Abstract
All-trans-retinoic acid (ATRA) and 1α,25-dihydroxyvitamin D (1,25D) are potent inducers of differentiation of myeloid leukemia cells. During myeloid differentiation specific transcription factors are expressed at crucial developmental stages. However, precise mechanism controlling the diversification of myeloid progenitors is largely unknown, CCAAT/enhancer-binding protein (C/EBP) transcription factors have been characterized as key regulators of the development and function of the myeloid system. Past data point at functional redundancy among C/EBP family members during myeloid differentiation. In this study, we show that in acute myeloid leukemia (AML) cells, high expression of vitamin D receptor gene (VDR) is needed for strong and sustained upregulation of CEBPB gene, while the moderate expression of VDR is sufficient for upregulation of CEBPD in response to 1,25D. The high expression level of the gene encoding for retinoic acid receptor α (RARA) allows for high and sustained expression of CEBPB, which becomes decreased along with a decrease of RARA expression. Expression of CEBPB induced by ATRA is accompanied by upregulated expression of CEBPE with similar kinetics. Our results suggest that CEBPB is the major VDR and RARA-responsive gene among the CEBP family, necessary for expression of genes connected with myeloid functions.
Keywords: nuclear receptors, CCAAT-enhancer-binding proteins, CEBP genes, vitamin D receptor, retinoic acid receptor α, differentiation, acute myeloid leukemia
1. Introduction
CCAAT/enhancer-binding proteins (C/EBPs) are transcription factors that activate the expression of target genes through interaction with response elements within their promoters [1]. There are six members of C/EBP family, and they regulate differentiation process in various tissues [2]. The first transcription factor in this family, C/EBPα, was isolated from the rat liver and it appeared to be important for adipocyte differentiation [3]. C/EBPs are modular proteins consisting of an activation domain, a DNA binding domain, and a leucine-rich dimerization domain that is responsible for forming dimers with other members of the family [4]. In order to activate transcription, the C/EBP dimers bind to the consensus sequence 5′-TT/GNNGNAAT/G-3′ in promoter regions of target genes. For three out of six genes encoding C/EBP family members, alternative protein products are translated, due to a leaky ribosomal scanning mechanism. Some of them lack the N-terminal activation domains and exert inhibitory functions, presumably by a dominant negative mechanism [5].
Hematopoiesis is a process in which all blood elements are formed from multipotential hematopoietic stem cells (HSCs). In the process of hematopoiesis, the HSCs and their progeny interact with the bone marrow stromal cells and they are stimulated by the numerous growth factors that are secreted in the bone marrow environment. The eventual cell fate during hematopoiesis is governed by spatiotemporal fluctuations in transcription factor concentrations, which either cooperate or compete in driving target gene expression [6]. Some members of C/EBP family of transcription factors are important at certain steps of hematopoiesis [7]. C/EBPα appears in differentiating cells at the stage of early progenitors with lymphoid and myeloid potential and then reappears only in the cells that are differentiating into granulocytes [8]. C/EBPα-deficient mice show disturbances in monocyte and neutrophil development [9,10]. High level of C/EBPβ leads to monocyte and macrophage development [11,12], while high level of C/EBPε leads to neutrophil differentiation [13]. The role of C/EBPδ in blood cells development is less defined, since CEBPD−/− mice did not reveal any blood disturbances [14]. It has been documented that C/EBPδ regulates expression of genes important for granulocyte function [15].
However, the most important factors that drive blood cells development are cytokines [16], some ligands for nuclear receptors can also modulate cell fate during hematopoiesis [7]. The best described in this respect are ligands for retinoic acid receptors (RARs). Active metabolites of vitamin A are natural ligands for RARs. A dominating retinoic acid (RA) metabolite is all-trans-RA (ATRA), which binds with high affinity to all RARs (α, β, and γ) [17]. During embryogenesis, ATRA causes the appearance of hematopoietic progenitors from the hemogenic endothelium [18], while in adults, it is important for the differentiation of granulocytes, as well as B and T lymphocytes [19]. This activity of ATRA has been used in clinics. The most clinically significant application of ATRA is to treat a rare subtype of an acute myeloid leukemia (AML), called acute promyelocytic leukemia (APL). At the first description this subtype was considered the most difficult to treat [20], while it is now considered as highly curable using the combination of ATRA and anthracycline-based chemotherapy [21]. Another ligand for the nuclear receptor which influences hematopoiesis is an active metabolite of vitamin D. The correct physiological concentrations of 1,25-dihydroxyvitamin D (1,25D), which is a natural ligand for vitamin D receptor (VDR), are necessary to induce markers of monocytic differentiation in HSCs [22]. The expression of the VDR gene is higher at the early steps of hematopoiesis than at later stages and in mature blood cells [23]. However, both these ligands do not seem indispensable for blood cells development since RARα-deficient and VDR-deficient mice show no defects in hematopoiesis [24,25]. The possibility that these nuclear receptors can, in some aspects, functionally compensate each other should be considered.
It has been documented in the past that members of C/EBP family of transcription factors can be upregulated in blood cells by an exposure to RA, 1,25D, or to their active analogs. For example, the expression of C/EBPε mRNA and protein increases in AML cells exposed to 9-cis–RA or 1,25D analog (KH1060) [26]. The gene encoding C/EBPβ has been shown to be a target for VDR regulation [27] and all isoforms of this transcription factor are increased in AML cells exposed to 1,25D or to analogs of 1,25D [11,28]. This gene is also strongly upregulated in AML cells that were exposed to ATRA [29]. 1,25D induces a transient increase of C/EBPα [11], which also participates in the ATRA-induced differentiation of AML cells [30].
In this study, we addressed a question of whether the lack of one of the nuclear receptors mentioned above could be compensated by the other in terms of CEBP activation. Therefore, we used four cell lines in our study, with different expression of retinoic acid receptor α (RARA) or VDR. In HL60 cells, VDR expression is on a high level and RARA is moderate [31]. For the purpose of this study, we silenced the expression of VDR in HL60 cells using shRNA. In contrast to HL60 cells, KG1 cell express high levels of RARA, but low of VDR [31]. The effects of RARA silencing were studied using a sub-line KG1-RARα(−).
