Abstract
BACKGROUND:
It is unclear whether inhaled corticosteroids (ICS) have chemopreventive effect on lung cancer (LC) development in humans. We investigated the association between the ICS use in asthma patients and the risk of LC.
METHODS:
We conducted a nationwide, population-based retrospective cohort study using the National Health Insurance database. We identified 4210 asthmatics who were initially free of LC and regularly used ICS between 2001 and 2005 and 37,228 asthmatics without regular ICS use. Patients with documented history of tobacco use were excluded from the analyses. Asthmatics were categorized into a mild and a severe asthma group. Each patient was tracked until the end of 2010 to identify incident cases of LC. Cox proportional hazards models were used to evaluate the effect of ICS on the risk of LC, further stratifying by asthma severity and comorbidities.
RESULTS:
During follow-up, we identified 747 incident cases of LC diagnosed in the asthma cohort. Compared with severe asthmatics without regular ICS use, the risk of LC for those with mild asthma with regular ICS use was lower (adjusted hazard ratio = 0.42, 95% confidence interval = 0.31–0.56, P < 0.0001). The risk of LC was calculated among the following rankings of risk severe asthma without regular ICS use, low severity without regular ICS, high severity with regular ICS, and low severity with regular ICS group showed a decreasing trend of LC incidence (P = 0.041). Analyses stratified by comorbidities revealed that the protective effect of ICS was assessed with better precision and more pronounced in those with renal diseases, stroke, and hyperlipidemia.
CONCLUSIONS:
For patients with asthma, regular ICS use might have a protective effect against LC. Further studies are required to assess this potential association from both immunohistopathological and clinical aspects.
Keywords: Asthma, chemopreventive effect, inhaled corticosteroids, lung cancer
Asthma is a substantial burden for individuals and families and often is a lifetime illness. Being a chronic inflammatory disorder of the respiratory system with long-lasting chronic inflammation, it may increase the risk of lung cancer (LC) or promote the genesis of cancer.[1] Previous reports have suggested that asthma is associated with an increased risk of LC in population-based cohort studies.[2,3,4,5] In addition, atopic constitution, including different manifestations of allergy and asthma, is a possible risk factor for LC, particularly in never-smokers.[6] Recent human studies have also demonstrated that asthmatics are at high risk of developing LC through chronic inflammation (the antigenic stimulation theory).[1,7,8] Given the high prevalence of asthma and incidence of LC, this association has important public health implications.
Worldwide, LC accounts for 17% of all new cancer cases and 23% of total cancer deaths, and it is the leading cause of cancer death.[9] Despite improvements in traditional therapies such as surgery, radiation, and chemotherapy, the current 5-year survival rate of LC is only 15%.[10] It has been considered that this is related to the observation that the majority of LC patients present with late-stage disease.[11] Therefore, additional strategies and prevention programs to reduce the burden of LC are required, and a promising approach is chemoprevention.
Chemoprevention is an intervention using natural or synthetic compounds before the onset of cancer or in the early precancerous stages of carcinogenesis. It aims to prevent, arrest, or reverse either the initiation phase of carcinogenesis or the progression of neoplastic cells to cancer. Since chemoprevention targets healthy people at high risk of developing LC, efficacy and safety are two key criteria for the selection of chemopreventive agents.[12]
Regarding inhaled corticosteroids (ICS), compared to other means of administration, aerosol delivery has distinct advantages as it provides a direct route of delivery to the lungs and it has been used successfully to treat asthma for decades. Although ICS are associated with adverse effects such as oral candidiasis, dysphonia, osteoporosis, reduction in growth velocity, and cataracts, ICS have been proven to be safe even with long-term use.[13,14] Given that chronic inflammation has been implicated in the pathogenesis of LC, inhaled budesonide (a corticosteroid) was reported to be effective for a chemopreventive effect on lung carcinogenesis in mice.[14] ICS have been reported to inhibit all stages of progression from hyperplasia formation of the lung to LC in mice without systemic toxicity and in transgenic mice treated with benzo[a] pyrene.[15,16] However, whether ICS have a chemopreventive effect on LC in humans remains unclear. In addition, approaches to identify individuals who benefit the most from ICS interventions are still lacking. Therefore, aims of this study were first to examine the association between ICS use in asthmatics and the risk of LC in a nationwide dataset and second to test whether clinical subpopulations with other comorbidities are less or more likely to benefit from ICS chemoprevention.
