Abstract
CONTEXT AND AIM:
The skeleton is a frequent site for metastasis in patients with breast, lung, and prostate cancer. Bone metastasis compromises skeletal integrity leading to skeletal-related events (SREs). This study aims at estimating the prevalence of bone metastasis in lung cancer and describing types of bone involvement, management, outcomes, and overall survival.
METHODS:
We retrospectively reviewed the charts of 259 patients with nonsmall cell lung cancer who consulted the Department of Medical Oncology at our institution between January 2002 and December 2012. We documented their lung cancer characteristics, presence of skeletal metastases, management types, outcome parameters, and survival status.
RESULTS:
A total of 116 patients (58.6%) were diagnosed with bone metastasis. The most common site of metastasis was the spine. The most common SREs were bone pain (44%) and need for radiotherapy (25.9%). Patients with adenocarcinoma (P = 0.002) and concomitant liver metastasis (P = 0.013) tended to have more incidence of bone metastasis. Survival rates were (36%) at 1 year, and (3%) at 5 years. Metastasis to the bone did not impact patients' survival. Patients tended to have worse survival in the presence of concomitant bone and liver metastases (P = 0.012), older age (P = 0.024), lower limb metastasis (P = 0.014), hypercalcemia (P = 0.001), and not receiving calcium therapy (P = 0.011).
CONCLUSION:
Metastatic bone disease is considered a huge burden on patients, clinicians, and the society. The majority of bone metastasis patients will experience SREs. Most SREs predict poor prognosis. Supportive therapy to overcome the reasons for poor prognosis may improve patients' survival and quality of life.
Keywords: Adenocarcinoma, bone metastasis, nonsmall cell lung cancer, skeletal-related events
Metastatic bone disease is the spread of cancerous cells, through the blood or the lymph, from a tumor in a distant part of the body to the bone.[1] The bone is considered a common target for metastatic cancer cells due to many factors. High blood flow directed toward the bone, and the many growth factors stored in the bone's microenvironment both make it a fertile soil for cancer cells to grow, and therefore, a preferred site for metastases.[1] Bone metastases can be osteolytic (bone destructing) or osteoblastic (bone forming), both of which cause interruption of the normal bone remodeling process, and require different treatment options.[1,2]
Bone metastasis causes compromise of the skeletal integrity, which can lead to numerous and serious skeletal-related events (SRE) such as bone pain, cord compression, hypercalcemia, and pathological fractures.[3] All these events, in addition to many others, will inevitably reduce the functional independence of affected patients, consequently leading to deterioration in their quality of life and shortening of their survival.[3] Treatment required for bone metastasis is usually palliative; it is aimed at reducing skeletal pain and preventing skeletal-related complications.[4] Treatment options include the use of chemotherapy, irradiation of bone lesions, orthopedic surgery, and use of bisphosphonates.[3] Bisphosphonates are a group of medications that work by inducing apoptosis in osteoclasts, consequently inhibiting bone resorption and thereby preserving bone strength, alleviating bone pain, and reducing the risk of fractures.[3,4] The use of bisphosphonate in treating skeletal metastasis has dramatically grown in popularity in the last decade and proven to be effective at reducing skeletal-related symptoms and preventing skeletal complications.[3] There are three known generations of bisphosphonate currently in use; the most potent of which is the third generation, with Zoledronic acid being the most widely used in bone metastasis.[3]
Bone metastasis is a frequent consequence of numerous cancers; it is noted to be most prevalent in breast, prostate, and lung cancer according to many studies.[2,3,4] In 2008, approximately 280,000 US citizens were living with bone metastasis from all cancer types; breast, lung, and prostate cancers marked 68% of the cases.[4] Lung cancer is the most commonly diagnosed cancer worldwide.[5] In the 2009 Saudi Cancer Incidence Report, lung cancer constituted of almost 4% of all cancer cases.[6] The prevalence of lung cancer is significantly growing over the years, which can attribute to an increased prevalence of bone metastasis.[7]
There are no well-characterized local studies on the prevalence of bone metastases from lung cancer, and the burden of bone metastasis and its outcomes in the Saudi population is also not well understood. Therefore, this study aims at estimating the prevalence of bone metastasis from lung cancer in the Saudi population. Secondary aims of our study are to report the types of bone involvement, management options, and disease outcomes in our population and to assess prognostic factors of survival.
