Abstract
In this paper, the influences of the graphite precursor and the oxidation method on the resulting reduced graphene oxide (especially its composition and morphology) are shown. Three types of graphite were used to prepare samples for analysis, and each of the precursors was oxidized by two different methods (all samples were reduced by the same method of thermal reduction). Each obtained graphite oxide and reduced graphene oxide was analysed by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy (RS).
Keywords: graphite, graphite oxide, reduced graphene oxide, modified Hummers method, oxidation, reduction, Raman spectroscopy
1. Introduction
Carbon-based materials are very interesting due to their stable physicochemical properties and low cost, as well as other characteristics [1]. These materials have been utilised in many applications, e.g., supercapacitors and thin film transistors [1,2,3]. One of the most interesting types of these carbon-based materials is reduced graphene oxide (rGO). This material can be used for many purposes e.g., in gas sensing structures [2,4,5]. However, the properties of the gas sensors depend on the properties of sensor layers [6], which are related to the preparation method of the material.
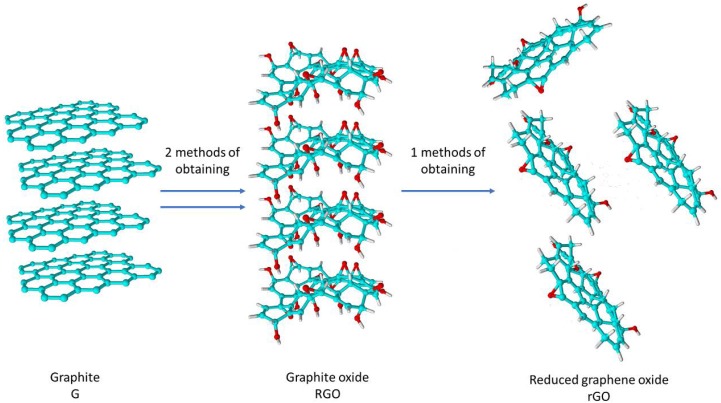
rGO is obtained by the oxidation of graphite (the obtained material is called graphite oxide (GRO)) followed by reduction and exfoliation [4,7]. Currently, one of the most popular methods of obtaining graphene oxide is the method proposed by Hummers [8,9,10,11], but other methods (e.g., that proposed by Staudenmeier and Brodie) are also well-known [8,10]. The reduction step can also be completed through several methods, such as the environmentally methods of flash photoreduction, hydrothermal, catalytic reduction and thermal annealing [7,12,13,14].
Based on the literature [5,7,8,15,16,17] the kind of graphite and the oxidation/reduction method have large impacts on the kind of functional groups present in the final product (in rGO and GRO). Materials prepared through different methods will have difference properties. For this reason, it is necessary to characterize products from different preparation methods. The characterization of carbon materials can be performed using many methods, but one of the more interesting methods is Raman spectroscopy (RS). This method gives a large amount of information about the testing materials and, importantly, is a nondestructive method [18,19]. The typical Raman spectrum of carbon materials contains bands marked as D, G and 2D [20]. The D band (located near 1350 cm−1) results from the presence of vacancies or dislocations in the graphene layer and at the edge of this layer. This band is also related to the presence of defects in the material [20,21,22]. The next band, the G peak, is related to the in-plane vibration of sp2 hybridized carbon atoms and is located near 1580 cm−1 [21,23]. The last peak (2D) is related to the number of graphene layers. This band is sometimes also marked as G’ and is located near 2700 cm−1 [23,24]. Based on the data obtained from the Raman spectra, additional information about the carbon materials can be obtained by analysing the ratio of the intensities of the individual peaks. As an example, the ID/IG ratio (intensity of the D peak to the intensity of the G peak) is related to the amount of defects present in the material [25,26], while the I2D/IG ratio (intensity of the 2D peak to the intensity of the G peak) is related to the number of graphene layers in the material [27]; when the G band increases and the 2D band decreases, the number of layers in the material is assumed to increase [20].
The literature indicates that additional peaks may be visible in the Raman spectra of some graphene-based materials after deconvolution. In our previous work [28], we presented a method for deconvoluting Raman spectra. After deconvolution, a peak near 1620 cm−1 (marked as D’) and two (according to other papers- three) additional bands above 2440 cm−1 become visible. There are discrepancies in the name of these latter peaks, where the first peak above 2440 cm−1 is marked as D* or G* and the next peaks are marked as D+D’ or D+G and 2D’ or G+D’ [20,22,28]. Analysis of the intensity ratios of these peaks also gives additional information about the materials, e.g., an increase in the I2D/ID+G (I2D/ID+D’) ratio indicates the restoration of the sp2 hybridized structure [22].
