of heterocyclic amines [43].
In this study, we hypothesized that ethanolic extracts from Azadirachta indica leaves (EENL) obtained by dichloromethane (DCM) or ethyl acetate (EA) extraction could modulate the expression of estrogen and androgen receptors, thus promoting molecular changes that would hinder the mammary tumor activity. Therefore, our goal was to evaluate the cytotoxic and mutagenic effects of the extracts and their effect on the expression of genes coding for the hormonal receptors in the lineages MCF 10A (non-tumorigenic), MCF7 (ESR + BC), and MDA-MB-231 (triple-negative BC [TNBC]).
2. Results
2.1. Bioactive Compounds and Antiproliferative Effects of EENL
Total phenols of DCM and EA extracts were calculated according to the standard curve of gallic acid equivalents (GAE) subjected to a linear regression. Concentrations of this bioactive compound were 40.415 ± 0.566 mg GAE/g and 45.200 ± 0.569 mg GAE/g for EA and DCM, respectively (P < 0.01). Antiproliferative activity of EENL extracts was further investigated in three breast lineages (MCF 10A, MCF7, and MDA-MB-231) through MTT assay.
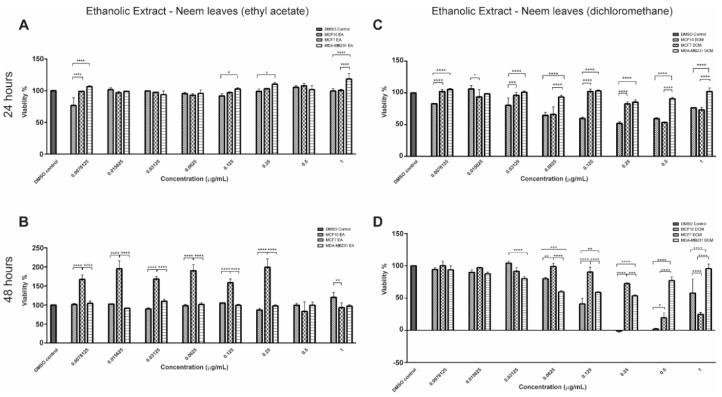
EENL–EA did not reduce the proliferation of breast cancer cell lines (Figure 1A) after 24 h of treatment. The non-tumorigenic lineage was more sensitive to the EA extract at 0.0078125 μg/mL, 0.125 μg/mL, 0.25 μg/mL, and 1.0 μg/mL. In addition, the viability of MCF7 increased after treatment at 0.0078125 μg/mL up until 0.25 μg/mL for 48 h (Figure 1B). However, in the highest concentration, MCF7 viability decreased compared to MCF 10A (P < 0.001).
Figure 1.
Effect of Ethanolic Extract of Neem Leaves (EENL) prepared from A. indica on breast cells (MCF 10A, MCF7, and MDA-MB-231) proliferation. (A,B) Treatment with EENL obtained with Ethyl Acetate (EENL–EA) extract for 24 and 48 h, respectively. (C,D) Treatment with EENL extract obtained using dichloromethane (EENL–DCM) for 24 and 48 h respectively. * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001.
The treatment with EENL–DCM extract for 24 h reduced the proliferation of MCF7 compared to MCF 10A (P < 0.05) at 0.015625 μg/mL (Figure 1C). After 48 h (Figure 1D), DCM extract inhibited the proliferation of the triple-negative tumor cell line (MDA-MB-231), with extracts’ concentrations ranging from 0.0625 to 0.25 μg/mL, when compared to MCF7 lineage. Compared to MCF 10A, the ESR + tumor cell line (MCF7) showed a reduction in viability at a concentration of 1.0 μg/mL. Only at the concentration 0.03125 μg/mL and 0.0625 were there significant differences (P < 0.001) between the non-tumorigenic lineage (MCF 10A) and the triple-negative cells’ (MDA-MB-231) viability.
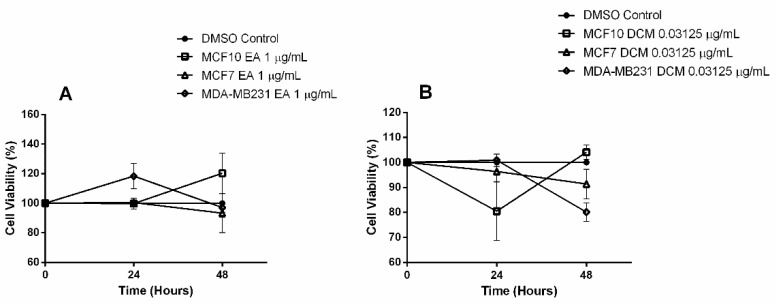
Based on these results, the concentrations that showed antiproliferative effect on tumor cell lines, but did not decrease MCF 10A viability, were chosen for molecular assays. For EENL–EA extract we used 1.0 µg/mL (P < 0.001), and for EENL–DCM extract 0.03125 µg/mL (P < 0.0001) after 48 h of treatment, as demonstrated in the time-course Figure 2.
Figure 2.
Time-course response to Ethanolic Extract of Neem Leaves (EENL). Treatment with 1.0 μg/mL of EENL obtained with ethyl acetate (EENL–EA) (A), and EENL obtained with dichloromethane (EENL–DCM) (B).
2.2. Transcriptional Profile of Hormone Receptors after Treatment with EENL
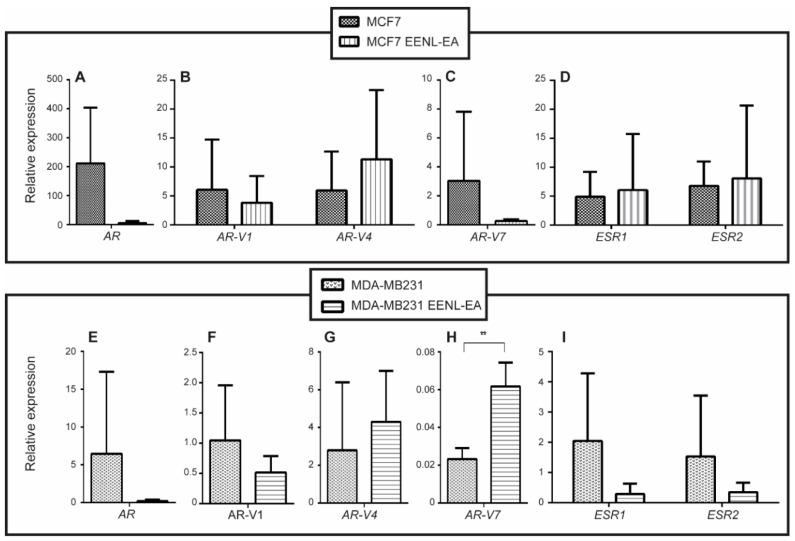
Gene expression of ESR1, ESR2, AR, AR-V1, AR-V4, and AR-V7 in breast cells was evaluated before and after 48 h of treatment with 1.0 μg/mL of EENL–EA extract (Figure 3). AR-V7 expression increased 2.85-fold (P < 0.01) in treated MDA-MB-231 cells after 48 h (Figure 3H). The remaining genes in MDA-MB-231, and all genes in MCF7, did not display statistically significant differences in gene expression levels. Although not significant, the mean AR, AR-V1, ESR1, and ESR2 relative gene expression levels were higher in 48 h-treated MDA-MB-231 cells (31.74, 2.03, 7.07, and 4.37-fold, respectively).