Methods
Data source
Data were obtained from the National Health Insurance Research Database (NHIRD) released by the National Health Research Institutes in Taiwan. Taiwan began its National Health Insurance program in 1995 to finance health care for all residents. The coverage rate was >99% of the total population of about 23 million residents in 2012. The database includes comprehensive information on insured individuals, such as demographic data, dates of clinical visits, diagnostic codes, details of prescriptions, and expenditure amounts. The current study was approved by the Institutional Review Board of the Hospital (THIRB-10-13).
Study design
We conducted a population-based retrospective cohort study using the National health insurance research database (NHIRD). People aged ≥40 years who were first diagnosed with asthma (International Classification of Disease 9th edition, Clinical Modification [ICD-9-CM] code 493) between 2001 and 2005 and initially free of LC were enrolled in this study. We excluded patients <40 years of age since the risk of LC was minimal in this group and the time window of follow-up (maximal 2001–2010) would not allow sufficient follow-up time. We excluded patients older than 70 years of age since the new occurrence of asthma is very low in this group. At least one of the following criteria had to be met for inclusion: (1) one or more inpatient admissions with a diagnosis of asthma, or (2) three or more outpatient visits within a 3-month period, each with a diagnosis of asthma. This index date represents the date when asthma was first diagnosed. Patients with a history of tobacco use (ICD-9-CM codes V15.82) and current tobacco use (ICD-9-CM codes 305.1) were also excluded to reduce the confounding effect of smoking on LC risk.[17] We followed all patients until the first diagnosis of LC, withdrawal from the National Health Insurance program, or until the end of 2010. Each patient was observed for a minimum of 2 years and a maximum of 9 years. Patients who developed LC within the first 2 years of the index day were excluded. Patients with a prescription of ICS prior to the first diagnosis of asthma were also excluded from this study.
Exposure to inhaled corticosteroids
The duration of steroid use and the formulations (inhaler, tablet, or injection) were recorded for each patient over the entire follow-up period. Exposure to ICS was determined by prescription of inhaled beclomethasone, fluticasone, budesonide, triamcinolone, and flunisolide.
The asthma cohort consisted of two subcohorts: those who regularly received prescription of steroid inhalers (regular inhaler cohort) after diagnosis of asthma and the other without regular prescription (irregular inhaler cohort). The patients who regularly received prescriptions of ICS (>28 days/month) and continuously for >4 months during the follow-up period after the index day were defined as regular inhaler users. A 4-month cut point was used because the step-down treatment after asthma well control was 3 months in the GINA guideline.
Asthma
Allergic and nonallergic asthma were defined as ICD-9-CM codes 493.00, 493.01, and 493.02 as extrinsic asthma and ICD-9-CM codes 493.10, 493.11, and 493.12 as intrinsic asthma. We divided the patients into four groups: severe asthma without regular ICS use, low severity without regular ICS use, high severity with regular ICS use, and low severity with regular ICS use group, with the low severity with regular ICS use group as the reference group. Severity of asthma was based on drug prescription according to GINA guideline in the follow-up period.[18] The disease status in patients with only short-acting β2-agonist (SABA), ICS plus as-needed SABA, or leukotriene receptor antagonist was defined as having mild asthma. Alternatively, patients receiving ICS treatment plus long-acting β2-agonist as-needed, or ICS plus theophylline or tiotropium, or omalizumab, or steroid tablets or injections, were defined as having severe asthma. The following comorbidities were considered if they occurred prior to the index date and included, listed by ICD-9 code, atrial fibrillation (427.31), diabetes mellitus (250), renal disease (580–589), stroke (430–438), coronary artery disease (410–414), hyperlipidemia (272–277), gout (274.9), and peripheral neuropathy (354 and 355).
Lung cancer
Records from the Registry of Catastrophic Illness Patient Database were used to identify LC. Application for a cancer certificate requires that the applicant provides medical records including cytologic or pathologic reports or evidence such as additional laboratory and imaging studies supporting the diagnosis of cancer, which should include tumor markers, X-rays, bone scans, and computed tomography (CT) or magnetic resonance imaging scans. A minimum of two oncologists examine the patients' information. Certificates are issued to patients who met the criteria of the diagnosis. As patients with in situ malignancies do not qualify for a catastrophic illness certificate, they are not included in the registry. LC was defined as ICD-9-CM code 162.x.
