Abstract
In recent years, organs-on-chips (OOCs) have been developed to meet the desire for more realistic in vitro cell culture models. These systems introduce microfluidics, mechanical stretch and other physiological stimuli to in vitro models, thereby significantly enhancing their descriptive power. In most OOCs, porous polymeric membranes are used as substrates for cell culture. The polymeric material, morphology and shape of these membranes are often suboptimal, despite their importance for achieving ideal cell functionality such as cell–cell interaction and differentiation. The currently used membranes are flat and thus do not account for the shape and surface morphology of a tissue. Moreover, the polymers used for fabrication of these membranes often lack relevant characteristics, such as mechanical properties matching the tissue to be developed and/or cytocompatibility. Recently, innovative techniques have been reported for fabrication of porous membranes with suitable porosity, shape and surface morphology matching the requirements of OOCs. In this paper, we review the state of the art for developing these membranes and discuss their application in OOCs.
Keywords: membranes, biomaterials, polymers, microfabrication, organs-on-chips
1. Introduction: the need for organs-on-chips
The development of new drugs is becoming more difficult, time-consuming and costly. Despite all the trials done before approval of a drug, reactions of patients to the drug can differ greatly. In fact, individuals can even react adversely to a drug. These responses do not always become apparent in the first stages of clinical trials. Participants are often selected on characteristics such as sex, age or ethnicity and thus do not represent the entire population. Also, due to ethical reasons, the effects of drugs on children are hardly investigated. As a result of these limitations, clinical trials often do not show the entire spectrum of responses to a drug. Moreover, many drugs fail during clinical trials, despite showing efficacy during preclinical trials. The notion that we cannot fully rely on animal models is growing. Despite a considerable resemblance in both genetics and physiology between animal models and humans, animal models often do not accurately predict how drugs will perform in humans.
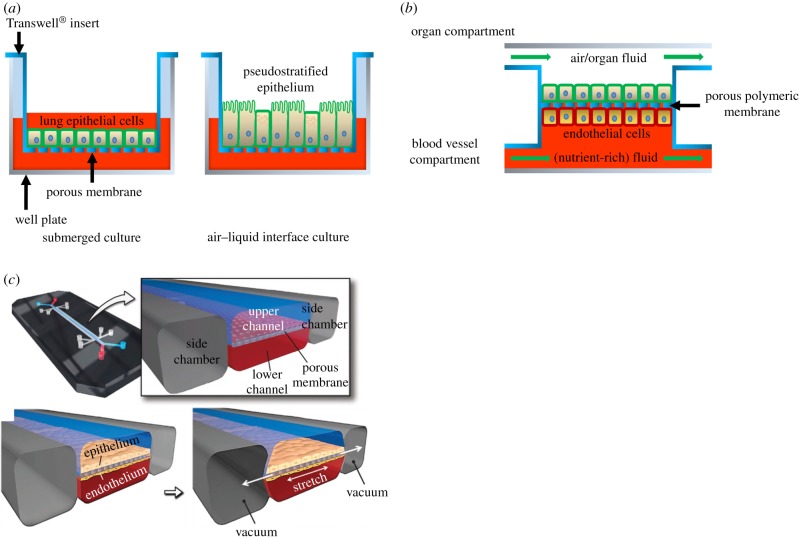
In vitro models have the advantage that they often use only human cells. However, they lack the physiology of a tissue, and thus cell behaviour may differ from the in vivo situation. Transwell® inserts consist of small baskets with a porous, permeable membrane underneath which can be placed in a well plate. This way, the membrane is suspended above the bottom of the well (figure 1a). Lung cells, for example, can be placed on the membrane and exposed to air (figure 1a), while receiving nutrients through the membrane. This already allows more realistic cell culturing than traditional systems. However, the cells do not experience stimuli such as air flow or mechanical stretch.
Figure 1.
Schematic representation of lung cell culture systems. (a) Lung epithelial cells are cultured in a conventional manner on a porous poly(bisphenol-A carbonate) (PC) or poly(ethylene terephthalate) (PET) membrane using a commercially available Transwell® system. Initially, cell culture medium is provided both on top of the cells and below the insert, i.e. submerged culture. Subsequently, the medium on top of the cells is removed to expose the cells to air, i.e. air–liquid interface culture. This causes the cells to transition to a pseudostratified epithelium. Nutrients from the cell culture medium pass through the membrane. (b) OOCs generally comprise two compartments that are connected via a porous, polymeric membrane. The blood vessel compartment contains a flowing fluid which substitutes the blood in the particular organ, often containing the nutrients and other factors which the cells need, such as cell culture medium. Endothelial cells can be cultured on the membrane to represent the blood vessel wall. The organ compartment holds cells of the organ of interest, for example liver, kidney or lung epithelial cells which are grown on the other side of the membrane. The compartment is usually filled with a medium mimicking the fluid in the organ or, for example, air, in the case of the lung or skin. In most OOCs, this medium flows to mimic the flow of air or liquid in the organ. (c) A lung-on-a-chip consisting of a microfluidic device with three channels. The middle channel contains two compartments, separated by a porous poly(dimethyl siloxane) (PDMS) membrane. Lung epithelial cells are cultured on the top side, while endothelial cells are cultured on the bottom side of the membrane. Air and cell culture medium flow through the top and bottom compartment, respectively. A vacuum can be applied in the adjacent two channels which provide mechanical stretch to the membrane and cells. Adapted from Huh et al. [1]. (Online version in colour.)
In vitro models that better predict drug responses and can account for individual differences would address many of the issues in drug development. Because of the need of such in vitro models, organs-on-chips (OOCs) have been developed [1–48]. These are small microfluidic devices that mimic a particular organ by introducing organ-like features such as fluid flow and mechanical stress (figure 1b,c). Cell lines are used in most OOCs, but the need for the development of personalized therapies leads to the desire of using human-induced pluripotent stem cells (hiPSCs). With iPSC technology, differentiated cells can be dedifferentiated to an embryonic stem cell-like state, after which they can be redifferentiated to any cell type. The development of OOCs and iPSC technology could result in powerful tools for developing personalized therapies. Moreover, iPSC technology could also provide an opportunity to test drugs on cells derived from children or other parts of the population which are generally not investigated. By giving a more accurate prediction of drug responses in humans, fewer drugs would fail in clinical trials, thus decreasing overall development costs and time. Moreover, because of their small size, OOCs only need small amounts of resources and thus are not expensive. OOCs could also lead to a decrease in animals needed for research, reducing the ethical difficulties related to drug development. Ultimately, they can provide patients with more effective drugs faster, cheaper and with a lower ethical impact.
Although each type of OOC is different in order to mimic a specific organ properly, many of their characteristics are the same (figure 1b). They often consist of a ‘blood vessel compartment’ containing endothelial cells and an ‘organ compartment’ containing cells of the tissue or organ of interest. Between the two compartments, generally, a porous polymeric membrane is applied for achieving cell adhesion and cell separation as well as cell communication between the two compartments. In this review, membranes are defined as interfaces between two phases. Membranes have a selective permeability, based on the membrane structure among other factors. Therefore, a distinction can be made between porous membranes which contain microscopic pores and non-porous membranes which only contain molecular pores, i.e. intermolecular spaces through which gases can diffuse. This is based on the definition of a membrane given by Mulder [49]. In OOCs, the different cell types are grown on opposite sides of the membrane. The blood vessel compartment can be perfused with aqueous solutions of nutrients, such as cell culture medium or a blood substitute (figure 1b,c). The nutrients will need to pass the endothelial cells and the porous membrane to supply the cells of interest that are cultured on the other side of the membrane. Similar to the blood vessel compartment, the organ compartment can often be perfused, for example, with a nutrient-rich fluid in a gut-on-a-chip [8,9,18,19,29,39,50,51] (figure 1b) or ventilated with air as would be appropriate for a lung-on-a-chip [11,12,31,36,42] (figure 1b,c). In addition, applying mechanical forces to the cells by stretching the cell-covered membranes can simulate the peristalsis in the intestines or the respiratory movement in the lungs [1,10–13,18,19,42].
Despite the drastic advancement in OOC technologies in general (better sensors, pumps and microfluidics) with a rapidly expanding library of OOCs of different organs or redesigns of already existing ones, the advancements in the development of appropriate porous, polymeric membranes are very slow. Only a handful of materials are applied for membranes in OOCs (table 1). These membranes and the materials they are made of are very relevant in these models because they provide structural support for the different cells while also allowing nutrient transport to the separate compartments. It is also known that cells react to environmental cues like chemistry, stiffness, topography and curvature of their substrate. However, important aspects of the membranes used in OOCs and other in vitro models, such as their material and shape, are often neglected. In fact, several OOCs simply use membranes found in commercial inserts [2,15,21,26,29,39,43,48,53] or filter membranes [4,16,23,25,27,41,43,44,51]. Many are made of polymers that have suboptimal cytocompatibility and often do not have suitable mechanical properties matching the tissue to be developed. To accurately simulate in vivo situations, the membranes used should stimulate natural behaviour of cells and should be made with a surface structure that mimics the architecture of the tissue.
Table 1.
