Abstract
The vitamin D receptor (VDR) is a nuclear receptor that mediates the biological action of the active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], and regulates calcium and bone metabolism. Lithocholic acid (LCA), which is a secondary bile acid produced by intestinal bacteria, acts as an additional physiological VDR ligand. Despite recent progress, however, the physiological function of the LCA−VDR axis remains unclear. In this study, in order to elucidate the differences in VDR action induced by 1,25(OH)2D3 and LCA, we compared their effect on the VDR target gene induction in the intestine of mice. While the oral administration of 1,25(OH)2D3 induced the Cyp24a1 expression effectively in the duodenum and jejunum, the LCA increased target gene expression in the ileum as effectively as 1,25(OH)2D3. 1,25(OH)2D3, but not LCA, increased the expression of the calcium transporter gene Trpv6 in the upper intestine, and increased the plasma calcium levels. Although LCA could induce an ileal Cyp24a1 expression as well as 1,25(OH)2D3, the oral LCA administration was not effective in the VDR target gene induction in the kidney. No effect of LCA on the ileal Cyp24a1 expression was observed in the VDR-null mice. Thus, the results indicate that LCA is a selective VDR ligand acting in the lower intestine, particularly the ileum. LCA may be a signaling molecule, which links intestinal bacteria and host VDR function.
Keywords: vitamin D receptor, vitamin D, lithocholic acid, bile acid, CYP24A1, TRPV6, calcium metabolism, ileum
1. Introduction
The vitamin D receptor (VDR) mediates the physiological functions of the active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], including calcium and bone metabolism, immunity, and cardiovascular function [1,2]. 1,25(OH)2D3 and its synthetic derivatives also exhibit many pharmacological effects, including the regulation of cellular proliferation and differentiation and lipid metabolism, through VDR activation [3]. VDR undergoes a ligand-dependent conformational change that results in a dynamic interaction with the heterodimer partner retinoid X receptor (RXR) and an exchange of cofactor complexes [4]. The RXR−VDR heterodimer binds preferentially to a specific DNA element that consists of a two-hexanucleotide (AGGTCA or a related sequence) direct repeat motif separated by three nucleotides, known as a direct repeat 3 element, in target genes, such as cytochrome P450 (CYP) 24A1 (gene symbol, CYP24A1), and the transient receptor potential vanilloid (TRPV) type 6 (gene symbol, TRPV6). CYP24A1 catabolizes 25-hydroxyvitamin D3 and 1,25(OH)2D3 into biological inactive metabolites through 24-hydroxylatoin, a negative feedback mechanism in vitamin D signaling [2,4]. VDR is also activated by bile acids, such as lithocholic acid (LCA) and its metabolite, 3-ketocholanic acid. The VDR activation by these bile acids and 1,25(OH)2D3 induces the expression of CYP3A enzymes, which metabolize drugs and secondary bile acids in the xenobiotic metabolism pathway [5,6]. Human CYP3A4 can inactivate 1,25(OH)2D3 [7,8]. LCA treatment also induces the expression of CYP24A1 in intestinal cells and osteoblasts [9,10]. The VDR activation by LCA may induce vitamin D insufficiency or deficiency by enhancing vitamin D catabolism.
Bile acids are the major metabolic products of cholesterol and are essential for the intestinal digestion and absorption of hydrophobic nutrients, such as triglycerides, fatty acids, cholesterol, and lipid-soluble vitamins, including vitamin D [11]. The primary bile acids, cholic acid and chenodeoxycholic acid, are generated from cholesterol by the sequential action of the liver enzymes and are secreted in the bile as glycine or taurine conjugates [12]. After assisting in the digestion and absorption of lipid compounds, most of the bile acids are reabsorbed in the ileum and enter the enterohepatic circulation. Bile acids that escape reabsorption are converted to the secondary bile acids, deoxycholic acid and LCA, by the intestinal microflora [13]. While deoxycholic acid is avidly accumulated in the enterohepatic circulation pool, a small amount of LCA is absorbed in the ileum, sulfated in the liver, excreted into bile, and then lost in feces [14].
VDR can be activated by 1,25(OH)2D3, synthetic vitamin D derivatives, LCA, and its derivatives [15,16]. The principal physiological effect of vitamin D is to enhance calcium absorption in the upper intestine [2]. Many synthetic vitamin D derivatives induce hypercalcemia, a potential adverse effect which must be addressed to allow for a broader clinical application [3,17]. Apart from the direct effect of vitamin D absorption, a physical link between bile acids and calcium metabolism has not been demonstrated. The secondary bile acids are produced by the intestinal microflora in the lower intestine, particularly the ileum and colon, sites that are not a location of vitamin D-induced calcium absorption. In this study, we investigated whether 1,25(OH)2D3 and LCA induce selective VDR activation in the intestine of mice.
