Abstract
(1) Background: Arteriosclerosis is associated with high levels of low-density lipoprotein (LDL) cholesterol. O-methylated catechins in “Benifuuki” green tea are expected to reduce cholesterol levels, although there is limited research regarding this topic; (2) Methods: This trial evaluated 159 healthy volunteers who were randomized to receive ice cream containing a high-dose of “Benifuuki” extract including 676 mg of catechins (group H), a low-dose of “Benifuuki” extract including 322 mg of catechins (group L), or no “Benifuuki” extract (group C). Each group consumed ice cream (with or without extract) daily for 12 weeks, and their lipid-related parameters were compared; (3) Results: A significant reduction in the level of lectin-like oxidized LDL receptor-1 ligand containing ApoB (LAB) was detected in group H, compared to groups L and C. No significant differences between the three groups were detected in their levels of total cholesterol, triglycerides, and LDL cholesterol; (4) Conclusions: “Benifuuki” extract containing O-methylated catechins may help prevent arteriosclerosis.
Keywords: dyslipidemia, Benifuuki, O-methylated catechin, Lectin-like oxidized LDL receptor-1, LOX-1 ligand
1. Introduction
Lifestyle changes, such as overeating and lack of exercise, have caused global increases in the rates of obesity, hypertension, dyslipidemia, and cardiovascular diseases [1]. As hyperlipidemia is one of the most important risk factors for cardiovascular disease, reductions in serum low-density lipoprotein (LDL) cholesterol levels can help prevent cardiovascular disease [2]. This is because oxidized LDL injures the vascular endothelium, which promotes platelet aggregation and thrombus formation. Furthermore, macrophage uptake of oxidized LDL leads to conversion into foam cells and the promotion of plaque formation, which can lead to stenosis, vascular obstruction, plaque rupture, and cardiovascular events [3,4]. Oxidized LDL is generated when LDL in the blood enters the vascular endothelium, and there is a clear relationship between increased serum levels of oxidized LDL and metabolic syndrome, such as hypertriglyceridemia [5]. Moreover, arteriosclerosis is ameliorated by reduced serum levels of oxidized LDL and the ligand, even in the presence of high levels of LDL and triglycerides [6]. Therefore, prevention of LDL oxidization, regardless of blood LDL levels, may be useful for preventing arteriosclerosis onset, progression, and cardiovascular diseases.
Green tea is a popular beverage in Japan, and previous studies have indicated that the consumption of green tea can help prevent cardiovascular events [7]. In particular, the oxidization of LDL is strongly suppressed by (−)-epigallocatechin-3-O-gallate (EGCG) and (−)-epicatechin-3-O-gallate (ECG) [8,9]. Green tea contains catechins, which are polyphenols that reduce lipase activity during the digestive process and prevent adipose micellization. These mechanisms lead to decreases in the serum levels of cholesterol and triglycerides [10,11]. Therefore, green tea catechins may reduce lipid levels and help prevent cardiovascular events.
The main catechins in green tea are (−)-epicatechin (EC), (−)-epigallocatechin (EGC), and EGCG, with EGCG providing the greatest reduction in serum cholesterol levels [11]. In addition, O-methylated catechins, such as (−)-epigallocatechin-3-O-(3-O-methyl)-gallate (EGCG3”Me) and (−)-gallocatechin-3-O-(3-O-methyl)-gallate (GCG3”Me), are contained in specific types of green tea (e.g., “Benifuuki” green tea), and not contained in standard green tea (e.g., “Yabukita” green tea that is most commonly consumed in Japan). These O-methylated catechins may have greater physiological activity than EGCG, because their high blood concentration is maintained by relatively good absorption through the gastrointestinal tract, compared to EGCG [12]. Moreover, our previous report has indicated that the consumption of “Benifuuki” green tea containing O-methylated catechins was associated with significantly lower serum LDL levels, compared to standard green tea containing no O-methylated catechins [13]. Therefore, O-methylated catechins may be more useful for modulating lipid metabolism, compared to standard catechins, although there is insufficient research regarding this topic.
Although green tea is a very popular beverage in Japan, its bitterness has made it relatively unpopular in Europe and America. Therefore, we propose that green tea catechins could be included in ice cream, which would mask their bitterness and improve acceptance among non-Asian populations. This randomized controlled trial examined whether ice cream containing green tea-derived O-methylated catechins could affect the lipid metabolism of healthy Japanese volunteers.
2. Materials and Methods
2.1. Participants and Enrolment
This study was performed in accordance with the principles of the Declaration of Helsinki, and all participants provided their written informed consent. The randomized double-blind placebo-controlled design was approved by the ethics committees of Osaka Medical College (1285-04; approval dates: 2 September 2013 to 31 March 2016) and the Osaka Medical College Health Science Clinic (2011-CR-10; approval date: 29 June 2013). Furthermore, the trial was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000011901). We approached 10,009 healthy individuals to recruit them for the study, in cooperation with the Osaka Medical College Health Science Clinic and using local advertisements. All potentially eligible individuals completed a careful examination that included a medical history, physical examination, and clinical testing (including blood chemistry testing). The inclusion criteria were healthy individuals who were 20–80 years old, had serum LDL cholesterol levels of >3.10 mmol/L, and had a body mass index (BMI) of >18.5 kg/m2. The exclusion criteria were treatment for arrhythmia, liver disorders, chronic renal diseases, cerebrovascular disorders, rheumatism, diabetes, lipid abnormalities, a history of anemia, severe allergies to specific foods and drugs, heart failure, myocardial infarction, pregnancy or a desire to become pregnant in the near future, lactation, and other conditions that were judged by the physician to preclude inclusion (e.g., the use of dietary supplements). Based on the inclusion and exclusion criteria, a total of 159 individuals were considered eligible for inclusion (56 men and 103 women who were 23–80 years old). The mean age was 53.7 ± 10.4 years and the median age was 53 years. All participants were recruited between late August of 2014 and September of the year. All participants lived in Osaka, Kyoto, or Hyogo (Japan), and underwent baseline evaluations between September 2014 and December of the year. The participants were followed for 12 weeks after the baseline evaluation.
