Abstract
This study attempted to investigate whether nutrient and food intake were related with mild cognitive impairment (MCI) in adults and elderly over 50 years of age in Korea. Questionnaires and anthropometric measurements were conducted on general aspects of the research, and food frequency questionnaires (FFQs) were conducted to determine nutritional status. The relative theta power (RTP) through electroencephalography (EEG) measurements, neurocognitive function test (NFT; CNS Vital Signs), and cognitive function was measured. The MCI group consumed significantly lower C18:4, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) among the N-3 fatty acids, N-6 fatty acids dihomo-γ-linolenic acid (DGLA), mono unsaturated fatty acids, C22:1, biotin, vitamin D in the nutrients, and sweet potato (12.35g/day, p = 0.015), mackerel (3.38g/day, p = 0.017), mandarin orange (p = 0.016), persimmon (p = 0.013) and apple (p = 0.023) in the food than the normal group did. And the MCI group consumed salted fish (3.14g/day, p = 0.041) and ice-cream (5.01g/day, p = 0.050) at a significantly higher level. Delayed verbal score, delayed visual score, and verbal memory score of the NFT and RTP values of the prefrontal cortex among the EEGs were significantly lower in the MCI group compared to those in the normal group. From this study, we found that nutrient and food intake are closely related to MCI in Korean aged 50 years and older, but more human studies are needed to verify these findings.
Keywords: Nutrient, Food, Cognition, Electroencephalography, Koreans
INTRODUCTION
Dementia is defined as multiple disorders of cerebral function including memory, thinking ability, orientation, comprehension, calculation, learning ability, language and judgment [1]. The prevalence of dementia, a major disease associated with aging, is rapidly increasing worldwide. Korea is listed among the aged society in August of 2017 with the fastest aging rate in the world, and it is expected to become a super aged society in 2025. The prevalence of dementia over 65 years of age also increased to 9.8% in 2015, and is estimated to reach 1 million (prevalence 10.3%) by 2024. Dementia affects not only the quality of life of an individual but also the lives of others around the patient, as the cost of medical treatment per dementia patient is much higher than that of chronic diseases. Such circumstances around dementia continuously increase the national and socioeconomic burden. So the effective strategies for early prevention of dementia and delaying the onset of dementia are needed [2,3].
Mild cognitive impairment (MCI) is an intermediate stage between normal and dementia. It is a clinically important step in maximizing the prevention effect of Alzheimer's disease (AD) in the earliest stages [4]. The absence of effective drugs which proved the prevention of dementia progression has raised the importance of maintaining physical fitness and lifestyle management. According to numerous epidemiological studies, homocysteine concentration, smoking, and drinking were reported as definite risk factors for cognitive dysfunction and dementia. Education level, degree of physical activity, consumption of caffeine, antioxidant nutrients and N-3 fatty acids have been suggested as protective factors for reducing cognitive dysfunction and dementia risk [5].
N-3 fatty acids, phosphatidylcholine, and essential amino acids, which are components of brain membrane and neurotransmitter, have been reported as typical nutrients related to cognitive function. Increased levels of homocysteine in the blood that are associated with the onset of dementia, is responsible for accumulation of amyloid β (Aβ, β-amyloid and plaque peptide) in the brain, which results in loss of neurons in the hippocampus by forming plaques and tangles, making recovery of damaged DNA difficult and leading to apoptosis [6]. It has been reported that B vitamins such as vitamin B6, vitamin B12, folate, and choline indirectly inhibit the increase of Aβ by decreasing homocysteine concentration [2]. AD is partially caused by inflammation and oxidative stress resulting from aging [7]. The brain is rich in unsaturated fatty acids, is always exposed to oxygen, and thereby easily peroxidized. Increased oxidative stress causes damages to fat, protein, DNA, and RNA, causing AD [8]. Although many epidemiological studies and clinical studies have been carried out on N-3 fatty acids, consistent results have not been drawn on their effectiveness and relevance. Recently, epidemiological studies and clinical studies have been conducted on the effects of vitamin C, vitamin E, manganese, copper, and zinc on cognitive function [3].
Many studies have used Mini-Mental State Examination (MMSE) questionnaire which is a widely used subjective assessment tool to evaluate cognitive function, is less sensitive as the process relies on the strength of participation or concentration ability of the subjects. However, there have been no studies on the relationship in Koreans above 50 years old between nutritional status and cognitive abilities using cognitive function evaluation index (electroencephalography; EEG), which is an objective cognitive function measurement index.
Thus, this study investigated the difference in the nutritional status, dietary intakes, cognitive function tests, and EEG between the normal group and MCI group classified by neurocognitive function test (NFT) in Koreans 50 years of age and over.
MATERIALS AND METHODS
Study period and participants
This study was conducted in compliance with the clinical trial ethical standards based on the Helsinki Declaration, and was approved by the Institutional Review Board of Seoul National University Medical School and Seoul National University Hospital (No. 2013-93). The study was conducted at the Data Center for Korean EEG, during the period from December 2013 to March 2018. The subjects of this study were Korean adults and elderly between 50 and 90 years old. Total of 242 healthy adults who did not have severe behavioral disorders and did not have history of head trauma or epilepsy were enrolled for the study. As for additional exclusion criteria the subjects are excluded when one Z score is less than −2, or 3 or more Z scores are less than −1.5 among verbal memory test, visual memory test, reasoning test and shift attention test in the package of NFT. The general information was also investigated by the questionnaires included gender, age, education level, and occupation etc.
Anthropometric survey
To measure the subject's body, height was measured with a height measure scale, and Inbody H20 (Biospace, Ltd., Seoul, Korea) was used to measure body weight and body composition, such as muscle mass and body fat percentage. Body mass index (BMI) was calculated to assess weight status.