2. Results
2.1. Activation of Expression of CEBP Transcription Factor’s Genes in AML Cells with High Level of VDR and Low Level of RARα
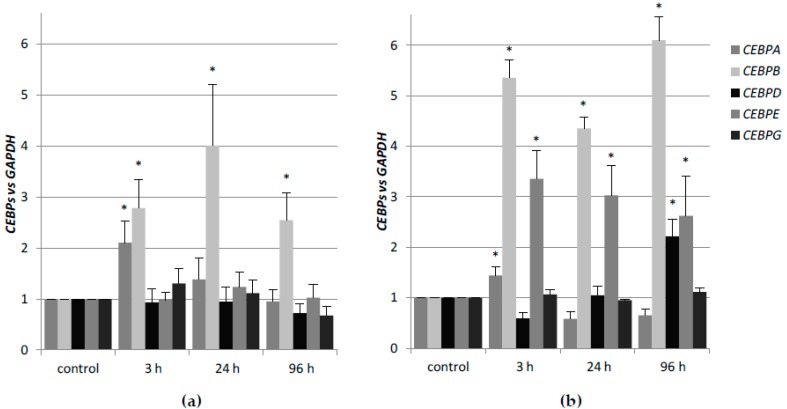
In previous studies, we have shown that different AML cell lines have variable sensitivity to 1,25D− and ATRA-induced differentiation [32]. HL60 cell line responded to 1,25D with robust monocytic differentiation and to ATRA with moderate granulocytic differentiation. That corresponded to high basal level of expression of VDR and low basal level of expression of RARA [31]. In view of demonstrated regulation of differentiation of myeloid leukemia cells by these two compounds, it was of interest to determine the expression profiles of CEBP genes in response to 1,25D and ATRA in HL60 cells. Therefore, the expression of CEBPA, CEBPB, CEBPD, CEBPE, and CEBPG in HL60 cells that were exposed to 1 μM ATRA or to 10 nM 1,25D for different time periods was tested. As depicted in Figure 1a, transient upregulation of CEBPA was detected in HL60 cells stimulated with 1,25D, followed by fast decline. This was in concordance with an earlier observed transient upregulation of C/EBPα protein in HL60 cells after exposure to 1,25D [11]. The increase in expression of CEBPB was more sustained, with a peak at 24 h and more gradual decline. As presented before, protein level of C/EBPβ follows this sustained expression pattern and it peaks between two and three days of exposure to 1,25D [11]. CEBPD, CEBPE, and CEBPG were not upregulated in response to 1,25D exposure of HL60 cells. As presented in Figure 1b in HL60 cells that were exposed to ATRA, CEBPA was upregulated weakly and transiently. Expression of CEBPB and CEBPE was stimulated by ATRA stronger and in a sustained manner. Modest upregulation of CEBPD was observed at 96 h from exposure to ATRA. Again, no stimulation of CEBPG was observed. Values of mRNA expression obtained using comparative quantification algorithm are presented in Table A1.
Figure 1.
Regulation of CEBP genes in HL60 exposed to 1,25D or all-trans-retinoic acid (ATRA). HL60 cells were exposed to 10 nM 1,25D (a) or 1 µM ATRA (b) and after desired time the expression of CEBP genes was measured by Real-time PCR. The bars represent mean values (±standard error of the mean (SEM)) of the fold changes in mRNA levels relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. Expressions in control cells were treated as calibrators. Values significantly different from those obtained from respective controls cells are marked with an asterisk (* p < 0.05).
2.2. Activation of Expression of CEBP Transcription Factor’s Genes in AML Cells with Low Level of VDR and High Level of RARα
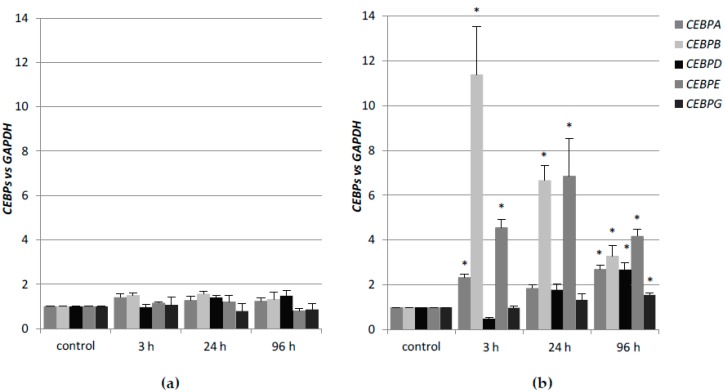
In contrast to HL60 cells, KG1 cells are not responsive to 1,25D and they have a low level of VDR protein, whilst being susceptible to ATRA-driven granulocytic differentiation [33]. This corresponds with the high basal level of expression of RARA gene and high constitutive content of RARα protein [31]. In the next series of experiments, KG1 cells were exposed to 1 μM ATRA or to 10 nM 1,25D for different time periods. In KG1 cells, the transcript levels of CEBP genes remained unchanged after exposure to 1,25D (Figure 2a). In contrast, significant changes in expression of CEBP genes after exposure of KG1 cells to ATRA were observed. Modest upregulation of CEBPA was detected at 3 h and 96 h from exposure. CEBPB was the most responsive to ATRA out of the genes studied, the expression upregulation was fast and long-lasting. The second ATRA-responsive gene was CEBPE, where the expression peaked at 24 h. The expression of CEBPD and CEBPG was modest with a peak at 96 h (Figure 2b). Values of mRNA expression that were obtained using comparative quantification algorithm are presented in Table A2.
Figure 2.
Regulation of CEBP genes in KG1 exposed to 1,25D (a) or to ATRA (b). KG1 cells were exposed to 10 nM 1,25D or 1 µM ATRA and after desired time the expression of CEBP genes was measured by Real-time PCR. The bars represent mean values of the fold changes (±SEM) in mRNA levels relative to GAPDH mRNA levels. Expressions in control cells were treated as calibrators. Values significantly different from those that were obtained from respective controls cells are marked with asterisk (* p < 0.05).