Statistical analysis
Baseline characteristics and comorbidities for the two cohorts were analyzed first, and steroid prescription (inhaler, days/year) was calculated using data from the follow-up period. We assessed the effect of inhaler use on the risk of LC using Cox proportional hazards models, adjusting for age, gender, allergic status, and comorbidities. If the proportional hazard assumption was fulfilled, we estimated the hazard ratio (HR) and their 95% confidence interval (CI) using the proportional hazards model, adjusting for confounders (adjusted HR [aHR]). Then, we stratified by comorbidities and compared HR in the various strata to calculate the risk of LC according to strata. All analyses were performed using the SAS software (version 9.3; SAS Institute, Cary, NC, USA).
Results
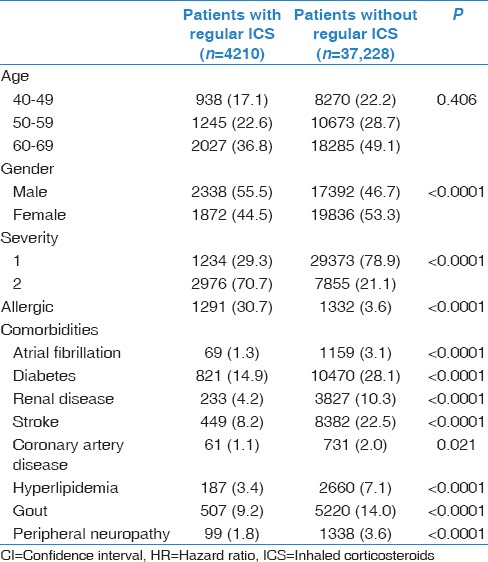
We identified 4210 asthma patients with regular ICS use (regular inhaler cohort) and 37,228 asthma patients without regular ICS use (irregular inhaler cohort) after the first diagnosis of asthma. Figure 1 shows the patient selection flow chart. Each patient was individually tracked from the index date until the end of 2010 to identify incident cases of LC. There were 747 incident cases of LC in the asthma cohort during the follow-up period. Baseline characteristics of the asthma patients with and without regular use an inhaler are shown in Table 1. There was no significant age difference between those with and without regular use an inhaler.
Figure 1.

Flow chart of data collection
Table 1.
Baseline characteristics of the asthmatic patients with and without the regular use of an inhaler
Compared with severe asthmatics without regular ICS inhaler use, the risk of LC for those with mild asthma with regular ICS use was statistically significantly lower (aHR = 0.42, 95% CI: 0.31–0.56, P < 0.0001) [Table 2]. The risk of the development of LC showed a decreasing trend in the following groups: “high severity without regular ICS use,” “low severity without regular ICS use,” “high severity with regular ICS use,” and “low severity with regular ICS use” (P for trend = 0.0414). The overall survival was lower in those with high severity without regular ICS inhaler use than those with low severity with regular ICS inhaler use [Figure 2]. Men had a significant higher HR of 2.36 (95% CI: 1.87–2.98) than women.
Table 2.
The risk of developing lung cancer between those with and without the regular use of an inhaler and with high and low severity of asthma
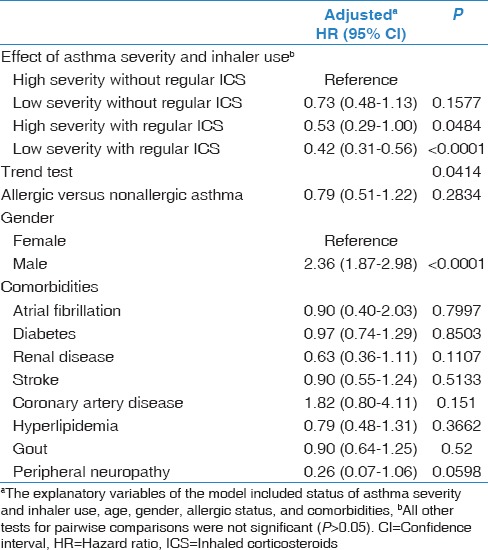
Figure 2.