2.2. Randomization
The participants were randomized 1:1:1 using a computer-generated randomization sequence, with the block size kept constant. The groups were assigned non-specific identifiers, which corresponded to group H, high-dose “Benifuuki” extract containing 676 mg of catechins (314 mg of epigallocatechin gallate (EGCG) and 66 mg of O-methylated catechins) and 66 mg of caffeine in ice cream; group L, low-dose “Benifuuki” extract containing 322 mg of catechins (138 mg of EGCG, 32 mg of O-methylated catechins) and 33 mg of caffeine in ice cream; and group C, no “Benifuuki” extract in ice cream. All participants received individually wrapped packages of ice cream with or without the extract, which were provided by an unrelated third party and were only labelled with the corresponding group identifier. Thus, the investigators and participants were blinded to the group assignments and amounts of extract in the ice cream packages.
2.3. Sample Size
Previous studies have evaluated 22–240 participants (group sizes of 22–120 participants) to determine whether daily consumption of catechins was associated with significant reductions in LDL cholesterol and oxidized LDL cholesterol [14,15,16]. Based on the results of those studies, we set the target sample size to 150 participants.
2.4. Restrictions during the Study Period
The participants were instructed to avoid excessive overeating and overdrinking starting at 1 week before the baseline evaluation, and not to modify their lifestyle (e.g., dietary habits, smoking, and exercise). Furthermore, the participants were instructed not to consume unregulated drugs and supplements that could influence their serum lipid levels. Moreover, the participants were instructed to fast starting at 10 PM on the nights before the study measurements, although they were allowed to drink water during the fasting period. Finally, the participants were instructed to refrain from smoking and extreme exercise until completion of the study, and to record their physical condition and compliance eating the ice cream (daily life record).
A limitation of our study is that polyphenols contained in chocolate, wine, and certain other foods were not evaluated in the Food Frequency Questionnaire because only the consumption of polyphenols present in tea and coffee were restricted.
2.5. Outcomes and Measures
The primary outcomes were defined as serum levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. In addition, we evaluated lipid metabolism based on the lectin-like oxidized LDL receptor-1 index (LOX index), which is calculated by multiplying the LOX-1 ligand including ApoB (LAB) concentration by the soluble LOX-1 concentration. LAB concentration was measured using sandwich enzyme-linked immunosorbent assay. The secondary outcomes were defined as (1) body weight, waist circumference, and blood pressure; (2) fasting plasma glucose, glycated hemoglobin (HbA1c), glycoalbumin (GA), and insulin (IRI) levels; (3) pentosidine and urinary 8-hydroxydeoxyguanosine levels; (4) adiponectin levels; (5) aspartate transaminase, alanine transaminase, and γ-glutamyltransferase levels; (6) serum iron and ferritin levels; (7) urinalysis results; and (8) dietary survey responses.
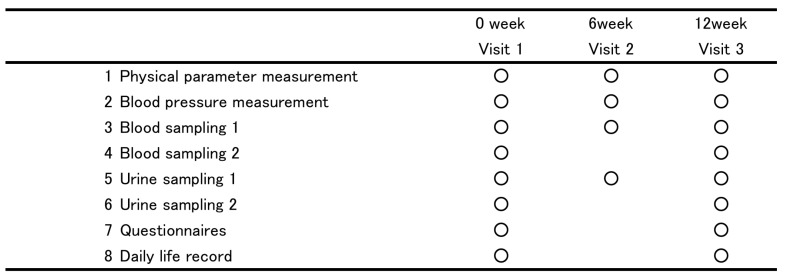
The participants’ body weights were measured using bioelectricity impedance analysis (InnerScan®50V BC-621-SS; Tanita Corporation, Tokyo, Japan). Blood pressure was measured twice, before and after a 3-min rest, using a sphygmomanometer (HEM-7200®; Omron Corporation, Kyoto, Japan). Biochemical tests were performed by SRL Inc. (Tokyo, Japan). The participants also completed questionnaires regarding their health, lifestyle, food intake, type of green tea that they consumed, and the frequency of green tea consumption. Intake of tea catechins was evaluated using the food frequency questionnaire from the Osaki National Health Insurance cohort study [7]. We show the study’s protocol in Figure 1.
Figure 1.
The schematic representation of study protocol. 1. the measure of height, body weight, waist circumference. 2. the measure of systolic blood pressure, diastolic blood pressure, heart rate. 3. total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting plasma glucose, glycated hemoglobin (HbA1c), glycoalbumin (GA), immunoreactive insulin (IRI), aspartate transaminase (AST), alanine transaminase (ALT) and γ-glutamyltransferase (γ-GT), blood cell count, hemoglobin, hematocrit, platelet. 4. soluble lectin-like oxidized LDL receptor-1 (sLOX-1), LOX-1 ligand including ApoB(LAB), LOX index, pentosidine, adiponectin, serum iron and ferritin. 5. Urinalysis. 6. urinary 8-hydroxydeoxyguanosine (8-OH-dG) level. 7. the Food Frequency Questionnaire. 8. records of their individual adherence to eating test ice cream and their physical conditions.
2.6. Ice Cream and Intervention
Ice cream packages (125 mL (110 g), 138 kcal/package) with no extract, as well as packages with high or low levels of “Benifuuki” extract, were manufactured by MORINAGA & Co., Ltd. (Tokyo, Japan). The nutritional profile and the content of catechins and caffeine of the test ice cream is shown in Table 1. The analysis of catechins and caffeine in “Benifuuki” extract was performed as previously described [17]. All participants received packages of ice cream that corresponded to their blinded group assignment and were instructed to consume one package per day for 12 weeks.
Table 1.
Nutritional profile and catechins and caffeine content of the test ice cream provided per day.
| Placebo | Benifuuki Extract Low-Dose | Benfuuki Extract High-Dose | |
|---|---|---|---|
| calories (kcal) | 138 | 138 | 138 |
| protein (g) | 3.3 | 3.3 | 3.3 |
| fat (g) | 6.9 | 6.9 | 6.9 |
| carbohydrate (g) | 21.1 | 20.1 | 18.8 |
| Sodium choloride (mg) | 58 | 56 | 56 |
| total catechins (mg) | 0 | 322 | 676 |
| total O-methyltated catechins ((−)-epigallocatechin-3-O-(3-O-methyl) gallate + (−)-gallocatechin-3-O-(3-O-methyl) gallate) (mg) | 0 | 32 | 66 |
| (−)-epigallocatechin-3-O-gallate + (−)-gallocatechin-3-O-gallate (mg) | 0 | 138 | 314 |
| caffeine | 0 | 33 | 66 |
2.7. Statistical Methods
Differences between groups H, L, and C were evaluated based on the intention-to-treat principle and using the Kruskal-Wallis test, the Steel–Dwass test, the Steel test, and the paired t-test, as appropriate. All analyses were performed using JMP software (version 11.2.1; SAS Research Institute Corporation, Carey, NC, USA). Differences were considered statistically significant at a p-value of <0.05.