Methods
Study population
This study took place at one of the largest tertiary care centers in Saudi Arabia's capital city of Riyadh. From our oncology department's database, we identified all patients with confirmed pathological diagnosis of nonsmall cell lung cancer (NSCLC) during the period between January 2002 and December 2012, treated at our institution and aged 18 and above. The primary source of data was the patients' medical records, radiology reports, and pharmacy archives. Consent was deemed unnecessary by the ethical committee considering that the identities of our patients were not disclosed. Our patients' confidentiality was maintained using serial numbers to replace names and medical record numbers during data collection. The variables considered for analysis included patients' demographics (i.e., age, gender, marital status). In addition, NSCLC characteristics (i.e., date of diagnosis, topography, histological reports, laterality, staging, behavior, and chemotherapy regimens). NSCLC diagnosis was confirmed in our patients by histopathological reporting of lung tissue specimens. Specimens were obtained either by fine needle aspiration, bronchoscopy, computed tomography (CT)-guided core biopsy, or surgical biopsy. Subtyping of NSCLC cases was done according to the World Health Organization classification of NSCLC, which relays on thorough histological assessment of the resected specimen. Thus, a small percentage of NSCLC cases were labeled as unclassified NSCLC due to a small-sized specimen or because the primary diagnosis was done without subtyping in other institutions, and no further testing was done at our institution. Furthermore, all NSCLC patients with positive bony metastasis, confirmed by bone scan in majority of patients, positron emission tomography CT (PET CT) in some patients, or bone biopsy in a few cases, were reviewed for total numbers and sites of metastasis, SREs, and the type of therapy those patients received (i.e., chemotherapy, palliative irradiation, surgical fixation, bisphosphonate, calcium, and Vitamin D). The outcomes of bone metastasis in our patients (i.e., remission, progression to SRE, death) were also captured. This study gained ethical approval by the institutional review board of our institution. Funding was not required for the execution of this study.
Statistical analysis
All variables were summarized using descriptive analysis by reporting proportions for all categorical variables. In addition, inferential analysis was performed by applying univariate analysis to test the association between bone metastasis and demographic and clinical characteristics. The Chi-square test was used for comparing proportions of nominal categorical variables and the t-test for continuous variables. Multivariate logistic regression was used to investigate predictors of bone metastasis in NSCLC patients. Cox regression was used to predict prognostic factors of bone metastasis patients. Kaplan–Meier method was used to estimate overall survival (OS) and 95% confidence interval (CI) for median time of OS. Data were entered and analyzed using SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) version 21. A P < 0.05 was considered statistically significant.