2.3. Effects of Silencing High RARA on Expression of CEBP Transcription Factor’s Genes in KG1 Cells
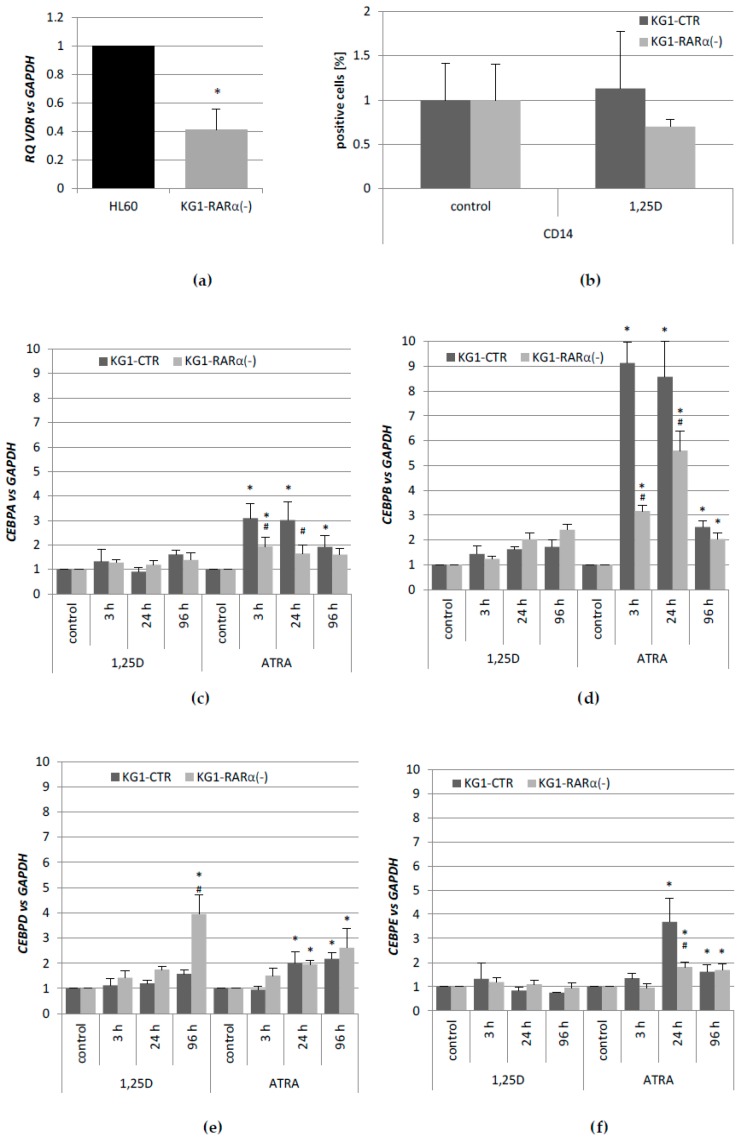
In an attempt to elucidate whether the lack of one of the nuclear receptors VDR and RARα could be compensated by the other in terms of CEBP activation, we used KG1 sublines with silenced RARA gene (KG1-RARα(−)) and KG1 control cells (KG1-CTR), which were obtained before [31]. These cells have substantially reduced level of RARA gene expression and RARα protein, but also exhibit the increased expression of VDR gene and VDR protein, when compared to wild-type KG1 and KG1-CTR [31]. It should be noted that the expression of VDR gene in KG1-RARα(−) is still lower than in HL60 cells (Figure 3a), and it was not sufficient to induce antigen CD14 typical for monocytes (Figure 3b). The KG1 sublines were stimulated with 1,25D or ATRA in a similar manner as before. As presented in Figure 3e, KG1-RARα(−) cells started to be responsive to 1,25D, however in a manner that was different from HL60 cells. Only CEBPD gene became responsive to 1,25D in RARA silenced KG1 cells, and the expression of CEBPB remained at a control level. As expected, expression levels of CEBPA, CEBPB, and CEBPE were reduced in KG1-RARα(−) when compared to KG1-CTR cells after ATRA stimulation, especially at the early hours from stimulation (Figure 3c–f). Values of mRNA expression that were obtained using comparative quantification algorithm are presented in Table A3.
Figure 3.
Responses to 1,25D and to ATRA in KG1 cells with silenced retinoic acid receptor α (RARA) gene. Expression of vitamin D receptor (VDR) gene in KG1-RARα(−) cells compared to HL60 cells, which were treated as calibrator (a). Differentiation of KG1 sublines in response to 1,25D. KG1-CTR and KG1-RARα(−) cells were stimulated with 10 nM 1,25D for 96 h and then the expression of CD14 differentiation marker was detected using flow cytometry (b). Expression of CEBPA (c), CEBPB (d), CEBPD (e), and CEBPE (f) genes in KG1-CTR and KG1-RARα(−). Cells were stimulated with 10 nM 1,25D or 1 µM ATRA and after desired time the expression of CEBP genes was measured by Real-time PCR. The bars represent mean values of the fold changes (±SEM) in mRNA levels relative to GAPDH mRNA levels. Expressions in control cells were treated as calibrators. Values that are significantly different from those obtained from respective controls cells are marked with asterisk (* p < 0.05); values that differ significantly from those obtained from respective KG1-CTR control cells are marked with hash (# p < 0.05).
2.4. Effects of Silencing High VDR on Expression of CEBP Transcription Factor’s Genes in HL60 Cells
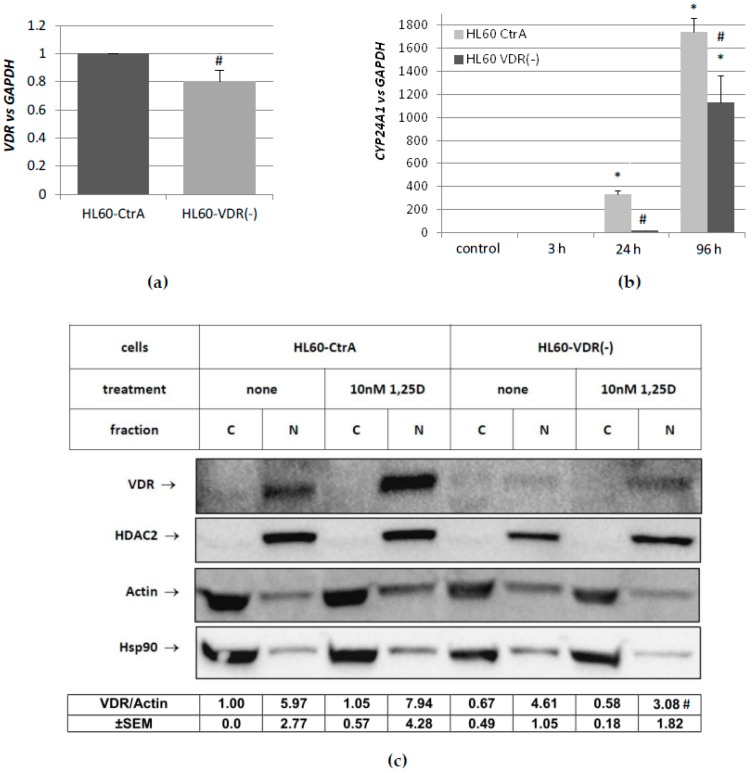
Having shown that KG1-RARα(−) cells demonstrate an altered CEBP expression profile, we decided to silence the expression of VDR gene in HL60 cells. The gene silencing was performed using shRNA plasmid and the scrambled shRNA plasmid, as described before [34]. This way, two HL60 sublines were generated: HL60-VDR(−) and HL60-CtrA. In order to validate whether the expression of VDR gene was indeed efficiently knocked down in HL60-VDR(−) cells, the mRNA and protein levels were compared to HL60-CtrA cells. Unfortunately, the silencing was far from complete and mRNA level was reduced to approximately 80% of the initial level (Figure 4a). In order to verify whether this reduction would lead to VDR-dependent effects, the expression of the gene that encodes 24-hydroxylase of 1,25D (CYP24A1) was tested in both HL60 sublines exposed to 10 nM 1,25D. CYP24A1 is the most strongly regulated out of all 1,25D-target genes and is the best measure of VDR’s activity [35]. 1,25D-induced expression of CYP24A1 was significantly reduced in HL60-VDR(−) cells when compared to HL60-CtrA cells. Figure 4c shows that VDR protein content was also significantly reduced in the nuclei of HL60-VDR(−) cells to 77% in control cells, and to 39% after 1,25D treatment when compared to HL60-CtrA cells.