Polymers used for fabrication of porous membranes in OOCs and microfluidic systems.
| polymer | application |
|---|---|
| poly(dimethyl siloxane) (PDMS) | artery [6] |
| erythrocyte oxygenation [7] | |
| gut [1,18,19] | |
| heart [40] | |
| kidney [13] | |
| liver [1,3,13,38] | |
| lung [1,11,13,42] | |
| multi-organ-on-chip (MOC) of liver and heart [47] | |
| new membranes for OOCs [52] | |
| poly(carbonate) (PC) | blood–brain barrier (BBB) [4,43,44] |
| bonding of membrane to chip [51] | |
| colon and breast cancer [27] | |
| gut [8,9] | |
| gut, liver and brain cancer [16] | |
| MOC of liver and intestine [29] | |
| MOC of liver and skin [29] | |
| multiple-layered cultures of fibroblasts, endothelial and mesenchymal cells [41] | |
| liver, lung and breast cancer and gut [25] | |
| skin [5,23] | |
| poly(ethylene terephthalate) (PET) | gut [39] |
| heart [28] | |
| kidney [15] | |
| liver [17,48] | |
| microfluidic barrier tissues [2] | |
| MOC of liver and cancer [21] | |
| white adipose tissue (fat) [26] | |
| aliphatic polyesters | |
| poly(lactic acid) (PLA) | endothelial barrier [33] |
| poly(ɛ-caprolactone) (PCL) | endothelial cell/pericyte/astrocyte cultures for future BBB [34] |
| others | |
| poly(amide) | gut [50] |
| parylene C (dichloro[2,2]paracyclophane) | liver [46] |
| poly(tetrafluoroethylene) | liver (hepatic sinusoid) [14] |
| poly(urethane acrylate) (PUA) | new membranes for OOCs [52] |
| poly(ethylene glycol)-diacrylate (PEG-DA) | new membranes for OOCs [52] |
This review focuses on the characteristics and fabrication of porous polymeric membranes used in OOCs, as well as the importance of their characteristics in relation to cell behaviour. The main polymers that have been used for membranes are presented, and the techniques used to introduce porosity in these membranes are discussed. We also highlight other factors affecting cell behaviour, mainly the topography of the surface the cells are growing on, and we include methods to manufacture porous polymeric membranes that more realistically mimic in vivo micro-environments. These membranes could further improve OOCs.
2. Material properties affecting cells
A very important requirement for producing optimal OOCs is the application of a polymeric membrane which supports the cells and functions as an equivalent of the extracellular matrix (ECM) or more specifically the cell basement membrane found in natural tissue. In this section, we will first briefly discuss important characteristics of the ECM and then focus on important polymeric membrane characteristics tailored to mimic the ECM.
2.1. Need for mimicking the extracellular matrix
Both the ECM and the basement membrane comprise a complex arrangement of components, mostly proteins such as collagens, fibronectin and vitronectin. These proteins contain amino acid sequences like arginine–glycine–aspartic acid (RGD), which are potent sites for cell adhesion [54,55]. Cells interact with these sites via integrins, transmembrane proteins which can also link to the actin cytoskeleton of the cells. Through the connection between the ECM, cell membrane and cytoskeleton, the ECM can affect the cells both mechanically and biochemically.
These interactions between cells and cell-adhesive proteins can also play a role in the case of cell substrates other than the ECM. The adsorption of cell-adhesive proteins containing RGD sequences onto a surface can stimulate cell attachment to that surface [54,56,57]. Moreover, the conformation of the adsorbed proteins is very important [54]. If the RGD sequences are denatured or not accessible to the cells, adherence may be compromised.
2.2. Surface roughness
The surface roughness of a material can significantly influence cell behaviour [57–60]. Lampin et al. [57] sandblasted the surface of poly(methyl methacrylate) films with alumina grains to alter the surface roughness. Adhesion and migration of vascular and corneal cells increased with higher surface roughness. Proliferation was, however, not affected. They suggested that the increased cell adhesion on films with a higher roughness was due to more adsorbed proteins, such as fibronectin and collagen I and III, on the surface of the films. Dowling et al. [58] cultured MG63 osteosarcoma cells on poly(styrene) with varying roughness. Adhesion of the cells was higher on rough films than on smooth films. Conversely, cell spreading decreased as surface roughness increased. Moreover, MG63 cells showed a decreased cell spreading and an increased integrin expression on titanium discs with rough surfaces compared to discs with smooth surfaces [60]. Kunzler et al. [59] showed that different cell types could be affected very differently by the surface. Rat calvarial osteoblasts (RCOs) and human gingival fibroblasts (HGFs) were cultured on aluminium sheets with a roughness gradient. Spreading of both cell types was reduced on rough surfaces. However, as roughness increased, the proliferation of RCOs increased while that of HGFs decreased.
2.3. Hydrophilicity
Hydrophilicity of a material is a strong determinant of protein adsorption and thus cell adhesion [56,57]. Wala et al. [61] prepared poly(dimethyl siloxane) (PDMS) films with different hydrophilicity (by either oxygen plasma or piranha treatment) and reported that cell attachment, spreading and growth of 3T3 fibroblasts and HaCaT keratinocytes increased with increasing hydrophilicity of the films. Others found that moderate hydrophilicity often yields the best cell response [56,62]. Lee et al. [56] increased the hydrophilicity of poly(ethylene) films by surface oxidation. Moderate hydrophilicity led to the highest cell adhesion, spreading and growth, while cells performed worse on films with low or very high hydrophilicity. Adsorption of serum proteins also showed an optimum on films with moderate hydrophilicity. The adsorbed proteins were probably the main cause of the increased cell response. Premnath et al. [62] used a laser to introduce nano-patterns in silicon wafers which made the surface more hydrophilic. Again, moderate hydrophilicity increased adhesion of HeLa cells as well as adsorption of cell-adhesive proteins, whereas high surface hydrophilicity decreased adsorption of the proteins and cell adhesion. They also suggested that the superior cell-adhesive properties of moderately hydrophilic surfaces are due to the higher adsorption of cell-adhesive proteins, when compared with hydrophobic or highly hydrophilic surfaces.
2.4. Mechanical properties
It is suggested that initial cell adhesion and spreading are regulated by material surface properties, such as hydrophilicity and roughness, while properties such as the stiffness of a cell substrate influence later stages of cell growth [61]. For example, Wala et al. [61] reported that 3T3 fibroblasts proliferated more on stiffer PDMS films (Young's modulus of 2.6–3.2 MPa), while HaCaT keratinocytes showed higher proliferation on softer PDMS films (Young's modulus of 0.6–1 MPa). Wen et al. [63] cultured adipose stem cells (ASCs) on poly(acrylamide) (PAA) hydrogels with varying stiffness. Soft hydrogels directed cells towards adipogenic differentiation, while stiff hydrogels led to osteogenic differentiation. Engler et al. [64] differentiated mesenchymal stem cells (MSCs) on collagen I-coated PAA substrates and showed that the lineage of differentiation depended on the stiffness of the substrate. Substrates with a stiffness corresponding to brain (0.1–1 kPa) and muscle (8–17 kPa) led to neurogenic and myogenic differentiation, respectively, while stiff collagen I substrates (25–40 kPa) led to osteogenic differentiation. When the cell culture medium was changed after one week of culturing on one of these substrates to a differentiation medium for a different lineage, many cells began showing traits of that lineage. However, when the medium was changed after three weeks of culturing, cells were much less plastic. The effect of the stiffness of the substrate was strong enough to lead cells towards a stable commitment for a certain lineage.
Ye et al. [55] looked at the mechanisms involved between substrate stiffness and differentiation by culturing MSCs on poly(ethylene glycol) (PEG) hydrogels with varying stiffness. The hydrophilicity of PEG avoided protein adsorption and cell adhesion, and thus cells could only attach to RGD peptides bound to the surface. Softer hydrogels stimulated adipogenesis, while stiffer hydrogels resulted in osteogenesis. Stiff hydrogels probably led to more focal adhesions and thus more cell tension, inducing osteogenic differentiation, while fewer focal adhesions and less cell tension on softer hydrogels stimulated adipogenesis. Interestingly, Casillo et al. [53] cultured endothelial cells on PDMS films with varying stiffness but did not see an influence of PDMS stiffness on the formation of focal adhesions. It seems that the effect of material stiffness depends on the cell type applied.
2.5. Porosity
Porosity is an important characteristic of the membranes in OOCs for achieving cell communication between the chip compartments as well as for achieving oxygen and nutrient transport to the cells. Casillo et al. [53] grew endothelial cells on non-porous and porous SiO2 membranes. The porous membranes had different pore sizes and corresponding pore spacings. Cells showed fewer focal adhesions and less fibronectin fibrillogenesis on porous membranes, and overall cells showed less interaction with the porous membranes. This effect was stronger on membranes with smaller pores, probably because the spacing between the pores, and thus the amount of continuous space for the cells to adhere was smaller. However, cell–cell interactions in the form of tight junctions measured by ZO-1 activity showed an opposite trend. Porosity, especially with small pore spacing, could thus limit cell adhesion, but enhance barrier function of cells. They concluded that weaker cell–substrate interaction might lead to stronger cell–cell interaction. Wen et al. [63], however, found no influence of the porosity on the differentiation of ASCs on PAA hydrogels, suggesting that the effect of porosity on cells may depend on multiple factors.
2.6. Microstructures
2.6.1. Microstructures of singular cell size
Hulsman et al. [65] prepared a cell culture chip from poly(lactic acid) (PLA) that contained a large number of differently designed surface microtopographies. Human MSCs responded differently to the different topographies. Especially the amount of open space between the microstructures was important. Cell and nucleus morphology was rounded when cells had enough space to spread, while they had an abnormal shape when they were forced to compact when the spacing between structures was small. Moreover, cells aligned in between structures with a specific spacing. The best alignment was found at spacings of 2.5–14.1 µm, while smaller spacings did not lead to proper alignment. These responses could have major implications for cell behaviour. Reimer et al. [66] confirmed this by using a similar chip prepared from PLA. They found that some topographies could stimulate Oct4 expression and proliferation of hiPSCs, while other topographies decreased it. In general, smaller feature sizes and higher densities of the shapes on the PLA surface stimulated pluripotency of the cells. These results imply that the topography of the surface can potentially trigger a cell to differentiate or remain in a proliferative state. The effect could be the result of mechanosensory pathways, which are activated by specific topographies.