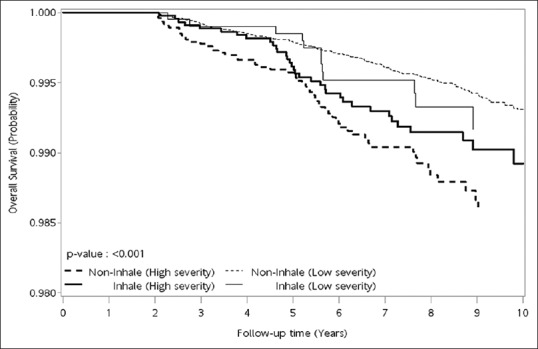
The overall survival was lower in those with high severity without regular inhaled corticosteroid inhaler use than those with low severity with regular inhaled corticosteroid inhaler use
We further stratified the analysis by comorbidities to assess the effect of ICS inhaler use on LC.
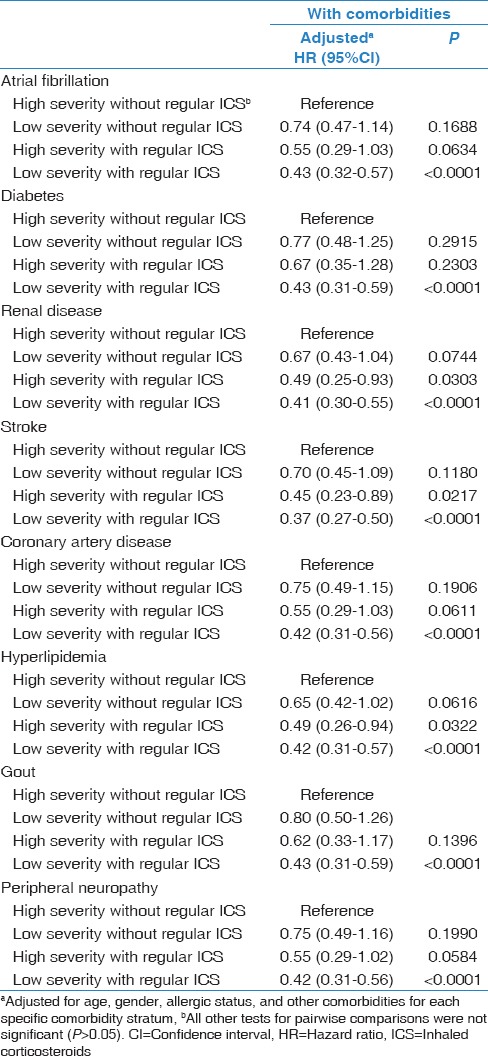
When considering different strata of the medications status with comorbidities, the protective effect of ICS inhaler use did not change much in the different strata [aHR in Table 2: 0.42 and between 0.37 and 0.43 in Table 3]. However, the variance was smaller in some strata, especially in those with renal disease, stroke, and hyperlipidemia [Table 3], resulting in statistically significant HR. Hence, for asthmatic patients with renal disease, stroke, and hyperlipidemia, the risk of LC in the high severity with regular ICS inhaler use compared with the high severity without regular ICS became statistically significantly (P = 0.0303, 0.0217, and 0.0322, respectively). The risk of the development of LC still showed a decreasing trend in the following groups: “high severity without regular ICS use,” “low severity without regular ICS use,” “high severity with regular ICS use,” and “low severity with regular ICS use.”
Table 3.
The risk of developing lung cancer between those with and without the regular use of an inhaler and with high and low severity of asthma or those with comorbidities
Discussion
Using a national dataset, we investigated the effect of prescription of ICS inhaler in asthmatic patients on the risk of LC. Our results show that ICS may have a protective effect against LC in asthma patients. After stratification by comorbidities, the protective effect showed less variation (more precision) among patients with comorbidities, such as renal disease, stroke, and hyperlipidemia. The study results suggest a chemopreventive effect of regular ICS use on LC in asthmatic patients.