Figure 4.
Generation of HL60 cells with reduced VDR expression. Constitutive expression of VDR gene in HL60-CtrA and HL60-VDR(−) cells. Expression in HL60-CtrA was treated as calibrator (a). Expression of CYP24A1 was measured in both cell lines exposed to 10 nM 1,25D for different times (b). The levels of VDR protein were determined in the cytosol and nuclei of HL60-CtrA and HL60-VDR(−) cells by western blots after 10 nM 1,25D stimulation for 24h (c). Cell lysates were tested while using anti-VDR. Proper cell fractionation was revealed using anti-histone deacetylase 2 (anti-HDAC2), while proper lane loading using anti-Hsp90 and anti-actin. Values below the blots are means (±SEM), as obtained from five experiments. The bars represent mean values of the fold changes (±SEM) in mRNA levels relative to GAPDH mRNA levels. Values that are significantly different from those obtained from respective controls cells are marked with asterisk (* p < 0.05); values that differ significantly from those obtained from respective HL60-CtrA cells are marked with hash (# p < 0.05).
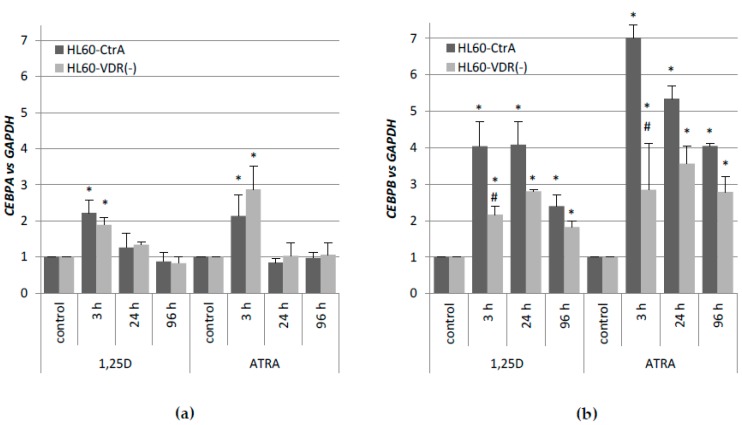
Even though HL60-VDR(−) subline was not entirely devoid of VDR, the cells were exposed 1 μM ATRA or 10 nM 1,25D in order to examine the expression of selected CEBP genes. CEBPA and CEBPB have been selected, since they are direct targets of VDR-dependent transcriptional regulation [36,37]. As presented in Figure 5a,b, the limited decrease of VDR expression level resulted in a reduced CEBPA and CEBPB expression levels in response to 1,25D when compared to HL60-CtrA cells. Interestingly, the response to ATRA in HL60-VDR(−) cells was different than in HL60-CtrA cells, and upregulated regarding CEBPA expression, while downregulated regarding CEBPB expression. Values of mRNA expression that were obtained using comparative quantification algorithm are presented in Table A4.
Figure 5.
Expression of CEBPA (a) and CEBPB (b) genes in HL60-CtrA and HL60-VDR(−). Cells were exposed to 10 nM 1,25D or 1 µM ATRA and after desired time the expression of CEBP genes was measured by Real-time PCR. The bars represent mean values of the fold changes (±SEM) in mRNA levels relative to GAPDH mRNA levels. Expressions in control cells were treated as calibrators. Values that are significantly different from those obtained from respective controls cells are marked with an asterisk (* p < 0.05); values that differ significantly from those obtained from respective HL60-CtrA cells are marked with hash (# p < 0.05).
3. Discussion
The process of hematopoiesis leads to the acquisition of immune functions by terminally differentiated cells. Lineage selection within hematopoiesis depends on the appropriate levels of key transcription factors, which are regulated in response to numerous hematopoietic cytokines and interactions with bone marrow environment [38]. Transcription factors C/EBPα, C/EBPβ, and C/EBPδ have been demonstrated in granulocytes, monocytes, and eosinophils, as well as in myeloid progenitor cells [39]. C/EBPε has been identified as a critical regulator of terminal granulopoiesis [13]. Many genes that are important for myeloid functions contain in their promoters binding sites for C/EBP transcription factors [5]. In normal hematopoiesis, C/EBP transcription factors are produced in response to coordinated actions of cytokines and upstream transcription factors, and their activity is further modulated by posttranslational modifications [5].
C/EBPα seems to be the most important for normal blood development, since mutations in CEBPA gene lead to AML. CEBPA is mutated in around 13% of all AML patients [40], and mutations in this gene appear early, indicating at the driver role in leukemogenesis [41]. This is why the expression of CEBPA in AML patients has been extensively studied, and it has been shown to be downregulated by another driver of leukemogenesis, namely the AML-ETO fusion protein, which is present in 5–10% of patients with AML [42]. Later studies have documented that the downregulation of CEBPA also accompanies AML cases with inv(16), which creates CBFB-MYH11 gene fusion and occurs in about 10% of AML patients [43]. All together, the above data show that more than 30% of patients with AML exhibit disturbances in expression of CEBPA.
It has been shown that both 1,25D and ATRA are able to upregulate expression of C/EBP factors without the addition of hematopoietic cytokines [11,26,27,29]. Whether all the CEBP genes are direct targets for either RARα or VDR is not clear. Retinoic acid response elements (RAREs) have been found in the promoter of CEBPE gene, and not in other genes of this family [44], but at present, we know that RARα can bind to big variety of RAREs [45], which are sometimes located in a long distance from the transcription start [44]. CEBPA and CEBPB are direct targets of VDR-dependent transcriptional regulation [36,37], but it is not sure whether such a mechanism occurs also for CEBPD.
We thus wanted to find out how variable levels of VDR and RARα proteins affect the expression of CEBP genes, and whether the lack of one of the nuclear receptors that are mentioned above, could be compensated by the other. In the first place, we determined the expression profiles of these genes after stimulation with 1,25D or ATRA in HL60 and KG1 cells. These cells differ in basal levels of expression of VDR and RARα, and in susceptibility to 1,25D-induced differentiation. The basal levels of VDR and RARA mRNA expression in all of the cell lines that were used for the purpose of this research is presented in Table A5. HL60 cells, which have high level of VDR protein, respond to 1,25D with transient upregulation of CEBPA, and strong and sustained upregulation of CEBPB. It appeared that KG1 cells that have low level of VDR protein do not express CEBP genes in response to 1,25D at all. After the silencing of the RARA gene in KG1, these cells reduced the responsiveness to ATRA, but started to be responsive to 1,25D, most probably because of an increased expression of VDR [31]. The restored VDR expression level was not high enough to upregulate the expression of CEBPB, but it was sufficient to upregulate CEBPD. As presented here, the upregulation of CEBPD alone was not sufficient to complete the myeloid differentiation process. KG1 cells and HL60 cells are both responsive to ATRA, however, due to higher basal expression of RARA, KG1 cells respond stronger than HL60. In KG1 cells, the upregulation of CEBPB and CEBPE is approximately two times higher than in HL60 cells in response to ATRA; however, the kinetics of expression is similar.