Food frequency questionnaires (FFQs)
The FFQ used in this study was modified and supplemented to fit the present study by using semi-quantitative FFQ [9] developed for elderly and Korean adults over 50 years. The FFQ used in this study consisted of 65 foods. FFQ analysis was performed using Can Pro version 4.0 (computer aided nutritional analysis program; Korea Nutrition Society, Seoul, Korea, 2011), and the coefficients for the frequency of food intake were calculated with 3 times/day (3), twice/day (2), once/day (1), 5–6 times/week (0.8), 3–4 times/week (0.5), 1–2 times/week (0.2), 2–3 times/month (0.083), and once/month (0.033).
EEG
MEGAV-202 (24 + 8) EEG system (Mitsar-Co., Ltd., Saint Petersburg, Russia) was used for EEG. A skin conductance measurement electrode is attached to the finger of the subject to monitor the awakening state, and EEG waveforms were measured and recorded at an international 10–20 electrode position during a 4-minute open eye. EEG specialist attached the EEG cap with electrode attached to 19 international channels to the scalp of the subject, and gel was injected into each channel so that the contact resistance of all electrodes was made 5 kΩ or less by using both earlobes as a reference electrode.
NFT (CNS Vital Signs)
The NFT was carried out by 4 tests including verbal memory test, which visualizes Korean words and learns and memorizes them, visual memory test, which visualizes geometric figures and learns and memorizes them, reasoning test (non-verbal reasoning test), in which geometric figures are arranged according to the rules of various difficulty levels, and a shift attention test that alternately selects the same color or shape. The subject score is the value calculated from the number of sources using the data of individual subjects. High score implies good neurocognitive function score as this is the response of the simple response, and the response time (0.001 seconds).
Korean version of Mini-Mental State Examination (K-MMSE)
MMSE is a rapid and simple test to assess the cognitive function devised by Folstein et al. [10] and is widely used clinically for assessing and studying cognitive dysfunction, including dementia patients. K-MMSE is an evaluation tool that has been verified and developed to fit the Korean population [11,12]. There are total of 27 items, and the score of each item was added to the total score. The total score was distributed from 0 to 30 points. The lower the score, the lower the cognitive function. Twenty-four points or more is classified as no cognitive impairment, 18–23 is classified as mild cognitive dysfunction, and 0–17 is classified as cognitive dysfunction.
Statistical analysis
The results of this study were statistically analyzed using Statistical Package for the Social Science (SPSS) version 23.0 program (IBM Corp., New York, NY, USA), and the mean and standard error were obtained. As for the general characteristics (sex, education level, and occupation) of the survey subjects, frequency analysis was performed by dividing the total and MCI (verbal/visual delay memory test average score percentile ≤ 16%). Significant differences in frequency were confirmed by the χ2 test. The mean age and body measurements (BMI) according to MCI were confirmed by the Student's t-test after the regularity test using Kolmogorov-Smirnov. As for body measurement (height, weight, and body fat percentage) after the regularity test using Kolmogorov-Smirnov, this study identified significant differences through the Mann-Whitney U test. Using GLM univariate analysis, this study confirmed the significant difference of age-adjusted nutrients and food intake, EEG, NFT, and K-MMSE mean. The statistical significance level of the study was all p <0.05.
RESULTS
General information
Table 1 summarizes all subjects' responses for general information. In detail, the average age was 64.86, and among the total 242 subjects, 64 subjects (26%) are men and 178 subjects (78%) are women, showing that the proportion of women was higher. In education level high school graduates (34.7%) and college graduates (30.5%) were the most common. With regard to occupation, housewives accounted for the highest percentage of 37.3%. This study divided the entire subjects into “MCI group” and the “normal group” based on less than 16 percentile of verbal delayed memory test and visual delayed memory test known as a highly correlated index of normal elderly, MCI patients, and mild AD among the neurocognitive assessment indices [4]. The age (p < 0.001) was significantly higher in the MCI group than in the normal group. There were no significant differences in height, weight, and BMI between the two groups. There was no significant difference in general information such as gender, academic level, and occupation (Table 1).
Table 1. General characteristics of study subjects.
| Characteristics | Total (n = 242) | MCI (n = 36) | Normal (n = 206) | p-value | |
|---|---|---|---|---|---|
| Gender | 0.067 | ||||
| Males (%) | 64 (26.4) | 14 (21.9) | 50 (78.1) | ||
| Females (%) | 178 (73.6) | 22 (12.4) | 156 (87.6) | ||
| Age | 64.86 ± 7.50 | 68.52 ± 6.93* | 64.22 ± 7.43 | 0.001 | |
| Height | 158.67 ± 7.54 | 158.70 ± 9.69 | 158.66 ± 7.12 | 0.890 | |
| Weight | 61.94 ± 9.78 | 64.08 ± 11.11 | 61.56 ± 9.50 | 0.057 | |
| BMI | 24.54 ± 3.02 | 25.41 ± 3.77 | 24.39 ± 2.86 | 0.061 | |
| Body fat (%) | 30.66 ± 7.13 | 30.02 ± 8.06 | 30.77 ± 6.97 | 0.452 | |
| Education (%) | 0.962 | ||||
| Illiterate | 17 (7.1) | 3 (17.6) | 14 (82.4) | ||
| Elementary school | 23 (9.6) | 4 (17.4) | 19 (82.6) | ||
| Middle school | 29 (12.1) | 5 (17.2) | 24 (82.8) | ||
| High school | 83 (34.7) | 12 (14.5) | 71 (85.5) | ||
| College/university | 73 (30.5) | 11 (15.1) | 62 (84.9) | ||
| Graduate school | 14 (5.9) | 1 (7.1) | 13 (92.9) | ||
| Employment status (%) | 0.408 | ||||
| Worker | 42 (17.7) | 7 (16.7) | 35 (83.3) | ||
| Owner-operator | 30 (12.7) | 3 (10.0) | 27 (90.0) | ||
| Office job | 32 (13.6) | 7 (21.9) | 25 (78.1) | ||
| Management | 2 (0.8) | 0 (0.0) | 2 (100.0) | ||
| Professional | 8 (3.4) | 0 (0.0) | 8 (100.0) | ||
| Others | 17 (7.2) | 4 (23.5) | 13 (76.5) | ||
| Homemaker | 88 (37.3) | 10 (11.4) | 78 (88.6) | ||
| Unemployed | 17 (7.2) | 5 (29.4) | 12 (70.6) | ||
MCI group is defined as the subjects with an average score of 16 percentiles or less between the delayed verbal scores and delayed visual scores. Data are means ± standard deviation and No. (%).