Figure 3.
Relative expression of hormone receptors transcripts after treatment with Ethanolic Extract of Neem Leaves (EENL). Cell lines were treated with 1.0 µg/mL of EENL obtained with ethyl acetate (EENL–EA) for 48 h. Relative gene expression levels were evaluated for AR (A), AR-V1, AR-V4 (B), AR-V7 (C), ESR1 and ESR2 (D) in MCF7 cell line. In MDA-MB-231 the gene expression levels were evaluated for AR (E), AR-V1 (F), AR-V4 (G), AR-V7 (H), ESR1 and ESR2 (I). The analyses were performed using the comparative Cq calibrated with data from MCF 10A and ** p < 0.01.
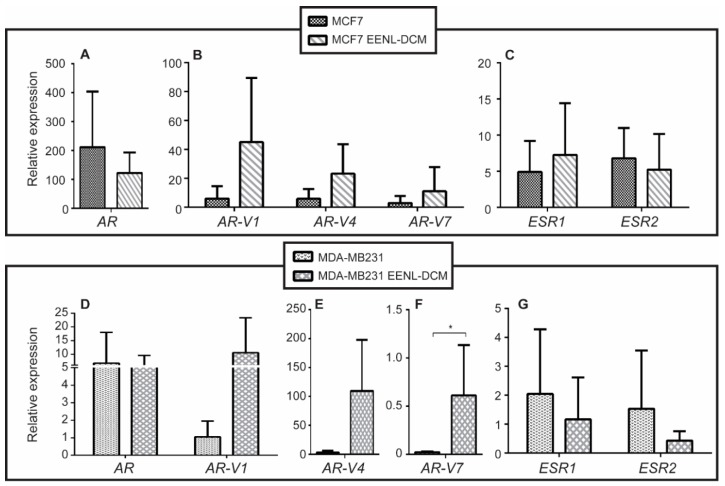
No significant gene expression changes were found in MCF7 cells treated with EENL–DCM extract at 0.03125 μg/mL for 48 h (Figure 4). However, the TNBC cell line MDA-MB-231 had a 28.41-fold increase on the expression of AR-V7 upon treatment (P < 0.05) (Figure 4F).
Figure 4.
Relative expression of hormone receptors genes after treatment with Ethanolic Extract of Neem Leaves (EENL). Cell lines were treated with 0.03125 µg/mL of EENL obtained using dichloromethane (EENL–DCM) for 48 h. Relative gene expression levels were evaluated for AR (A), AR-V1, AR-V4, and AR-V7 (B), ESR1 and ESR2 (C) in MCF7 cell line. In MDA-MB-231 the relative gene expression levels were evaluated for AR and AR-V1 (D), AR-V4 (E), AR-V7 (F), ESR1 and ESR2 (G). The analyses were performed using the comparative Cq calibrated with data from MCF10 * P < 0.05.
2.3. In Vivo Experiments with Drosophila Melanogaster
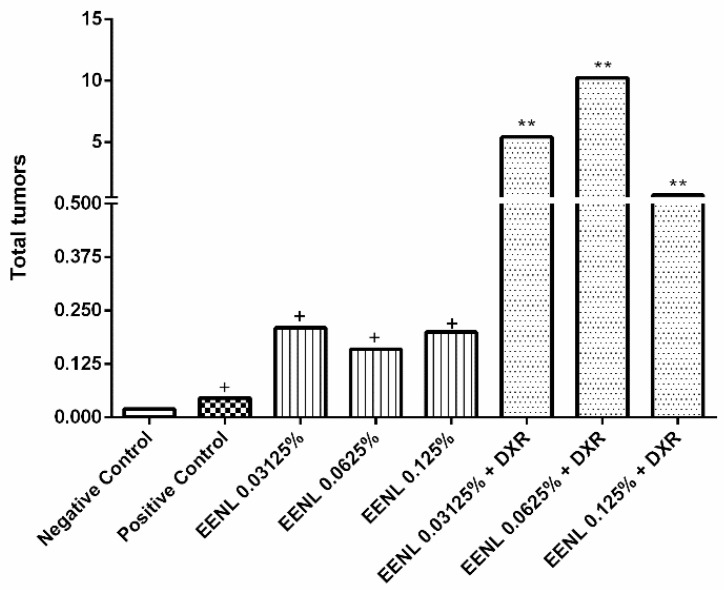
The results showed the carcinogenic action of EENL isolate at the concentrations 0.03125%, 0.0625%, and 0.125%, and the modulating effect of the EENL on the carcinogenic action of doxorubicin (DXR at 0.4 mM). DXR may intercalate on DNA and induce formation of DNA adducts at active promoter sites, increasing torsional stress and enhancing nucleosome turnover. Furthermore, it may trap topoisomerase II at breakage sites, causing double strand breaks. Enhanced nucleosome turnover might increase the exposure of DNA to reactive oxygen species (ROS) resulting in DNA damage and cell death [44]. The frequency of the tumor clone per segment of Drosophila melanogaster is demonstrated in Table 1 and Figure 5.
Table 1.
Frequency of tumors in the different segments of Drosophila melanogaster. The flies used in this study are heterozygous for the warts tumor suppressor gene and were treated with different concentrations of isolated Ethanolic Extract from Neem Leaves (EENL) and EENL associated with doxorubicin (DXR).