Our results suggest that the ability of 1,25D or ATRA to effectively force the final myeloid differentiation of AML cells strongly depends on effective levels of nuclear receptors for these compounds. It also seems that expression of CEBPB is indispensable for the final effect of myeloid differentiation, and that VDR and RARα do not compensate each other in terms of the induction of CEBP expression. Our data are in agreement with the earlier findings that strong and sustained expression of CEBPB, when accompanied by transient expression of CEBPA leads to the differentiation towards monocytes [11], while, when accompanied by the sustained expression of CEBPE, it leads the differentiation process to granulocytes [46].
4. Materials and Methods
4.1. Cell Lines and Cultures
HL60 cells were from the local cell bank at the Institute of Immunology and Experimental Therapy in Wrocław, and KG1 cells were purchased from the German Resource Center for Biological Material (DSMZ GmbH, Braunschweig, Germany). The cells were grown in RPMI-1640 medium with 10% fetal bovine serum, 100 units/ml penicillin, and 100 µg/mL streptomycin (Sigma, St. Louis, MO, USA), and maintained at standard cell culture conditions.
4.2. Chemicals and Antibodies
1,25D was purchased from Cayman Europe (Tallinn, Estonia) and ATRA was from Sigma-Aldrich (St. Louis, MO, USA). The compounds were dissolved in an absolute ethanol to 1000× final concentrations, and subsequently, diluted in the culture medium to the required concentration.
4.3. cDNA Synthesis and Real-Time PCR
Total RNA was extracted from the cells treated with 1 µM ATRA or 10 nM 1,25D for different time points (3 h, 24 h, 96 h). Briefly, the isolation of total RNA, reverse transcription into cDNA, and Real-time PCR reactions were performed as published before [33], using CFX Real-time PCR System (Bio-Rad Laboratories Inc., Hercules, CA, USA). The sequences of GAPDH, CYP24A1, VDR, and RARA primers, and the reaction conditions were described previously [31,47]. The CEBPA, CEBPB, CEBPD, CEBPE, and CEBPG primers were obtained from RealTimePrimers.com (Real Time Primers, LLC, PA, USA). Their sequences are as follows: CEBPA: forward 5′-TTGGTGCGTCTAAGATGAGG-3′, reverse 5′-GGCAGGAAACCTCCAAATAA-3′; CEBPB: forward 5′-AACTCTCTGCTTCTCCCTCTG-3′, reverse 5′-AAGCCCGTAGGAACATCTTT-3′; CEBPD: forward 5′-ATCGACTTCAGCGCCTACAT-3′, reverse 5′-GCCTTGTGATTGCTGTTGAA-3′; CEBPE: forward 5′-GAGGAGGTTGCTCAGAGTGG-3′, reverse 5′-TCCTGGCCTATTCAGCAGTT-3′; CEBPG: forward 5′-GAACAACCCATTTTGCACTC-3′, reverse 5′-TGAAAGCCAGGAACAAAAAG-3′; APDH: forward 5′-CATGAGAAGTATGACAACAGCCT-3′, reverse 5′-AGTCCTTCCACGATACCAAAGT-3′. Quantification of gene expression was analyzed with either the ∆Cq (to present comparative quantification of expression levels) or with the ∆∆Cq (to present changes in expression induced by treatment) methods using GAPDH as the endogenous control Primers efficiencies were measured in all of the cell lines using Real-time PCR reaction based on the slope of the standard curve. The results were normalized to primer efficiencies to compare gene expression in different cell lines [48]. Real-time PCR assays were performed at least in triplicate.
4.4. Flow Cytometry
The expression of CD14 was determined by flow cytometry. The cells were incubated with 10 nM 1,25D for 96 h, then washed, and stained with 1 µL of Phycoerythrin labeled antibody (or the appropriate control immunoglobulins; both from ImmunoTools, Friesoythe, Germany) for 1 h on ice. Next, they were washed with ice-cold PBS supplemented with 0.1% BSA and suspended in 0.5 mL of PBS supplemented with 0.1% BSA prior to analysis on FACS Calibur flow cytometer (Becton–Dickinson, San Jose, CA, USA). Experiments were repeated at least three times. The acquisition parameters were set for an isotype control. Data analysis was performed with the use of WinMDI 2.8 software (freeware by Joseph Trotter).
4.5. Western Blotting
In order to obtain cytosolic and nuclear extracts, 5 × 106 cells/sample were washed and lysed using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific Inc., Worcester, MA, USA), according to the user’s manual. Lysates were denatured by adding 5× sample buffer (1/4 volume of the lysate) and boiled for 5 min. 25 µL of each lysate were separated in SDS-PAGE and then electroblotted to PVDF membrane. The membranes were then dried and incubated sequentially with primary and a horseradish peroxidase-conjugated secondary antibody. The protein bands were visualized with a chemiluminescence. Then, the membranes were stripped, dried again, and probed with subsequent antibodies. Western blots were repeated five times.
4.6. Gene Silencing Reagents and Procedure
The RARA gene silencing in KG1 cells was described before [31]. The VDR gene silencing in HL60 cells was performed using shRNA plasmids and Neon® Transfection System (Invitrogen™, Carlsbad, CA, USA) using control shRNA plasmid-A (sc-108060) and the VDR shRNA plasmid (sc-106692-SH; both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The procedure of electrotransfection by Neon® Transfection System was described before [49].
4.7. Statistical Analysis
For statistical analysis one-way ANOVA was used to test the null hypothesis that the samples in two or more groups are drawn from populations with the same mean values. When the ANOVA test had shown that the null hypothesis is not true, the Student’s t-test for independent samples was used to analyze the differences between the pairs of groups (Excel, Microsoft Office and free ANOVA Calculator: http://www.danielsoper.com/statcalc3/calc.aspx?id=43).
Abbreviations
| ATRA | all-trans-retinoic acid |
| 1,25D | 1α,25-dihydroxyvitamin D |
| C/EBP | CCAAT/enhancer-binding protein |
| RARs | retinoic acid receptors |
| AML | acute myeloid leukemia |
| HSCs | hematopoietic stem cells |
| APL | acute promyelocytic leukemia |
| VDR | vitamin D receptor |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| SEM | standard error of the mean |
Appendix A
Table A1.