Ochsner et al. [67] cultured human umbilical vein endothelial cells (HUVECs) on PDMS films which contained fibronectin-coated microwells of different shapes and sizes to investigate the effect of the microwells on single-cell behaviour. The space between the microwells was coated with poly(l-lysine)-graft-poly(ethylene glycol) to avoid cell growth outside of the microwells. Smaller microwells confined the cell shape. This confinement led to a more compact cytoskeleton, while the cytoskeleton of cells grown in larger microwells did spread. Cells in smaller microwells often showed actin stress fibres parallel to the length of the microwell. Geometry can also result in survival or death of cells as shown by Chen et al. [68]. They grew human capillary endothelial cells on culture dishes coated with fibronectin (FN) patterns of various shapes and sizes, ranging from the size of single cells to small clusters. Cells preferred growing on the coated areas instead of the culture dish. Better cell spreading, either by bigger patches of fibronectin or spacing between patches, which allowed spreading from one patch to the other, decreased apoptosis, while survival and growth were promoted. The results were independent of the contact area between cells and the ECM, and thus cell shape seemed to be critical between survival and death.
2.6.2. Microstructures larger than a cell
The studies mentioned above mainly looked at surface topographies in the same size range as a single cell. Chen et al. [68] showed that larger topographies involving multiple cells also have significant influence on clusters of cells. Lee et al. cultured a murine cancer cell line on hydrazine-patterned PAA gels to which ECM proteins were bound in different geometries. In general, patterned gels raised tumorigenic and stem-cell-like behaviour, especially at the edges of the tumour. Their results pointed towards a potential role of geometry in the occurrence of metastasis [69]. Similar results were seen with bovine pulmonary artery endothelial cells grown on glass surfaces that contained patches coated with FN [70]. Cells covered the areas coated with FN. Proliferation of the cells was higher at the edges of the areas than at the centre. The geometry of the coated area, and thus the cell colony, influenced the proliferation. A higher amount of stress at the edges of the areas compared to the centre was probably the cause of the increased proliferation at the edges. Human MSCs have also been shown to change their differentiation depending on the substrate shape [71]. The MSCs were grown on FN-patterned glass surfaces. In general, the cells seemed to have a preference for osteogenic differentiation on shapes with sharper edges, while adipogenic differentiation was more common on squares as well as shapes with rounded corners.
Cells do not only respond to topographies as mentioned above. Large, three-dimensional microstructures with sizes of up to several hundreds of micrometres in both width and height can also direct cell behaviour. Papenburg et al. [72,73] showed the influence of microstructures on the orientation of cells growing on a microstructured membrane. Moreover, there is evidence that cells that are grown in microstructures also arrange their ECM in a more organized manner [74]. The mechanical strength of engineered tissues, consisting of cells and their ECM, improved when grown on microstructured films instead of flat films [74]. Soscia et al. [75] fabricated PDMS films containing microwells coated with poly(lactic-co-glycolic acid) (PLGA) nanofibres. They found that increasing the curvature of the microwells caused an increase of polarity in salivary gland cell lines and a higher expression of the tight junction protein occludin at the apical side. Also, the cells showed signs of differentiation. The magnitude of the curvature affected the strength of the response.
Esch et al. [76] grew HUVECs on PDMS films with square and semicircular microfluidic channels, both under static conditions and under flow. Cell–cell interactions were similar across the different culturing conditions as shown by expression of VE-cadherin. However, vinculin, a measure of focal adhesions, was less present in square channels than in semicircular ones, suggesting that semicircular channels led to a better cell-surface communication. Others have shown that stress fibres were primarily present at the edges of microstructures and that proliferation was also high at those locations [70,77], further elucidating the influence of the surface morphology on cells. Conversely, Hebeiss et al. [78] showed that endothelial cells produced fewer stress fibres and focal adhesions when cultured on semicircular porous channels with a 200 µm diameter when compared with flat membranes. Yamashita et al. [79] cultured human aortic endothelial cells and human aortic smooth muscle cells on concave surfaces. Retaining the concave shape of the cell layer became more difficult when contraction of the cells was stimulated by the addition of TGF-β, and thus cells detached. Cell detachment also increased when the concave shape of the substrate was larger. Additionally, Broaders et al. [80] showed that detachment of cells on PDMS substrates with concave channels is dependent on the cell type. For example, nearly all HUVECs detached from the channels, while Madin–Darby Canine Kidney-Ras cells remained attached to the substrate. Detachment of cells was susceptible to factors which affect contractility of the cells such as blebbistatin, which decreased detachment. Interestingly, larger flat areas between channels increased detachment. They suggested that the flat areas changed the tension in the cells residing in the channels. The machinery involved in cell–substrate and cell–cell interactions as well as cell contraction probably influences each other, causing cells to react to topography with which they are not directly in contact. Channel-like microstructures could potentially mimic other tissues such as small airways, kidney tubules or intestines and thus serve as a better in vitro model as it is clear that surface geometry influences cell behaviour. However, there is still little understanding of the exact mechanisms.
3. Polymers used in the preparation of membranes for organs-on-chips
Although several polymers have been used to fabricate porous membranes for OOCs and microfluidic systems, only a handful of polymers, i.e. PDMS, poly(carbonate) (PC), poly(ethylene terephthalate) (PET), PLA and poly(ɛ-caprolactone) (PCL), were used in the majority of the studies (table 1). This is often driven by practical reasons rather than properties of the polymer related to the application. For example, PDMS membranes are rather easy to make and they are transparent (and thus easily applied with microscopy) and flexible (and as a result can be easily handled). PC is very often used in Transwell® cell culture inserts, and therefore reference data are widely available. Using PC in OOCs thus seemed to be a natural way of transferring knowledge from the standard cell culture system. Besides, the notion that the interaction of the membrane with the cells can be tailored by application of surface treatments and/or bioactive coatings on the above widely used materials seems to limit the application of a broader range of materials. In the last few years, it has become more and more evident that also other characteristics of the membrane, such as the mechanical properties, have to be taken into account [55,61,63]. For example, in the case of membranes that need to be stretched, an elastic polymer is preferable because it has to withstand deformations for many cycles. Whether a polymer degrades and how it degrades is another important consideration as this would determine possible changes in the membrane properties in time and whether cytotoxicity would occur.
3.1. Poly(dimethyl siloxane)
PDMS (table 2) is often used in OOCs (tables 1 and 3) [1,3,5–8,10–13,15,16,18,19,23,25,26,28,29,33,35,36,38,42,47]. In fact, it is the state-of-the-art material in OOC technology, used for both the membrane for cell adhesion and for fabrication of the chip itself. It is a hydrophobic polymer which is flexible and transparent, which makes it a desirable material to work with for OOCs, as the chips [1,3,5–8,10–13,15,16,18,19,23,25,26,28,29,33,35,36,38,42,47] can withstand mechanical forces, and cells are easily visible. PDMS is inert and non-biodegradable. It is, however, known to absorb small molecules and drugs [87,88]. This is very relevant because drug studies are an important application for OOCs. One approach to limit the absorption is by coating PDMS, e.g. with a lipid-based coating [88]. In most cases, PDMS needs to be coated with proteins to improve cell adhesion and proliferation. It is also possible to alter the surface chemistry of PDMS to improve cell adhesion [61].
Table 2.
Chemical structures and properties of commonly used polymers for porous membranes in OOCs. The Tg is the temperature under which the material behaves like glass and thus is stiff. At temperatures above the Tg, polymers are more flexible. The Young's modulus is a measure of stiffness. A high modulus corresponds to high stiffness, while polymers with a low modulus are flexible. The water contact angle is the angle between a water droplet and a polymer film. Polymers with a high water contact angle are hydrophobic, while those with a low contact angle are hydrophilic. The values mentioned are contact angles measured with smooth polymer films as surface roughness changes the contact angle.
| polymer | chemical structure | Tg (°C) | Young's modulus | water contact angle |
|---|---|---|---|---|
| poly(dimethyl siloxane) (PDMS) | 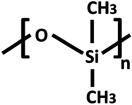 |
−125 | variable (kPa–MPa) [67] | 107.5° [81] |
| poly(bisphenol-A-carbonate) (PC) | 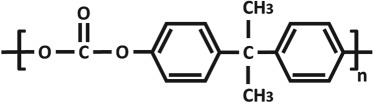 |
145 | 2–2.4 GPa | 85° [82] |
| poly(ethylene terephthalate) (PET) | 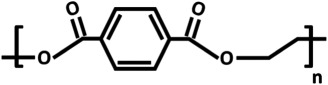 |
70 | 2–3 GPa | 82.6° [83] |
| poly(lactic acid) (PLA) | 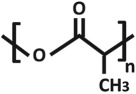 |
55–65 | 3–4 GPa | 61° [84] |
| poly(ɛ-caprolactone) (PCL) | 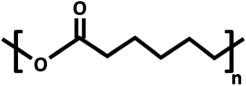 |
−60 | 400 MPa [85] | approximately 119° [86] |
Table 3.
Fabrication techniques used to prepare porous membranes for OOCs.
PDMS has a low glass transition temperature (Tg) of −125°C. To prepare creep-resistant PDMS structures, it is cross-linked. A mixture of PDMS prepolymer and a cross-linker is prepared, cast and afterwards exposed to elevated temperatures or UV light. The mechanical properties of PDMS can be tuned by changing the ratio of PDMS to curing agent [61,67]. Membranes with a Young's modulus as low as 4 kPa can be prepared [67], which makes it ideal for cells which prefer a soft substrate, such as cardiomyocytes, which need to contract their substrate. Higher amounts of curing agent can yield PDMS with a modulus of several MPa, suitable for cells which prefer a stiffer substrate. This method of casting and curing is often used together with soft lithography to prepare both PDMS membranes and microfluidic chips. These include OOCs mimicking heart [6,30,40], liver [1,3,13,38], lung [1,11,13,42], a multi-organ-on-chip of liver and heart [47], oxygenation of erythrocytes [7] and vascular networks [35].