| Treatments | Indiv. (N) | Tumors | ||||||
|---|---|---|---|---|---|---|---|---|
| Eyes | Head | Wings | Body | Legs | Halteres | Total Tumors | ||
| Negative Control | 203 | 0.00 (00) | 0.00 (01) | 0.00 (00) | 0.00 (01) | 0.01 (02) | 0.00 (01) | 0.02 (05) |
| Positive Control | 176 | 0.01 (02) | 0.04 (07) | 0.20 (36) | 0.15 (26) | 0.05 (09) | 0.01 (01) | 0.46 (81) + |
| EENL 0.03125% | 200 | 0.00 (00) | 0.01 (02) | 0.10 (20) | 0.04 (08) | 0.03 (06) | 0.03 (05) | 0.21 (41) + |
| EENL 0.0625% | 200 | 0.02 (03) | 0.01 (02) | 0.07 (13) | 0.03 (06) | 0.04 (07) | 0.01 (01) | 0.16 (32) + |
| EENL 0.125% | 218 | 0.00 (00) | 0.01 (02) | 0.04 (08) | 0.13 (29) | 0.01 (03) | 0.01 (05) | 0.20 (44) + |
| EENL 0.03125% + DXR | 200 | 0.01 (01) | 0.26 (52) | 2.08 (416) | 0.98 (196) | 1.92 (384) | 0.19 (37) | 5.43 (1086) ** |
| EENL 0.0624% + DXR | 200 | 0.09 (18) | 0.59 (117) | 3.65 (730) | 1.82 (363) | 3.65 (729) | 0.45 (90) | 10.24 (2047) ** |
| EENL 0.125% + DXR | 165 | 0.03 (05) | 0.06 (10) | 0.24 (39) | 0.28 (46) | 0.06 (10) | 0.02 (04) | 0.69 (114) ** |
Statistical diagnosis according to the Mann–Whitney U-Test. Level of significance P ≤ 0.05. + value considered statistically different from the negative control (P ≤ 0.05). ** value considered statistically different from the positive control (DXR 0.4 mM) (P ≤ 0.05). DXR = doxorubicin (0.4 mM). EENL = Ethanolic Extract from Neem Leaf.
Figure 5.
Frequency of tumors in Drosophila melanogaster. Drosophila melanogaster heterozygous for the warts tumor suppressor gene were treated with different concentrations of isolated Ethanolic Extract from Neem Leaves (EENL) (0.03125%, 0.0625%, and 0.125%) and associated with doxorubicin (DXR) (0.03125%, 0.625%, and 0.125%). The frequency of tumors was analyzed in different segments (eyes, head, wings, body, legs, and halteres). Statistical analysis was according to Mann–Whitney U-test with a significance level of P < 0.05. + value considered statistically different from the negative control (P < 0.05) and ** value considered statistically different from the positive control (DXR 0.4 mM) (P < 0.05).
The study design included a negative control, which was flies with mutated gene; and DXR as a positive control. For the negative control, the frequency of 0.02 of tumors per fly were observed, and this discrete tumor induction occurs due to the genetic predisposition of the test organism. On the other hand, the positive control induced a frequency of 0.46 of tumors per fly, proving that the organism lineage responded to tumor induction.
Larvae that were exposed to the EENL isolate, at concentrations of 0.03125%, 0.0625%, and 0.125% displayed frequencies of 0.21, 0.16, and 0.20 tumors per fly, respectively. Compared to the negative control, a statistically significant increase in tumor induction confirmed the carcinogenic effect of the extract on D. melanogaster. When 0.03125%, 0.0625%, and 0.125% of EENL were applied together with doxorubicin at 0.4 mM, the frequencies of tumors per fly were 5.43, 10.24, and 0.69, respectively. These results demonstrate that EENL with DXR increased tumor frequency at the lowest concentrations, when compared to the positive control. However, in the highest concentration used (0.125%), even when associated with DXR 0.4 mM, the tumor frequency decreased.
3. Discussion
The association between phenolic compounds in plants and antioxidant activity is due to phenolic hydroxyl groups that have a strong free radical scavenger activity [45,46,47,48,49,50]. Abdelhady et al. (2015) [40], during a phytochemical search for active substances, demonstrated that several phenolic compounds are present in Azadirachta indica.
In fact, natural phytochemicals contain phenolic compounds with the ability to prevent cancer metastasis [36]. Several studies have shown that different flavonoids and polyphenols exert an anti-metastatic effect [37,38,39]. Here we evaluated the cellular effects of ethanolic extracts of neem in breast cell lines. The presence of phenolic compounds in the EENL–DCM and EENL–EA demonstrate their potential antiproliferative activity, which may reduce tumor aggressiveness. The different cellular responses, evaluated by the MTT reduction, after treatments with EENL extracts obtained using DCM and EA, indicated this behavior. For the assays with Drosophila melanogaster, the EENL extracts induced higher tumors at a higher concentration when applied with DXR, probably due to the toxic effect of EENL together with the chemotherapeutic DXR, inducing cell death, which makes the appearance of tumors unfeasible.
According to Paul et al. (2011) [51], the major secondary metabolites present in the Azadirachta indica leaves are nimbolide, vilasinin, nimbinene, 6-deacetyl nimbinene, nimbandiol, nimocinol, β-sitosterol, β-sitosterol-β-d-glicoside, neem leaf glycoprotein, quercetin, glycoside of quercetin, glycoside of kaemferol, quercetin-3-galactoside (hyperin), and rutin. Nimbolide is the most abundant tetranortriterpenoid isolated from leaves of A. indica, showing apoptotic and antiproliferative activity acting in the following pathways: (1) oxidative stress and caspase activation; (2) reduction of the expression of anti-apoptotic proteins (Bcl-xl; Bcl-2) and increasing the expression of pro-apoptotic proteins (Bax, Bad, Bid, cytochrome c); (3) activation of the tumor suppressor p53; (4) activation of the extrinsic apoptosis pathway; (5) inhibition of IGF-1; (6) reducing levels of cyclin-dependent kinase (CDKs) and cyclins, promoting cell cycle arrest; and (7) inhibition of NFκB and its pro-tumorigenesis pathway [52,53,54]. The cytotoxicity profile verified in the MTT assays may also be attributed to the probable induction of apoptosis by the EENL. MCF7 cells were more sensitive to high concentrations of EENL. This behavior was previously observed in prostate cancer, in which hormone-responsive cells (LNCaP) were more sensitive to treatment with this extract [55]. Therefore, analysis of the gene expression, especially of hormone receptor genes, may indicate molecular patterns involved in EENL modulation in breast tumor cells.
The results of Aleskandarany et al. (2016) [56] have shown that there is an association between the expression of AR and good prognosis in BC. Comparing AR expression in HER+, TNBC, and luminal tumors, they observed that luminal BC presented a higher receptor expression. However, AR is an oncogene in triple-negative tumors by “replacing” the estrogen receptor and stimulating tumor growth [57]. After treatment with EENL we observed a decrease of the AR transcripts in the MDA-MB-231 line. This effect, therefore, strongly suggests a possible action of EENL in triple-negative tumors and patients treated with this phenolic compound would have a better prognosis considering the behavior of AR signaling in TNBC [58].
After treatment with EENL–EA and EENL–DCM we detected an increase in AR-V7 transcripts in MDA-M-231. The AR-V7 gene is expressed in primary BC and in breast tumor cell lines, which can promote growth and mediate resistance in androgen deprivation therapies (ADT) in BC subsets [59,60,61]. In this context, the expression of AR and its variants emerge as a new strategy for BC treatment.
The effects of AR are directly linked to the ESR pathway [62,63]. ESR+ breast tumor cells, such as MCF7, have the growth stimulated by androgens and inhibited by the antiandrogens [64,65]. AR antagonizes the ESR growth by: (1) directly inhibiting ESR target genes; (2) competing with ESR for binding in the estrogen responsive elements (ERE); (3) sequestering transcriptional factors (TFs); and (4) inducing apoptosis by direct negative regulation of cyclin D1 gene expression [62,66,67].