MCI, mild cognitive impairment; BMI, body mass index.
*Statistical difference between the MCI group and normal group by t-test at p < 0.05.
Assessment of cognitive function evaluation to MCI
To look at the neuro-cognitive scores, when the age was adjusted, the MCI group showed significantly lower scores on language delay recall (p < 0.001), sight delay recall (p < 0.001), and language memory (p = 0.004) compared to the normal group (Table 2).
Table 2. Evaluation standard of cognitive function in study subjects.
| Evaluation standard | Total (n = 242) | MCI (n = 36) | Normal (n = 206) | p-value | |
|---|---|---|---|---|---|
| K-MMSE | 26.12 ± 2.39 | 25.53 ± 0.38 | 26.23 ± 0.16 | 0.098 | |
| Neurocognitive assessment | |||||
| Delayed verbal score | 9.21 ± 3.49 | 4.72 ± 0.47* | 10.00 ± 0.19 | < 0.001 | |
| Delayed visual score | 10.54 ± 2.76 | 6.50 ± 0.37* | 11.25 ± 0.15 | < 0.001 | |
| Verbal score | 49.85 ± 5.95 | 47.12 ± 1.01* | 50.33 ± 0.41 | 0.004 | |
| Visual score | 45.44 ± 5.82 | 45.48 ± 0.98 | 45.44 ± 0.41 | 0.969 | |
| Executive score | 32.22 ± 21.57 | 30.44 ± 3.24 | 32.52 ± 1.32 | 0.555 | |
| Reasoning score | −0.25 ± 8.10 | −1.73 ± 1.37 | 0.00 ± 0.56 | 0.243 | |
MCI group is defined as the subjects with an average score of 16 percentiles or less between the delayed verbal scores and delayed visual scores. Data are least square means ± standard deviation.
MCI, mild cognitive impairment; K-MMSE, Korean version of Mini-Mental State Examination;
*Statistical difference between the MCI group and normal group adjusted age by GLM univariate analysis at p < 0.01.
EEG states according to MCI
To examine EEG, when the age was adjusted, the increased relative theta power (RTP) in early cognitive dysfunction of the MCI group was significantly higher (p =0.030, 0.004) in prefrontal (Fp1, Fp2) position than that of the normal group (Table 3).
Table 3. The EEG of RTP in study subjects with MCI.
| Area | Total (n = 242) | MCI (n = 36) | Normal (n = 206) | p-value | |
|---|---|---|---|---|---|
| Prefrontal lobe | |||||
| RTP_Fp1 | 0.170 ± 0.052 | 0.188 ± 0.009* | 0.167 ± 0.004 | 0.030 | |
| RTP_Fp2 | 0.168 ± 0.054 | 0.193 ± 0.009* | 0.164 ± 0.004 | 0.004 | |
| Frontal lobe | |||||
| RTP_F7 | 0.170 ± 0.052 | 0.180 ± 0.009 | 0.179 ± 0.009 | 0.939 | |
| RTP_F3 | 0.168 ± 0.054 | 0.205 ± 0.011 | 0.197 ± 0.005 | 0.489 | |
| RTP_Fz | 0.179 ± 0.051 | 0.245 ± 0.014 | 0.219 ± 0.006 | 0.094 | |
| RTP_F4 | 0.198 ± 0.065 | 0.211 ± 0.010 | 0.194 ± 0.004 | 0.131 | |
| RTP_F8 | 0.223 ± 0.082 | 0.177 ± 0.008 | 0.173 ± 0.003 | 0.618 | |
| Central lobe | |||||
| RTP_C3 | 0.174 ± 0.048 | 0.187 ± 0.009 | 0.175 ± 0.004 | 0.241 | |
| RTP_Cz | 0.173 ± 0.049 | 0.219 ± 0.011 | 0.200 ± 0.004 | 0.096 | |
| RTP_C4 | 0.178 ± 0.052 | 0.180 ± 0.009 | 0.175 ± 0.004 | 0.610 | |
| Temporal lobe | |||||
| RTP_T3 | 0.197 ± 0.061 | 0.170 ± 0.008 | 0.173 ± 0.003 | 0.764 | |
| RTP_T4 | 0.202 ± 0.064 | 0.184 ± 0.009 | 0.175 ± 0.004 | 0.331 | |
| RTP_T5 | 0.176 ± 0.053 | 0.176 ± 0.009 | 0.172 ± 0.004 | 0.684 | |
| RTP_T6 | 0.156 ± 0.047 | 0.186 ± 0.010 | 0.175 ± 0.004 | 0.307 | |
| Parietal lobe | |||||
| RTP_P3 | 0.176 ± 0.050 | 0.163 ± 0.008 | 0.154 ± 0.003 | 0.285 | |
| RTP_Pz | 0.172 ± 0.053 | 0.177 ± 0.008 | 0.168 ± 0.003 | 0.284 | |
| RTP_P4 | 0.169 ± 0.048 | 0.160 ± 0.008 | 0.154 ± 0.003 | 0.513 | |
| Occipital lobe | |||||
| RTP_O1 | 0.177 ± 0.059 | 0.197 ± 0.010 | 0.188 ± 0.004 | 0.384 | |
| RTP_O2 | 0.189 ± 0.058 | 0.201 ± 0.010 | 0.194 ± 0.004 | 0.514 | |
MCI group is defined as the subjects with an average score of 16 percentiles or less between the delayed verbal scores and delayed visual scores. Data are least square means ± standard deviation.