Results
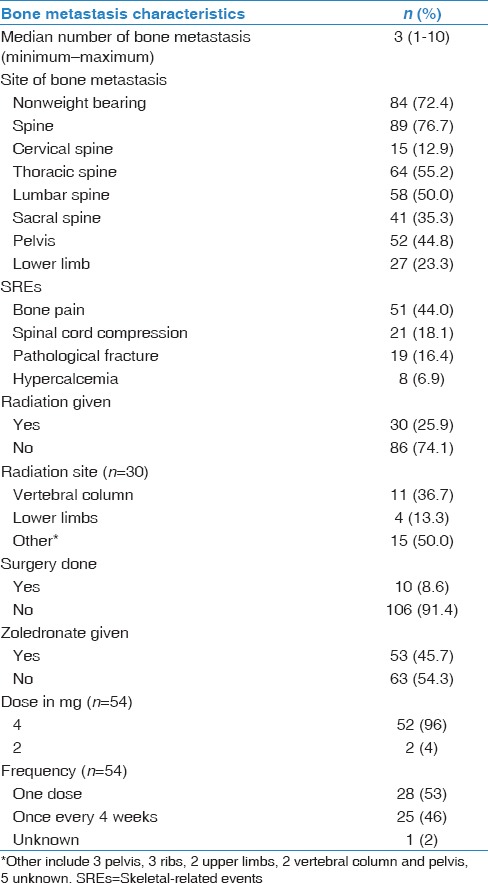
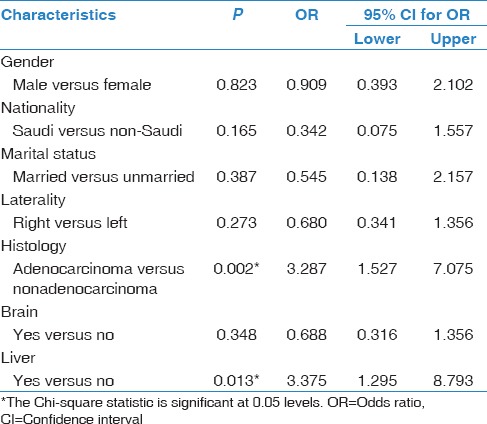
Two hundred fifty-nine unique NSCLC patients were included in this study. Median age of our patients was 65 years at diagnosis, with a range of 34–98 years, and 186 (71.8%) were men. The histologic subtypes of NSCLC were adenocarcinoma [Figure 1], squamous cell carcinoma, nonspecified NSCLC, and large cell carcinoma in 175 (67.6%), 64 (24.7%), 12 (14.6%), and 8 (3.1%) cases, respectively. Demographics and disease characteristics of our patients are shown in Table 1. Out of 259 NSCLC patients, 198 (76.4%) were diagnosed with Stage IV distant metastasis. Irrespective of bone, the major sites of metastasis were brain (25.5%), liver (18.9%), and adrenal gland (15.4%). As for bone, 116 patients (58.6%) developed bone metastasis throughout their clinical course. Median number of metastatic bone lesions seen on either bone scans or PET/CT scans was 3 (1–10). On revealing bone lesion sites, most of the lesions tended to appear on the weight-bearing skeleton such as spine (76.6%), pelvis (44.8%), and lower limb (23.3%). Spine metastasis tended to appear more in thoracic vertebrae (55.2%), followed by lumbar vertebrae (50%). Out of 116 NSCLC patients with bone metastasis, 69 (59.5%) developed 1 or more SRE during their clinical course. The rates of SREs in our patients were as follows: bone pain (44%), need for radiotherapy (25.9%), spinal cord compression (18.1%), pathological fracture (16.4%), and malignant hypercalcemia (6.9%). More characteristics of bone metastasis are shown in Table 2. In terms of bone metastasis treatment, 54 patients (46.6%) received bisphosphonate in the form of Zoledronic acid (Zoledronate). Almost half of our patients received zoledronate as a single IV dose (53%), while the other half received Zoledronate once every 4 weeks (46%). Thirty patients (25.9%) received palliative external beam radiation to one or multiple sites in the skeleton. The most commonly irradiated site was the vertebral column (36.7%). Ten patients (8.6%) had either decompressive or fixative surgery done to the skeleton following a pathologic fracture or cord compression.
Figure 1.
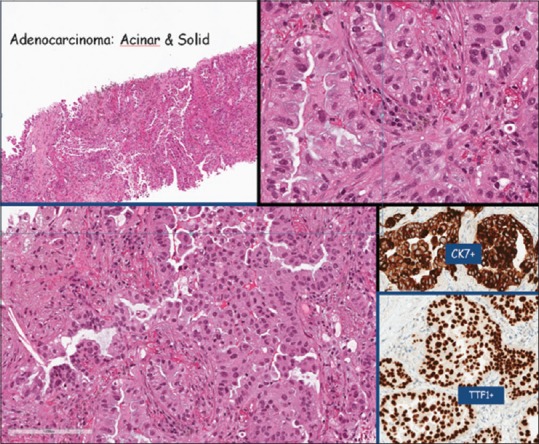
Adenocarcinoma of Lung (thyroid transcription factor 1 positive) with predominant micro-papillary pattern (75%; lepidic 10%, solid 5%, papillary 5%, and acinar 5%)
Table 1.