Taken together, our results demonstrate a differential effect of EENL in breast tumor cell lines (MCF7 and MDA-MB-231), specially modulating nuclear receptor expression. The behavior of AR-V7 in the MDA-MB-231 tumor cell line indicates new pathways involved in tumor biology, especially as a therapeutic target. Further studies are needed to better understand the role of these compounds in the modulation of such receptors.
4. Materials and Methods
4.1. Plant Material
The Azadirachta indica (neem) leaves were collected at latitude 18°34′13.99″ S and longitude 46°29′52.57″. The present study was registered in the Genetic Patrimony Management Board (CGEN) (number A2FFD84) and a voucher specimen was recorded in the Herbarium of Institute of Biology of the Federal University of Uberlandia with the number 71869. After identification, the neem leaves were sanitized and oven-dried (Lucaderma), for 72 h at 40 °C. The dry leaves were further milled in a knife mill (Solab).
4.2. Ethanolic Extraction and Determination of Phenolic Compounds
Flavonoids and phenolic compounds were extracted according to Cechinel-Filho and Yunes method (1998) [68]. Dried and milled leaves (100 g) were stirred for homogenization in a solution of methanol 50% and protected from light for 20 days.
Extractions were further performed with triple addition of 30 mL of ethyl acetate (EA) P.A. (Proquímicos), and dichloromethane (DCM) P.A. (Alphatec, Carlsbad, CA, USA), separately. After filtration, the filtrates were evaporated in rotary evaporator at 55 °C with reduced pressure and resuspended with DMSO (Sigma Aldrich, St. Louis, MO, USA). All experiments were conducted in six replicates. For in vivo assays, EENL were obtained according to Silva (2010) [69].
Total phenolic compounds (mg gallic acid equivalents (GAE)/g DW) were determined by the Folin–Ciocalteu method [70]. Absorbance analysis was performed in spectrophotometer UV-VIS at the wavelength of 760 nm and dosage was adopted from Swain and Hillis (1959) [71].
4.3. In Vitro Assays
4.3.1. Breast Cell Lines
MCF 10A (non-tumorigenic), MCF7 (ESR + BC), and MDA-MB-231 (triple-negative phenotype) breast cells lines were obtained from ATCC and cultured with Dulbecco’s Modified Eagle Medium (DMEM) (Cultilab), Iscove’s Modified Dulbecco’s Medium (IMDM) (Cultilab), and Leibovitz’s L-15 Medium (Sigma Aldrich), respectively. MCF 10A was supplemented with 10% of fetal bovine serum (FBS), 20 ng/mL of epidermal growth factor (EGF), 500 ng/mL of hydrocortisone, 10 µg/mL of insulin, and 50 μg/L of gentamycin. The malignant lineages were supplemented with 10% of FBS and 50 μg/L of gentamycin. All lineages were incubated at 37 °C in a humidified atmosphere containing 5% CO2 atmosphere until they reached 80% of confluence.
4.3.2. Antiproliferative Assay
The antiproliferative effect of the Ethanolic Extract from Neem Leaves (EENL) on the three lineages was evaluated by MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) according to Mosmann (1983) [72]. After confluence, 104 cells per well were seeded onto 96 well plates and incubated for 24 h at 37 °C and 5% CO2 atmosphere for adherence.
Treatments were performed in eight concentrations of EENL–EA and EENL–DCM (1 μg/mL; 0.5 μg/mL; 0.25 μg/mL; 0.125 μg/mL; 0.0625 μg/mL; 0.03125 μg/mL; 0.015625 μg/mL; and 0.0078125 μg/mL) for 24 and 48 h. Controls included groups treated with DMSO according to each concentration of the extracts, untreated cells (positive control), and wells with only medium (negative control).
After incubation, 10 µL of MTT (5 mg/mL in PBS) (Sigma Chemical Co., St. Louis, MO, USA) was added in each well and incubated for 4 h. Formazan crystals were dissolved in a Sodium Dodecyl Sulfate (SDS) solution containing N-dimethylformamide at 50%. The absorbance was measured at the wavelength of 570 nm in a Thermo Plate Reader (TP-Reader), and the percentage of viable cells was calculated by using the formula in Equation (1).
| (1) |
SA = Sample A570nm (treated cells)
PC = Positive Control A570nm (untreated cells)
NC = Negative Control A570nm (culture medium only)
DMSO = DMSO Control A570nm
4.3.3. Quantitative Real-Time PCR
Total RNA was extracted before and after treatments with EENL–EA (1.0 μg/mL) and EENL–DCM (0.03125 μg/mL) for 48 h. Trizol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) was used for extraction according to the manufacturer’s recommendations. After isolation, RNA quality was electrophoretic confirmed (1.5% agarose gel) and the concentration was determined using the NanoDrop System (Thermo Fisher Scientific). Reverse transcription was performed as previously described [73].
Quantitative qPCR was performed to evaluate gene expression profiles of ESR1, ESR2, AR, AR-V1, AR-V4, and AR-V7. For normalization, Beta-2-microglobulin (B2M) gene was used and Cq method was validated through relative standard curve construction.
Mix solutions containing 5 µL of Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA), 5 pmol/µL of primers (Table 2), and 2 µL of cDNA were prepared and gene expression profiles were analyzed in a 7300 Real Time PCR System (Applied Biosystems). Data from MCF 10A was used as calibrator.
Table 2.
Oligonucleotide sequences used for qPCR assays.
| GENE | Primers sequence (Forward/Reverse) (5′–3′) |
|---|---|
| β2M [73] | F: CCTGCCGTGTGAACCATGT/R: GCGGCATCTTCAAACCTCC |
| ESR1 | F: CTAACTTGCTCTTGGACAGGAAC/R: GATTTGAGGCACACAAACTCCTC |
| ESR2 | F: GGGAATGGTGAAGTGTGGCT/R: TCATGTGTACCAACTCCTTGTCGG |
| AR | F: CATGTGGAAGCTGCAAGGTCT/R: GTGTAAGTTGCGGAAGCCAGG |
| AR-V1 [74] | F: CTACTCCGGACCTTACGGGGACATGCG/R: GATTCTTTCAGAAACAACAACAGCTGCT |
| AR-V4 [74] | F: CTACTCCGGACCTTACGGGGACATGCG/R: CTTTTAATTTGTTCATTCTGAAAAATCCTC |
| AR-V7 [74] | F: CTACTCCGGACCTTACGGGGACATGCG/R: TGCCAACCCGGAATTTTTCTCCC |
4.4. In Vivo Assays
Drosophila melanogaster is a well-established insect model for human diseases and toxicological research due to its well-documented genetics, developmental biology, and capacity to activate enzymatically promutagens and procarcinogens in vivo [75].