3.2. Poly(carbonate)
PC (table 1) is widely used in OOCs (tables 1 and 3) [4,8,9,16,23,27,29,41,43,44,51]. It is also one of the most commonly used polymers for porous filter membranes and the porous membranes in Transwell® inserts. In fact, several OOCs implemented PC filter membranes [4,16,23,25,27,41,43,44,51] or PC membranes taken from Transwell® inserts [29,43]. PC is hydrophobic, transparent, inert and non-biodegradable. For cells to properly adhere and grow on PC, the surface of PC is often altered by protein coating [4,9,23,44] or gas plasma treatment [43] similar to PDMS. PC, however, is a very stiff polymer and has a Tg of 145°C and a Young's modulus of 2–2.4 GPa. As a result, PC is not suitable for OOCs which require membrane stretching and for culturing of tissues which need a soft substrate. Nevertheless, PC has been used in many different OOCs, including studies on the blood–brain barrier (BBB) [4,43,44], cancer [16,25,27] and liver [29]. Surprisingly, however, it has also been used for skin [23,29] and gut [8,29] OOCs, which would ideally require the cells to be mechanically stimulated by a flexible membrane.
3.3. Poly(ethylene terephthalate)
PET (table 2) is another popular polymer for commercial membranes and inserts. Several OOCs and microfluidic cell culture systems included PET membranes (tables 1 and 3), often PET membranes which were taken directly from inserts [2,15,21,26,39,48,53] or porous filter membranes [28]. PET is transparent, inert and non-biodegradable and also requires treatment to improve cell adhesion, such as plasma treatment. It has a Tg of 70°C and a Young's modulus of approximately 2–3 GPa. Thus, like PC, PET is not suitable for OOCs which require mechanical strain on the cells. It has been used, however, for OOCs mimicking gut [39] and studies on endothelial cells [2,28] as well as fat [26], liver [17,21,48] and kidney [15].
3.4. Aliphatic polyesters: poly(lactic acid) and poly(ɛ-caprolactone)
The aliphatic polyesters PLA [33] and PCL [34] (table 2) have been used in in vitro models mimicking the BBB (table 1). Both polymers are hydrophobic and biodegradable. This could hold potential for functioning as a temporary membrane, which is later replaced by the ECM of the cells to create an entirely natural cell layer. It has to be taken into account, however, that due to the degradation, the membrane pores might change over time. Moreover, the degradation products of these esters are acidic and thus could affect cells.
The Tg of PLA and PCL differs significantly at approximately 55–65°C and −60°C, respectively. As a result, PCL is in a more deformable state at room or body temperature than PLA. This is accompanied by a Young's modulus of 400 MPa for PCL [85] and 3–4 GPa for PLA. The high modulus makes PLA unsuitable for membranes which should be exposed to mechanical strain.
4. Preparation of porous polymeric membranes for organs-on-chips
The porous membranes in OOCs should not only provide support for the cells, but should also allow communication of the cells between the chip compartments and allow nutrients and other factors to reach all the cells. For example, in the lung-on-a-chip from Huh et al. [12], porous membranes were applied, so neutrophils could pass the membrane and reach the lung cells on the other side of the membrane to initiate an immune response against Escherichia coli. In general, membranes in OOCs are made porous by two methods that allow for good control of the pore size, shape and distribution (table 3).
4.1. Soft lithography
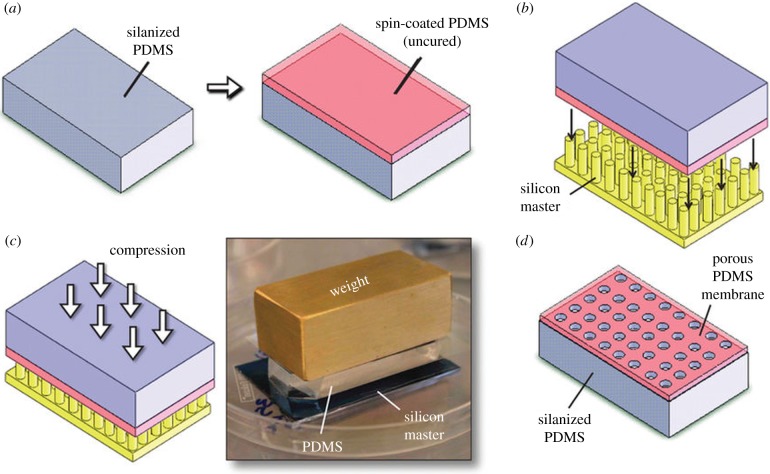
The most common method to fabricate components of the OOCs is soft lithography. It is used for both the ‘rigid’ chip itself [1,2,5–7,9,11–13,15,16,18,19,21–26,28,29,33,36,38,40,47] and the flexible porous membrane that supports the cells [1,6,7,10,11,13,18,19,33,38,42,47]. Soft lithography is very suitable to prepare porous membranes for OOCs (table 3). Huh et al. [1] pushed the micropillars of a silicon master through an uncured PDMS film (figure 2). The punctured film was cured to create a porous PDMS membrane. The size, distribution and amount of the pores were thus all controlled by the micropillars on the silicon master. This method results in a low pore tortuosity as the pores are always perpendicular to the membrane surface. This is a great advantage because the migration and transport of cells and nutrients are optimized. A similar approach was used by Pensabene et al. [33], who used a patterned mould with microneedles made of poly(vinyl alcohol) to prepare porous PLA membranes for PDMS-based OOCs containing endothelial cells.
Figure 2.
Preparation of porous PDMS membranes by soft lithography. (a) A slab of PDMS is cured and silanized. Uncured PDMS is spin-coated on top of the silanized side to form a 10 μm thin film. (b) The PDMS is placed on top of a silicon master containing micropillars with the diameter of the pores. (c) The entire assembly is compressed, in order for the micropillars to completely penetrate the uncured PDMS. The PDMS is cured and (d) after removing the silicon master and silanized PDMS, a porous membrane remains. Adapted from Huh et al. [1]. (Online version in colour.)
4.2. Track etching
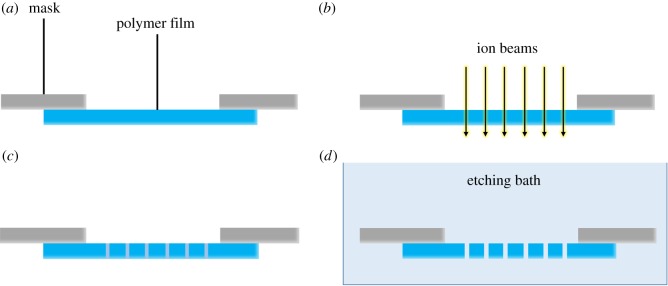
Many of the porous membranes from OOCs originate from commercial inserts or filter membranes made of PC and PET which were prepared by track etching [2,4,5,21,23,25–27,29,39,41,43,44,51]. Track etching involves applying either electrons, heavy ions, X-ray irradiation or UV light, which pass at predefined spaces of a mask. For example, ion track etching (figure 3) uses heavy ions to cleave polymer chains at specific spots to form cylindrical tracks of degraded polymer, perpendicular to the surface of the film. After irradiation, the tracks have to be opened. A conventional method is wet-chemical etching which uses chemicals or etchants, often acids or bases such as sodium hydroxide, which may not be preferable for some polymers [78,89–91]. By changing the etchant concentration, etching temperature and etching duration, pore sizes can be tailored from nanometres to micrometres (figure 3d). With the polymer removed, cylindrical pores remain with low tortuosity. The control over pore size, shape and density makes track etching a very relevant method to produce porous polymer membranes which are very suitable for transport or migration studies.
Figure 3.
Ion track etching. (a) A polymer film is covered by a mask. (b) Ion beams pass through the opening(s) in the mask and locally penetrate the polymer film. (c) Polymer chains have been locally degraded by the ion beams. (d) By placing the film in a bath with etching agents, the degraded polymer is removed, and pores are formed. (Online version in colour.)
5. Microfabrication of membranes for use in future organs-on-chips
As we discussed earlier, to create reliable and representative OOCs, we need to apply membranes with surface topography closely mimicking the topography of the tissue/organ to be investigated. In this section, we will discuss the state of the art in developing porous, microstructured membranes which could replace the flat, non-microstructured membranes currently used in OOCs. Realistic cell behaviour could be stimulated by culturing cells on porous membranes that also include microstructures which resemble the configuration of the cells in the body. To prepare such porous, microstructured membranes, the mentioned methods to produce porous membranes can be combined with microfabrication. Microfabrication consists of a set of techniques to prepare micrometre-scale structures. Microfabrication is already applied in OOC technology to prepare the chips [1,18,19], but not used to fabricate porous, microstructured membranes. Taking advantage of microfabrication as it is used in other fields of biomedical engineering could accomplish the three-dimensional organization of cells. Microfabrication could provide a robust, reproducible micro-environment that is independent of the culturing conditions of the cells. Microfabrication would make it possible to prepare structures that mimic different versions of a particular tissue, such as large or small blood vessels, duodenum and small intestine, or specific pathologies where the shape of the tissue is abnormal.