Comparative quantification of CEBP genes related to a reference GAPDH gene in HL60 cells.
| HL60 | CEBPA | SEM | CEBPB | SEM | CEBPD | SEM | CEBPE | SEM | CEBPG | SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| control | 0.16 | 0.06 | 0.07 | 0.02 | 0.004 | 0.001 | 0.004 | 0.002 | 0.068 | 0.026 | |
| 1,25D | 3 h | 0.33 | 0.07 | 0.19 | 0.03 | 0.003 | 0.001 | 0.003 | 0.002 | 0.085 | 0.051 |
| 24 h | 0.22 | 0.03 | 0.27 | 0.03 | 0.003 | 0.001 | 0.004 | 0.002 | 0.072 | 0.042 | |
| 96 h | 0.15 | 0.05 | 0.18 | 0.02 | 0.003 | 0.001 | 0.004 | 0.001 | 0.046 | 0.030 | |
| ATRA | 3 h | 0.18 | 0.04 | 0.06 | 0.03 | 0.003 | 0.001 | 0.002 | 0.002 | 0.058 | 0.014 |
| 24 h | 0.25 | 0.07 | 0.30 | 0.14 | 0.002 | 0.000 | 0.007 | 0.008 | 0.061 | 0.020 | |
| 96 h | 0.13 | 0.04 | 0.25 | 0.07 | 0.004 | 0.001 | 0.006 | 0.0071 | 0.055 | 0.012 | |
Table A2.
Comparative quantification of CEBP genes related to a reference GAPDH gene in KG1 cells.
| KG1 | CEBPA | SEM | CEBPB | SEM | CEBPD | SEM | CEBPE | SEM | CEBPG | SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| control | 0.025 | 0.016 | 0.039 | 0.000 | 0.0006 | 0.000 | 0.00003 | 0.0000 | 0.0037 | 0.0009 | |
| 1,25D | 3 h | 0.035 | 0.022 | 0.060 | 0.000 | 0.0006 | 0.000 | 0.00003 | 0.0000 | 0.0041 | 0.0010 |
| 24 h | 0.032 | 0.015 | 0.063 | 0.000 | 0.0008 | 0.000 | 0.00003 | 0.0000 | 0.0031 | 0.0009 | |
| 96 h | 0.031 | 0.058 | 0.053 | 0.000 | 0.0009 | 0.002 | 0.00002 | 0.0000 | 0.0034 | 0.0018 | |
| ATRA | 3 h | 0.026 | 0.011 | 0.039 | 0.000 | 0.0006 | 0.000 | 0.00002 | 0.0000 | 0.0023 | 0.0011 |
| 24 h | 0.063 | 0.030 | 0.496 | 0.000 | 0.0003 | 0.000 | 0.00009 | 0.0000 | 0.0023 | 0.0012 | |
| 96 h | 0.050 | 0.027 | 0.284 | 0.000 | 0.0011 | 0.000 | 0.00013 | 0.0001 | 0.0031 | 0.0018 | |
Table A3.
Comparative quantification of CEBP genes related to a reference GAPDH gene in KG1-CTR and KG1-RARα(−) cells.
| KG1 CTR | CEBPA | SEM | CEBPB | SEM | CEBPD | SEM | CEBPE | SEM | |
| control | 0.0061 | 0.0022 | 0.0199 | 0.0016 | 0.00002 | 0.0000 | 0.00003 | 0.0022 | |
| 1,25D | 3 h | 0.0056 | 0.0019 | 0.0197 | 0.0013 | 0.00002 | 0.0000 | 0.00003 | 0.0018 |
| 24 h | 0.0048 | 0.0011 | 0.0282 | 0.0008 | 0.00002 | 0.0000 | 0.00002 | 0.0012 | |
| 96 h | 0.0106 | 0.0017 | 0.0373 | 0.0012 | 0.00003 | 0.0000 | 0.00002 | 0.0016 | |
| ATRA | 3 h | 0.0153 | 0.0056 | 0.1484 | 0.0040 | 0.00001 | 0.0000 | 0.00003 | 0.0056 |
| 24 h | 0.0133 | 0.0080 | 0.1242 | 0.0056 | 0.00002 | 0.0000 | 0.00008 | 0.0079 | |
| 96 h | 0.0134 | 0.0007 | 0.0577 | 0.0005 | 0.00003 | 0.0000 | 0.00005 | 0.0007 | |
| KG1 RARα(−) | CEBPA | SEM | CEBPB | SEM | CEBPD | SEM | CEBPE | SEM | |
| control | 0.0082 | 0.0023 | 0.0261 | 0.0016 | 0.00003 | 0.0000 | 0.00002 | 0.0022 | |
| 1,25D | 3 h | 0.0123 | 0.0034 | 0.0377 | 0.0024 | 0.00003 | 0.0000 | 0.00003 | 0.0033 |
| 24 h | 0.0102 | 0.0034 | 0.0548 | 0.0024 | 0.00003 | 0.0000 | 0.00003 | 0.0033 | |
| 96 h | 0.0120 | 0.0042 | 0.0660 | 0.0030 | 0.00007 | 0.0000 | 0.00002 | 0.0041 | |
| ATRA | 3 h | 0.0140 | 0.0038 | 0.0729 | 0.0027 | 0.00002 | 0.0000 | 0.00002 | 0.0037 |
| 24 h | 0.0135 | 0.0025 | 0.1457 | 0.0018 | 0.00003 | 0.0000 | 0.00004 | 0.0025 | |
| 96 h | 0.0154 | 0.0036 | 0.0619 | 0.0025 | 0.00004 | 0.0000 | 0.00005 | 0.0034 | |
Table A4.
Comparative quantification of CEBP genes related to a reference GAPDH gene in HL60-CtrA and HL60-VDR(−) cells.
| HL60 CtrA | CEBPA | SEM | CEBPB | SEM | HL60 VDR(−) | CEBPA | SEM | CEBPB | SEM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| control | 0.06228 | 0.0214 | 0.0753 | 0.0125 | control | 0.09500 | 0.0318 | 0.08958 | 0.0099 | ||
| 1,25D | 3 h | 0.05003 | 0.0089 | 0.3774 | 0.2312 | 1,25D | 3 h | 0.07767 | 0.0227 | 0.28449 | 0.0147 |
| 24 h | 0.07403 | 0.0172 | 0.2351 | 0.0788 | 24 h | 0.12883 | 0.0455 | 0.41116 | 0.2031 | ||
| 96 h | 0.14063 | 0.0437 | 0.1749 | 0.0622 | 96 h | 0.17163 | 0.0468 | 0.24263 | 0.0734 | ||
| ATRA | 3 h | 0.06025 | 0.0180 | 0.1710 | 0.0118 | ATRA | 3 h | 0.09056 | 0.0082 | 0.24728 | 0.0580 |
| 24 h | 0.05366 | 0.0167 | 0.2221 | 0.0188 | 24 h | 0.09029 | 0.0166 | 0.40953 | 0.0871 | ||
| 96 h | 0.13521 | 0.0491 | 0.6720 | 0.0612 | 96 h | 0.24610 | 0.0229 | 0.358334 | 0.2188 | ||
Table A5.