The Drosophila tumor suppressor gene warts (wts) encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Homozygous loss of the wts gene of Drosophila, caused by mitotic recombination in somatic cells, leads to the formation of tumors, with aspects of warts appearing on the body of adult flies [76].
4.4.1. Chemical Agents
Doxorubicin (DXR; CAS 25316-40-9) was used as positive control, commercially known as ADRIBLASTINA®, manufactured and packaged by Actavis Italy S.p.A–Nerviano, Milan–Italy; registered, imported and distributed by Laboratories Pfizer LTDA, Pharmacist responsible—José Cláudio Bumerad—CRF-SP n° 43746, CNPJ n° 46070 868/0001-69. Ethanol 2.38% was used as the negative control and to dilute the EENL. DXR, in the concentration used in this work, previously generated reactive oxygen species, induced homologous recombination in D. melanogaster, and tumor formation [77,78,79,80,81].
4.4.2. Test for the Detection of Epithelial Tumor Clones (Warts)
The test warts (wts) was performed using two strains of Drosophila melanogaster (Diptera: Drosophilidae): (1) Warts lineage (wts/TM3, Sb1) presents a lethal allele wts, on chromosome three, balanced by a chromosome TM3, which is characterized by multiple inversions and marked by dominant mutated stubble (Sb). The stubble mutation is phenotypically characterized by short and thicker hair identified all over the body of the fly. This lineage was kindly provided by the Bloomington Drosophila Stock Center, from Indiana University-USA, registered: Bloomington/7052; (2) Lineage flies multiple wing hairs (mwh) has the marker gene on the chromosome 3 (3-0.3) in a distal position, which is characterized by expressing three or more hairs in each cell. This lineage was kindly provided by Dr. Ulrich Graf (Physiology and Animal Husbandry, Institute of Animal Science, ETH Zurich, Schwerzenbach, Switzerland) [75].
Stocks are grown at the Laboratory of Cytogenetics and Mutagenesis (University Center of Patos de Minas-UNIPAM, Brazil), kept in jars with culture medium for D. melanogaster. The medium is composed of 820 mL of water, 25 g of yeast powder (Sacchoromyces cerevisae), 11 g of agar, 156 g of banana, and 1 g of nipagin. The lineages are maintained inside an incubator B.O.D. 411 D, under light/dark cycles (12 h/12 h), at 25 ± 1 °C and approximately 60% humidity.
4.4.3. Experimental Procedure
To obtain wts +/+ mwh heterozygotic larvae, virgin females wts/TM3, Sb1 [82] are crossed with mwh/mwh males [83]. The eggs of the descendants were collected for 8 h in flasks containing a culture medium suitable for posture (4% w/v agar-agar based, topped with a thick layer of live baker’s yeast supplemented with sucrose). After 72 ± 4 h, third-instar larvae were washed in reverse osmosis water and collected using a fine mesh sieve. The larvae were transferred to a 25 mL vial containing 1.5 g of instant mashed potatoes (HIARI® brand, São Paulo, Brazil) culture medium. Five milliliters of EENL (in the concentrations 0.03125%, 0.0625%, and 0.125%), diluted in 2.38% ethanol, were added to each tube on its own as well as together with DXR. A co-treatment system was used to apply DXR and EENL. Ethanol 2.38% was used as negative control, and DXR 0.4 mM as the positive control (Table 3). The treatments were performed in quadruplicate.
Table 3.
Drosophila melanogaster larvae treated with different concentrations of EENL. EENL were used isolated and associated with doxorubicin.
| Treatments | Concentrations | Composition |
|---|---|---|
| Negative control (−) | - | MP + 5 mL ROW + 2.38% of ethanol P.A |
| Positive control (+) | - | MP + DXR 0.4 mM |
| T1 | 0.03% | MP + 5 mL of EENL 0.03125% |
| T2 | 0.06% | MP + 5 mL of EENL 0.0625% |
| T3 | 0.13% | MP + 5 mL of EENL 0.125% |
| T4 | 0.03125% + DXR | MP + 5 mL of EENL 0.03125% + DXR 0.4 mM |
| T5 | 0.0625% + DXR | MP + 5 mL of EENL 0.0625% + DXR 0.4 mM |
| T6 | 0.125% + DXR | MP + 5 mL of EENL 0.125% + DXR 0.4 mM |
MP = Mashed Potatoes. ROW = Reverse Osmose Water. T1-6 = Treatments. EENL = Ethanolic Extract from Neem Leaves. DXR = Doxorubicin. P.A = Pure for Analysis.
These larvae were submitted to a chronic treatment for 48 h. After 120 h of the larval feeding, following metamorphosis, the flies were collected and preserved in 70% ethanol.
4.4.4. Analysis of the Flies
Adult flies of the wts +/+ mhw genotype with long hairs were analyzed for the presence of tumor (wart). The adults with short and thick hairs were discarded, since they did not have the gene being studied. The flies were observed using a stereoscopic magnifying glass and entomological tweezers. Only tumors large enough to be unequivocally classified were recorded. The tumor frequency was calculated as the number of tumors divided by number of wts +/+ mwh flies [75]. A total of 1600 flies were analyzed, 200 flies for each control (positive or negative), 200 flies for each isolated concentration, and 200 flies for each associated group.
4.5. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered significant. Paired Student t test was used to compare gene expression and the unpaired for phenols dosage. MTT assays were tested using ANOVA. Finally, the carcinogenic potential of EENL in the D. melanogaster model was validated by the Mann–Whitney and Wilcoxon nonparametric U test, using α = 0.05 level of significance.
Acknowledgments
This work was supported by the following grants: Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Abbreviations
| BC | Breast cancer |
| neem | Azadirachta indica |
| DCM | Dichloromethane |
| EA | Ethyl acetate |
| EENL | Ethanolic extracts of neem leaves |
| DXR | Doxorubicin |
| ESR1 | Estrogen receptor alpha |
| ESR2 | Estrogen receptor beta |
| AR | Androgen receptor |
| wts | Test warts |
Author Contributions
Designed the study, D.L.B., L.R.G. and T.G.A.; performance of cellular assays, D.L.B., S.T.S.M., M.A.P.Z.; performance of in vivo assays, D.L.B., P.M.A.P.L., P.C.O., J.C.N.; performance of statistical analysis, D.L.B., L.V., C.R.F.; revision of the manuscript, T.G.A., L.V., Y.C.P.M. and L.R.G. All authors read and approved the final manuscript.