2. Results
2.1. LCA Induces CYP24A1 mRNA Expression in the Ileum but Not in the Duodemum or Jejunum
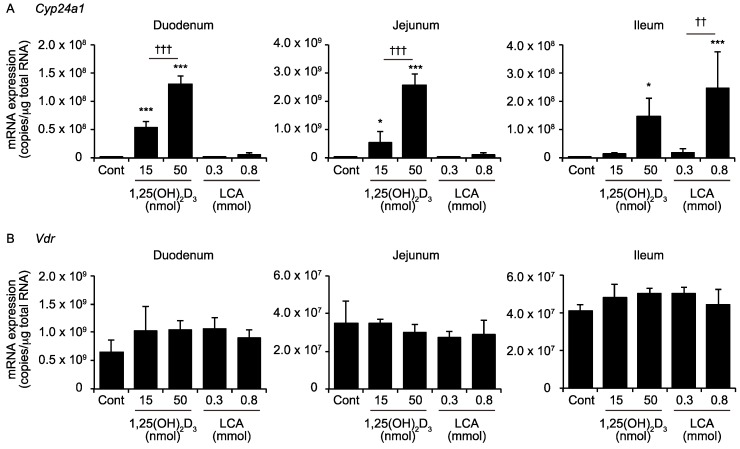
CYP24A1 encodes vitamin D 24-hydroxylase and is the VDR target that is induced by VDR activation in many of the VDR-expressing cells [16]. We treated mice with 1,25(OH)2D3 (15 or 50 nmol/kg) or LCA (0.3 or 0.8 mmol/kg) by oral gavage twice (14 and 2 h before euthanization), according to our previous reports, with minor modification [9,16,18], and compared the effect of these compounds on CYP24A1 mRNA expression in the duodenum, jejunum, and ileum. The 1,25(OH)2D3 treatment increased the Cyp24a1 mRNA levels in the duodenum, jejunum, and ileum (Figure 1A). LCA was not effective in the duodenum or jejunum, but increased the Cyp24a1 mRNA levels as effectively as 1,25(OH)2D3 in the ileum. The 1,25(OH)2D3 treatment did not change the Vdr mRNA levels in the duodenum, jejunum, or ileum (Figure 1B). These findings suggest that LCA is selectively active in the ileum.
Figure 1.
mRNA expression of cytochrome P450 24A1 (Cyp24a1) (A) and the vitamin D receptor (Vdr) (B) in the intestine. Wild-type mice were administered the vehicle control (Cont), 15 or 50 nmol/kg 1,25(OH)2D3, or 0.3 or 0.8 mmol/kg LCA via gavage. * p < 0.05; *** p < 0.001 versus Cont. †† p < 0.01. ††† p < 0.001.
2.2. LCA Does Not Induce Intestinal Trpv6 Expression or Increase Plasma Calcium Levels
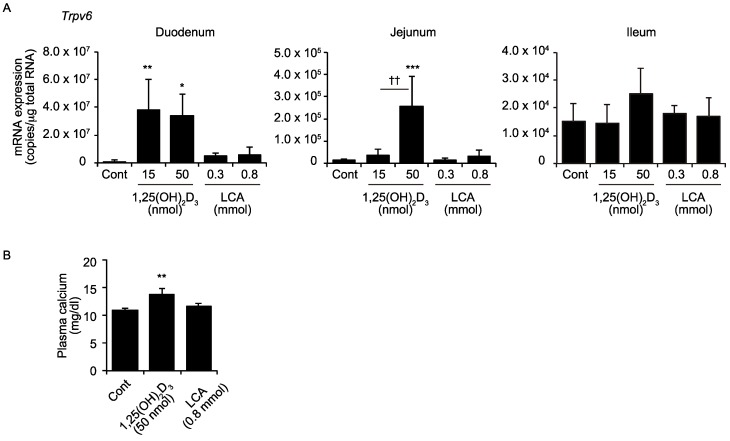
1,25(OH)2D3 exhibits its principal physiological action of calcium absorption by inducing the calcium channel Trpv6 in the duodenum [19]. We examined the effect of the oral LCA administration on the Trpv6 expression, in comparison to 1,25(OH)2D3. While the 1,25(OH)2D3 increased the Trpv6 expression in the duodenum and jejunum, but not the ileum, the LCA did not affect the Trpv6 expression in the duodenum, jejunum, or ileum (Figure 2). The LCA at 0.8 nmol/kg, which induced ileal Cyp24a1 expression to similar levels as 1,25(OH)2D3 at 50 nmol/kg, did not increase plasma calcium levels. The 1,25(OH)2D3 at 50 nmol/kg increased the calcium levels, consistent with the Trpv6 expression.