Demographic and disease characteristics (n=259)
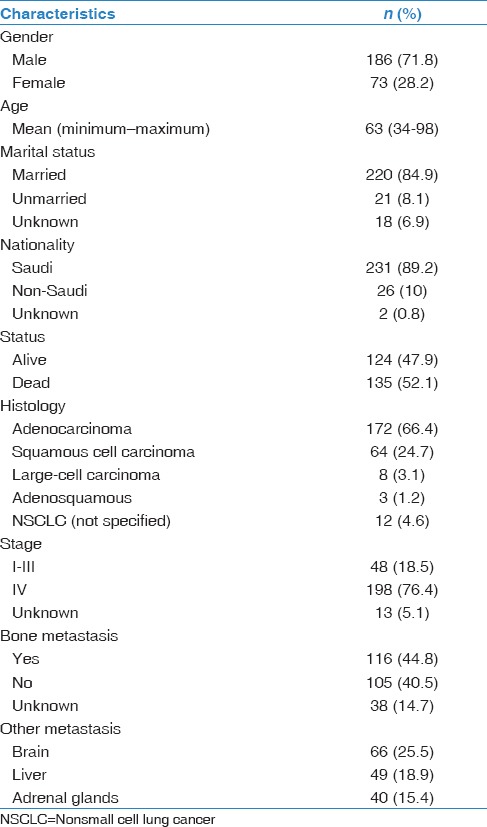
Table 2.
Characteristics of bone involvement (n=116)
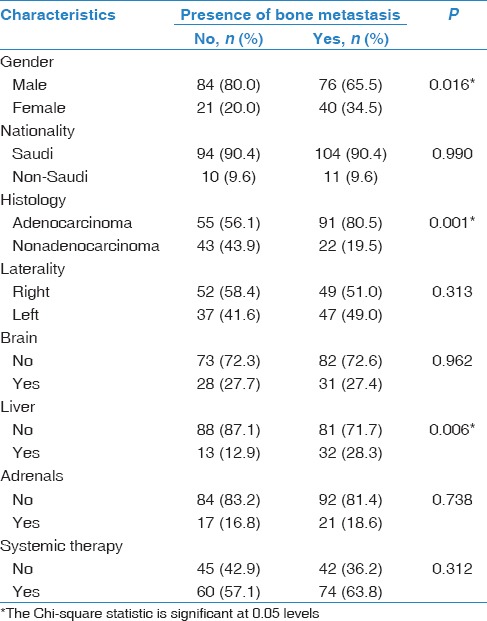
Univariate analysis showed a significant correlation between some of our NSCLC patients' demographic, histological, and clinical characteristics and the presence of bone metastasis. Being male (P = 0.016), presenting with the histologic subtype adenocarcinoma (P = 0.001) and concurrent diagnosis of liver metastasis (P = 0.006) were all highly correlated with the presence of bone metastasis in our patients [Table 3].
Table 3.
Univariate analysis of demographic and disease characteristics and presence of bone metastasis (n=259)
In multivariate analysis, NSCLC patients presenting with the histologic subtype adenocarcinoma were 3 times more likely to have bone metastasis than patients with nonadenocarcinoma subtype (OR: 3.3 95% CI: 1.5–7; P = 0.002). Patients who presented with liver metastasis were also 3 times more often presenting with bone metastasis than patients with no liver metastasis (OR: 3.4 95% CI: 1.3–8.8; P = 0.013) [Table 4].
Table 4.
Multivariate analysis of demographic and disease characteristics and presence of bone metastasis (n=259)
Analysis of overall survival and predictive factors affecting survival rates
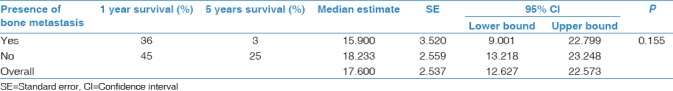
Survival analysis was conducted to estimate and compare median survival times in NSCLC patients with bone metastasis versus NSCLC patients with no bone metastasis. The cumulative survival rates in NSCLC patients with bone metastasis were (36%) at 1 year, and (3%) at 5 years. NSCLC patients with no bone metastasis survival rates were (45%) at 1 year, and (25%) at 5 years. The overall median survival obtained by the method of Kaplan–Meyer was 17.6 months (95% CI: 12.6–22.6). Median survival in bone metastasis patients is 15.9 months (95% CI: 9–22.8) while median survival in patients with no bone metastasis is 18.2 (95% CI: 13.2–23.2). However, this survival analysis did not reach statistical significance (P = 0.155) [Table 5 and Figure 1].
Table 5.