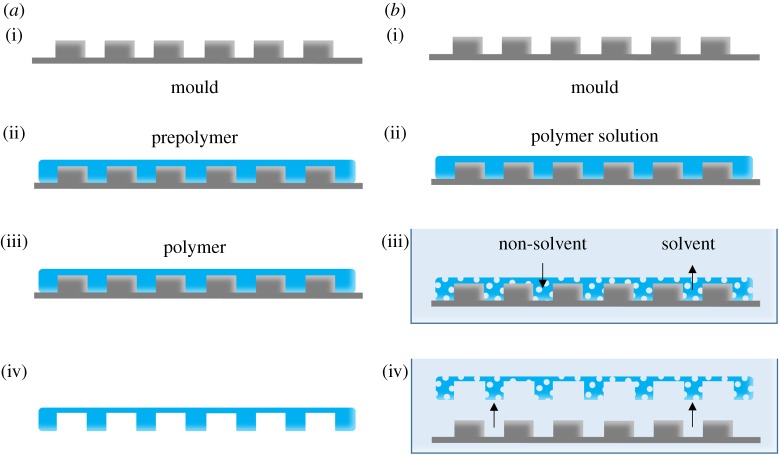
5.1. Soft lithography replica moulding
As mentioned before, soft lithography is used for fabrication of flat porous membranes, as well as the chips of OOCs. However, it can also be used for producing microstructured membranes. The most common soft lithography technique for OOCs is replica moulding (figure 4a) [1,15,18,19,33]. In the case of OOCs, uncured PDMS is deposited on a microstructured mould and cured (figure 4a(i–iii)). After removal from the mould, the PDMS contains negative copies of the microstructures of the mould (figure 4a(iv)).
Figure 4.
(a) Schematic representation of soft lithography replica moulding. (i) A microstructured mould. (ii) The mould is filled with prepolymer. (iii) The prepolymer is then cured. Note: depending on the polymer, curing may not be necessary. (iv) Removing the mould reveals a microstructured polymer film. (b) The principle of phase separation micromoulding. (i) A microstructured mould. (ii) The mould is filled with a polymer solution consisting of polymer dissolved in a solvent. (iii) The mould and polymer solution are submerged in a bath of non-solvent. Liquid-induced phase separation occurs through the exchange of non-solvent and solvent. This exchange solidifies the polymer solution and creates pores. (iv) The porous, microstructured membrane releases from the mould. (Online version in colour.)
Although simple and accurate, there are limitations to the capabilities of soft lithography. Microstructures will only appear on one side of the membrane, while the other side remains flat (figure 4a(iv)). Pores could be included, but their position and orientation would be limited. Using micropillars as discussed before, pores can be created perpendicular to the surface of a flat membrane. For the purpose of a porous membrane with microwells, the walls of the microwells would not contain pores as the mould cannot be made with micropillars in the horizontal direction.
Another approach is to combine soft lithography with other pore-forming methods such as particulate leaching. Vozzi et al. [92] prepared scaffolds of PLGA by different soft lithography techniques. By dispersing glucose grains in the polymer solution which were leached afterwards, it was possible to introduce pores into the scaffolds. Likewise, soft lithography could be used to prepare porous, microstructured membranes.
5.2. Phase separation micromoulding
Phase separation micromoulding (PSµM) (figure 4b) enables the introduction of porosity and patterns into membranes in one step by inducing phase separation, most often liquid-induced phase separation [72,73,93,94], of a polymer solution on a patterned mould. The appropriate polymer solution is cast onto a micro- and/or nanostructured master mould (prepared by technologies derived from microelectronics and photolithography). During liquid-induced phase separation, solvent and non-solvent liquid exchange initiate phase separation until the polymer solution contains sufficient non-solvent to precipitate. As a result, the porous membrane solidifies and releases from the mould. By selecting the right solvent/non-solvent system with the specific polymer, the porosity of the resulting membrane can be tuned. The shape, size and distribution of the microstructures that are imprinted in the membranes can be altered by changing the design of the mould to meet the requirements of a particular application [9,72,73,93].
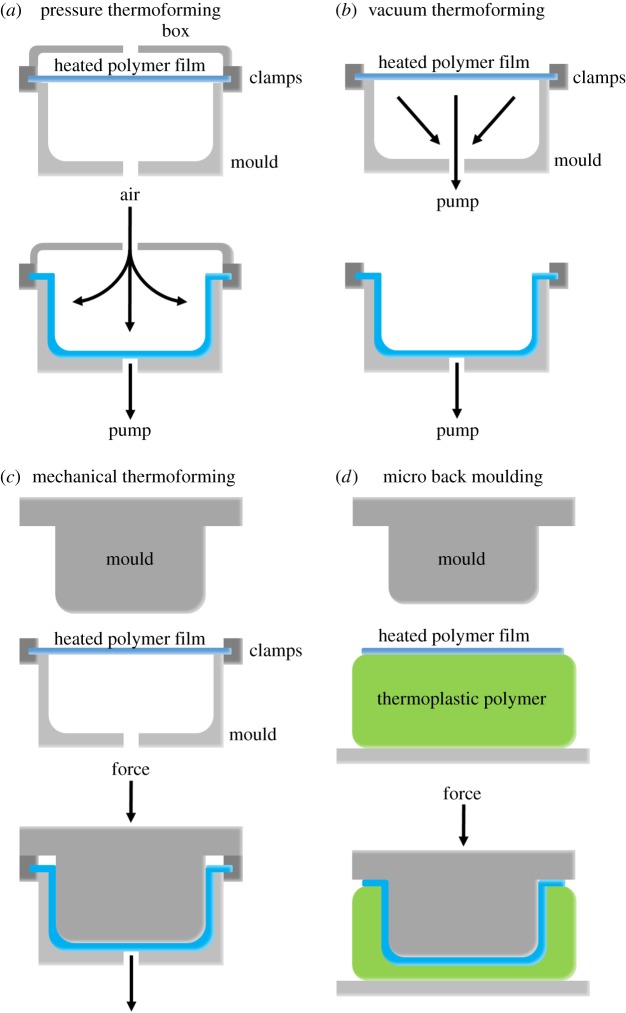
5.3. Thermoforming
Porous, microstructured membranes can be made by thermoforming [78,89–91,95]. With most thermoforming methods, a polymer film is placed on a plate which acts as the mould and contains the microstructures that have to be replicated [78,89–91,95]. It is then heated to a soft but still solid state, after which a force is applied to shape the film to the mould. This force can be applied in several ways, most commonly by air pressure (figure 5a), a vacuum (figure 5b) or a mechanical force (figure 5c,d). The properties of the film may affect the outcome. For example, densely cured polymer networks may not deform permanently by thermoforming.
Figure 5.
Schematic of thermoforming techniques. (a) Pressure thermoforming: a compressed gas can be released which exerts a pressure that pushes a preheated plastic sheet, such as a polymer film or membrane, against the mould. (b) Vacuum thermoforming: a vacuum can be applied on the other side of a preheated plastic sheet. The lower pressure forces the sheet towards the mould. (c) Mechanical thermoforming: a preheated plastic sheet is placed between complementary moulds, i.e. a positive and negative version of the mould, on both sides of the sheet. These are pressed against each other, shaping the plastic sheet to the moulds. (d) Micro back moulding: a preheated plastic sheet is placed on a thick layer of elastomeric or thermoplastic polymer. Upon compression by a mould, both the plastic sheet and the polymer layer beneath it are shaped to the mould. (Online version in colour.)
To create porous, microstructured membranes, thermoforming is often used in conjunction with ion track etching [78,89–91]. Usually, polymer films are first track-etched locally and are then thermoformed into a three-dimensional shape. Finally, the pores are opened by wet-chemical etching. Particular attention is needed because the films are stretched during thermoforming. By opening the pores afterwards, the effect of stretch on pore size and shape is minimal [78,89–91].
Thermoforming has been used to prepare an alternative Boyden chamber for studies on trans-endothelial transport [78]. By combining ion track etching with thermoforming, porous PC membranes with a semicircular microchannel were prepared which closely resembled the three-dimensional shape of a blood vessel. Indeed, these kinds of systems allow for a more realistic study of, for example, trans-endothelial transport of drugs [78].
Hou et al. [96] compression-moulded a mixture of dispersed salt particles in PCL. Compression moulding is very similar to thermoforming. However, a clear distinction has to be made. Often temperatures are much higher during compression moulding than thermoforming, heating a polymer above the melting point to a liquid state. When the polymer is in the liquid state, it is compressed into a film between two plates and afterwards cooled. By using microstructured moulds instead of flat plates during compression moulding, the films can be made to contain microstructures, and pores can be formed by particulate leaching.
Buitinga et al. [95] prepared flat films of poly(ethylene oxide terephthalate)–poly(butylene terephthalate) (PEOT/PBT) block copolymer by solvent casting. This was followed by evaporation of the solvent to form a dense film. The films were then thermoformed to obtain a microwell array where human islets of Langerhans were cultured separately without adhesion to the film or each other. They also prepared porous PEOT/PBT membranes via electrospinning which were afterwards thermoformed.
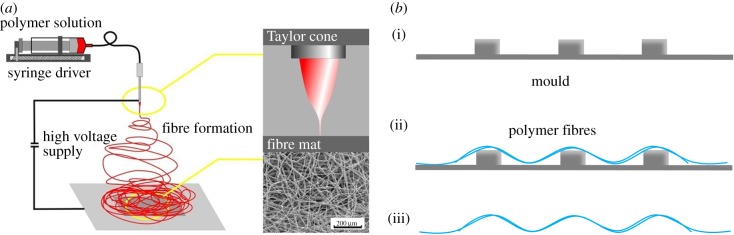
5.4. Electrospinning
Electrospinning can also yield porous, microstructured membranes in one step. Electrospinning involves charging a polymer solution or polymer melt by a high voltage (figure 6a). The charge attracts the polymer to a ground plate, where it is collected. Because changes in the charge occur between the needle and the collection substrate, the polymer flow will continuously change direction and thus spread over the ground plate, resulting in a fibre mesh. The amount of space between fibres can be changed by controlling the density and amount of the polymer solution that is deposited, effectively controlling the porosity, pore size and thickness of the membrane [98,99]. The same principle can be applied to fabricate porous, microstructured membranes by electrospinning over microstructured moulds (figure 6b). Cheng et al. [100] spun PLA fibres on PDMS moulds containing different pyramid-like microstructures. Distinct PLA structures were obtained by changing the dimensions of and spacing between the pyramids.