Comparative quantification of VDR and RARA genes related to a reference GAPDH gene in all cell lines used in this study.
| Cell Line | VDR | SEM | RARA | SEM |
|---|---|---|---|---|
| HL60 | 0.00048 | 0.00001 | 0.0679 | 0.001 |
| HL60 CtrA | 0.00045 | 0.00003 | 0.0600 | 0.003 |
| HL60 VDR(−) | 0.00035 | 0.00006 | 0.0638 | 0.003 |
| KG1 | 0.00002 | 0.00001 | 0.1241 | 0.008 |
| KG1CTR | 0.00005 | 0.00000 | 0.1792 | 0.002 |
| KG1 RARα(−) | 0.00018 | 0.00003 | 0.0563 | 0.003 |
Author Contributions
Both authors conceived and designed the experiments; analyzed the data; provided financial support and wrote the paper. E.M. conceived the general idea of the research and performed one western blot experiment. A.M. performed most of the experiments.
Funding
The research was funded by a grant PRELUDIUM No 2013/11/N/NZ3/00197 (A.M.), and grant OPUS No 2016/23/B/NZ5/00065 (E.M.), both from the National Science Centre in Poland. Publication cost was supported by Wroclaw Center of Biotechnology Program, The Leading National Research Center (KNOW) for years 2014–2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nerlov C. The C/EBP family of transcription factors: A paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Tsukada J., Yoshida Y., Kominato Y., Auron P. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine. 2011;54:6–19. doi: 10.1016/j.cyto.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Johnson P., Landschulz W., Graves B., McKnight S. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. Biol. 1987;2:133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- 4.Landshulz W., Johnson P., McKnight S. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989;243:1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- 5.Ramji D., Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 2002;365:561–575. doi: 10.1042/bj20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown G., Hughes P., Michell R., Rolink A., Ceredig R. The sequential determination model of hematopoiesis. Trends Immunol. 2007;28:442–448. doi: 10.1016/j.it.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Studzinski G., Marcinkowska E. Intracellular signaling for granulocytic and monocytic differentiation. In: Brown G., Ceredig R., editors. Cell Determination during Hematopoiesis. Nova Science Publishers; Hauppauge, NY, USA: 2009. pp. 53–77. [Google Scholar]
- 8.Friedman A. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 9.Heath V., Suh H., Holman M., Renn K., Gooya J., Parkin S., Klarmann K., Ortiz M., Johnson P., Keller J. C/EBPα deficiency results in hyperproliferation of hematopoietic progenitor cells and disrupts macrophage development in vitro and in vivo. Blood. 2004;104:1639–1647. doi: 10.1182/blood-2003-11-3963. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D., Zhang P., Wang N., Hetherington C., Darlington G., Tenen D. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcinkowska E., Garay E., Gocek E., Chrobak A., Wang X., Studzinski G. Regulation of C/EBPβ isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp. Cell Res. 2006;312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham T., Langmann S., Schwarzfischer L., El Chartouni C., Lichtinger M., Klug M., Krause S., Rehli M. CCAAT enhancer-binding protein β regulates constitutive gene expression during late stages of monocyte to macrophage differentiation. J. Biol. Chem. 2007;282:21924–21933. doi: 10.1074/jbc.M611618200. [DOI] [PubMed] [Google Scholar]
- 13.Lekstrom-Himes J. The role of C/EBPepsilon in the terminal stages of granulocyte differentiation. Stem Cells. 2001;19:125–133. doi: 10.1634/stemcells.19-2-125. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T., Yoshida N., Kishimoto T., Akira S. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford A., Bennett C., Healy L., Towatari M., Greaves M., Enver T. Regulation of the myeloperoxidase enhancer binding proteins Pu1, C-EBP α, -β, and -δ during granulocyte-lineage specification. Proc. Natl. Acad. Sci. USA. 1996;93:10838–10843. doi: 10.1073/pnas.93.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieger M.A., Hoppe P.S., Smejkal B.M., Eitelhuber A.C., Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 17.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. doi: 10.1096/fasebj.10.9.8801176. [DOI] [PubMed] [Google Scholar]
- 18.Gritz E., Hirschi K. Specification and function of hemogenic endothelium during embryogenesis. Cell. Mol. Life Sci. 2016;73:1547–1567. doi: 10.1007/s00018-016-2134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cañete A., Cano E., Muñoz-Chápuli R., Carmona R. Role of Vitamin A/Retinoic Acid in Regulation of Embryonic and Adult Hematopoiesis. Nutrients. 2017;9:159. doi: 10.3390/nu9020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillestad L. Acute promyelocytic leukemia. Acta Med. Scand. 1957;159:189–194. doi: 10.1111/j.0954-6820.1957.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 21.Adès L., Guerci A., Raffoux E., Sanz M., Chevallier P., Lapusan S., Recher C., Thomas X., Rayon C., Castaigne S., et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–1696. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 22.Grande A., Montanari M., Tagliafico E., Manfredini R., Zanocco Marani T., Siena M., Tenedini E., Gallinelli A., Ferrari S. Physiological levels of 1α, 25 dihydroxyvitamin D3 induce the monocytic commitment of CD34+ hematopoietic progenitors. J. Leukoc. Biol. 2002;71:641–651. [PubMed] [Google Scholar]
- 23.Janik S., Nowak U., Łaszkiewicz A., Satyr A., Majkowski M., Marchwicka A., Śnieżewski Ł., Berkowska K., Gabryś M.C.M., Marcinkowska E. Diverse Regulation of Vitamin D Receptor Gene Expression by 1,25-Dihydroxyvitamin D and ATRA in Murine and Human Blood Cells at Early Stages of Their Differentiation. Int. J. Mol. Sci. 2017;18:1323. doi: 10.3390/ijms18061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kastner P., Chan S. Function of RARα during the maturation of neutrophils. Oncogene. 2001;20:7178–7185. doi: 10.1038/sj.onc.1204757. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizawa T., Handa Y., Uematsu Y., Takeda S., Sekine K., Yoshihara Y., Kawakami T., Arioka K., Sato H., Uchiyama Y., et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 26.Morosetti R., Park D., Chumakov A., Grillier I., Shiohara M., Gombart A., Nakamaki T., Weinberg K., Koeffler H. A novel, myeloid transcription factor, C/EBPepsilon, is upregulated during granulocytic, but not monocytic, differentiation. Blood. 1997;90:2591–2600. [PubMed] [Google Scholar]