Kaplan–Meier test estimating median survival times in nonsmall cell lung cancer patients with bone metastasis versus no bone metastasis (n=259)
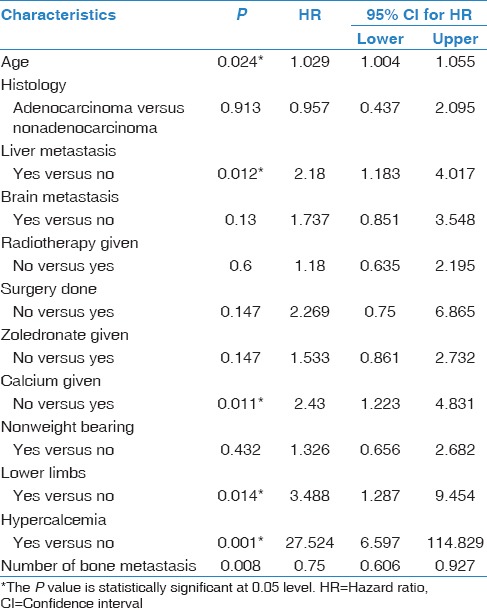
Many predictive factors were explored for their effect on survival in our NSCLC patients [Table 6]; only four factors were associated with significant nonfavorable change on survival. First, increase in age by 1 year decreases patients' survival by 2.9%. Patients with concomitant bone and liver metastases have significantly decreased survival (hazard ratio [HR] =1.9 95% CI: 1–3.5; P = 0.041). In addition, patients with bone metastasis to the lower limbs have significantly decreased survival (HR = 3.488 95% CI: 1.3–9.5; P = 0.014). Patients who were diagnosed with malignant hypercalcemia have dramatic decrease in survival (HR = 27.5 95% CI: 6.6–114.8; P = 0.001).
Table 6.
Prognostic factors affecting survival of patients with bone metastasis
Discussion
Bone metastasis occurs in many tumor types. According to many studies, it occurs most commonly in lung, breast, and prostate cancer.[2,3,4] This study estimated the rate of bone metastasis from lung cancer in Saudi adults over a 10-year period and found it to be (58.6%). This result can be compared with a local study conducted at a large university hospital that focused on discussing the different parameters of lung cancer in Saudi Arabia over a 4-year period.[7] The authors of the aforementioned study found the rate of bone metastasis in lung cancer patients to be (49.1%).[7] A larger scale study that estimated the number of prevalent cases of metastatic bone disease in the US adult population found the rate of bone metastasis in lung cancer to be (15.6%).[8]
The incidence of adenocarcinoma is rising in recent years according to many epidemiological reports.[9,10] In our study, we found that adenocarcinoma was the most common histological subtype in lung cancer patients at a rate of (66.4%). Approximately similar high rates have been reported in recent studies.[11,12] We also found in univariate and multivariate analyses that patients with adenocarcinoma were more likely to have bone metastasis. This shows that metastatic spread to the bone can be influenced by histologic subtype of the tumor, and adenocarcinomas have a higher tendency to disseminate to the bone.[13] According to “The Textbook of Metastasis,” (45%) of patients with adenocarcinomas, in general, metastasize to the bone.[13]
We found that bone lesions appeared more on the weight-bearing skeleton, with the most common site of metastasis being the spine (76.7%). A Japanese retrospective cohort of 259 NSCLC patients has demonstrated a closely similar result in which spinal metastasis was the most prevalent among their patients at a rate of (50%).[14] A multicentric Italian study of NSCLC patients also showed the spine to be the site with most metastatic bony lesions in their patients at a rate of (74.9%).[15]
Univariate analysis showed that male gender was associated with more incidence of bone metastasis. This result has not been reported in the literature. In fact, a study conducted in Japan on the incidence of bone metastasis in lung cancer found the association between bone metastasis and gender to be nonsignificant (P = 0.95).[16]
We found in univariate and multivariate analysis that concomitant bone and visceral metastasis to the liver was associated with higher incidence of bone metastasis. To the best of our knowledge, a similar result was not described in the literature.