3. Results
3.1. Participants and Follow-Up
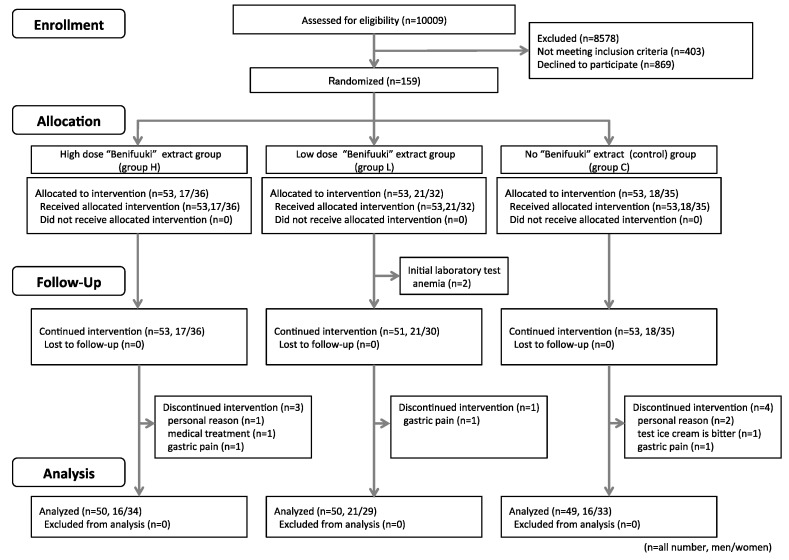
The 159 participants were allocated to group H (n = 53, 17 men and 36 women), group L (n = 53, 21 men and 32 women), and group C (n = 53, 18 men and 35 women), although ten participants were subsequently excluded from the analysis. At the baseline evaluation, two participants from group L were disqualified because they had developed anemia. During the study period, three participants in group H were excluded (withdrawal for personal reasons, gastric pain, and treatment of a previous illness). One participant in group L was excluded because of gastric pain. One participant in group C was disqualified because they could not tolerate the daily ice cream consumption, two participants withdrew because of personal reasons, and one participant withdrew because of gastric pain. Thus, 149 participants (53 men and 96 women) completed the study, with 50 participants in group H (16 men and 34 women), 50 participants in group L (21 men and 29 women), and 49 participants in group C (16 men and 33 women). We show the flowchart of the study in Figure 2.
Figure 2.
Flowchart of the study.
3.2. Baseline Data and Outcomes
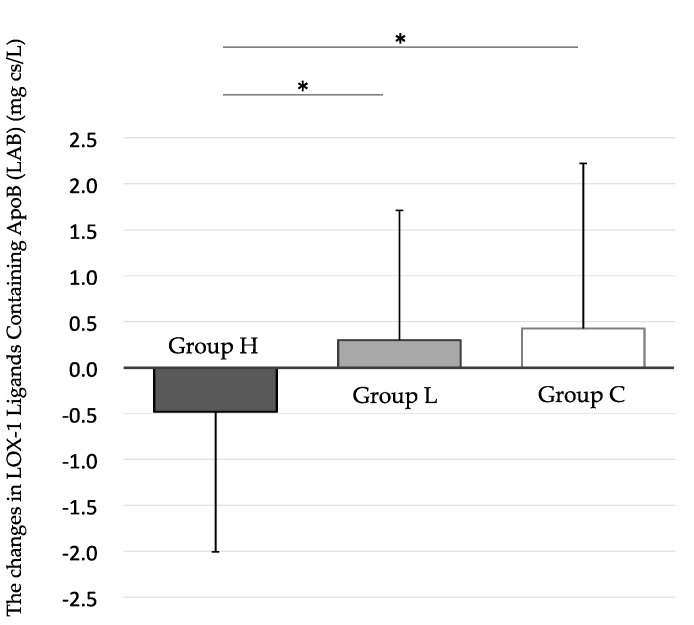
The overall compliance rate for the ice cream consumption was 96.0 ± 6.1%, and there was no significant inter-group difference in the compliance rates (group H, 96.2 ± 5.5%; group L, 94.5 ± 7.8%; group C, 97.5 ± 4.2%). No significant differences were observed between the three groups in their baseline values for weight, BMI, abdominal circumference, and γ-glutamyltransferase levels. By the paired t test, a significant decrease in group H’s LAB level was detected at the 12-week follow-up, compared to the baseline value in that group. However, no meaningful reductions were observed in the week 12 values for LAB in groups L and C, compared to their respective baseline values. And, by the Steel–Dwass test, a significant reduction in the level of LAB was detected in group H, compared to both groups L and C, at the 12-week follow-up (Figure 3) (Table 2).
Figure 3.
The changes in LOX-1 ligands containing ApoB (LAB) level from 0 to 12 weeks. The Steel–Dwass test shows significant differences in the Δ value at 12 weeks between group H and group L, and between group H and group C. Data are shown as mean ± SD, * p < 0.05. Black bar: High-dose “Benifuuki” extract ice cream group (group H). Gray bar: Low-dose “Benifuuki” extract ice cream group (group L). White bar: No “Benifuuki” extract ice cream (control) group (group C).
Table 2.