- 27.Christakos S., Barletta F., Huening M., Dhawan P., Liu Y., Porta A., Peng X. Vitamin D target proteins: function and regulation. J. Cell. Biochem. 2003;88:238–244. doi: 10.1002/jcb.10349. [DOI] [PubMed] [Google Scholar]
- 28.Corcoran A., Bermudez M., Seoane S., Perez-Fernandez R., Krupa M., Pietraszek A., Chodyński M., Kutner A., Brown G., Marcinkowska E. Biological evaluation of new vitamin D2 analogues. J. Steroid Biochem. Mol. Biol. 2016;164:66–71. doi: 10.1016/j.jsbmb.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Duprez E., Wagner K., Koch H., Tenen D. C/EBPβ: A major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. EMBO J. 2003;22:5806–5816. doi: 10.1093/emboj/cdg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiki A., Imamura T., Sakamoto K., Kawashima S., Yoshida H., Hirashima Y., Miyachi M., Yagyu S., Nakatani T., Sugita K., et al. All-trans retinoic acid combined with 5-Aza-2′-deoxycitidine induces C/EBPα expression and growth inhibition in MLL-AF9-positive leukemic cells. Biochem. Biophys. Res. Commun. 2012;428:216–223. doi: 10.1016/j.bbrc.2012.09.131. [DOI] [PubMed] [Google Scholar]
- 31.Marchwicka A., Cebrat M., Łaszkiewicz A., Śnieżewski Ł., Brown G., Marcinkowska E. Regulation of vitamin D receptor expression by retinoic acid receptor α in acute myeloid leukemia cells. J. Steroid Biochem. Mol. Biol. 2016;159:121–130. doi: 10.1016/j.jsbmb.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Gocek E., Kielbinski M., Baurska H., Haus O., Kutner A., Marcinkowska E. Different susceptibilities to 1,25-dihydroxyvitamin D3-induced differentiation of AML cells carrying various mutations. Leuk. Res. 2010;34:649–657. doi: 10.1016/j.leukres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Gocek E., Marchwicka A., Baurska H., Chrobak A., Marcinkowska E. Opposite regulation of vitamin D receptor by ATRA in AML cells susceptible and resistant to vitamin D-induced differentiation. J. Steroid Biochem. Mol. Biol. 2012;132:220–226. doi: 10.1016/j.jsbmb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Marchwicka A., Corcoran A., Berkowska K., Marcinkowska E. Restored expression of vitamin D receptor and sensitivity to 1,25-dihydroxyvitamin D3 in response to disrupted fusion FOP2-FGFR1 gene in acute myeloid leukemia cells. Cell Biosci. 2016;6:7. doi: 10.1186/s13578-016-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahlen J., Carlberg C. Identification of a vitamin D receptor homodimer-type response element in the rat calcitriol 24-hydroxylase gene promoter. Biochem. Biophys. Res. Commun. 1994;202:1366–1372. doi: 10.1006/bbrc.1994.2081. [DOI] [PubMed] [Google Scholar]
- 36.Dhawan P., Peng X., Sutton A., MacDonald P., Croniger C., Trautwein C., Centrella M., McCarthy T., Christakos S. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol. Cell. Biol. 2005;25:472–487. doi: 10.1128/MCB.25.1.472-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhawan P., Wieder R., Christakos S. CCAAT enhancer-binding protein α is a molecular target of 1,25-dihydroxyvitamin D3 in MCF-7 breast cancer cells. J. Biol. Chem. 2009;284:3086–3095. doi: 10.1074/jbc.M803602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown G., Hughes P., Michell R., Rolink A., Ceredig R. Ordered Commitment of Hematopoietic Stem Cells to Lineage Options. Nova Science Publishers Inc.; Hauppauge, NY, USA: 2008. [Google Scholar]
- 39.Scott L., Civin C., Rorth P., Friedman A. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–1735. [PubMed] [Google Scholar]
- 40.Taskesen E., Bullinger L., Corbacioglu A., Sanders M., Erpelinck C., Wouters B., van der Poel-van de Luytgaarde S., Damm F., Krauter J., Ganser A., et al. Prognostic impact, concurrent genetic mutations, and gene expression features of AML with CEBPA mutations in a cohort of 1182 cytogenetically normal AML patients: Further evidence for CEBPA double mutant AML as a distinctive disease entity. Blood. 2011;117:2469–2475. doi: 10.1182/blood-2010-09-307280. [DOI] [PubMed] [Google Scholar]
- 41.Bullinger L., Döhner K., Döhner H. Genomics of Acute Myeloid Leukemia Diagnosis and Pathways. J. Clin. Oncol. 2017;35:934–946. doi: 10.1200/JCO.2016.71.2208. [DOI] [PubMed] [Google Scholar]
- 42.Pabst T., Mueller B., Harakawa N., Schoch C., Haferlach T., Behre G., Hiddemann W., Zhang D., Tenen D. AML1-ETO downregulates the granulocytic differentiation factor C/EBPα in t(8;21) myeloid leukemia. Nat. Med. 2001;7:444–451. doi: 10.1038/86515. [DOI] [PubMed] [Google Scholar]
- 43.Cilloni D., Carturan S., Gottardi E., Messa F., Messa E., Fava M., Diverio D., Guerrasio A., Lo-Coco F., Saglio G. Down-modulation of the C/EBPα transcription factor in core binding factor acute myeloid leukemias. Blood. 2003;102:2705–2706. doi: 10.1182/blood-2003-07-2256. [DOI] [PubMed] [Google Scholar]
- 44.Balmer J., Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J. Steroid Biochem. Mol. Biol. 2005;96:347–354. doi: 10.1016/j.jsbmb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Moutier E., Ye T., Choukrallah M., Urban S., Osz J., Chatagnon A., Delacroix L., Langer D., Rochel N., Moras D., et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J. Biol. Chem. 2012;287:26328–26341. doi: 10.1074/jbc.M112.361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gombart A., Kwok S., Anderson K., Yamaguchi Y., Torbett B., Koeffler H. Regulation of neutrophil and eosinophil secondary granule gene expression by transcription factors C/EBPepsilon and PU.1. Blood. 2003;101:3265–3273. doi: 10.1182/blood-2002-04-1039. [DOI] [PubMed] [Google Scholar]
- 47.Baurska H., Kłopot A., Kiełbiński M., Chrobak A., Wijas E., Kutner A., Marcinkowska E. Structure-function analysis of vitamin D2 analogs as potential inducers of leukemia differentiation and inhibitors of prostate cancer proliferation. J. Steroid Biochem. Mol. Biol. 2011;126:46–54. doi: 10.1016/j.jsbmb.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gocek E., Marchwicka A., Bujko K., Marcinkowska E. NADPH-cytochrome p450 reductase is regulated by all-trans retinoic acid and by 1,25-dihydroxyvitamin D3 in human acute myeloid leukemia cells. PLoS ONE. 2014;9:e91752. doi: 10.1371/journal.pone.0091752. [DOI] [PMC free article] [PubMed] [Google Scholar]