Figure 6.
Schematic representation of electrospinning (a) a porous, flat and (b) a porous, microstructured membrane. (a) A polymer solution is charged with a high voltage. Thin fibres are spun from the polymer solution, which are attracted to a substrate where they are collected. Adapted from Wallace et al. [97]. (b) (i) A microstructured silicon wafer mould. (ii) Fibres are electrospun on top of the mould. (iii) The mould is removed, and a microstructured mesh of polymer fibres remains. (Online version in colour.)
6. Conclusion
There are significant issues concerning the development of new drugs. In vitro and in vivo models often do not predict drug responses in humans and clinical trials do not account for individual differences in response to drugs. Recently, OOCs have been developed to overcome these issues. OOCs are small microfluidic chips that contain cells and feature characteristics of an organ, such as fluid flow and mechanical forces, in order to closely mimic organ function. Indeed, OOCs have proved that cells show more realistic behaviour in an environment which resembles their native tissue than in traditional static culturing systems. In most OOCs, cells are cultured on a porous polymer membrane. Although OOCs confirm that mimicking the cell environment is beneficial, there is little attention paid to the membranes used in OOCs. Membrane properties such as stiffness and surface roughness can greatly influence cells, such as their adherence and growth. Moreover, cells react to the shape of the membrane, such as the presence of microstructures.
Most membranes in OOCs are made from only a few polymers, which often require treatments or coatings to allow cells to adhere and grow. Moreover, most of the polymers cannot be stretched, and thus many OOCs cannot provide mechanical strain to the cells, despite the often dynamic nature of the native tissues the OOCs try to mimic.
The pores in the membranes are fabricated with two methods in particular, i.e. soft lithography using a micropillar mould or ion track etching of a polymer film. Both offer good control over pore size and density, and result in low pore tortuosity. In fact, commercial membranes made by ion track etching are used in many OOCs.
There is compelling evidence that surface microstructures have a significant effect on cells and can stimulate realistic cell behaviour. Despite this, membranes in current OOCs are flat, while there is great potential for microfabrication of three-dimensional environments. Microfabrication provides simple processing techniques for the preparation of microstructured membranes from a wide variety of materials.
Soft lithography and thermoforming have already been used to prepare porous, microstructured membranes for purposes outside the field of OOCs. In these cases, thermoforming is combined with ion track etching to create the microstructures and pores, respectively. Soft lithography can be used to produce microstructures and has to be complemented with techniques such as particulate leaching to prepare porous, microstructured membranes. PSµM is very similar to soft lithography replica moulding. However, PSµM can create both pores and microstructures in a single step. Porous membranes with microstructures can also be produced by electrospinning in conjunction with thermoforming or electrospinning alone.
Thus far, evidence concerning the effects of membrane characteristics, polymer properties and surface microstructures on cells has been neglected in the field of OOCs. However, the desire to improve OOCs demands that all elements of the system are optimized, including the membranes. To do so, we need to understand more about the underlying mechanisms of cell–substrate interactions and how they affect cell behaviour. Moreover, although it is becoming clear that each cell type has preferences, it is not known what those ideal substrate properties are. By mimicking the stiffness of the native ECM, while providing a collagen coating to stimulate cell adhesion, it is already possible to direct MSC differentiation [64], indicating that properly mimicking the ECM is beneficial. Conversely, not having the correct properties of a substrate might give interfering cues to a cell. While most studies make use of only a few polymers, often with different properties from the tissue of interest, other studies show less common polymers with superior cytocompatibility and/or mechanical properties, which should be explored. Surface roughness, porosity, cell adhesive properties and the inclusion of microstructures spanning multiple cells should also be optimized. However, these studies could very easily become impractical with many variables to consider.
Therefore, systematic studies with high-throughput screening are needed, such as those using chips with numerous geometries to analyse cell responses to surface topography [65,66]. This could also provide information about the preferred spacing of topographies which could be used when considering the surface roughness, pore size and porosity. Systems such as that of Lee et al. [69], where different ECM proteins can be attached to the substrate surface, could provide tools to better investigate cell responses to substrate stiffness for example, with limited influence of the substrate chemistry. Combining these kinds of methods could provide high-throughput chips which can systematically screen for the best topography, stiffness, surface roughness and other properties to come closer to the native ECM. Lastly, incorporating microstructures with similar shapes and dimensions as the native tissue would further mimic the spatial organization of cells in the body.
Microfabrication can be applied to create a wide range of porous, microstructured membranes acting as controllable, physiologically accurate representations of native tissue morphology. Depending on the requirements for the membrane or limitations of the polymer, microfabrication offers different techniques for creating both the pores and microstructures to suit these needs. The relative simplicity of microfabrication techniques makes them viable tools for the preparation of a new generation of membranes to be used for further improvement of OOCs.
Data accessibility
This article has no additional data.
Authors' contributions
T.P. drafted the manuscript. D.G., D.S. and A.P. helped with the design and drafted the manuscript. All the authors gave their final approval for publication.
Competing interests
The authors are part of a consortium developing a microstructured membrane made of poly(trimethylene carbonate) for a lung-on-a-chip. We do not have competing financial interests to declare.
Funding
The Netherlands Lung Foundation is gratefully acknowledged for funding this review as part of project no. 6.1.14.010.
References
- 1.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. et al. 2013. Microfabrication of human organs-on-chips. Nat. Protoc. 8, 2135–2157. ( 10.1038/nprot.2013.137) [DOI] [PubMed] [Google Scholar]
- 2.Abhyankar VV, Wu M, Koh CY, Hatch AV. 2016. A reversibly sealed, easy access, modular (SEAM) microfluidic architecture to establish in vitro tissue interfaces. PLoS ONE 11, e0156341 ( 10.1371/journal.pone.0156341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavli D, Prill S, Ezra E, Levy G, Cohen M, Vinken M, Vanfleteren J, Jaeger M, Nahmias Y. 2016. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl Acad. Sci. USA 113, E2231–E2E40. ( 10.1073/pnas.1522556113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JA, et al. 2016. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 13, 306 ( 10.1186/s12974-016-0760-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Mehl BT, Sell SA, Martin RS. 2016. Use of electrospinning and dynamic air focusing to create three-dimensional cell culture scaffolds in microfluidic devices. Analyst 141, 5311–5320. ( 10.1039/C6AN01282E) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YF, Chan HN, Michael SA, Shen YS, Chen Y, Tian Q, Huang L, Wu H. 2017. A microfluidic circulatory system integrated with capillary-assisted pressure sensors. Lab Chip 17, 653–662. ( 10.1039/C6LC01427E) [DOI] [PubMed] [Google Scholar]
- 7.Di Caprio G, Stokes C, Higgins JM, Schonbrun E. 2015. Single-cell measurement of red blood cell oxygen affinity. Proc. Natl Acad. Sci. USA 112, 9984–9989. ( 10.1073/pnas.1509252112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eain MMG, Baginska J, Greenhalgh K, Fritz JV, Zenhausern F, Wilmes P. 2017. Engineering solutions for representative models of the gastrointestinal human-microbe interface. Engineering 3, 60–65. ( 10.1016/J.ENG.2017.01.011) [DOI] [Google Scholar]
- 9.Gao D, Liu H, Lin JM, Wang Y, Jiang Y. 2013. Characterization of drug permeability in Caco-2 monolayers by mass spectrometry on a membrane-based microfluidic device. Lab Chip 13, 978–985. ( 10.1039/c2lc41215b) [DOI] [PubMed] [Google Scholar]
- 10.Huh D, Hamilton GA, Ingber DE. 2011. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754. ( 10.1016/j.tcb.2011.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. et al. 2012. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Trans. Med. 4, 159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. 2010. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668. ( 10.1126/science.1188302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. 2012. Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12, 2156–2164. ( 10.1039/c2lc40089h) [DOI] [PubMed] [Google Scholar]