Consistent with our results, Lee et al. reported that ICS use was associated with a reduced risk of LC by analyzing the Health Insurance Review and Assessment Service database in Korea.[19] The protective effect may extend to other obstructive airway diseases. An epidemiologic study exploring the association between the use of ICS and the development of LC in a cohort of 10,474 US veterans with chronic obstructive pulmonary disease (COPD) also suggested a reduction in the risk of LC with the use of ICS.[20] In the EUROSCOP study, 4% of the study participants who received 800 mcg of inhaled budesonide daily for 3 years developed LC, compared with 6% of patients who were given a placebo.[21] Budesonide was also reported to have inhibitory effects on B[a] P-induced carcinogenesis of the lung in A/J mice at all stages of progression from hyperplasia formation to carcinoma.[22] However, a clinical trial of 6 months of inhaled budesonide treatment in high-risk smokers showed no effect on bronchial dysplasia, although a significantly greater number of CT-detected peripheral lung nodules decreased in size after treatment.[23] Similarly, a clinical trial of fluticasone for 6 months in patients with squamous dysplasia showed that more patients showed a decrease and fewer an increase in the number of nodules detected on chest CT in the treatment arm, although this failed to reach statistical significance.[24] It is reported that budesonide was quite effective in protecting against pulmonary carcinogenicity caused by cigarette smoke.[25] Against that, treatment with inhaled budesonide for 1 year in smokers did not significantly affect the size of CT-detected peripheral lung nodules in a randomized placebo-controlled trial.[26] Regarding squamous cell carcinomas, in hamsters, inhaled budesonide was also found to be ineffective in preventing this type of LC, which was in contrast to previous studies using adenocarcinoma models.[27] The reason for these variations may be due to different histologic abnormalities (bronchial dysplasia or squamous metaplasia) or the different time frames of the clinical trials. In contrast to animal studies in which the intervention occurs early after carcinogen exposure, human interventions are delivered relatively late, and sometimes after the presence of preneoplastic lesions. It is possible that earlier interventions may be more efficacious; however, a future task is to identify high-risk populations that would benefit most. To improve the efficiency of clinical trials, genetic and epigenetic markers may be used to identify subgroups that are at a higher risk. In addition, the potentially chemopreventive effects of ICS on different histological cell types of LC and its stage need further investigations.
On the one hand, it has been reported that allergic respiratory inflammation promotes the recruitment of circulating tumor cells in mice, thus promoting lung metastasis.[28] On the other hand, it has been hypothesized that a higher dose of ICS reduces local airway inflammation, cell turnover, and propagation of genetic errors, which may lead to a subsequent reduction in the risk of LC.[23] ICS have also been demonstrated to suppress proto-oncogene expression in human smokers.[23] In models of chemically induced lung tumorigenesis, budesonide has been shown to markedly decrease tumor formation, potentially by modifying the gene expression involved with cell cycle, signal transduction, and apoptosis.[15,22] In addition, budesonide treatment may be linked to a decrease in the size of lung tumors and reverse DNA demethylation and gene expression in lung tumors induced by vinyl carbamate in A/J mice.[29] It has also been hypothesized that budesonide may affect mouse lung tumorigenesis through modulating the expressions of genes in the Bcl-2- and caspase-2-regulated apoptosis pathways and the Mad2/3-regulated mitotic checkpoint.[30] However, the possible mechanism by which ICS diminishes the risk of LC is not clear.
We found that patients who regularly received ICS prescription and had low asthma severity had a lower risk of LC than those who did not use ICS and had high asthma severity. Thus, regular prescription of ICS may decrease inflammation and reduce the risk of LC.[20] However, Ji et al. reported that patients with asthma with multiple hospital admissions (high severity) had a high risk for stomach and colon cancers.[3] Nevertheless, the study by Ji et al. did not investigate other cancer localizations. As not every patients respond to ICS, a more efficient approach when prescribing ICS is to focus on patients who respond well to treatment. Therefore, we stratified all patients by gender and comorbidity. Although estrogens may play a role in the development of LC,[31] we could not identify a gender difference in the effect of ICS (data not shown). Interestingly, we found that the protective effect of ICS was significant in those with stroke, renal disease, and hyperlipidemia. In patients with these diseases, the effect of ICS was not stronger but more consistent (less variance). We currently have no explanation why these diseases but not others provide a better precision of the protective effect. It could be the use of other medications (e.g., hyperlipidemia) or it could be that the disease process provides some additional protection. Moreover, the effectiveness of ICS may be influenced by several factors such as oral bioavailability, different pharmacokinetics, and on-site activation in the lung.[32] ICS have variable potent binding activities to specific glucocorticoid receptors by either binding to DNA and inducing anti-inflammatory genes, or by repressing the induction of pro-inflammatory mediators.[13] Further studies are needed to improve the identification of individuals who will respond better to application of ICS medication strategies and their mechanisms.