Funding
This research was funded by Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization (WHO) Breast Cancer: Prevention and Control. [(accessed on 14 April 2018)];2018 Available online: http://www.who.int/cancer/detection/breastcancer/en/
- 2.World Health Organization (WHO) Media Centre-Cancer. [(accessed on 18 April 2018)];2018 Available online: http://www.who.int/mediacentre/factsheets/fs297/en/
- 3.Araujo T.G., Paiva C.E., Rocha R.M., Maia Y.C., Sena A.A., Ueira-Vieira C., Carneiro A.P., Almeida J.F., de Faria P.R., Santos D.W., et al. A novel highly reactive Fab antibody for breast cancer tissue diagnostics and staging also discriminates a subset of good prognostic triple-negative breast cancers. Cancer Lett. 2014;343:275–285. doi: 10.1016/j.canlet.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 6.Schulz W.A. Molecular Biology of Human Cancers. An Advanced Student’s Textbook. Springer; Dordrecht, The Netherlands: 2005. pp. 12–14. [Google Scholar]
- 7.Suter R., Marcum J.A. The molecular genetics of breast cancer and targeted therapy. Biologics. 2007;1:241–258. [PMC free article] [PubMed] [Google Scholar]
- 8.Frei A., Macdonald G., Lund I., Gustafsson J.-A., Hynes N.E., Nalvarte I. Memo interacts with c-Src to control Estrogen Receptor α sub-celular localization. Oncotarget. 2016;7:56170–56182. doi: 10.18632/oncotarget.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia M., Dahlman-Wright K., Gustafsson J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29:557–568. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Osborne C.K., Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosselman S., Polman J., Dijkema R. ERbeta: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- 12.Yim O.S., Zhang K., Shalev I., Monakhov M., Zhong S., Hsu M., Chew S.H., Lai P.S., Ebstein R.P. Delay discouting, genetic sensitivity, and leukocyte telomete length. Proc. Natl. Acad. Sci. USA. 2016;113:2780–2785. doi: 10.1073/pnas.1514351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng N., Liu L., Liu W., Zhang P., Huang H., Zang L., Hayashi T., Tashiro S., Onodera S., Xia M., et al. ERbeta up-regulation was involved in silibinin-induced growth inhibition of human breast cancer MCF-7 cells. Arch. Biochem. Biophys. 2016;591:141–149. doi: 10.1016/j.abb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Chang C.S., Kokontis J., Liao S.T. Molecular cloning of human and rat complementary DNA econding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 15.Trapman J., Klaassen P., Kuiper G.G., van der Korput J.A., Faber P.W., Van Rooij H.C., Geurts van Kessel A., Voorhorst M.M., Mulder E., Brinkmann A.O. Cloning, structure and expression of cDNA econdiing the human androgen receptor. Biochem. Biophys. Res. Commun. 1988;153:241–248. doi: 10.1016/S0006-291X(88)81214-2. [DOI] [PubMed] [Google Scholar]
- 16.Lu C., Luo J. Decoding the androgen receptor splice variants. Transl. Adrol. Urol. 2013;2:178–186. doi: 10.3978/j.issn.2223-4683.2013.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu J., van der Steen T., Tindall D.J. Are androgen receptor variants a substitute for the full-length receptor? Nat. Rev. Urol. 2015;12:137–144. doi: 10.1038/nrurol.2015.13. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H., Coram M.A., Nolley R., Reese S.W., Young S.R., Peehl D.M. Transcript levels of androgen receptor variant AR-V1 or AR-V7 do not predict recurrence in patients with prostate cancer at indeterminate risk for progression. J. Urol. 2012;188:2158–2164. doi: 10.1016/j.juro.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Wadosky K.M., Koochekpour S. Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget. 2017;14:18550–18576. doi: 10.18632/oncotarget.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey T.E., Robinson J.L., Carroll J.S., Tilley W.D. Minireview: The androgen receptor in breast tissues: Growth inhibitor, tumor suppressor, oncogene? Mol. Endocrinol. 2012;26:1252–1267. doi: 10.1210/me.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agoff S.N., Swanson P.E., Linden H., Hawes S.E., Lawton T.J. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am. J. Clin. Pathol. 2003;120:725–731. doi: 10.1309/42F00D0DJD0J5EDT. [DOI] [PubMed] [Google Scholar]
- 22.Park S., Koo J., Park H.S., Kim J.H., Choi S.Y., Lee J.H., Park B.W., Lee K.S. Expression of androgen receptors in primary breast cancer. Ann. Oncol. 2010;21:488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 23.Asano Y., Kashiwagi S., Onoda N., Kurata K., Morisaki T., Noda S., Takashima T., Ohsawa M., Kitagawa S., Hirakawa K. Clinical verification of sensitivity to preoperative chemotherapy in cases of androgen receptor-expressing positive breast cancer. Br. J. Cancer. 2016;114:14–20. doi: 10.1038/bjc.2015.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moinfar F., Okcu M., Tsybrovskyy O., Regitning P., Lax S.F., Weybora W., Ratscheck M., Tavassoli F.A., Denk H. Androgen receptors frequently are expressed in breast carcinomas: Potential relevance to new therapeutic strategies. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 25.Leite J.P.V. Fitoterapia: Bases Científicas e Tecnológicas. 1st ed. Atheneu; São Paulo, Brazil: 2009. [Google Scholar]
- 26.Fan S., Zhang J., Nie W., Zhou W., Jin L., Chen X., Lu J. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem. Toxicol. 2017;102:53–62. doi: 10.1016/j.fct.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Xie J.H., Liu X., Shen M.Y., Nie S.P., Zhang H., Li C., Gong D.M., Xie M.Y. Purification: Physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013;136:1453–1460. doi: 10.1016/j.foodchem.2012.09.078. [DOI] [PubMed] [Google Scholar]
- 28.Yu Q., Nie S.P., Wang J.Q., Huang D.F., Li W.J., Xie M.Y. Toll like receptor 4 mediates the antitumor host response induced by Ganoderma atrum polysaccharide. J. Agric. Food Chem. 2015;63:517–525. doi: 10.1021/jf5041096. [DOI] [PubMed] [Google Scholar]
- 29.Zong A., Cao H., Wang F. Anticancer polysaccharides from natural resources: A review of recent research. Carb. Pol. 2012;90:1395–1410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Locke J.C. Fungi. In: Schmutterer H., editor. The Neem Tree: Source of Unique Natural Products for Integrated Pest Management, Medicine, Industry and Other Purposes. VCH; Weinheim, Germany: 1995. pp. 118–125. [Google Scholar]
- 31.Verkerk R.H.J., Wright D.J. Biological activity of neem seed kernel extracts and synthetic azadirachtin against larvae of Plutella xylostella L. Pest Manag. Sci. 1993;37:83–91. doi: 10.1002/ps.2780370113. [DOI] [Google Scholar]
- 32.Santos L.U., Andrade C.F.S. Azadiractha Indica—A Árvore do Nim e o Controle de Piolhos. Department of Zoology of the State University of Campinas-UNICAMP. Marc, 2000. [(accessed on 18 April 2018)]; Available online: http://www.piolho.org.br/artigos/arvoredonim.pdf.