- 14.Illa X, Vila S, Yeste J, Peralta C, Gracia-Sancho J, Villa R. 2014. A novel modular bioreactor to in vitro study the hepatic sinusoid. PLoS ONE 9, e111864 ( 10.1371/journal.pone.0111864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung SY, Suh KY, Ingber DE. 2013. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. 5, 1119–1129. ( 10.1039/c3ib40049b) [DOI] [PubMed] [Google Scholar]
- 16.Jie M, Li H-F, Lin L, Zhang J, Lin J-M. 2016. Integrated microfluidic system for cell co-culture and simulation of drug metabolism. RSC Adv. 6, 54 564–54 572. ( 10.1039/C6RA10407J) [DOI] [Google Scholar]
- 17.Kang YB, Sodunke TR, Lamontagne J, Cirillo J, Rajiv C, Bouchard MJ, Noh M. et al. 2015. Liver sinusoid on a chip: long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol. Bioeng. 112, 2571–2582. ( 10.1002/bit.25659) [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Huh D, Hamilton G, Ingber DE. 2012. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174. ( 10.1039/c2lc40074j) [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Ingber DE. 2013. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 5, 1130–1140. ( 10.1039/c3ib40126j) [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Cho DW. 2016. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 16, 2618–2625. ( 10.1039/C6LC00450D) [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Kim DS, Ha SK, Choi I, Lee JM, Sung JH. 2017. A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic-pharmacodynamic (PK-PD) model. Biotechnol. Bioeng. 114, 432–443. ( 10.1002/bit.26087) [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D. 2016. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J. Matern-Fetal Neo. Med. 29, 1046–1054. ( 10.3109/14767058.2015.1038518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Jin SP, Kim YK, Sung GY, Chung JH, Sung JH. 2017. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 19, 22 ( 10.1007/s10544-017-0156-5) [DOI] [PubMed] [Google Scholar]
- 24.Li X, Brooks JC, Hu J, Ford KI, Easley CJ. 2017. 3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling. Lab Chip 17, 341–349. ( 10.1039/C6LC01201A) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Guo Y, Yu Y, Xu C, Xu H, Qin J. 2016. Assessment of metabolism-dependent drug efficacy and toxicity on a multilayer organs-on-a-chip. Integr. Biol. 8, 1022–1029. ( 10.1039/C6IB00162A) [DOI] [PubMed] [Google Scholar]
- 26.Loskill P, et al. 2017. WAT-on-a-chip: a physiologically relevant microfluidic system incorporating white adipose tissue. Lab Chip 17, 1645–1654. ( 10.1039/C6LC01590E) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Pan JZ, Zhao SP, Lou Q, Zhu Y, Fang Q. 2016. Microdroplet chain array for cell migration assays. Lab Chip 16, 4658–4665. ( 10.1039/C6LC00823B) [DOI] [PubMed] [Google Scholar]
- 28.Maoz BM, et al. 2017. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 17, 2294–2302. ( 10.1039/C7LC00412E) [DOI] [PubMed] [Google Scholar]
- 29.Maschmeyer I, et al. 2015. Chip-based human liver-intestine and liver-skin co-cultures—a first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 95, 77–87. ( 10.1016/j.ejpb.2015.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCain ML, Sheehy SP, Grosberg A, Goss JA, Parker KK. 2013. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl Acad. Sci. USA 110, 9770–9775. ( 10.1073/pnas.1304913110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mermoud Y, Felder M, Stucki JD, Stucki AO, Guenat OT. 2018. Microimpedance tomography system to monitor cell activity and membrane movements in a breathing lung-on-chip. Sens. Actuators, B 255, 3647–3653. ( 10.1016/j.snb.2017.09.192) [DOI] [Google Scholar]
- 32.Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee SH. 2015. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer's disease. Lab Chip 15, 141–150. ( 10.1039/C4LC00962B) [DOI] [PubMed] [Google Scholar]
- 33.Pensabene V, Costa L, Terekhov AY, Gnecco JS, Wikswo JP, Hofmeister WH. 2016. Ultrathin polymer membranes with patterned, micrometric pores for organs-on-chips. ACS Appl. Mater. Interfaces 8, 22 629–22 636. ( 10.1021/acsami.6b05754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pensabene V, Crowder SW, Balikov DA, Lee JB, Sung HJ. 2016. Optimization of electrospun fibrous membranes for in vitro modeling of blood-brain barrier. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 125–128. [DOI] [PubMed] [Google Scholar]
- 35.Phan DTT, et al. 2017. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 17, 511–520. ( 10.1039/C6LC01422D) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Punde TH, et al. 2015. A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation. Integr. Biol. 7, 162–169. ( 10.1039/C4IB00239C) [DOI] [PubMed] [Google Scholar]
- 37.Qian F, et al. 2017. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab Chip 17, 1732–1739. ( 10.1039/C7LC00210F) [DOI] [PubMed] [Google Scholar]
- 38.Riahi R, et al. 2016. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep. 6, 24598 ( 10.1038/srep24598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shim KY, Lee D, Han J, Nguyen NT, Park S, Sung JH. 2017. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed. Microdevices 19, 37 ( 10.1007/s10544-017-0179-y) [DOI] [PubMed] [Google Scholar]
- 40.Shin SR, et al. 2016. Aptamer-based microfluidic electrochemical biosensor for monitoring cell-secreted trace cardiac biomarkers. Anal. Chem. 88, 10 019–10 027. ( 10.1021/acs.analchem.6b02028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sticker D, Rothbauer M, Lechner S, Hehenberger MT, Ertl P. 2015. Multi-layered, membrane-integrated microfluidics based on replica molding of a thiol-ene epoxy thermoset for organ-on-a-chip applications. Lab Chip 15, 4542–4554. ( 10.1039/C5LC01028D) [DOI] [PubMed] [Google Scholar]
- 42.Stucki AO, Stucki JD, Hall SR, Felder M, Mermoud Y, Schmid RA, Geiser T, Guenat OT. 2015. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 15, 1302–1310. ( 10.1039/C4LC01252F) [DOI] [PubMed] [Google Scholar]
- 43.van der Helm MW, Odijk M, Frimat JP, van der Meer AD, Eijkel JCT, van den Berg A, Segerink LI. et al. 2016. Direct quantification of transendothelial electrical resistance in organs on-chips. Biosens. Bioelectron. 85, 924–929. ( 10.1016/j.bios.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 44.Wang YI, Abaci HE, Shuler ML. 2017. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 114, 184–194. ( 10.1002/bit.26045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weibel DB, Diluzio WR, Whitesides GM. 2007. Microfabrication meets microbiology. Nat. Rev. Microbiol. 5, 209–218. ( 10.1038/nrmicro1616) [DOI] [PubMed] [Google Scholar]
- 46.Yu F, Deng R, Hao Tong W, Huan L, Chan Way N, IslamBadhan A, Iliescu C, Yu H. 2017. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 7, 14528 ( 10.1038/s41598-017-13848-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang YS, et al. 2017. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl Acad. Sci. USA 114, E2293–EE302. ( 10.1073/pnas.1612906114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu L, et al. 2016. A vertical-flow bioreactor array compacts hepatocytes for enhanced polarity and functions. Lab Chip 16, 3898–3908. ( 10.1039/C6LC00811A) [DOI] [PubMed] [Google Scholar]
- 49.Mulder M. 1996. Basic principles of membrane technology. Dordrecht, The Netherlands: Springer Netherlands. [Google Scholar]
- 50.Marzorati M, et al. 2014. The HMI module: a new tool to study the host-microbiota interaction in the human gastrointestinal tract in vitro. BMC Microbiol. 14, 133 ( 10.1186/1471-2180-14-133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pocock KJ, Gao XF, Wang CX, Priest C, Prestidge CA, Mawatari K, Kitamori T, Thierry B. et al. 2016. Low-temperature bonding process for the fabrication of hybrid glass-membrane organ-on-a-chip devices. J. Micro-Nanolith Mem. 15, 044502 ( 10.1117/1.JMM.15.4.044502) [DOI] [Google Scholar]
- 52.Tahk D, Paik SM, Lim J, Bang S, Oh S, Ryu H, Jeon NL. 2017. Rapid large area fabrication of multiscale through-hole membranes. Lab Chip 17, 1817–1825. ( 10.1039/C7LC00363C) [DOI] [PubMed] [Google Scholar]
- 53.Casillo SM, Peredo AP, Perry SJ, Chung HH, Gaborski TR. 2017. Membrane pore spacing can modulate endothelial cell-substrate and cell-cell interactions. ACS Biomater. Sci. Eng. 3, 243–248. ( 10.1021/acsbiomaterials.7b00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. 2005. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 11, 1–18. ( 10.1089/ten.2005.11.1) [DOI] [PubMed] [Google Scholar]
- 55.Ye K, Wang X, Cao LP, Li SY, Li ZH, Yu L, Ding J. 2015. Matrix stiffness and nanoscale spatial organization of cell-adhesive ligands direct stem cell fate. Nano Lett. 15, 4720–4729. ( 10.1021/acs.nanolett.5b01619) [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Khang G, Lee JW, Lee HB. 1998. Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci. 205, 323–330. ( 10.1006/jcis.1998.5688) [DOI] [PubMed] [Google Scholar]
- 57.Lampin M, Warocquier C, Legris C, Degrange M, Sigot-Luizard MF. 1997. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 36, 99–108. ( 10.1002/(SICI)1097-4636(199707)36:1%3C99::AID-JBM12%3E3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 58.Dowling DP, Miller IS, Ardhaoui M, Gallagher WM. 2011. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 26, 327–347. ( 10.1177/0885328210372148) [DOI] [PubMed] [Google Scholar]
- 59.Kunzler TP, Drobek T, Schuler M, Spencer ND. 2007. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 28, 2175–2182. ( 10.1016/j.biomaterials.2007.01.019) [DOI] [PubMed] [Google Scholar]