There are several limitations to this study. First, it is possible that patients with COPD may have been misclassified as having asthma. We addressed this concern by performing a series of analyses that restricted the cohort definition to a greater specificity for COPD and found that the cohort definition had a small effect on the point estimates (data not shown). Moreover, because ICS are less effective in patients with COPD and because patients with asthma may not have had a similar degree of tobacco exposure, this would bias the findings in the opposite direction of the described effect.[13] However, it is also possible that we did not exclude all patients with a smoking history in the clinical diagnosis.
Second, the follow-up period may be too short. LC occurs relatively rarely, and when used as the endpoint of a trial, it requires a large cohort and a long follow-up duration to ensure sufficient statistical power. We analyzed the overall incidence of cancer and used a large dataset including 23 million people. Each patient was observed for a minimum of 2 years and a maximum of 9 years. Hence, an extension of the follow-up in this cohort would be informative. Finally, we could not adjust for occupational history as a risk factor for LC because there is no information about occupational history in national health insurance data.
This study is strengthened by the large sample size. First, because of the large sample size, we were able to perform some subgroup investigations. The cohort is also based on a highly representative nationwide database, which reduces the likelihood of selection bias and may enhance the generalizability. In addition, this is the first study to report an association between the use of ICS and the risk of LC among patients with asthma and to identify subpopulations which would particularly benefit from ICS in preventing LC. The prospective characteristic of this cohort study design likely excluded the issue of reverse causation. Second, the definition of asthma was verified by physician-verified diagnoses according to insurance registry data from the Bureau of National Health Insurance, which is more reliable than self-reported questionnaires of asthma, which have been found to have satisfactory specificity (94%) but low sensitivity (68%).[33]
Conclusions
The regular prescription of ICS inhalers might have a protective effect against LC in patients with asthma, especially those with comorbidities, including renal diseases, stroke, and hyperlipidemia. In addition, this study suggests additional information for education of patients with asthma about the benefit of regular ICS use. Future studies may consider evaluating the chemopreventive effects of ICS on different histological cell types of LC and need to determine population who are at higher risk of LC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Engels EA. Inflammation in the development of lung cancer: Epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 2.Boffetta P, Ye W, Boman G, Nyrén O. Lung cancer risk in a population-based cohort of patients hospitalized for asthma in Sweden. Eur Respir J. 2002;19:127–33. doi: 10.1183/09031936.02.00245802. [DOI] [PubMed] [Google Scholar]
- 3.Ji J, Shu X, Li X, Sundquist K, Sundquist J, Hemminki K, et al. Cancer risk in hospitalised asthma patients. Br J Cancer. 2009;100:829–33. doi: 10.1038/sj.bjc.6604890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DW, Young KE, Anda RF, Giles WH. Asthma and risk of death from lung cancer: NHANES II mortality study. J Asthma. 2005;42:597–600. doi: 10.1080/02770900500216234. [DOI] [PubMed] [Google Scholar]
- 5.Brown DW, Young KE, Anda RF, Felitti VJ, Giles WH. Re: Asthma and the risk of lung cancer. Findings from the adverse childhood experiences (ACE) Cancer Causes Control. 2006;17:349–50. doi: 10.1007/s10552-005-0420-5. [DOI] [PubMed] [Google Scholar]
- 6.García Sanz MT, González Barcala FJ, Alvarez Dobaño JM, Valdés Cuadrado L. Asthma and risk of lung cancer. Clin Transl Oncol. 2011;13:728–30. doi: 10.1007/s12094-011-0723-9. [DOI] [PubMed] [Google Scholar]