Figure 2.
mRNA expression of transient receptor potential vanilloid type 6 (Trpv6) in the intestine (A) and the plasma calcium levels (B). Wild-type mice were administered the vehicle control (Cont), 15 or 50 nmol/kg 1,25(OH)2D3, or 0.3 or 0.8 mmol/kg LCA via gavage. * p < 0.05; ** p < 0.01; *** p < 0.001 versus Cont. †† p < 0.01.
2.3. Effects of 1,25(OH)2D3 And LCA on Expression of VDR Target Genes in the Kidney
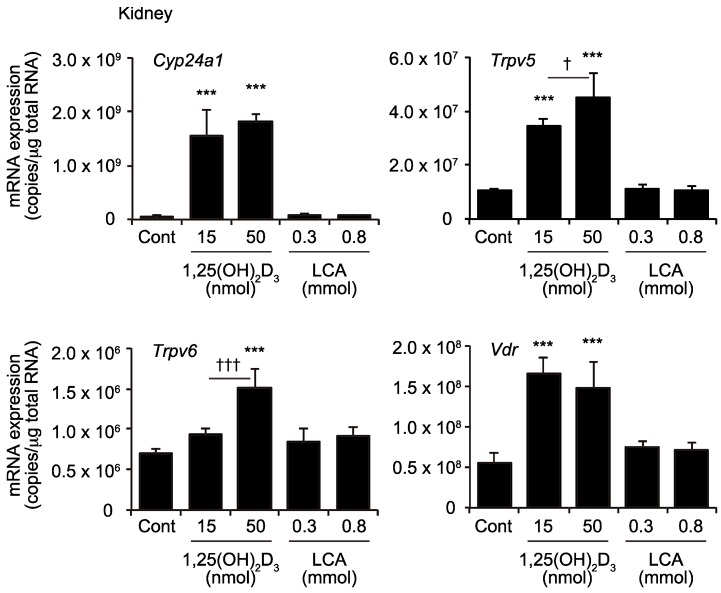
The kidney is also an important VDR target organ [20], and vitamin D signaling plays a role in calcium reabsorption by the renal tubule through the induced expression of Trpv5 and Trpv6 [21]. While oral 1,25(OH)2D3 treatment induced expressions of Cyp24a1, Trpv5, Trpv6, and in the kidney as reported previously [16,22], LCA was not effective in induction of these genes (Figure 3).
Figure 3.
mRNA expression of Cyp24a1, Trpv5, Trpv6, and Vdr mRNA in the kidney. Wild-type mice were administered the vehicle control (Cont), 15 or 50 nmol/kg 1,25(OH)2D3, or 0.3 or 0.8 mmol/kg LCA via gavage. *** p < 0.001 versus Cont. † p < 0.05; ††† p < 0.001.
2.4. Ileal Cyp24a1 Induction by LCA Is Mediated by VDR Activation
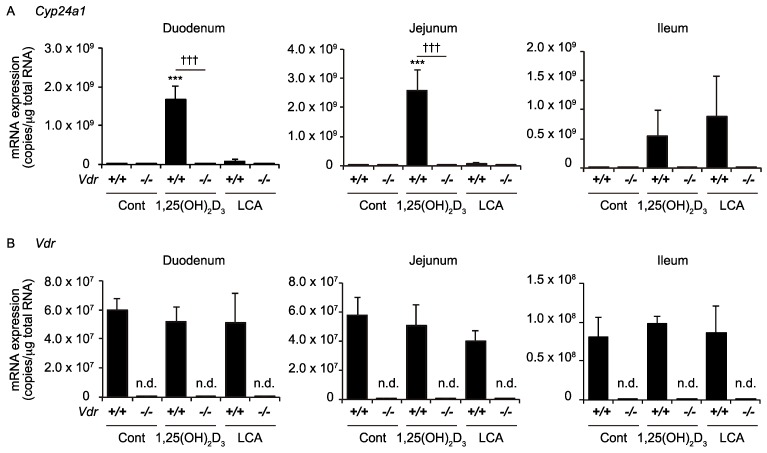
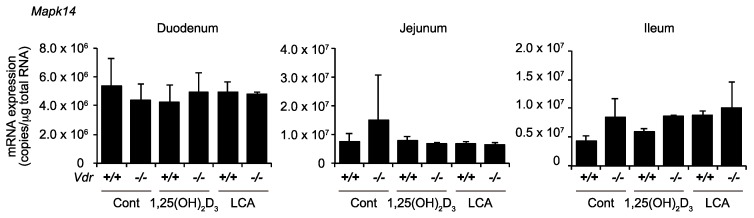
LCA is also a ligand for other nuclear receptors, particularly the farnesoid X receptor and pregnane X receptor [6,23,24]. We examined whether the effect of LCA on the ileal Cyp24a1 induction is mediated by VDR activation utilizing VDR knockout mice [25]. The effect of LCA on the ileal Cyp24a1 induction was abolished in the Vdr(−/−) mice, similar to the effect of 1,25(OH)2D3, although the effect of 1,25(OH)2D3 and LCA on the ileal Cyp24a1 expression did not reach statistical significance because of a large variation (Figure 4A). The Vdr mRNA expression was not detected in the Vdr(−/−) mice (Figure 4B). The Vdr(−/−) mice were generated by targeting exon 2 of the Vdr gene and showed no VDR protein expression [25]. The Cyp24a1 and Vdr mRNA levels in the wild-type mice (Figure 4) were different from those shown in Figure 1. This may be due to a difference in the diet conditions. The mitogen activated protein kinase p38α is involved in the intestinal VDR signaling [26]. There was no difference in the expression of Mapk14, which encodes p38α, among the experimental groups (Figure A1). We performed a microarray analysis to compare the RNAs purified from the ileum of the LCA-treated Vdr(+/+) and Vdr(−/−) mice, but found no difference in the expression of the Rxra, Rxrb, Rxrg, or lipid metabolism genes, including Srebf1 and Srebf2.