The characteristics and parameters at baseline (week 0) and week 12.
| Study Group | 0 Week | 12 Week | Δ Value at 12 Weeks a | Paired t Test b | Kruskal-Wallis Test c | Steel–Dwass Test d | Steel Test e | |
|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | p-Value | |||||
| Baseline characteristics | ||||||||
| Sex | H group | 16/34 | ||||||
| (male/female) | L group | 18/32 | ||||||
| C group | 19/30 | |||||||
| Age | H group | 52.1 ± 10.9 | ||||||
| (year) | L group | 54.4 ± 9.7 | ||||||
| C group | 53.8 ± 11.2 | |||||||
| Diet survey | ||||||||
| Total daily intake | H group | 2109.0 ± 1055.6 | 2160.0 ± 1178 | 51.0 ± 835.1 | 0.6676 | 0.4977 | §: 0.7108 | §: 0.6455 |
| (kcal/day) | L group | 2009.2 ± 539.7 | 2128.6 ± 946.1 | 119.3 ± 802.3 | 0.8101 | §§: 0.7322 | §§: 0.6696 | |
| C group | 2215.1 ± 903.9 | 2175.5 ± 902.6 | −39.5 ± 1145.1 | 0.2980 | §§§: 0.9759 | |||
| Anthropometric values | ||||||||
| Body weight | H group | 65.5 ± 10.6 | 66.2 ± 10.7 | 0.7 ± 1.3 | 0.0003 *** | 0.8074 | §: 0.6455 | §: 0.9777 |
| (kg) | L group | 60.9 ± 8.5 | 61.5 ± 8.6 | 0.6 ± 1.1 | <0.0001 **** | §§: 0.6696 | §§: 0.6695 | |
| C group | 62.0 ± 8.9 | 62.8 ± 9.0 | 0.8 ± 1.1 | <0.0001 **** | §§§: 0.6696 | |||
| Body Mass Index | H group | 24.9 ± 3.6 | 25.1 ± 3.7 | 0.3 ± 0.5 | 0.0002 *** | 0.8112 | §: 0.9618 | §: 0.9494 |
| (kg/m2) | L group | 23.5 ± 2.5 | 23.7 ± 2.5 | 0.2 ± 0.4 | <0.0001 **** | §§: 0.7615 | §§: 0.7032 | |
| C group | 23.5 ± 2.6 | 23.9 ± 2.5 | 0.3 ± 0.4 | <0.0001 **** | §§§: 0.9666 | |||
| Waist circumference | H group | 90.0 ± 8.5 | 89.2 ± 9.1 | −0.8 ± 2.1 | 0.0082 ** | 0.9879 | §: 0.9989 | §: 0.9985 |
| (cm) | L group | 85.9 ± 6.8 | 85.2 ± 6.9 | −0.7 ± 2.2 | 0.0328 * | §§: 0.9882 | §§: 0.9842 | |
| C group | 87.1 ± 7.2 | 86.4 ± 7.1 | −0.7 ± 2.2 | 0.0353 * | §§§: 0.9929 | |||
| SBP | H group | 122.0 ± 15.6 | 122.6 ± 13.8 | 0.7 ± 11.2 | 0.6687 | 0.4400 | §: 0.6998 | §: 0.6333 |
| (mmHg) | L group | 127.5 ± 19.6 | 126.9 ± 18.5 | −0.7 ± 11.5 | 0.6901 | §§: 0.8209 | §§: 0.7731 | |
| C group | 120.6 ± 14.0 | 119.2 ± 15.7 | −1.3 ± 13.4 | 0.4991 | §§§: 0.4532 | |||
| DBP | H group | 75.5 ± 10.1 | 76.9 ± 9.1 | 1.4 ± 6.3 | 0.1312 | 0.5862 | §: 0.5870 | §: 0.5118 |
| (mmHg) | L group | 78.4 ± 10.6 | 79.2 ± 10.0 | 0.8 ± 5.0 | 0.2731 | §§: 0.9996 | §§: 0.9994 | |
| C group | 75.1 ± 9.1 | 75.4 ± 8.6 | 0.4 ± 5.4 | 0.6465 | §§§: 0.7092 | |||
| Pulse | H group | 70.1 ± 12.0 | 71.8 ± 11.6 | 1.8 ± 8.1 | 0.1311 | 0.2087 | §: 0.3121 | §: 0.2484 |
| (b.p.m.) | L group | 72.0 ± 8.9 | 70.8 ± 8.9 | −1.9 ± 9.6 | 0.3122 | §§: 0.9908 | §§: 0.9877 | |
| C group | 72.1 ± 11.8 | 70.2 ± 10.3 | −1.2 ± 8.4 | 0.1796 | §§§: 0.2516 | |||
| Lipid parameters | ||||||||
| Total cholesterol | H group | 236.2 ± 26.5 | 246.5 ± 26.3 | 10.3 ± 36.2 | 0.0493 * | 0.3176 | §: 0.8395 | §: 0.7955 |
| (mg/dL) | L group | 247.1 ± 30.0 | 248.6 ± 27.7 | 1.5 ± 42.0 | 0.7992 | §§: 0.6007 | §§: 0.5261 | |
| C group | 236.7 ± 24.2 | 245.2 ± 27.7 | 8.6 ± 32.7 | 0.0734 | §§§: 0.3061 | |||
| Triglyceride | H group | 112.9 ± 51.2 | 103.0 ± 48.2 | −9.9 ± 41.6 | 0.0981 | 0.3753 | §: 0.8152 | §: 0.7663 |
| (mg/dL) | L group | 116.1 ± 60.6 | 112.3 ± 48.7 | −3.8 ± 42.8 | 0.5289 | §§: 0.8711 | §§: 0.8342 | |
| C group | 105.8 ± 51.4 | 103.1 ± 54.3 | −2.7 ± 33.9 | 0.5886 | §§§: 0.2781 | |||
| HDL cholesterol | H group | 58.0 ± 13.5 | 62.6 ± 15.3 | 4.6 ± 6.8 | <0.0001 **** | 0.1637 | §: 0.6373 | §: 0.5649 |
| (mg/dL) | L group | 61.8 ± 13.5 | 66.5 ± 14.3 | 4.7 ± 5.6 | <0.0001 **** | §§: 0.1486 | §§: 0.1113 | |
| C group | 59.7 ± 12.8 | 65.7 ± 13.9 | 6.0 ± 6.0 | <0.0001 **** | §§§: 0.5472 | |||
| LDL choresterol | H group | 155.6 ± 25.9 | 163.3 ± 29.3 | 7.7 ± 36.1 | 0.1399 | 0.5772 | §: 0.6604 | §: 0.6503 |
| (mg/dL) | L group | 162.1 ± 26.2 | 159.7 ± 30.7 | −2.4 ± 42.4 | 0.6870 | §§: 0.6183 | §§: 0.6960 | |
| C group | 155.8 ± 20.2 | 158.9 ± 28.6 | 3.1 ± 32.2 | 0.5030 | §§§: 0.9862 | |||