- 33.Mossini S.A.G., Kemmelmeir C. A árvore nim (Azadirachta indica. A. Juss.): Múltiplos usos. Acta Farm. Bonaerense. 2005;24:139–148. [Google Scholar]
- 34.Lee S.M., Olsen J.I., Schewizer M.P., Klocke J.A. 7-Deacetyl-17β-hydroxyazadiradione, a new limonoid insect growth inhibitor from Azadirachta indica. Phytochemistry. 1988;27:2773–2775. doi: 10.1016/0031-9422(88)80661-7. [DOI] [Google Scholar]
- 35.Martinez S.S. O Nim–Azadirachta indica Natureza: Usos Múltiplos, Produção. IAPAR; Londrina, Brazil: 2002. [Google Scholar]
- 36.Weng C.J., Yen G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Dai Z.J., Wang B.F., Lu W.F., Wang Z.D., Ma X.B., Min W.L., Kang H.F., Wang X.J., Wu W.Y. Total Flavonoids of Scutellaria barbata Inhibit Invasion of Hepatocarcinoma via MMP/TIMP in vitro. Molecules. 2013;18:934–950. doi: 10.3390/molecules18010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng C.J., Yen G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012;31:323–351. doi: 10.1007/s10555-012-9347-y. [DOI] [PubMed] [Google Scholar]
- 39.Chien S.C., Wu Y.C., Chen Z.W., Yang W.C. Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes. Evid. Based Complement. Alternat. Med. 2015;2015:357357. doi: 10.1155/2015/357357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelhady M.I.S., Bader A., Shaheen U., El-Malah Y., Abourehab M.A.S., Barghash M. Azadirachta indica as source for Antioxidant and Cytotoxic Polyphenolic Compounds. Biosci. Biotechnol. Res. Asia. 2015;12:1209–1222. doi: 10.13005/bbra/1774. [DOI] [Google Scholar]
- 41.Balasenthil S., Arivazhagan S., Ramachandran C.R., Ramachandran V., Nagini S. Chemopreventive potential of neem (Azadirachta indica) on 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J. Ethnopharmacol. 1999;67:189–195. doi: 10.1016/S0378-8741(99)00015-X. [DOI] [PubMed] [Google Scholar]
- 42.Baral R., Chattopadhyay U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 2004;4:355–366. doi: 10.1016/j.intimp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Nakahara K., Roy M.K., Ono H., Maeda I., Kameyama M.O., Yoshida M., Trakoontivakorn G. Prenylated flavanones isolated from flowers of Azadirachta indica (the neem tree) as antimutagenic constituents against heterocyclic amines. J. Agric. Food Chem. 2003;51:6456–6460. doi: 10.1021/jf034666z. [DOI] [PubMed] [Google Scholar]
- 44.Yang F., Teves S.S., Kemp C.J., Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta. 2014;1845:84–89. doi: 10.1016/j.bbcan.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yildirim A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of Rumex cripus L. extracts. J. Agric. Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 46.Bendini A., Cerretani L., Pizzolante L., Gallina-Toschi T., Guzzo F., Ceoldo S., Marconi A.M., Andreetta F., Levi M. Phenol Content Related to Antioxidant and An-timicrobial Activity of Passiflora spp. Extracts. Eur. Food Res. Technol. 2006;223:102–109. doi: 10.1007/s00217-005-0150-7. [DOI] [Google Scholar]
- 47.Dlugosz A., Lembas-Bogaczyk J., Lamer-Zaraw-Ska Antoxid Increases Ferric Reducing Antioxidant Po-wer (FRAP) even Stronger than Vitamin C. Acta Pol. Pharm. 2006;63:446–448. [PubMed] [Google Scholar]
- 48.Wojdylo A., Oszmianski J., Czemerys R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- 49.Mohamed H., Masmoudi O., Yosra E.-T., Rayda S., Gharsallah N., Moncef N. Chemical composition and antioxidant and radical scavenging activities of Periploca laevigata rook bark extracts. J. Sci. Food Agric. 2008;89:897–905. doi: 10.1002/jsfa.3532. [DOI] [Google Scholar]
- 50.Singh U.P., Maurya S., Singh D.P. Phenolic acids in neem (Azadirachta indica): A major preexisting secondary metabolite. J. Herb. Pharm. 2005;5:35–43. [PubMed] [Google Scholar]
- 51.Paul R., Prasad M., Sah N.K. Anticancer biology of Azadirachta indica L (neem): A mini review. Cancer Biol. Ther. 2011;12:467–476. doi: 10.4161/cbt.12.6.16850. [DOI] [PubMed] [Google Scholar]
- 52.Wang L., Phan D.D.K., Zhang J., Ong P.-S., Thuya W.L., Soo R., Wong A.L.-A., Yong W.P., Lee S.C., Ho P.C.-L., et al. Anticancer properties of nimbolide and pharmacokinetic considerations to accelerate its development. Oncotarget. 2016;7:44790–44802. doi: 10.18632/oncotarget.8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmieri G., Capone M., Ascierto M.L., Gentilcore G., Stroncek D.F., Casula M., Sini M.C., Palla M., Mozzilli N., Ascierto P.A. Main roads to melanoma. J. Transl. Med. 2009;7:86. doi: 10.1186/1479-5876-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy M.K., Kobori M., Takenaka M., Nakahara K., Shinmoto H., Tsushida T. Inhibition of colon cancer (HT-29) cell proliferation by a triterpenoid isolated from Azadirachta indica is accompanied by cell cycle arrest and up-regulation of p21. Planta Med. 2006;72:917–923. doi: 10.1055/s-2006-946694. [DOI] [PubMed] [Google Scholar]
- 55.Wu Q., Kohli M., Bergen H.R., Cheville J.C., Karnes R.J., Cao H., Young C.Y., Tindall D.J., Mcniven M.A., Donkena K.V. Preclinical evaluation of the supercritical extract of Azadirachta indica (neem) leaves in vitro and in vivo on inhibition of prostate cancer tumor growth. Mol. Cancer Ther. 2014;13:1067–1077. doi: 10.1158/1535-7163.MCT-13-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aleskandarany M.A., Abduljabbar R., Ashankyty I., Elmouna A., Jerjees D., Ali S., Buluwela L., Diez-Rodriguez M., Caldas C., Green A.R., et al. Prognostic significance of androgen receptor expression in invasive breast cancer: Transcriptonic and protein expression analysis. Breast Cancer Res. Treat. 2016;159:215–227. doi: 10.1007/s10549-016-3934-5. [DOI] [PubMed] [Google Scholar]
- 57.Hickey T.E., Irvine C.M., Dvinge H., Tarulli G.A., Hanson A.R., Ryan N.K., Pickering M.A., Birrell S.N., Hu D.G., Mackenzie P.I., et al. Expression of androgen receptor splice variants in clinical breast Cancers. Oncotarget. 2015;6:44728–44744. doi: 10.18632/oncotarget.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rampurwala M., Wisinski K.B., O’Regan R. Role of the androgen receptor in triple-negative breast cancer. Clin. Adv. Hematol. Oncol. 2016;14:186–193. [PMC free article] [PubMed] [Google Scholar]