Figure 4.
mRNA expression of Cyp24a1 (A) and Vdr (B) in the intestine of Vdr(+/+) mice and Vdr(−/−) mice. Mice were administered vehicle control (Cont), 50 nmol/kg 1,25(OH)2D3, or 0.8 mmol/kg LCA via gavage. *** p < 0.001 versus Cont. ††† p < 0.001. n.d., not detected.
3. Discussion
The secondary bile acid LCA acts as a VDR ligand, is produced by intestinal microflora, and is present mainly in the lower intestine [5,13]. These properties of LCA are different from those of 1,25(OH)2D3, the essential signaling molecule in the maintenance of calcium homeostasis. The principal site for the 1,25(OH)2D3 action in calcium absorption is the upper intestine, particularly the duodenum [27]. Consistent with their biological characteristics, our results show that 1,25(OH)2D3 and LCA induce the VDR target gene Cyp24a1 preferentially in the upper intestine and lower intestine, respectively (Figure 1).
Dietary vitamin D is absorbed via passive diffusion and through cholesterol transporters, such as Niemann-Pick C1-like 1, scavenger receptor class B type 1, and cluster determinant 36, in the upper intestine [28,29]. Vitamin D is hydroxylated at the 25-position in the liver and then at the 1α-position, to yield the 1,25-dihydroxylated active form in the kidney [2]. 1,25(OH)2D3, whether it is generated endogenously or taken by oral administration, induces the expression of genes involved in calcium import, such as Trpv6, to enhance calcium absorption in the duodenum. 1,25(OH)2D3 also induces the Cyp24a1 expression in the upper intestine, and is then inactivated by CYP24A1, leading to decreased activity in the lower intestine. The vitamin D prodrug 1,25(OH)2D3-25β-glucuronide, which cannot activate VDR or be absorbed in the upper intestine, is converted to free 1,25(OH)2D3 by intestinal bacteria in the lower intestine [30]. This compound, similar to LCA, exhibits a Cyp24a1 expression selectively in the lower intestine. LCA is generated by intestinal bacteria and has little reabsorption in the enterohepatic circulation [13]. The orally administered LCA did not induce the expression of the VDR target genes in the kidney (Figure 3), likely because only a small amount of LCA reaches the circulation. The dietary supplementation of LCA induces little change in the blood bile acid composition [31]. Unlike vitamin D, LCA is suggested to be biologically inactive in the upper intestine and to play a role selectively in the lower intestine.
TRPV6 is necessary to mediate the vitamin D-stimulated calcium absorption [19]. In contrast to 1,25(OH)2D3, LCA did not increase the intestinal Trpv6 expression or plasma calcium levels (Figure 2). These findings are consistent with an absence of a physiological link connecting the LCA and calcium metabolism. The LCA administration elevates the serum calcium levels and induces renal Cyp24a1 expression, only in vitamin D-deficient rats [32]. Thus, LCA has only a limited function as a VDR ligand in the body. LCA effectively induces Cyp24a1 in the ileum (Figure 1). LCA activates other nuclear receptors, farnesoid X receptor and pregnane X receptor, as well as the G protein-coupled receptor, TGR5 [6,23,24,33,34]. The induction of Cyp24a1 by LCA was not observed in the VDR-null mice (Figure 4), indicating that VDR, but not the other receptors, mediates the effect of LCA on Cyp24a1 induction.