EEG, electroencephalography; MCI, mild cognitive impairment; RTP, relative theta power
*Statistical difference between the MCI group and normal group adjusted age by GLM univariate analysis at p < 0.05.
Nutrient intake status according to MCI
Table 4 shows the results of nutrient intake for all subjects after age adjustment. Energy, carbohydrate, protein, and lipid intake were not significantly different between the 2 groups, but the calorie, carbohydrate, protein, and lipid intake were lower in the MCI group. The intake of N-3 fatty acids C18:4 (p = 0.016), C20:5 (eicosapentaenoic acid; EPA) (p = 0.016), C22:6 (docosahexaenoic acid; DHA) (p = 0.016) C22:6 of unsaturated fatty acids, intake of N-6 fatty acids C20:3 dihomo-γ-linolenic acid (DGLA) (p = 0.039), and intake of C22:1 (p = 0.021) of mono unsaturated fatty acids in the MCI group were significantly lower than those in the normal group. When it comes to vitamin intake only, the intake of biotin and vitamin D were significantly lower in the MCI group than in the normal group.
Table 4. The nutrients intakes of subjects with MCI.
| Subjects | 2015 DRIs | MCI (n = 36) | Normal (n = 206) | p-value | |
|---|---|---|---|---|---|
| Energy (kcal) | 1,600–2,200* | 1,833.30 ± 96.69† | 1,887.60 ± 39.82 | 0.606 | |
| Carbohydrate (%) | 55–65 | 69.47 ± 17.58 | 68.48 ± 0.43 | 0.381 | |
| Protein (%) | 7–20 | 13.64 ± 0.36 | 13.89 ± 0.15 | 0.517 | |
| Fat (%) | 15–30 | 16.89 ± 0.79 | 17.63 ± 0.32 | 0.391 | |
| C18:4 (N-3) (g) | 0.005 ± 0.002§ | 0.010 ± 0.001 | 0.016 | ||
| C20:3 (N-6) (g) | 0.041 ± 0.006§ | 0.055 ± 0.002 | 0.039 | ||
| C20:5 (N-3) (g) | 0.041 ± 0.015§ | 0.081 ± 0.006 | 0.016 | ||
| C22:1 (g) | 0.005 ± 0.001§ | 0.008 ± 0.000 | 0.021 | ||
| C22:6 (N-3) (g) | 0.092 ± 0.035§ | 0.185 ± 0.014 | 0.016 | ||
| Vitamin D (ug) | 10–15‡ | 1.62 ± 0.24§ | 2.31 ± 0.10 | 0.010 | |
| Vitamin E (mg) | 12‡ | 13.71 ± 1.15 | 15.42 ± 0.47 | 0.173 | |
| Vitamin C (mg) | 100† | 227.66 ± 26.83 | 280.45 ± 11.05 | 0.072 | |
| Folate (ug) | 400† | 745.02 ± 56.67 | 786.20 ± 23.34 | 0.505 | |
| Vitamin B12 (ug) | 2.4† | 6.38 ± 1.05 | 8.46 ± 0.43 | 0.068 | |
| Biotin (ug) | 30‡ | 14.60 ± 1.62 | 19.17 ± 0.70 | 0.010§ | |
| Magnesium (mg) | 280–370† | 75.65 ± 7.49 | 91.52 ± 3.08 | 0.052 | |
| Zinc (mg) | 7–9† | 10.63 ± 0.62 | 10.89 ± 0.26 | 0.701 | |
MCI group is defined as the subjects with an average score of 16 percentiles or less between the delayed verbal scores and delayed visual scores. Data are least square means ± standard deviation.
MCI, mild cognitive impairment; DRI, Dietary Reference Intake.
*Estimated energy requirement (EER); †Recommended nutrition intake (RNI); ‡Adequate intake (AI); §Statistical difference between the MCI group and normal group adjusted age by GLM univariate analysis at p < 0.05.