| LOX index-associated parameters | ||||||||
| sLOX-1 | H group | 271.8 ± 135.8 | 232.5 ± 101.4 | −39.3 ± 110.1 | 0.0150 * | 0.4188 | §: 0.4656 | §: 0.3901 |
| (ng/L) | L group | 297.3 ± 137.7 | 231.3 ± 94.8 | −65.8 ± 103.7 | <0.0001 **** | §§: 0.9839 | §§: 0.9786 | |
| C group | 285.2 ± 181.3 | 207.6 ± 76.6 | −77.5 ± 161.2 | 0.0015 ** | §§§: 0.5272 | |||
| LAB | H group | 6.2 ± 2,2 | 5.7 ± 2.5 | −0.5 ± 1.5 | 0.0296 * | 0.0120 * | §: 0.0337 * | §: 0.0238 * |
| (mg cs/L) | L group | 6.1 ± 2.7 | 6.4 ± 3.4 | 0.3 ± 1.4 | 0.1421 | §§: 0.9983 | §§: 0.9977 | |
| C group | 6.3 ± 2.7 | 6.7 ± 3.3 | 0.4 ± 1.8 | 0.1042 | §§§: 0.0228 * | |||
| LOX index | H group | 1682.3 ± 994.5 | 1312.3 ± 764.8 | −370.0 ± 624.9 | 0.0001 *** | 0.7130 | §: 0.7778 | §: 0.7222 |
| L group | 1807.1 ± 1122.9 | 1455.9 ± 924.4 | −351.2 ± 537.2 | <0.0001 **** | §§: 0.7511 | §§: 0.6913 | ||
| C group | 1785.6 ± 1454.1 | 1388.2 ± 797.9 | −397.3 ± 1025.6 | 0.0093 ** | §§§: 0.9710 | |||
| Glycometabolism-associated parameters | ||||||||
| HbA1c | H group | 5.28 ± 0.26 | 5.32 ± 0.25 | 0.04 ± 0.03 | 0.0701 | 0.6052 | §: 0.7259 | §: 0.6625 |
| (%) | L group | 5.24 ± 0.25 | 5.31 ± 0.29 | 0.07 ± 0.24 | 0.0529 | §§: 0.9890 | §§: 0.9853 | |
| C group | 5.22 ± 0.29 | 5.30 ± 0.32 | 0.08 ± 0.21 | 0.0115 * | §§§: 0.6140 | |||
| GA | H group | 14.15 ± 1.10 | 13.77 ± 1.13 | −0.38 ± 0.33 | <0.0001 **** | 0.0022 * | §:0.0191 * | §:0.0133 * |
| (%) | L group | 14.01 ± 0.85 | 13.82 ± 0.82 | −0.19 ± 0.32 | <0.0001 **** | §§: 0.7859 | §§: 0.7316 | |
| C group | 14.13 ± 1.08 | 13.91 ± 1.11 | −0.22 ± 0.31 | <0.0001 **** | §§§: 0.0035 ** | |||
| Blood glucose | H group | 89.9 ± 6.4 | 90.6 ± 9.0 | 0.7 ± 6.7 | 0.4394 | 0.9474 | §: 0.9790 | §: 0.9721 |
| (mg/dL) | L group | 89.6 ± 7.5 | 90.0 ± 7.2 | 0.4 ± 5.4 | 0.6405 | §§: 0.9357 | §§: 0.9157 | |
| C group | 89.2 ± 8.7 | 89.3 ± 6.9 | 0.4 ± 7.1 | 0.7037 | §§§: 0.9987 | |||
| IRI | H group | 6.5 ± 4.2 | 6.9 ± 4.2 | 0.4 ± 2.3 | 0.2674 | 0.2453 | §: 0.5301 | §: 0.4536 |
| (μU/mL) | L group | 5.1 ± 2.6 | 5.8 ± 3.6 | 0.7 ± 2.5 | 0.0589 | §§: 0.2505 | §§: 0.1951 | |
| C group | 6.3 ± 6.9 | 5.5 ± 2.4 | −0.8 ± 6.6 | 0.4081 | §§§: 0.7493 | |||
| HOMA-IR | H group | 1.4 ± 0.9 | 1.6 ± 1.0 | 0.1 ± 0.6 | 0.1602 | 0.2848 | §: 0.5213 | §: 0.4449 |
| L group | 1.1 ± 0.6 | 1.3 ± 0.8 | 0.1 ± 0.6 | 0.0714 | §§: 0.2708 | §§: 0.2124 | ||
| C group | 1.5 ± 2.1 | 1.1 ± 0.6 | −0.2 ± 2.0 | 0.4049 | §§§: 0.9057 | |||
| Cytokines | ||||||||
| Adiponectin | H group | 3.6 ± 2.3 | 3.9 ± 2.5 | 0.3 ± 0.7 | 0.0025 ** | 0.8585 | §: 0.8859 | §: 0.8525 |
| (μg/mL) | L group | 4.0 ± 2.3 | 3.8 ± 2.0 | 0.3 ± 0.5 | 0.0016 ** | §§: 0.8875 | §§: 0.8545 | |
| C group | 3.5 ± 1.9 | 4.3 ± 2.5 | 0.3 ± 0.6 | 0.0017 ** | §§§: 0.9890 | |||
| A biomarker for advances glycation end products | ||||||||
| Pentosidine | H group | 0.037 ± 0.010 | 0.045 ± 0.013 | 0.008 ± 0.016 | 0.0018 ** | 0.6181 | §: 0.9736 | §: 0.9649 |
| (μg/mL) | L group | 0.037 ± 0.009 | 0.046 ± 0.013 | 0.009 ± 0.013 | <0.0001 **** | §§: 0.5763 | §§: 0.5008 | |
| C group | 0.038 ± 1.000 | 0.044 ± 0.013 | 0.007 ± 0.014 | 0.0022 ** | §§§: 0.8089 | |||
| Complete blood count | ||||||||
| Hb | H group | 14.2 ± 1.0 | 14.5 ± 1.2 | 0.3 ± 0.6 | 0.0038 * | 0.9913 | §: 0.9913 | §: 0.9883 |
| (g/dL) | L group | 14.0 ± 1.3 | 14.3 ± 1.5 | 0.3 ± 0.6 | 0.0019 * | §§: 0.9983 | §§: 0.9977 | |
| C group | 14.1 ± 1.1 | 14.4 ± 1.2 | 0.3 ± 0.6 | 0.0015 * | §§§: 0.9971 | |||
| Hct | H group | 42.5 ± 3.2 | 43.5 ± 3.5 | 0.9 ± 1.9 | 0.0015 * | 0.8793 | §: 0.9103 | §: 0.8832 |
| (%) | L group | 41.9 ± 3.7 | 42.9 ± 4.2 | 1.0 ± 1.8 | 0.0002 * | §§: 0.9985 | §§: 0.9980 | |
| C group | 43.1 ± 3.2 | 43.1 ± 3.6 | 1.0 ± 2.0 | 0.0008 * | §§§: 0.8936 | |||
| RBC | H group | 459.9 ± 35.4 | 466.2 ± 33.5 | 6.3 ± 20.8 | 0.0364 * | 0.8523 | §: 0.9913 | §: 0.9884 |
| (×104/μL) | L group | 453.4 ± 33.7 | 460.3 ± 38.4 | 6.9 ± 18.7 | 0.0122 * | §§: 0.9479 | §§: 0.9314 | |