1,25(OH)2D3 is also inactivated by CYP3A4, another VDR target gene product, in human cells [8]. LCA induces the CYP3A4 promoter activity in a VDR-dependent manner in human intestinal LS174T cells [35]. The LCA accumulation may induce vitamin D deficiency or insufficiency by enhancing vitamin D inactivation. The VDR deletion induces a change in the intestinal microbial flora [36], suggesting an interaction between the intestinal microflora and the intestine through the VDR activation by LCA. VDR plays a role in innate immunity and protection against antimicrobial infection [37]. LCA-producing bacteria may induce a favorable environment by regulating host immunity though VDR activation. The VDR regulates the intestinal proliferation, barrier function, and immunity, and plays a protective role in inflammatory bowel diseases [38]. We suggest two possible roles of the LCA−VDR axis. First, LCA induces vitamin D-mimic effects to maintain intestinal homeostasis, a beneficial effect. Second, the LCA decreases the vitamin D signaling by inducing vitamin D catabolism, a pathological effect. Vitamin D deficiency and insufficiency cause rickets and osteomalacia and are also associated with an increased risk of osteoporosis, cancer, autoimmune disease, infection, cardiovascular disease, obesity, and diabetes [1]. Decreased vitamin D signaling induces the differentiation of mesenchymal stem cells into adipocytes not osteoblasts [39,40]. The gene silencing experiments of the VDR and RXR in the intestinal cells and culture experiments using primary cells from Vdr(−/−) mice will clarify the LCA−VDR axis.
VDR regulates the target gene expression by forming a heterodimer with RXR [4]. Although the VDR–RXR heterodimer is not permissive to RXR ligand activation, heterodimer allosteric communication is required for activation of VDR by LCA and not by 1,25(OH)2D3 [41]. LCA induces the interaction of VDR with RXR and cofactors, such as the steroid receptor coactivator 1, silencing mediator of retinoic acid and thyroid hormone receptor, and Hairless, in a manner distinct from 1,25(OH)2D3 [42,43,44]. The vitamin A derivative, 9-cis retinoic acid (9-cis RA), has been identified as a natural RXR ligand [45], but it can be detected only in the pancreas [46]. Although fatty acids, such as docosahexaenoic acid and the long chain fatty acid C24:5, have been shown as natural RXR ligands [47,48], their role in intestinal VDR signaling remains unknown. The permissiveness of RXR in the VDR–RXR heterodimer has been investigated using 9-cis RA or synthetic ligands, and there may be a natural ligand that can exhibit a permissive or conditionally permissive RXR activation in VDR–RXR. The lower intestine-selective cofactor(s) and/or natural RXR ligand(s) may be involved in LCA-mediated VDR signaling.
We used male mice in this study. Sex-related differences have been reported in the phenotypes of the VDR knockout mice in lipid metabolism and resistance to obesity, and in skeletal structures [49,50], in VDR single nucleotide polymorphisms with human immune, as well as intestinal pathology [38], and also in bile acid metabolism and gut microbiota [51]. Further studies are needed to elucidate the physiological and pathological roles of LCA as a VDR ligand in the intestine.
4. Materials and Methods
4.1. Animal Experiments
The VDR-null (Vdr(−/−)) mice and wild-type mice (Vdr(+/+)) mice were obtained by breeding Vdr(+/−) mice on a pure C57BL/6J background [52]. Original Vdr(−/−) mice were kindly provided by Shigeaki Kato and Chugai Pharmaceutical Co. [25]. These mice were raised on a high-calcium and high-lactose diet to normalize the blood calcium levels in the Vdr(−/−) mice, and were maintained under a controlled temperature (23 ± 1 °C) and humidity (45–65%), with free access to water and chow as reported previously [22]. The experiments were conducted with male mice at 8–12 weeks of age. The mice were treated with corn oil containing the vehicle control (ethanol; Kanto Chemical Co., Tokyo, Japan), 1,25(OH)2D3 (15 or 50 nmol/kg; Wako Pure Chemicals, Osaka, Japan), or LCA (0.3 or 0.8 mmol/kg; Nacalai Tesque, Kyoto, Japan) via oral gavage, twice (14 and 2 h before euthanization), as reported previously, with minor modifications [9,16,18]. The plasma, kidney, and intestine samples were collected after euthanization with carbon dioxide. All of the tissue samples were snap-frozen in liquid nitrogen or on dry ice, and sorted until analysis. The plasma calcium concentrations were quantified with Calcium C Testwako (Wako Pure Chemicals, Osaka, Japan) [16]. The experimental protocol adhered to the Guidelines for Animal Experiments of the Nihon University School of Medicine, and was approved by the Ethics Review Committee for Animal Experimentation of the Nihon University School of Medicine (AP10M096, 3 September 2010; AP12M029, 5 October 2012).