Dietary Reference Intake (DRI) versus nutrient intake by MCI
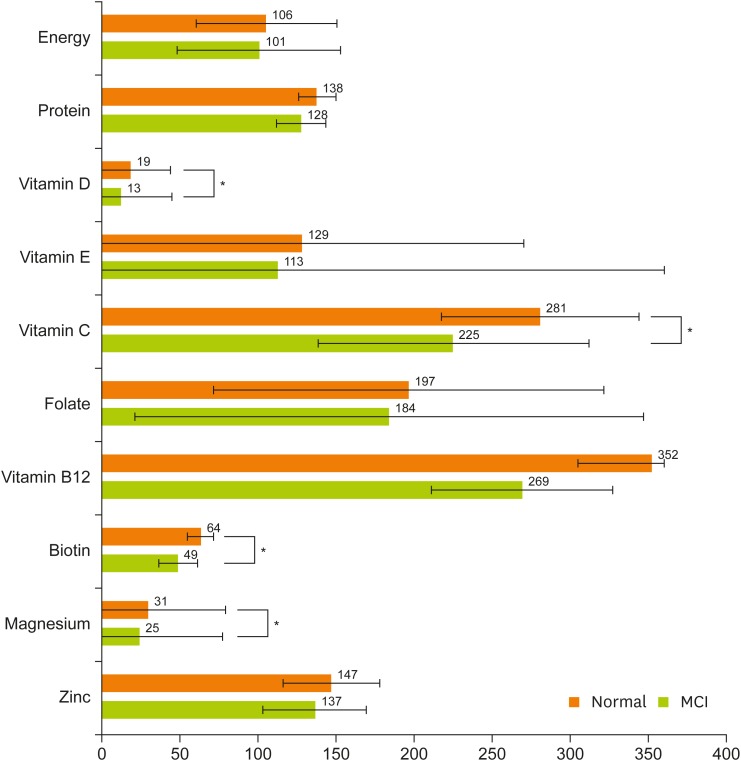
The distribution of nutrient intake is shown in Figure 1 based on the percentage of intake of nutrients (% DRI) compared to the nutritional intake standard suggested by Korean DRI . Estimated calorie intake, vitamin E, biotin intake, and intake of the remaining nutrients were compared based on the recommended intake. Most nutrients were consumed well in excess of 100% of the DRI. Of these, vitamin D, biotin, and magnesium were under supply, with less than 75% of the DRI. When the MCI group was compared with the normal group, vitamin D, vitamin C, biotin, and magnesium intake were significantly lower in the MCI group.
Figure 1. Percentage of nutrient intakes compared with DRI for Koreans 2015 of the subjects. DRI standards estimated energy requirements (energy) or adequate intake (vitamin E and biotin) or recommended dietary allowances (other nutrients). Data are least square means ± standard deviation.
DRI, Dietary Reference Intake; MCI, mild cognitive impairment.
*Significant difference between MCI group and normal group by Mann-Whitney U test.
Food intake status according to MCI
Table 5 shows the results of food intake for all subjects adjusted for age. The intake level of sweet potato (p = 0.015) was significantly lower in the MCI group than that in the normal group. To examine fish food group, the MCI group had significantly less intake of mackerel (p = 0.017) than the normal group. The intake level of salted fish (p = 0.041) was significantly higher in the MCI group than that in the normal group. To examine fruits, the intake levels of mandarin orange (p = 0.016), persimmon (p = 0.013), and apple (p = 0.023) in the MCI group were significantly lower than those in the normal group. To examine fast foods, the intake level of ice cream (p = 0.050) was significantly higher in the MCI group than that in the normal group. There was no significant difference between the 2 groups in terms of vegetable intake.
Table 5. Dietary intakes based on food group in study subjects with MCI.
| Food group | MCI (n = 36) | Normal (n = 206) | p-value | |
|---|---|---|---|---|
| Grains | ||||
| Sweet potato | 12.35 ± 6.03* | 28.41 ± 2.48 | 0.015 | |
| Multi-Grain rice | 170.94 ± 15.06 | 144.82 ± 6.20 | 0.851 | |
| White rice | 39.92 ± 11.06 | 37.66 ± 4.55 | 0.112 | |
| Meats | ||||
| Beef | 8.65 ± 2.26 | 10.66 ± 0.93 | 0.430 | |
| Pork | 9.35 ± 1.83 | 9.13 ± 0.76 | 0.910 | |
| Mackerel | 3.38 ± 1.35* | 6.90 ± 0.556 | 0.017 | |
| Fish cake | 2.20 ± 1.30 | 3.85 ± 0.53 | 0.242 | |
| Egg | 20.00 ± 4.22 | 27.42 ± 1.74 | 0.107 | |
| Salted seafood | 3.14 ± 0.87* | 1.19 ± 0.36 | 0.041 | |
| Vegetables | ||||
| Cabbage (include kimchi) | 143.75 ± 13.98 | 116.00 ± 5.76 | 0.069 | |
| Carrot | 12.17 ± 4.90 | 17.34 ± 2.02 | 0.332 | |
| Mushrooms | 15.88 ± 5.88 | 21.52 ± 2.42 | 0.378 | |
| Fruits (during past 3 month) | ||||
| Mandarin | 56.10 ± 16.51* | 99.82 ± 6.80 | 0.016 | |
| Persimmon | 17.38 ± 6.05* | 33.85 ± 2.49 | 0.013 | |
| Apple | 59.57 ± 13.09* | 92.12 ± 5.39 | 0.023 | |
| Grape | 40.22 ± 10.51 | 59.38 ± 6.90 | 0.095 | |
| Tomato | 20.26 ± 5.27 | 23.71 ± 2.17 | 0.548 | |
| Indulgence foods | ||||
| Rice wine | 5.69 ± 6.68 | 12.99 ± 2.75 | 0.316 | |
| Coffee | 210.06 ± 32.02 | 226.82 ± 13.19 | 0.631 | |
| Milk | 64.62 ± 14.65 | 69.17 ± 6.03 | 0.775 | |
| Ice cream | 11.56 ± 2.84* | 5.47 ± 1.17 | 0.050 | |
| Snack foods | 5.01 ± 1.20 | 3.13 ± 0.49 | 0.151 | |
MCI group is defined as the subjects with an average score of 16 percentiles or less between the delayed verbal scores and delayed visual scores. Data are least square means ± standard deviation.
MCI, mild cognitive impairment
*Statistical difference between the MCI group and normal group adjusted age by GLM univariate analysis at p < 0.05.
DISCUSSION
The purpose of this study was to investigate the nutritional status of the MCI group in regards to population aging and increasing prevalence of dementia in Korean society.