| C group | 454.7 ± 37.6 | 462.8 ± 42.1 | 8.2 ± 26.0 | 0.0328 * | §§§: 0.8182 | |||
| Plt | H group | 24.9 ± 5.0 | 27.9 ± 13.8 | 3.1 ± 12.7 | 0.0940 | 0.4660 | §: 0.9931 | §: 0.9908 |
| (×104/μL) | L group | 25.6 ± 5.9 | 25.9 ± 5.9 | 0.3 ± 2.5 | 0.3404 | §§: 0.4383 | §§: 0.3640 | |
| C group | 25.5 ± 5.3 | 26.1 ± 4.6 | 0.6 ± 2.6 | 0.0921 | §§§: 0.6535 | |||
| WBC | H group | 5298.0 ± 1307.0 | 5616.0 ± 1553.8 | 318.0 ± 1422.8 | 0.1204 | 0.2471 | §: 0.3171 | §: 0.2528 |
| (/μL) | L group | 5068.0 ± 1299.1 | 5200 ± 1149.1 | 132.0 ± 1080.9 | 0.3921 | §§: 0.3268 | §§: 0.2615 | |
| C group | 5187.8 ± 1426.5 | 5183.7 ± 1339.4 | −4.1 ± 938.5 | 0.9758 | §§§: 0.9656 | |||
| Liver functions | ||||||||
| AST | H group | 22.5 ± 6.7 | 21.7 ± 5.4 | −0.9 ± 6.3 | 0.3378 | 0.8213 | §: 0.9651 | §: 0.9538 |
| (U/L) | L group | 24.9 ± 10.6 | 24.7 ± 9.5 | −0.0 ± 3.2 | 0.8932 | §§: 0.9044 | §§: 0.8758 | |
| C group | 22.0 ± 5.3 | 22.0 ± 5.3 | −0.1 ± 7.3 | 0.9298 | §§§: 0.8312 | |||
| ALT | H group | 25.4 ± 15.8 | 25.3 ± 13.0 | −0.1 ± 11.8 | 0.9618 | 0.2705 | §: 0.2441 | §: 0.1897 |
| (U/L) | L group | 22.7 ± 14.8 | 23.1 ± 13.6 | 0.5 ± 10.6 | 0.7597 | §§: 0.4827 | §§: 0.4067 | |
| C group | 21.7 ± 11.5 | 22.0 ± 10.7 | 0.3 ± 4.7 | 0.6749 | §§§: 0.9817 | |||
| γGTP | H group | 30.1 ± 22.3 | 31.7 ± 27.2 | 1.6 ± 8.1 | 0.1653 | 0.4453 | §: 0.3913 | §: 0.3199 |
| (U/L) | L group | 41.0 ± 35.6 | 42.2 ± 38.3 | 1.2 ± 13.8 | 0.5484 | §§: 0.6982 | §§: 0.6314 | |
| C group | 23.8 ± 14.7 | 23.9 ± 14.9 | 0.1 ± 4.6 | 0.8516 | §§§: 0.9617 | |||
| Iron metabolism | ||||||||
| Fe | H group | 19.3 ± 6.0 | 18.8 ± 7.1 | −0.5 ± 7.3 | 0.6542 | 0.3897 | §: 0.6716 | §: 0.6021 |
| (μmol/L) | L group | 18.6 ± 6.7 | 19.2 ± 6.5 | 0.6 ± 7.2 | 0.5488 | §§: 0.3785 | §§: 0.3081 | |
| C group | 19.1 ± 5.4 | 17.6 ± 5.7 | −1.6 ± 6.2 | 0.0772 | §§§: 0.8403 | |||
H group: “Benifuuki” green tea extract high component ice cream group. L group: “Benifuuki” green tea extract low component ice cream group. C group: “Benifuuki” green tea extract non-component ice cream (control) group. All data are mean ± standard deviation. a: The value is the change from 0 to 12 weeks. b: Paired t test analyzed in each group to compare the data at 0 week (baseline) and 12 weeks (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). c: Kruskal–Wallis test analyzed the statistical difference of the Δ value at 12 weeks between all groups. (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). d: Steel–Dwass test analyzed the statistical difference of the Δ value at 12 weeks in H group. or L group compared to C group. (§: between H group and C group, §§: between L group and C group, §§§: between H group and L group, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). e: Steel test analyzed the statistical difference of the Δ value at 12 weeks in H group or L group compared to C group. (§: between H group and C group, §§: between L group and C group, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). SBP: systolic blood pressure, DBP: diastolic blood pressure, sLOX-1: soluble lectin-like oxidized LDL receptor-1, LAB: LOX-1 ligand including ApoB, LOX index: lectin-like oxidized LDL receptor-1 index, HbA1c: glycated hemoglobin, GA: glycoalbumin, IRI: immunoreactive insulin, HOMA-IR: Homeostatic Model Assessment for Insulin Resistance, Hb: hemoglobin, Hct: hematocrit, RBC: red blood cell, Plt: platelet, WBC: white blood cell, AST: aspartate transaminase, ALT: alanine transaminase, γ-GT: γ-glutamyltransferase.
No significant inter-group differences were observed in the levels of total cholesterol and LDL cholesterol when we compared the baseline and week 12 values (Table 2).
In addition, the Steel–Dwass test showed significant differences in the Δ value of GA at 12 weeks between group H and group L, and between group H and group C, but showed no significant differences in the Δ value of HbA1c (Table 2).