4.2. Reverse Transcrption and Real-Time Quantitative Polymerase Chain Reaction
The tissues were crushed using a Bessman Tissue Pulverizer (Spectrum Laboratories, Racho Dominguez, CA, USA), and the total RNA extraction was performed using the acid guanidium thiocyanate/phenol/chloroform method [52]. The cDNAs were synthesized using the ImProm-II Reverse Transcription system (Promega, Madison WI, USA), and the real time polymerase chain reaction was performed on the ABI PIRSM 7000 Sequence Detection System (Life Technologies Corporation, Carlsbad, CA, USA) using Power SYBR Green PCR Master Mix (Life Technologies Corporation) and primers reported previously [16,22]. The mRNA copy numbers were calculated with standard curves that were linear over a range of 0.02–200 ng/mL for the corresponding cloned cDNAs inserted into pcDNA3.1 plasmids (Life Technologies Corporation), as reported previously [26].
4.3. Statistical Analysis
Data are presented as means ± standard deviation (SD). We performed one-way ANOVA followed by Tukey’s multiple comparisons to assess significant differences.
Acknowledgments
The authors thank Shigeaki Kato and Chugai Pharmaceutical Co. for providing Vdr(−/−) mice, Shigeyuki Uno and other members of Makishima lab for technical assistance and helpful comments, and Andrew I. Shulman for editorial assistance.
Abbreviations
| VDR | vitamin D receptor |
| 1,25(OH)2D3 | 1α,25-dihydroxyvitamin D3 |
| RXR | retinoid X receptor |
| CYP | cytochrome P450 |
| TPPV | transient receptor potential vanilloid |
| LCA | lithocholic acid |
Appendix A
Figure A1.
mRNA expression of Mapk14 in the intestine of Vdr (+/+) mice and Vdr (−/−) mice. Mice were administered vehicle control (Cont), 50 nmol/kg 1,25(OH)2D3, or 0.8 mmol/kg LCA via gavage.
Author Contributions
M.I. performed experiments, analyzed the data, and wrote the manuscript; D.A. performed experiments; and M.M. supervised experiments and wrote the manuscript.
Funding
This word was supported by JSPS KAKENHI Grant Numbers JP 22590294, JP 25460394 and JP 16K08632 (to M.M.), and JSPS KAKENHI Grant Numbers JP 09J0328 (JP21-3828), JP 26860222 and JP 16K19062 (to M.I.). M.I. was a Research Fellow of the Japan Society for the Promotion of Science (JSPS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rosen C.J., Adams J.S., Bikle D.D., Black D.M., Demay M.B., Manson J.E., Murad M.H., Kovacs C.S. The nonskeletal effects of vitamin d: An endocrine society scientific statement. Endocr. Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick M.F. Vitamin d deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Plum L.A., DeLuca H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 4.Haussler M.R., Whitfield G.K., Kaneko I., Haussler C.A., Hsieh D., Hsieh J.C., Jurutka P.W. Molecular mechanisms of vitamin d action. Calcif. Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 5.Makishima M., Lu T.T., Xie W., Whitfield G.K., Domoto H., Evans R.M., Haussler M.R., Mangelsdorf D.J. Vitamin d receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 6.Xie W., Radominska-Pandya A., Shi Y., Simon C.M., Nelson M.C., Ong E.S., Waxman D.J., Evans R.M. An essential role for nuclear receptors sxr/pxr in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawada N., Kusudo T., Sakaki T., Hatakeyama S., Hanada M., Abe D., Kamao M., Okano T., Ohta M., Inouye K. Novel metabolism of 1α,25-dihydroxyvitamin D3 with C24-C25 bond cleavage catalyzed by human CYP24A1. Biochemistry. 2004;43:4530–4537. doi: 10.1021/bi030207f. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y., Hashizume T., Shuhart M.C., Davis C.L., Nelson W.L., Sakaki T., Kalhorn T.F., Watkins P.B., Schuetz E.G., Thummel K.E. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1α,25-dihydroxyvitamin D3: Implications for drug-induced osteomalacia. Mol. Pharmacol. 2006;69:56–65. doi: 10.1124/mol.105.017392. [DOI] [PubMed] [Google Scholar]
- 9.Adachi R., Honma Y., Masuno H., Kawana K., Shimomura I., Yamada S., Makishima M. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J. Lipid Res. 2005;46:46–57. doi: 10.1194/jlr.M400294-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Gaspà S., Guañabens N., Enjuanes A., Peris P., Martinez-Ferrer A., Martinez de Osaba M.J., Gonzalez B., Alvarez L., Monegal A., Combalia A., et al. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur. J. Clin. Investig. 2010;40:25–34. doi: 10.1111/j.1365-2362.2009.02230.x. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann A.F. Bile acids: Trying to understand their chemistry and biology with the hope of helping patients. Hepatology. 2009;49:1403–1418. doi: 10.1002/hep.22789. [DOI] [PubMed] [Google Scholar]