The neuropsychological scores were significantly lower in the MCI group than those in the normal group in terms of language delayed recall scores, visual delayed recall scores, and language memory scores. Clinically, verbal memory scores in MCI patients are known to be very sensitive indicators [13]. Previous studies have consistently reported delayed recall test, language fluency, name-standing ability, and attention loss in MCI patients compared with normal elderly subjects [14,15,16], and among them, the decline of anecdotal memory is known as a cognitive index to distinguish MCI at every clinical diagnostic stage. The decline of anecdotal memory is mainly due to poor performance in tasks requiring new learning or delayed recall abilities, and such memory decline is often reported in normal aging. Lambon et al. [17] also reported progressive neuropsychological deficits in cross-sectional studies, and in amnestic MCI patients, which begins with an ankle memory defect and gradually spreads to semantic memory and language domain execution defects. As the moderate AD progresses, they reported a defect in the perception and attention area.
K-MMSE, which is used clinically as a screening test for dementia, is not a sensitive indicator of cognitive impairment in dementia patients, but this is known to be a useful tool for detecting cognitive impairment and quantitatively assessing its extent [12]. The mean score of K-MMSE in the MCI group of this study was 25.19 points lower than that of the normal group (26.29 points), but there was no statistically significant difference, which shows the limitation of using MMSE as a sensitive indicator.
In this study, it is known that the EEG used as an objective cognitive function measurement index that reflects the electrical changes are generated by the activity of the brain-specific neurons in the scalp. The relative value of EEG indicates the percentage of a given frequency band relative to the absolute value. In the MCI group of this study, relative theta wave was significantly higher in the frontal lobe (Fp1, Fp2) than in the normal group. This is consistent with the increase in theta wave, which is a relatively consistent result in previous studies of patients with mild dementia [18], and the frontal lobe is known to preserve memory and thinking power. There is the hypothesis that the left prefrontal region is involved in memory coding and that the right prefrontal cortex is involved in memory retrieval [19]. In 1998, Science introduced the concept of psychometrics and neurometrics, and in a study by Prichep [20] from New York University, known as the pioneer of quantitative EEG (QEEG), the increase of theta wave over a wide range of locations was known to be an indicator of progression to MCI and AD after 7 years in the normal elderly with memory discomfort.
To investigate food and nutrient intakes, semi-quantitative FFQ was modified and developed to reflect dietary habits of elderly and adults over 50 years old in Korea, and it was developed to provide a relatively simple way to estimate the average annual intake of nutrients and food (fruit: 3 months) over the age of 50 years.
In terms of energy, carbohydrate, protein, lipid intake, and intake ratio of the total subjects' nutrient intake, there was no significant difference between the 2 groups, but the MCI group had lower average value of caloric, carbohydrate, protein, and lipid intake, the carbohydrate intake average rate was low and the lipid intake average rate was high. In Lee's [21] study, there was no significant difference in the rate of nutrients ingestion of male elderly in Korea. However, the rate of carbohydrate intake was significantly higher in female elderly compared to calories, and the fat intake rate was significantly lower.
In this study, the intake of N-3 fatty acids, C18:4, C20:5 (eicosapentaenoic acid; EPA), C22:6 (docosahexaenoic acid; DHA) among unsaturated fatty acids, and the intake of N-6 fatty acids, C20:3 dihomo-γ-linolenic acid (DGLA), and the intake of mono unsaturated fatty acids, C22:1 in the MCI group were significantly lower than those of the normal group. N-3 fatty acids are known to exert beneficial effects on atherosclerosis by eliminating risk factors for circulatory system disease or by altering lipid composition or platelet aggregation function in serum. In particular, DHA is a constituent of brain phospholipids and contributes to brain function improvement, including learning ability of experimental animals [22]. Chronic DHA intake has been shown to improve memory and reduce lipid peroxide content in the brain, thus favoring the environment of the nervous system. In addition, in the animal experiment, when the maze memory learning ability was measured after 4 months of feeding with 2% DHA in old mice, the time taken to reach the water exit was shorter than that of the N-3 fatty acid-depleted diet group, and the number of staying in the middle block of the maze was also small [23]. DGLA-derived eicosanoids (prostaglandin E1; PGE1) are associated with anti-inflammatory effects. DGLA is arachidonic acid (AA, C20:4) by Δ-5 desaturase, and AA is a precursor of PGE2 that causes inflammation. AA reacts with cyclooxygenase (COX)-1, COX-2 enzyme to become PGE2. In this process, EPA competitively inhibits the reaction of AA with COX-1 and COX-2. EPA also causes PGE3 associated with anti-inflammation. That is, if EPA is adequately ingested anti-inflammatory action can be exerted.
In this study, intake of biotin and vitamin D were significantly lower in the MCI group than the normal group. Biotin is a vitamin B complex, a crystalline vitamin required for cell growth, fatty acid production, metabolism, and vitamin B activation. In the present study, subjects consumed more than the recommended intake of other vitamin B, but biotin was consumed under sufficient intake, and the recommended intake of MCI group was significantly lower than that of the normal group. Therefore, it can be expected that the deficient intake of biotin has affected the metabolism of vitamin B group. On the subjects of thirty MCI patients (59 to 81 years) with high serum homocysteine concentration, in a study in which a high content of vitamin B (vitamin B12, vitamin B6, and folic acid) was supplied for 270 days, serum homocysteine concentration was normalized, and the cerebrospinal fluid-tau and albumin ratio of cerebrospinal fluid decreased and the blood-brain barrier function improved. No patients progressed to dementia during the experiment [24]. However, on the subjects of 409 patients with mild to moderate dementia with normal plasma vitamin B12, homocysteine concentration, when 18 months of high-dose folic acid, vitamin B6, and vitamin B12 were given to the experimental groupand placebo was given to the control group, the homocysteine concentration in the experimental group decreased, but there was no delayed decline in cognitive function, but rather side effects such as depression were found [25]. From the above results, in the subjects with high homocysteine concentration, the intake of vitamin B group had beneficial effects related to cognitive function, but future follow-up research is required.