Furthermore, no significant differences were observed in the participants’ questionnaire responses and life records. Moreover, based on the food frequency questionnaire responses, no significant differences were observed in the participants’ daily caloric intakes when we compared the baseline and week 12 values.
3.3. Ancillary Analysis
A subgroup analysis was performed between the two participant groups with (n = 105) and without (n = 44) a habit of daily tea drinking. In the both groups, no endpoints had any significant change, suggesting that the division of the study population weakened the detection power.
On the other hand, the differences adjusted for age and sex between groups H, L, and C were evaluated using the analyses of covariance (ANCOVA) test. The analyses adjusted for age and sex showed significant differences in the Δ value of LAB level at 12 weeks between three groups. We show the result of the analyses in Table 3.
Table 3.
The age- and sex-adjusted analyses in the Δ value of sLOX-1, LAB and LOX index at 12 weeks between three groups.
| Kruskal–Wallis Test a | ANCOVA Test b (Adjusted for Age) | ANCOVA Test b (Adjusted for Sex) | |
|---|---|---|---|
| p-Value | p-Value | p-Value | |
| the Δ value of sLOX-1 (ng/L) | 0.4188 | 0.3432 | 0.2901 |
| the Δ value of LAB (mg cs/L) | 0.0120 * | 0.0149 * | 0.0122 * |
| the Δ value of LOX index | 0.7130 | 0.9543 | 0.9378 |
a: Kruskal-Wallis test analyzed the statistical difference of the Δ value at 12 weeks between all groups (* p < 0.05). b: ANCOVA test analyzed the statistical difference of the Δ value at 12 weeks between all groups (* p < 0.05), the Δ value: the increase or decrease level compared to the baseline value; sLOX-1: soluble lectin-like oxidized LDL receptor-1; LAB: LOX-1 ligand including ApoB; LOX index: lectin-like oxidized LDL receptor-1 index.
3.4. Adverse Events
None of the participants reported experiencing adverse events that could be attributed to the ice cream or catechins, although a previous report has indicated that green tea catechin consumption can be associated with liver damage or low levels of iron [18].
4. Discussion
The present study revealed that consumption of “Benifuuki” extract in ice cream was associated with a significant reduction in LAB levels. The LAB levels reflect various types of modification (e.g., oxidization, malondialdehyde modification, acetylation, carbamylation, and glycation) of LDL [19]. Progression of arteriosclerosis is driven by the binding of modified LDL to LOX-1 in the vascular endothelium. This promotes endothelial injury through apoptosis, release of inflammatory cytokines, production of active oxygen, and reduction of nitric oxide levels [20]. In addition, LOX-1 is expressed in smooth muscle cells, macrophages, and platelets, where it can cause smooth muscle cell proliferation, foam cell formation, and aggregation of activated platelets [21]. In this context, LAB levels are suggested to be a new biomarker for evaluating the progression of arteriosclerosis and related diseases. For example, recent studies have suggested that LAB and the carotid intima-media thickness were significantly and positively correlated [22,23]. In addition, a recent study reported that LAB showed significant positive correlations with history of smoking, waist circumference, triglycerides [24]. In the present study, the Δ value of LAB at 12 weeks showed significant positive correlations with the Δ value of body weight, the Δ value of BMI, and the Δ value of triglyceride by the Spearman’s rank correlation coefficient test. However, the Δ values of body weight and BMI at 12 weeks were not significantly different between the three groups, suggesting that no significant correlations exist between the Δ value of LAB and the Δ value of body weight and BMI. Significantly positive correlations between the Δ value of triglyceride and the Δ value of LAB at 12 weeks were detected only in group C, and was not detected either in group H or L (the data shown in Supplementary material Table S1). However, the Δ value of triglyceride at 12 weeks had no significant difference between the three groups, suggesting that a significant decrease in group H’s LAB level at the 12 weeks was not related with triglyceride.
On the other hand, consumption of “Benifuuki” extract in ice cream was not associated with a reduction in LDL cholesterol levels. One possible explanation is that significant changes in cholesterol levels were not clear, in the participants without lipid abnormalities and without overweight. Alternatively, the doses of catechin may have been insufficient to affect cholesterol levels in such participants. Finally, all of the participants had a pre-existing habit of drinking green tea, which might have obscured any effects of the catechins in the ice cream.
In addition, by the result of this study, the Steel–Dwass test showed significant differences in the Δ value of GA at 12 weeks between group H and group L, and between group H and group C, but showed no significant differences in the Δ value of HbA1c. These results suggested that high-dose “Benifuuki” extract might have some influences on the participants’ glycemic control, compared to both low-dose “Benifuuki” extract and Control. However, the influence could be too minimal or short-lasting to significantly change HbA1c level, and may have no clinical significance, because GA is a useful clinical biomarker for glycemic control and reflects a short-term glycemic control, compared to HbA1c, which reflects long-term glycemia.
5. Conclusions
In the present study, daily consumption of “Benifuuki” extract (676 mg of catechins per day) was associated with a significant decrease in serum LAB levels, compared to the group that received less extract (322 mg of catechins per day) or no extract. We attribute this effect to the “Benifuuki” extract containing O-methylated catechins, which may help suppress the progression of arteriosclerosis.
Acknowledgments
We thank the Osaka Medical College Health Science Clinic for support with participant recruitment. We also thank Yukie Nakatsubo, Sayaka Ikeda, and Shinobu Mitsui (Osaka Medical College, Department of Internal Medicine (I)) for regularly following-up with the study participants. Furthermore, we thank Eisaku Nishimura (MORINAGA & Co., Ltd., Tokyo, Japan) for providing the test ice cream. And, we would like to thank Editage (www.editage.jp) for English language editing.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/10/7/924/s1, Table S1: The analysis of correlation between Lipid parameters and LAB at 12 weeks.
Author Contributions
Conceptualization, H.S., H.I., J.T., M.M.-Y., H.T. and T.H.; Methodology, H.S., H.I., J.T., M.M.-Y. and T.H.; Software, M.M.; Validation, M.M., H.S., R.F. and K.T.; Formal Analysis, M.M.; Investigation, M.M.; Resources, M.M.; Data Curation, M.M.; Writing-Original Draft Preparation, M.M.; Writing-Review & Editing, H.S., H.I., M.M.-Y., T.H. and A.I.; Visualization, M.M.; Supervision, J.T. and T.H.; Project Administration, M.M.; Funding Acquisition, M.M.-Y.