- 12.Russell D.W. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 2009;50:S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Li T., Chiang J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makishima M., Yamada S. Targeting the vitamin D receptor: Advances in drug discovery. Expert Opin. Ther. Pat. 2005;15:1133–1145. doi: 10.1517/13543776.15.9.1133. [DOI] [Google Scholar]
- 16.Ishizawa M., Matsunawa M., Adachi R., Uno S., Ikeda K., Masuno H., Shimizu M., Iwasaki K., Yamada S., Makishima M. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J. Lipid Res. 2008;49:763–772. doi: 10.1194/jlr.M700293-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Yamada S., Makishima M. Structure-activity relationship of nonsecosteroidal vitamin d receptor modulators. Trends Pharmacol. Sci. 2014;35:324–337. doi: 10.1016/j.tips.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Ozeki J., Choi M., Endo-Umeda K., Sakurai K., Amano S., Makishima M. Enhanced transcription of pancreatic peptide yy by 1α-hydroxyvitamin D3 administration in streptozotocin-induced diabetic mice. Neuropeptides. 2013;47:329–332. doi: 10.1016/j.npep.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Benn B.S., Ajibade D., Porta A., Dhawan P., Hediger M., Peng J.B., Jiang Y., Oh G.T., Jeung E.B., Lieben L., et al. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-d9k. Endocrinology. 2008;149:3196–3205. doi: 10.1210/en.2007-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer M.B., Zella L.A., Nerenz R.D., Pike J.W. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J. Biol. Chem. 2007;282:22344–22352. doi: 10.1074/jbc.M703475200. [DOI] [PubMed] [Google Scholar]
- 21.Nijenhuis T., Hoenderop J.G., Bindels R.J. Trpv5 and trpv6 in Ca2+ (re)absorption: Regulating Ca2+ entry at the gate. Pflugers Arch. 2005;451:181–192. doi: 10.1007/s00424-005-1430-6. [DOI] [PubMed] [Google Scholar]
- 22.Ishizawa M., Iwasaki K., Kato S., Makishima M. Hypergravity modulates vitamin D receptor target gene mrna expression in mice. Am. J. Physiol. Endocrinol. Metab. 2009;297:E728–E734. doi: 10.1152/ajpendo.00168.2009. [DOI] [PubMed] [Google Scholar]
- 23.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 24.Staudinger J.L., Goodwin B., Jones S.A., Hawkins-Brown D., MacKenzie K.I., LaTour A., Liu Y., Klaassen C.D., Brown K.K., Reinhard J., et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshizawa T., Handa Y., Uematsu Y., Takeda S., Sekine K., Yoshihara Y., Kawakami T., Arioka K., Sato H., Uchiyama Y., et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 26.Ishizawa M., Akagi D., Yamamoto J., Makishima M. 1α,25-Dihydroxyvitamin D3 enhances TRPV6 transcription through p38 mapk activation and GADD45 expression. J. Steroid Biochem. Mol. Biol. 2017;172:55–61. doi: 10.1016/j.jsbmb.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Bouillon R., Van Cromphaut S., Carmeliet G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J. Cell. Biochem. 2003;88:332–339. doi: 10.1002/jcb.10360. [DOI] [PubMed] [Google Scholar]
- 28.Hollander D., Muralidhara K.S., Zimmerman A. Vitamin D-3 intestinal absorption in vivo: Influence of fatty acids, bile salts, and perfusate ph on absorption. Gut. 1978;19:267. doi: 10.1136/gut.19.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmanuelle R., Aurélie G., Christine C., Romain B., Marion N., Jean-François L., Dominique J.R., Claire D., Xavier C., Patrick B. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011;55:691–702. doi: 10.1002/mnfr.201000553. [DOI] [PubMed] [Google Scholar]
- 30.Goff J.P., Koszewski N.J., Haynes J.S., Horst R.L. Targeted delivery of vitamin d to the colon using β-glucuronides of vitamin D: Therapeutic effects in a murine model of inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G460–G469. doi: 10.1152/ajpgi.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida S., Ozeki J., Makishima M. Modulation of bile acid metabolism by 1α-hydroxyvitamin D3 administration in mice. Drug Metab. Dispos. 2009;37:2037–2044. doi: 10.1124/dmd.109.027334. [DOI] [PubMed] [Google Scholar]