In this study, vitamin D intake of Koreans above 50 years old was less than the recommended intake equivalent to existing studies, and the recommended intake of MCI group was significantly lower than that of normal group. When vitamin D receptors are distributed in the hypothalamus and hippocampus of the brain, it acts on the synthesis of brain nerve growth promoting and inhibiting substances and it has also been reported that cortisol-induced neurotoxicity can be beneficial to dementia. Experimental studies and animal studies have been reported to be associated with decreased cognitive function, dementia and vitamin D, but there is a lack of consistency between the results of the research, and further research is needed [26,27].
In both of the study groups, magnesium was consumed below the recommended intake, and the recommended intake of MCI group was lower than that of normal group. Magnesium is known to help inhibit nerve damage by acting as an antagonist of glutamate (neurotransmitter) NMDA receptors in the nervous system.
In the results of food intake of the whole subjects adjusted for age, grains, sweet potato intake of the MCI group was significantly lower than that of the normal group. Sweet potatoes are high carbohydrate foods, but are rich in dietary fiber, potassium, and vitamins. Low glycemic index (GI) food among cereals group foods, which helps to control blood sugar. Several studies have reported that glucose intake improves memory, depending on the individual's ability to control glucose. If glucose regulation is impaired, anecdotal memory and cognitive abilities are impaired, and it is larger in the elderly over 65 years old. The average postprandial cognitive abilities of a 65-year-old adult with type 2 diabetes were significantly higher when taking carbohydrate meals with lower GI than those with higher GI [28]. In this study, the intake of sweet potato, a food of hypoglycemic index, may have contributed to controlling insulin resistance, which is one of the causes of cognitive dysfunction. Sweet potato also contains antioxidant nutrients such as beta carotene.
Of the MCI group had less intake than the normal group did, and mackerel intake showed a positive correlation with alpha peak frequency (APF) of the occipital lobe which is known to be highly correlated with cognitive function and memory. Mackerel is a food source of N-3 and N-6 fatty acids.
The intake of salted fish was significantly higher in the MCI group than that in the normal group. Salted fish is a salty food. According to a report in Nature Neuroscience [29] by a team of researchers at Weill Cornell Medicine, it has been found that cognitive function is significantly lowered when cerebral blood flow is reduced when a very high salt intake is taken. In that study, from the results of comparing rats fed a diet containing 4%–8% salt, which induced 16 times more salty taste than normal diet with the control group, it was confirmed that approximately 28% of the cerebral cortical blood flow decreased and that hippocampus was reduced by 25%. The mice with reduced cerebral blood flow showed a significant decrease in cognitive ability tests such as finding a maze. The researchers found that the mechanism of cerebral blood flow decline was due to the lack of nitrogen oxide (NO), a gas secreted by endothelial cells. Because it is the result of animal experiments and the amount of salt intake is very small compared to humans, it is difficult to immediately apply it to clinical practice, so further research is needed.
In the fruit group, the consumption of mandarin orange, persimmon and apple in the MCI group was significantly lower than that of normal group. Tangerines and persimmons are a food containing a lot of antioxidant vitamins and phytochemicals such as vitamin A, beta-carotene, vitamin C, and vitamin E, etc. In the elderly group of 815 elderly people aged 65 years or older without dementia, the dementia occurrence of the group with the high intake of vitamin E for average 3.9 years was significantly lower than that of the low intake group, and these effects were only seen in people who were not APOE ε4 alleles. There was no significant relationship between the intake of vitamin C and beta-carotene, the intake of vitamin E supplements, and the risk of dementia [30]. In a study comparing plasma antioxidant levels in normal group, MCI group and dementia elderly patients, MCI group and dementia group had lower plasma levels of vitamin C, vitamin E, vitamin A, lutein, zeaxanthin, α-carotene, β-cryptoxanthin, and lycopene than normal group. The plasma levels of vitamin A, zeaxanthin, β-cryptoxanthin, and lycopene were significantly lower in patients with dementia than MCI group. Therefore, it was estimated that increasing the level of antioxidants in plasma could help prevent dementia. However, in the result of a systematic review of eight cross-sectional studies and 13 prospective studies, there was no significant correlation in whether the intake of antioxidant nutrients from the diet improves cognitive ability or lowers the risk of dementia [3,31]. In this study, the ratio of vitamin C intake to the recommended intake of MCI group was significantly lower than that of the normal group.
So far, this study has confirmed that the nutritional intake of Korean over 50 years of age is related to cognitive function. In that we used EEG measurements for more objective confirmation as well as evaluation index in cognitive function measurement, this study can be regarded as a study that differs from the previous studies in Korea.
However, due to the limitations of this study, followed up research was not made, and the female ratio and the distribution of education level among subjects of this study were higher than those of the average Koreans over 50 years of age. Because of the good overall nutritional status of subjects in this study, it was difficult to generalize Koreans above 50 years old as a whole.
To reduce the risk of future dementia and improve cognitive function, through long-term prospective studies and nutritional intervention studies, it is considered necessary to study specific nutritional factors affecting cognitive function such as meal frequency, meal and snack consumption time, homocysteine concentration, and glucose control effect.