- 59.Hu D.G., Hickey T.E., Irvine C., Wijayakumara D.D., Lu L., Tilley W.D., Selth L.A., Mackenzie P.I. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Horm. Cancer. 2014;5:61–71. doi: 10.1007/s12672-014-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kono M., Fujii T., Lyons G.R., Huo L., Bassett R., Gong Y., Karuturi M.S., Tripathy D., Ueno N.T. Impact of androgen receptor expression. In fluoxymesterone-treated estrogen receptor-positive metastatic breast cancer refractory to contemporary hormonal therapy. Breast Cancer Res. Treat. 2016;160:101–109. doi: 10.1007/s10549-016-3986-6. [DOI] [PubMed] [Google Scholar]
- 61.Pietri E., Conteduca V., Andreis D., Masa I., Melegari E., Sarti S., Cecconetto L., Schirone A., Bravaccini S., Serra P., et al. Androgen receptor signaling pathways as a target for breast cancer treatment. Endocr. Relat. Cancer. 2016;23:R485–R498. doi: 10.1530/ERC-16-0190. [DOI] [PubMed] [Google Scholar]
- 62.Chia K., O’brien M., Brown M., Lim E. Targeting the androgen receptor in breast cancer. Curr. Oncol. Rep. 2015;17:4. doi: 10.1007/s11912-014-0427-8. [DOI] [PubMed] [Google Scholar]
- 63.Fioretti F.M., Sita-Lumsden A., Bevan C.L., Brooke G.N. Revising the role of the androgen receptor in breast cancer. J. Mol. Endocrinol. 2014;52:R257–R265. doi: 10.1530/JME-14-0030. [DOI] [PubMed] [Google Scholar]
- 64.Birrell S.N., Bentel J.M., Hickey T.E., Ricciardelli C., Weger M.A., Horsfall D.J., Tilley W.D. Androgens induce divergent proliferative responses in human breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1995;52:459–467. doi: 10.1016/0960-0760(95)00005-K. [DOI] [PubMed] [Google Scholar]
- 65.Cochrane D.R., Bernales S., Jacobsen B.M., Cittelly D.M., Howe E.N., D’Amato N.C., Spoelstra N.S., Edgerton S.M., Jean A., Guerrero J., et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16:R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim E., Ni M., Hazra A., Tamimi R., Brown M. Elucidating the role of androgen receptor in breast cancer. J. Clin. Investig. 2012;2:1003–1011. doi: 10.4155/cli.12.88. [DOI] [Google Scholar]
- 67.Lanzino M., Sisci D., Morelli C., Garofalo C., Catalano S., Casaburi I., Capparelli C., Giordano C., Giordano F., Maggiolini M., et al. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells—Identification of novel androgen response elemento. Nucleic Acids Res. 2010;38:5351–5365. doi: 10.1093/nar/gkq278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cechinel-Filho V., Yunes R.A. Estratégias para a obtenção de compostos farmacologicamente ativos a partir de plantas medicinais: Conceitos sobre modificação estrutural para otimização da atividade. Quím. Nova. 1998;21:99–105. doi: 10.1590/S0100-40421998000100015. [DOI] [Google Scholar]
- 69.Silva V.C.L. Master’s Thesis. Rural Federal University of Pernambuco; Recife, Brazil: 2010. Avaliação da Toxicidade Reprodutiva de Ratas Wistar Submetidas à Ingestão do Extrato Etanólico das Folhas de Nim (Azadirachta indica A. Jus) [Google Scholar]
- 70.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–158. doi: 10.1016/S0076-687999017-1. [DOI] [Google Scholar]
- 71.Swain T., Hills W.E. The phenolic constituents of Punnus domestica. Iquantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- 72.Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 73.Araujo T.G., Marangoni K., Rocha R.M., Maia Y.C.P., Araujo G.R., Alcântara T.M., Alves P.T., Calábria L., Neves A.F., Soares F.A., et al. Dynamic dialog between cytokeratin 18 and annexin A1 in breast cancer: A transcriptional disequilibrium. Acta Histochem. 2014;116:1178–1184. doi: 10.1016/j.acthis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Guo Z., Yang X., Sun F., Jiang R., Linn D.E., Chen H., Chen H., Kong X., Melamed J., Tepper C.G., et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nepomuceno J.C. Using the Drosophila melanogaster to assessment carcinogenic agents through the test for detection of epithelial tumor clones. Adv. Tech. Biol. Med. 2015;3:149. doi: 10.4172/2379-1764.1000149. [DOI] [Google Scholar]
- 76.Justice R.W., Zilian O., Woods D.F., Noll M., Bryant P.J. The Drosophila tumor suppressor gene warts encodes a homolog o-f human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 77.Orsolin P.C., Silva-Oliveira R.G., Nepomuceno J.C. Modulating effect of Modulating effect of synthetic statins against damage induced by doxorubicin in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2015;81:111–119. doi: 10.1016/j.fct.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Orsolin P.C., Silva-Oliveira R.G., Nepomuceno J.C. Modulating effect of simvastatin on the DNA damage induced by doxorubicin in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2016;90:10–17. doi: 10.1016/j.fct.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Vasconcelos M.A., Orsolin P.C., Silva-Oliveira R.G., Nepomuceno J.C., Spanó M.A. Assessment of the carcinogenic potential of high intense-sweeteners through the test for detection of epithelial tumor clones (warts) in Drosophila melanogaster. Food Chem. Toxicol. 2017;101:1–7. doi: 10.1016/j.fct.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira V.C., Constante S.A.R., Orsolin P.C., Nepomuceno J.C., Rezende A.A.A., Spanó M.A. Modulatory effects of metformin on mutagenicity and epithelial tumor incidence in doxorubicin-treated Drosophila melanogaster. Food Chem. Toxocol. 2017;106:283–291. doi: 10.1016/j.fct.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 81.Lima P.M.A.P., Orsoli P.C., Araújo T.G., Brandão D. Effects of a Carbonated Soft Drink on Epitheial Tumor Incidence in Drosophila melanogaster. J. Pharm. Pharmacol. 2018;6:240–247. doi: 10.17265/2328-2150/2018.03.005. [DOI] [Google Scholar]
- 82.Eeken J.C.J., Klink I., Veen B.L.V., Pastink A., Ferro W. Induction of epithelial tumors in Drosophila melanogaster heterozygous for the tumor suppressor gene wts. Environ. Mol. Mutagen. 2002;40:277–282. doi: 10.1002/em.10119. [DOI] [PubMed] [Google Scholar]
- 83.Costa W.F., Oliveira A.B., Nepomuceno J.C. Lapachol as an epithelial tumor inhibitor agent in Drosophila melanogaster heterozygote for tumor suppressor gene wts. Genet. Mol. Res. 2012;10:3236–3245. doi: 10.4238/2011.December.22.1. [DOI] [PubMed] [Google Scholar]