- 32.Nehring J.A., Zierold C., DeLuca H.F. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl. Acad. Sci. USA. 2007;104:10006–10009. doi: 10.1073/pnas.0703512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Nakamura T., Itadani H., Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem. Biophys. Res. Commun. 2002;298:714–719. doi: 10.1016/S0006-291X(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 34.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 35.Matsubara T., Yoshinari K., Aoyama K., Sugawara M., Sekiya Y., Nagata K., Yamazoe Y. Role of vitamin D receptor in the lithocholic acid-mediated CYP3A induction in vitro and in vivo. Drug Metab. Dispos. 2008;36:2058–2063. doi: 10.1124/dmd.108.021501. [DOI] [PubMed] [Google Scholar]
- 36.Wu S., Zhang Y.G., Lu R., Xia Y., Zhou D., Petrof E.O., Claud E.C., Chen D., Chang E.B., Carmeliet G., et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082–1094. doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 38.Bakke D., Sun J. Ancient nuclear receptor VDR with new functions: Microbiome and inflammation. Inflamm. Bowel Dis. 2018;24:1149–1154. doi: 10.1093/ibd/izy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou S., Glowacki J. Chronic kidney disease and vitamin D metabolism in human bone marrow-derived mscs. Ann. N. Y. Acad. Sci. 2017;1402:43–55. doi: 10.1111/nyas.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basoli V., Santaniello S., Cruciani S., Ginesu G.C., Cossu M.L., Delitala A.P., Serra P.A., Ventura C., Maioli M. Melatonin and vitamin D interfere with the adipogenic fate of adipose-derived stem cells. Int. J. Mol. Sci. 2017;18:981. doi: 10.3390/ijms18050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shulman A.I., Larson C., Mangelsdorf D.J., Ranganathan R. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell. 2004;116:417–429. doi: 10.1016/S0092-8674(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh J.-C., Sisk J.M., Jurutka P.W., Haussler C.A., Slater S.A., Haussler M.R., Thompson C.C. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J. Biol. Chem. 2003;278:38665–38674. doi: 10.1074/jbc.M304886200. [DOI] [PubMed] [Google Scholar]
- 43.Choi M., Yamada S., Makishima M. Dynamic and ligand-selective interactions of vitamin D receptor with retinoid X receptor and cofactors in living cells. Mol. Pharmacol. 2011;80:1147–1155. doi: 10.1124/mol.111.074138. [DOI] [PubMed] [Google Scholar]
- 44.Chuma M., Endo-Umeda K., Shimba S., Yamada S., Makishima M. Hairless modulates ligand-dependent activation of the vitamin D receptor-retinoid x receptor heterodimer. Biol. Pharm. Bull. 2012;35:582–587. doi: 10.1248/bpb.35.582. [DOI] [PubMed] [Google Scholar]
- 45.Heyman R.A., Mangelsdorf D.J., Dyck J.A., Stein R.B., Eichele G., Evans R.M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-V. [DOI] [PubMed] [Google Scholar]
- 46.Kane M.A., Folias A.E., Pingitore A., Perri M., Obrochta K.M., Krois C.R., Cione E., Ryu J.Y., Napoli J.L. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc. Nat. Acad. Sci. USA. 2010;107:21884–21889. doi: 10.1073/pnas.1008859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Urquiza A.M., Liu S., Sjoberg M., Zetterstrom R.H., Griffiths W., Sjovall J., Perlmann T. Docosahexaenoic acid, a ligand for the retinoid x receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 48.Niu H., Fujiwara H., di Martino O., Hadwiger G., Frederick T.E., Menéndez-Gutiérrez M.P., Ricote M., Bowman G.R., Welch J.S. Endogenous retinoid X receptor ligands in mouse hematopoietic cells. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aan1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber K., Erben R.G. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J. Anim. Physiol. Anim. Nutr. 2013;97:675–683. doi: 10.1111/j.1439-0396.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 50.Ryan J.W., Starczak Y., Tsangari H., Sawyer R.K., Davey R.A., Atkins G.J., Morris H.A., Anderson P.H. Sex-related differences in the skeletal phenotype of aged vitamin D receptor global knockout mice. J. Steroid Biochem. Mol. Biol. 2016;164:361–368. doi: 10.1016/j.jsbmb.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Jena P.K., Sheng L., Liu H.X., Kalanetra K.M., Mirsoian A., Murphy W.J., French S.W., Krishnan V.V., Mills D.A., Wan Y.Y. Western diet-induced dysbiosis in farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment. Am. J. Pathol. 2017;187:1800–1813. doi: 10.1016/j.ajpath.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishizawa M., Ogura M., Kato S., Makishima M. Impairment of bilirubin clearance and intestinal interleukin-6 expression in bile duct-ligated vitamin D receptor null mice. PLoS ONE. 2012;7:e51664. doi: 10.1371/journal.pone.0051664. [DOI] [PMC free article] [PubMed] [Google Scholar]