CONCLUSION
The importance of dietary therapy for the prevention and management of MCI groups is emerging, according to the recent increase in elderly population and prevalence of dementia. Thus, this study was to compare the nutritional status of MCI group and normal group. In the MCI group of this study, language delay recall score, visual delay recall score, and language memory score were lower in the neurocognitive function evaluation. In the EEG, the relative theta wave of the prefrontal cortex were increased, indicating a tendency to represent the background slowing. The MCI group showed a significantly lower intake of N-3 fatty acid (C18: 4, EPA, DHA), N-6 fatty acid (DGLA), mono unsaturated fatty acids (C22:1), biotin, and vitamin D than the normal group. In addition, the intake of vitamin D, vitamin C, biotin, and magnesium was significantly lower in the MCI group than in the normal group in 2015 for the Korean nutrition standard. In addition, intakes of mackerel, tangerine, persimmon, apple, and sweet potato were significantly lower than those of the normal group, and intakes of salted fish and ice cream were significantly higher. The results of this study showed the necessity of high-quality dietary management including supplementation of N-3 fatty acid, N-6 fatty acid, biotin, and vitamin D in MCI patients. Also, there is a difference in the intake of foods rich in N-3 fatty acid, antioxidant nutrients, complex saccharide, and salt etc. These results demonstrate the nutritional problems of MCI patients, and it is expected that it will be the basis of future nutrition study and nutrition education data development for MCI patients, and that Korean adults over 50 years old are expected to require specific nutrients and food intake.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993. [Google Scholar]
- 2.Kim HJ. Correlation between cognitive function and food intake and plasma homocysteine, folate, and vitamin B12 contents in the elderly [master's thesis] Seoul: The Graduate School Ewha Womans University; 2011. [Google Scholar]
- 3.Yang YJ. Mild cognitive impairment and nutrition in old adults. Hanyang Med Rev. 2014;34:53–59. [Google Scholar]
- 4.Han E. Neuropsychological differentiation of cognitive aging and predictors of progression from mild cognitive impairment to Alzheimer's disease [dissertation] Gwangju: Chonnam National University; 2010. [Google Scholar]
- 5.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Praticò D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Park SJ, Kim JH, Kim CI, Chang KJ, Yim KS, Kim KW, Choi HM. Development and validation of a computerized semi-quantitative food frequency questionnaire program for evaluating the nutritional status of the Korean elderly. Korean J Community Nutr. 2002;7:277–285. [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Kwon YC. Modification of the Mini-Mental State Examination for use in the elderly in a non-western society. Part 1. Development of Korean version of Mini-Mental State Examination. Int J Geriatr Psychiatry. 1990;5:381–387. [Google Scholar]
- 12.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 13.CNS Vital Signs. CNS Vital Signs interpretation guide. Morrisville (NC): CNS Vital Signs; 2003. [Google Scholar]
- 14.Fabrigoule C, Rouch I, Taberly A, Letenneur L, Commenges D, Mazaux JM, Orgogozo JM, Dartigues JF. Cognitive process in preclinical phase of dementia. Brain. 1998;121:135–141. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Knapen J, David A, Van Gool M, Van de Vilet P, Van Coppenolle H, Peuskens J, Knapen K. Likelihood of drop out during a graded exercise test in non-psychotic psychiatric patients. Br J Ther Rehabil. 2003;10:305–309. [Google Scholar]
- 17.Lambon Ralph MA, Patterson K, Graham N, Dawson K, Hodges JR. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: a cross-sectional and longitudinal study of 55 cases. Brain. 2003;126:2350–2362. doi: 10.1093/brain/awg236. [DOI] [PubMed] [Google Scholar]
- 18.Kwak YT, Suk SH, Son IH. Usefulness of occipital EEG spectral profile in the differential diagnosis of Alzheimer type and vascular dementia. J Korean Neurol Assoc. 2000;18:292–297. [Google Scholar]
- 19.Lee CY, Kim ES, Lee S, Ko B, Kim JS, Zhang BT. Properties of human cognitive learning in a movie scene-dialogue memory game using EEG-based brain function analysis; Proceedings of Korea Computer Congress 2011; 2011 Jun 29–Jul 1; Gyeongju, Korea. Seoul: Korean Institute of Information Sciences and Engineers; 2011. pp. Volume 38–210. 213. [Google Scholar]
- 20.Prichep LS, John ER, Ferris SH, Rausch L, Fang Z, Cancro R, Torossian C, Reisberg B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol Aging. 2006;27:471–481. doi: 10.1016/j.neurobiolaging.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Lee L. Associations between dietary intake and health status in Korean elderly population. J Nutr Health. 2002;35:124–136. [Google Scholar]
- 22.Kim YK, Joo KJ. EPA, DHA and tocopherols contents in fish oil products and fishes. J Korean Soc Food Nutr. 1994;23:68–72. [Google Scholar]
- 23.Lim S. Effect of treatment with docosahexaenoic acid into N-3 fatty acid adequate diet on learning related brain function in rat. J Life Sci. 2009;19:917–922. [Google Scholar]
- 24.Lehmann M, Regland B, Blennow K, Gottfries CG. Vitamin B12-B6-folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2003;16:145–150. doi: 10.1159/000071002. [DOI] [PubMed] [Google Scholar]
- 25.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, Thal LJ Alzheimer Disease Cooperative Study. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ministry of Health and Welfare, The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2015. Seoul: The Korean Nutrition Society; 2015. [Google Scholar]
- 27.Lee IH, Kang HS. Association of health physical fitness and serum vitamin d with global cognitive performance in older persons. J Korean Soc Living Environ Syst. 2016;23:217–224. [Google Scholar]
- 28.Kim WK, Cho S. Sugar and cognitive performance. J Nutr Health. 2007;40(Suppl):50–65. [Google Scholar]
- 29.Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, Buendia I, Santisteban MM, Segarra SG, Koizumi K, Sugiyama Y, Murphy M, Voss H, Anrather J, Iadecola C. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci. 2018;21:240–249. doi: 10.1038/s41593-017-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 31.Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia--a systematic review. Plant Foods Hum Nutr. 2013;68:279–292. doi: 10.1007/s11130-013-0370-0. [DOI] [PubMed] [Google Scholar]