The median survival in patients without bone metastasis was 18.2 months, while in patients with bone metastasis median survival was 15.9 months. Our study failed to reach significance regarding patient survival. Prognostic factors that significantly demonstrated lower survival in our patients were: age, visceral metastasis to liver, bone metastasis to the lower limb, hypercalcemia, and not receiving calcium therapy. Visceral metastasis was also associated with worse survival in NSCLC patients in an Italian multi-centric study (univariate P = 0.001; multivariate P = 0.002).[15] Another study demonstrated that the absence of visceral metastasis was a significant prognostic factor for longer survival (HR = 1.8; P = 0.025).[11] In terms of lower limb metastasis, no other study reported a worse outcome with lower limb involvement.
Hypercalcemia is a well-known nonfavorable prognostic factor in bone metastasis; it was confirmed in this study that the presence of hypercalcemia was associated with significantly poor prognosis (HR = 20.7; P = 0.001). Similarly, a French retrospective study described the presence of hypercalcemia in NSCLC patients to significantly lower the OS (HR = 2.38; P = 0.008).[11]
Conclusion
In conclusion, our study revealed the huge burden of bone metastasis in NSCLC patients, with adenocarcinoma and visceral metastasis being predictors of bone involvement. The worse outcome is correlated with age, liver metastasis, hypercalcemia, lower limb involvement, and lack of calcium therapy. These factors should be taken into account when managing patients to optimize patient care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roodman GD. Mechanism of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Dennis K, Vassiliou V, Balboni T, Chow E. Management of bone metastases: Recent advances and current status. J Radiat Oncol. 2012;1:201–10. [Google Scholar]
- 4.Li S, Peng Y, Weinhandl ED, Blaes AH, Cetin K, Chia VM, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87–93. doi: 10.2147/CLEP.S28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Al-Eid HS, García AD. Saudi Cancer Registry: Cancer Incidence Report 2009. Saudi Arabia: Kingdom of Saudi Arabia, Ministry of Health; 2012. pp. 11–2. [Google Scholar]
- 7.Alamoudi OS. Lung cancer at a university hospital in Saudi Arabia: A four-year prospective study of clinical, pathological, radiological, bronchoscopic, and biochemical parameters. Ann Thorac Med. 2010;5:30–6. doi: 10.4103/1817-1737.58957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–42. doi: 10.1002/cncr.22678. [DOI] [PubMed] [Google Scholar]
- 9.Charloux A, Quoix E, Wolkove N, Small D, Pauli G, Kreisman H, et al. The increasing incidence of lung adenocarcinoma: Reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol. 1997;26:14–23. doi: 10.1093/ije/26.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Charloux A, Rossignol M, Purohit A, Small D, Wolkove N, Pauli G, et al. International differences in epidemiology of lung adenocarcinoma. Lung Cancer. 1997;16:133–43. doi: 10.1016/s0169-5002(96)00623-x. [DOI] [PubMed] [Google Scholar]
- 11.Deberne M, Ropert S, Billemont B, Daniel C, Chapron J, Goldwasser F, et al. Inaugural bone metastases in non-small cell lung cancer: A specific prognostic entity? BMC Cancer. 2014;14:416. doi: 10.1186/1471-2407-14-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He YF, Luo HQ, Wang W, Chen J, Yao YW, Cai SB, et al. Clinical features and prognosis-associated factors of non-small cell lung cancer exhibiting symptoms of bone metastasis at the time of diagnosis. Oncol Lett. 2015;9:2706–12. doi: 10.3892/ol.2015.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasmin C, Coleman RE, Coia LR, Capanna R, Saillant G. Lung cancer. In: Roos DE, editor. Textbook of Bone Metastases. New York: John Wiley and Sons; 2005. [Google Scholar]
- 14.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: A retrospective study. Lung Cancer. 2007;57:229–32. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Santini D, Barni S, Intagliata S, Falcone A, Ferraù F, Galetta D, et al. Natural history of non-small-cell lung cancer with bone metastases. Sci Rep. 2015;5:18670. doi: 10.1038/srep18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katakami N, Kunikane H, Takeda K, Takayama K, Sawa T, Saito H, et al. Prospective study on the incidence of bone metastasis (BM) and skeletal-related events (SREs) in patients (pts) with stage IIIB and IV lung cancer-CSP-HOR 13. J Thorac Oncol. 2014;9:231–8. doi: 10.1097/JTO.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]