Funding
This work was supported by a grant (A11) from the Research Project on Development of Agricultural Products and Foods with Health Promoting Benefits, Japan.
Conflicts of Interest
The authors declare no conflict of interest to disclose for this study.
References
- 1.Braitman L.E., Adlin E.V., Stanton J.L., Jr. Obesity and caloric intake: The national health and nutrition examination survey of 1971–1975 (HANES I) J. Chronic Dis. 1985;38:727–732. doi: 10.1016/0021-9681(85)90114-6. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen T.R., Kjekshus J., Berg K., Haghfelt T., Faergeman O., Faergeman G., Pyorala K., Miettinen T., Wilhelmsen L., Olsson A.G., et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The scandinavian simvastatin survival study (4S). 1994. Atheroscler. Suppl. 2004;5:81–87. doi: 10.1016/j.atherosclerosissup.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D., Parthasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 4.Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holvoet P., Lee D.H., Steffes M., Gross M., Jacobs D.R., Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishigaki Y., Katagiri H., Gao J., Yamada T., Imai J., Uno K., Hasegawa Y., Kaneko K., Ogihara T., Ishihara H., et al. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118:75–83. doi: 10.1161/CIRCULATIONAHA.107.745174. [DOI] [PubMed] [Google Scholar]
- 7.Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., Tsubono Y., Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 8.Miura S., Watanabe J., Tomita T., Sano M., Tomita I. The inhibitory effects of tea polyphenols (flavan-3-ol derivatives) on Cu2+ mediated oxidative modification of low density lipoprotein. Biol. Pharm. Bull. 1994;17:1567–1572. doi: 10.1248/bpb.17.1567. [DOI] [PubMed] [Google Scholar]
- 9.Miura S., Watanabe J., Sano M., Tomita T., Osawa T., Hara Y., Tomita I. Effects of various natural antioxidants on the Cu(2+)-mediated oxidative modification of low density lipoprotein. Biol. Pharm. Bull. 1995;18:1–4. doi: 10.1248/bpb.18.1. [DOI] [PubMed] [Google Scholar]
- 10.Koo S.I., Noh S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda I., Imasato Y., Sasaki E., Nakayama M., Nagao H., Takeo T., Yayabe F., Sugano M. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim. Biophys. Acta. 1992;1127:141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- 12.Maeda-Yamamoto M., Ema K., Shibuichi I. In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement. Cytotechnology. 2007;55:135–142. doi: 10.1007/s10616-007-9112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imbe H., Sano H., Miyawaki M., Fujisawa R., Miyasato M., Nakatsuji F., Haseda F., Tanimoto K., Terasaki J., Maeda-Yamamoto M., et al. “Benifuuki” green tea, containing O-methylated EGCG, reduces serum low-density lipoprotein cholesterol and lectin-like oxidized low-density lipoprotein receptor-1 ligands containing apolipoprotein B: A double-blind, placebo-controlled randomized trial. J. Funct. Foods. 2016;25:25–37. doi: 10.1016/j.jff.2016.05.004. [DOI] [Google Scholar]
- 14.Hirano-Ohmori R., Takahashi R., Momiyama Y., Taniguchi H., Yonemura A., Tamai S., Umegaki K., Nakamura H., Kondo K., Ohsuzu F. Green tea consumption and serum malondialdehyde-modified LDL concentrations in healthy subjects. J. Am. Coll. Nutr. 2005;24:342–346. doi: 10.1080/07315724.2005.10719483. [DOI] [PubMed] [Google Scholar]
- 15.Inami S., Takano M., Yamamoto M., Murakami D., Tajika K., Yodogawa K., Yokoyama S., Ohno N., Ohba T., Sano J., et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int. Heart J. 2007;48:725–732. doi: 10.1536/ihj.48.725. [DOI] [PubMed] [Google Scholar]
- 16.Nagao T., Hase T., Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 17.Maeda-Yamamoto M., Ema K., Monobe M., Tokuda Y., Tachibana H. Epicatechin-3-O-(3″-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. J. Agric. Food Chem. 2012;60:2165–2170. doi: 10.1021/jf204497b. [DOI] [PubMed] [Google Scholar]
- 18.Sarma D.N., Barrett M.L., Chavez M.L., Gardiner P., Ko R., Mahady G.B., Marles R.J., Pellicore L.S., Giancaspro G.I., Low Dog T. Safety of green tea extracts: A systematic review by the us pharmacopeia. Drug Saf. 2008;31:469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- 19.Sato Y., Nishimichi N., Nakano A., Takikawa K., Inoue N., Matsuda H., Sawamura T. Determination of LOX-1-ligand activity in mouse plasma with a chicken monoclonal antibody for ApoB. Atherosclerosis. 2008;200:303–309. doi: 10.1016/j.atherosclerosis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. LOX-1-mediated effects on vascular cells in atherosclerosis. Cell. Physiol. Biochem. 2016;38:1851–1859. doi: 10.1159/000443123. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka H., Kume N., Miyamoto S., Minami M., Moriwaki H., Murase T., Sawamura T., Masaki T., Hashimoto N., Kita T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.CIR.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 22.Okamura T., Miura K., Sawamura T., Kadota A., Hisamatsu T., Fujiyoshi A., Miyamatsu N., Takashima N., Miyagawa N., Kadowaki T., et al. Serum level of LOX-1 ligand containing ApoB is associated with increased carotid intima-media thickness in Japanese community-dwelling men, especially those with hypercholesterolemia LOX-1 ligand and IMT in Japanese. J. Clin. Lipidol. 2016;10:172–180. doi: 10.1016/j.jacl.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Okamura T., Sekikawa A., Sawamura T., Kadowaki T., Barinas-Mitchell E., Mackey R.H., Kadota A., Evans R.W., Edmundowicz D., Higashiyama A., et al. LOX-1 ligands containing apolipoprotein B and carotid intima-media thickness in middle-aged community-dwelling US Caucasian and Japanese men. Atherosclerosis. 2013;229:240–245. doi: 10.1016/j.atherosclerosis.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida K., Suehiro A., Nakanishi M., Sawamura T., Wakabayashi I. Associations of atherosclerotic risk factors with oxidized low-density lipoprotein evaluated by LOX-1 ligand activity in healthy men. Clin. Chim. Acta. 2011;412:1643–1647. doi: 10.1016/j.cca.2011.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.