- 7.Sherman PW, Holland E, Sherman JS. Allergies: Their role in cancer prevention. Q Rev Biol. 2008;83:339–62. doi: 10.1086/592850. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719–26. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 11.Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78:1004–10. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–88. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880–4. doi: 10.1136/thorax.57.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu H, Zhang J, Pan J, Zhang Q, Lu Y, Wen W, et al. Chemoprevention of lung carcinogenesis by the combination of aerosolized budesonide and oral pioglitazone in A/J mice. Mol Carcinog. 2011;50:913–21. doi: 10.1002/mc.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wattenberg LW, Estensen RD. Studies of chemopreventive effects of budesonide on benzo[a] pyrene-induced neoplasia of the lung of female A/J mice. Carcinogenesis. 1997;18:2015–7. doi: 10.1093/carcin/18.10.2015. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang Z, Kastens E, Lubet RA, You M. Mice with alterations in both p53 and ink4a/Arf display a striking increase in lung tumor multiplicity and progression: Differential chemopreventive effect of budesonide in wild-type and mutant A/J mice. Cancer Res. 2003;63:4389–95. [PubMed] [Google Scholar]
- 17.Wiley LK, Shah A, Xu H, Bush WS. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20:652–8. doi: 10.1136/amiajnl-2012-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pocket Guide for Asthma Management and Prevention. A Pocket Guide for Physicians and Nurses; Updated 2017. [Last updated on 2017 Jan 01; Last accessed on 2017 Jun 09]. Available from: http://www.ginasthma.org/download/520/
- 19.Lee CH, Hyun MK, Jang EJ, Lee NR, Kim K, Yim JJ, et al. Inhaled corticosteroid use and risks of lung cancer and laryngeal cancer. Respir Med. 2013;107:1222–33. doi: 10.1016/j.rmed.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH, et al. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:712–9. doi: 10.1164/rccm.200608-1125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking.European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 22.Estensen RD, Jordan MM, Wiedmann TS, Galbraith AR, Steele VE, Wattenberg LW, et al. Effect of chemopreventive agents on separate stages of progression of benzo[alpha] pyrene induced lung tumors in A/J mice. Carcinogenesis. 2004;25:197–201. doi: 10.1093/carcin/bgg196. [DOI] [PubMed] [Google Scholar]
- 23.Lam S, leRiche JC, McWilliams A, Macaulay C, Dyachkova Y, Szabo E, et al. Arandomized phase IIb trial of Pulmicort Turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;10:6502–11. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg RM, Teertstra HJ, van Zandwijk N, van Tinteren H, Visser C, Pasic A, et al. CT detected indeterminate pulmonary nodules in a chemoprevention trial of fluticasone. Lung Cancer. 2008;60:57–61. doi: 10.1016/j.lungcan.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Balansky R, Ganchev G, Iltcheva M, Steele VE, De Flora S. Prevention of cigarette smoke-induced lung tumors in mice by budesonide, phenethyl isothiocyanate, and N-acetylcysteine. Int J Cancer. 2010;126:1047–54. doi: 10.1002/ijc.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veronesi G, Szabo E, Decensi A, Guerrieri-Gonzaga A, Bellomi M, Radice D, et al. Randomized phase II trial of inhaled budesonide versus placebo in high-risk individuals with CT screen-detected lung nodules. Cancer Prev Res (Phila) 2011;4:34–42. doi: 10.1158/1940-6207.CAPR-10-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira MA, Li Y, Gunning WT, Kramer PM, Al-Yaqoub F, Lubet RA, et al. Prevention of mouse lung tumors by budesonide and its modulation of biomarkers. Carcinogenesis. 2002;23:1185–92. doi: 10.1093/carcin/23.7.1185. [DOI] [PubMed] [Google Scholar]
- 28.Taranova AG, Maldonado D, 3rd, Vachon CM, Jacobsen EA, Abdala-Valencia H, McGarry MP, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 2008;68:8582–9. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira MA, Tao L, Liu Y, Li L, Steele VE, Lubet RA, et al. Modulation by budesonide of DNA methylation and mRNA expression in mouse lung tumors. Int J Cancer. 2007;120:1150–3. doi: 10.1002/ijc.22468. [DOI] [PubMed] [Google Scholar]
- 30.Yao R, Wang Y, Lemon WJ, Lubet RA, You M. Budesonide exerts its chemopreventive efficacy during mouse lung tumorigenesis by modulating gene expressions. Oncogene. 2004;23:7746–52. doi: 10.1038/sj.onc.1207985. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz AG, Wenzlaff AS, Prysak GM, Murphy V, Cote ML, Brooks SC, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non-small cell lung cancer in women. J Clin Oncol. 2007;25:5785–92. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 32.Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28:1042–50. doi: 10.1183/09031936.00074905. [DOI] [PubMed] [Google Scholar]
- 33.Kilpeläinen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy. 2001;56:377–84. doi: 10.1034/j.1398-9995.2001.056005377.x. [DOI] [PubMed] [Google Scholar]