- 60.Lange R, Luthen F, Beck U, Rychly J, Baumann A, Nebe B. 2002. Cell-extracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material. Biomol. Eng. 19, 255–261. ( 10.1016/S1389-0344(02)00047-3) [DOI] [PubMed] [Google Scholar]
- 61.Wala J, Maji D, Das S. 2017. Influence of physico-mechanical properties of elastomeric material for different cell growth. Biomed. Mater. 12, 065002 ( 10.1088/1748-605X/aa7e81) [DOI] [PubMed] [Google Scholar]
- 62.Premnath P, Tavangar A, Tan B, Venkatakrishnan K. 2015. Tuning cell adhesion by direct nanostructuring silicon into cell repulsive/adhesive patterns. Exp. Cell Res. 337, 44–52. ( 10.1016/j.yexcr.2015.07.028) [DOI] [PubMed] [Google Scholar]
- 63.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. 2014. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13, 979–987. ( 10.1038/nmat4051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. ( 10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 65.Hulsman M, Hulshof F, Unadkat H, Papenburg BJ, Stamatialis DF, Truckenmuller R, van Blitterswijk C, de Boer J, Reinders MJ. 2015. Analysis of high-throughput screening reveals the effect of surface topographies on cellular morphology. Acta Biomater. 15, 29–38. ( 10.1016/j.actbio.2014.12.019) [DOI] [PubMed] [Google Scholar]
- 66.Reimer A, Vasilevich A, Hulshof F, Viswanathan P, van Blitterswijk CA, de Boer J, Watt FM. et al. 2016. Scalable topographies to support proliferation and Oct4 expression by human induced pluripotent stem cells. Sci. Rep. 6, 18948 ( 10.1038/srep18948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ochsner M, Dusseiller MR, Grandin HM, Luna-Morris S, Textor M, Vogel V, Smith ML. 2007. Micro-well arrays for 3D shape control and high resolution analysis of single cells. Lab Chip 7, 1074–1077. ( 10.1039/b704449f) [DOI] [PubMed] [Google Scholar]
- 68.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. 1997. Geometric control of cell life and death. Science 276, 1425–1428. ( 10.1126/science.276.5317.1425) [DOI] [PubMed] [Google Scholar]
- 69.Lee J, Abdeen AA, Wycislo KL, Fan TM, Kilian KA. 2016. Interfacial geometry dictates cancer cell tumorigenicity. Nat. Mater. 15, 856–862. ( 10.1038/nmat4610) [DOI] [PubMed] [Google Scholar]
- 70.Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. 2005. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl Acad. Sci. USA 102, 11 594–11 599. ( 10.1073/pnas.0502575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kilian KA, Bugarija B, Lahn BT, Mrksich M. 2010. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877. ( 10.1073/pnas.0903269107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papenburg BJ, Schuller-Ravoo S, Bolhuis-Versteeg LAM, Hartsuiker L, Grijpma DW, Feijen J, Wessling M, Stamatialis D. 2009. Designing porosity and topography of poly(1,3-trimethylene carbonate) scaffolds. Acta Biomater. 5, 3281–3294. ( 10.1016/j.actbio.2009.05.017) [DOI] [PubMed] [Google Scholar]
- 73.Papenburg BJ, Vogelaar L, Bolhuis-Versteeg LAM, Lammertink RGH, Stamatialis D, Wessling M. 2007. One-step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials 28, 1998–2009. ( 10.1016/j.biomaterials.2006.12.023) [DOI] [PubMed] [Google Scholar]
- 74.Guillemette MD, et al. 2009. Surface topography induces 3D self-orientation of cells and extracellular matrix resulting in improved tissue function. Integr. Biol. 1, 196–204. ( 10.1039/b820208g) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soscia DA, Sequeira SJ, Schramm RA, Jayarathanam K, Cantara SI, Larsen M, Castracane J. 2013. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials 34, 6773–6784. ( 10.1016/j.biomaterials.2013.05.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esch MB, Post DJ, Shuler ML, Stokol T. 2011. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A 17, 2965–2971. ( 10.1089/ten.tea.2010.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rumpler M, Woesz A, Dunlop JWC, van Dongen JT, Fratzl P. 2008. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 5, 1173–1180. ( 10.1098/rsif.2008.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hebeiss I, Truckenmuller R, Giselbrecht S, Schepers U. 2012. Novel three-dimensional Boyden chamber system for studying transendothelial transport. Lab Chip 12, 829–834. ( 10.1039/c2lc20733h) [DOI] [PubMed] [Google Scholar]
- 79.Yamashita T, Kollmannsberger P, Mawatari K, Kitamori T, Vogel V. 2016. Cell sheet mechanics: how geometrical constraints induce the detachment of cell sheets from concave surfaces. Acta Biomater. 45, 85–97. ( 10.1016/j.actbio.2016.08.044) [DOI] [PubMed] [Google Scholar]
- 80.Broaders KE, Cerchiari AE, Gartner ZJ. 2015. Coupling between apical tension and basal adhesion allow epithelia to collectively sense and respond to substrate topography over long distances. Integr. Biol. 7, 1611–1621. ( 10.1039/C5IB00240K) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He ZK, Ma M, Lan XR, Chen F, Wang K, Deng H, Zhang Q, Fu Q. 2011. Fabrication of a transparent superamphiphobic coating with improved stability. Soft Matter 7, 6435–6443. ( 10.1039/c1sm05574g) [DOI] [Google Scholar]
- 82.Lee JH, Lee SJ, Khang G, Lee HB. 1999. Interaction of fibroblasts on polycarbonate membrane surfaces with different micropore sizes and hydrophilicity. J. Biomater. Sci. Polym. Ed. 10, 283–294. ( 10.1163/156856299X00351) [DOI] [PubMed] [Google Scholar]
- 83.Gotoh K, Yasukawa A, Taniguchi K. 2011. Water contact angles on poly(ethylene terephthalate) film exposed to atmospheric pressure plasma. J. Adhes. Sci. Technol. 25, 307–322. ( 10.1163/016942410X511114) [DOI] [Google Scholar]
- 84.Koo G-H, Jang J. 2008. Surface modification of poly(lactic acid) by UV/ozone irradiation. Fibers Polym. 9, 674–678. ( 10.1007/s12221-008-0106-1) [DOI] [Google Scholar]
- 85.Pego AP, Poot AA, Grijpma DW, Feijen J. 2001. Copolymers of trimethylene carbonate and epsilon-caprolactone for porous nerve guides: synthesis and properties. J. Biomater. Sci. Polym. Ed. 12, 35–53. ( 10.1163/156856201744434) [DOI] [PubMed] [Google Scholar]
- 86.Janvikul W, Uppanan P, Thavornyutikarn B, Kosorn W, Kaewkong P. 2013. Effects of surface topography, hydrophilicity and chemistry of surface-treated PCL scaffolds on chondrocyte infiltration and ECM production Procedia Eng. 59, 158–165. ( 10.1016/j.proeng.2013.05.106) [DOI] [Google Scholar]
- 87.Toepke MW, Beebe DJ. 2006. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 6, 1484–1486. ( 10.1039/b612140c) [DOI] [PubMed] [Google Scholar]
- 88.van Meer BJ, et al. 2017. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 482, 323–328. ( 10.1016/j.bbrc.2016.11.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giselbrecht S, Gietzelt T, Gottwald E, Guber AE, Trautmann C, Truckenmuller R, Weibezahn KF. et al. 2004. Microthermoforming as a novel technique for manufacturing scaffolds in tissue engineering (CellChips). IEE Proc. Nanobiotechnol. 151, 151–157. ( 10.1049/ip-nbt:20040824) [DOI] [PubMed] [Google Scholar]
- 90.Giselbrecht S, et al. 2006. 3D tissue culture substrates produced by microthermoforming of pre-processed polymer films. Biomed. Microdevices 8, 191–199. ( 10.1007/s10544-006-8174-8) [DOI] [PubMed] [Google Scholar]
- 91.Truckenmuller R, et al. 2008. Flexible fluidic microchips based on thermoformed and locally modified thin polymer films. Lab Chip 8, 1570–1579. ( 10.1039/b803619e) [DOI] [PubMed] [Google Scholar]
- 92.Vozzi G, Flaim C, Ahluwalia A, Bhatia S. 2003. Fabrication of PLGA scaffolds using soft lithography and microsyringe deposition. Biomaterials 24, 2533–2540. ( 10.1016/S0142-9612(03)00052-8) [DOI] [PubMed] [Google Scholar]
- 93.Girones M, Akbarsyah IJ, Nijdam W, van Rijn CJM, Jansen HV, Lammertink RGH, Wessling M. 2006. Polymeric microsieves produced by phase separation micromolding. J. Membr. Sci. 283, 411–424. ( 10.1016/j.memsci.2006.07.016) [DOI] [Google Scholar]
- 94.Vogelaar L, Lammertink RGH, Barsema JN, Nijdam W, Bolhuis-Versteeg LAM, van Rijn CJM, Wessling M. 2005. Phase separation micromolding: a new generic approach for microstructuring various materials. Small 1, 645–655. ( 10.1002/smll.200400128) [DOI] [PubMed] [Google Scholar]
- 95.Buitinga M, Truckenmuller R, Engelse MA, Moroni L, Ten Hoopen HWM, van Blitterswijk CA, de Koning EJ, van Apeldoorn AA, Karperien M. 2013. Microwell scaffolds for the extrahepatic transplantation of islets of Langerhans. PLoS ONE 8, e64772 ( 10.1371/journal.pone.0064772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hou QP, Grijpma DW, Feijen J. 2002. Preparation of porous poly(epsilon-caprolactone) structures. Macromol. Rapid Commun. 23, 247–252. ( 10.1002/1521-3927(20020301)23:4%3C247::AID-MARC247%3E3.0.CO;2-5) [DOI] [Google Scholar]
- 97.Wallace GG, Higgins MJ, Moulton SE, Wang C. 2012. Nanobionics: the impact of nanotechnology on implantable medical bionic devices. Nanoscale 4, 4327–4347. ( 10.1039/c2nr30758h) [DOI] [PubMed] [Google Scholar]
- 98.Rnjak-Kovacina J, Weiss AS. 2011. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 17, 365–372. ( 10.1089/ten.teb.2011.0235) [DOI] [PubMed] [Google Scholar]
- 99.Soliman S, Sant S, Nichol JW, Khabiry M, Traversa E, Khademhosseini A. 2011. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J. Biomed. Mater. Res. A 96a, 566–574. ( 10.1002/jbm.a.33010) [DOI] [PubMed] [Google Scholar]
- 100.Cheng Q, Lee BLP, Komvopoulos K, Li S. 2013. Engineering the microstructure of electrospun fibrous scaffolds by microtopography. Biomacromolecules 14, 1349–1360. ( 10.1021/bm302000n) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.