2. Materials and Methods
2.1. The Selection of the Graphites
Two types of natural graphite, scale graphite (S) and flake graphite (F), and one synthetic graphite; electrode graphite (E), were used for the measurements. The contents of carbon in these analytical samples in the dry and ashless state are 99.2, 99.5 and 99.5, respectively. These graphites are characterized by a high degree of crystallinity and large crystallite dimensions; however, their properties differ between materials. Due to the spatial arrangement of the flake graphite structure, the bonds between neighbouring planes can be easily broken, resulting in significant anisotropy in this graphite. The natural scale graphite has a coarse crystal structure with large crystals. The synthetic graphite is characterized by a high purity and close similarity to natural graphite; however, it is obtained in a synthetic method from a properly prepared mass in an Acheson electric resistance furnace at a temperature of 2500–3000 °C [29]. The differences in the structural parameters of the examined graphites will be discussed in more detail in later sections of this paper. Nevertheless, these differences will affect the properties of the materials (graphite oxides and reduced graphene oxides) obtained from such graphites (a scheme of the preparation method and nomenclature is shown in Figure 1).
Figure 1.
Scheme of the preparation of graphite oxide and reduced graphene oxide from graphite.
2.2. The Preparation of Graphite Oxide and Reduced Graphene Oxide
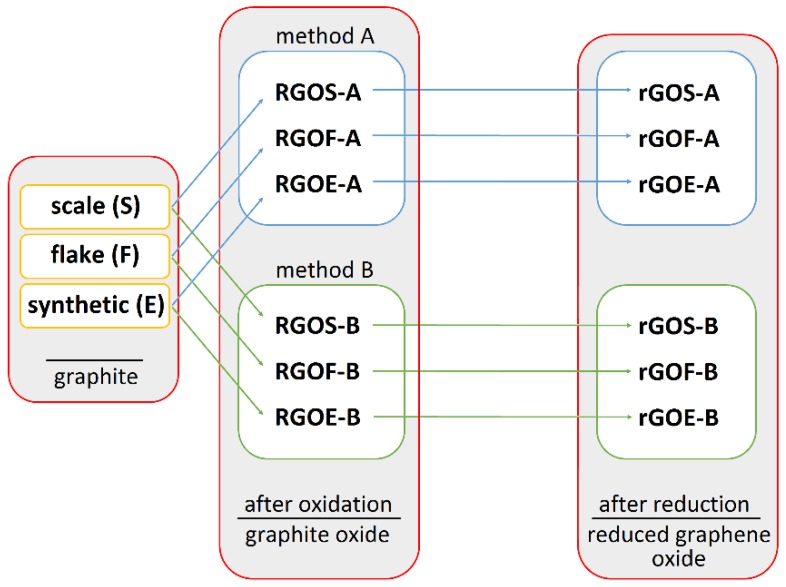
Graphite oxides were obtained from scale, flake and synthetic graphites by two methods denoted A and B (commercial graphite powders were ground in a planetary ball mill and sieved to a particle size < 20 µm and then oxidized according to the methods described in Table 1). Method A (Hummers’ method) is popular method which is used to obtain graphites oxides while method B is an original modification of Tour’s method. We will show (in this paper) that method B proved to be more effective in oxidation of graphites of various origins. The obtained products are denoted GROS-A, GROF-A, GROE-A, GROS-B, GROF-B and GROE-B according to the type of graphite used and the method of oxidation. The diagram showing used abbreviations (both for graphite, graphite oxide and reduced graphene oxide) is shown in Figure 2.
Table 1.
Conditions of graphite oxide preparation.
| Symbol | Condition of Preparation |
|---|---|
| Method A | graphite (1 g); H2SO4 (30 mL); NaNO3 (3 g); KMnO4 (3 g); 2 h |
| Method B | graphite (1 g); H2SO4 (45 mL); H3PO4 (5 mL); KNO3 (1.5 g); KMnO4 (5 g); 5 h |
Figure 2.
Diagram showing the abbreviations depending on the preparation step.
Reduced graphene oxides were obtained using high-temperature exfoliation/reduction according to the following process: the graphite oxide was blown under a flow of nitrogen for 30 min (5 mL/min) and subsequently annealed at 900 °C for 5 min. The obtained rGOs are denoted rGOS-A, rGOF-A, rGOE-A, rGOS-B, rGOF-B and rGOE-B according to the GRO used.
2.3. Characterization Methods of the Obtained Materials
2.3.1. X-ray Diffraction (XRD)
X-ray diffraction measurements were conducted using an X’Pert PRO PW 3040/60 diffractometer (PANalytical, Quebec, Canada). The samples were deposited onto glass and analysed by using Cu Ka1 radiation with a voltage of 45 kV and a current of 30 mA.
2.3.2. X-ray Photoelectron Microscopy (XPS)
XPS was performed using a PHI 500 VersaProbe spectrometer from ULVAC (Chigasaki, Japan) using an Al Kα anode radiation beam (1486.6 eV).
2.3.3. Raman Spectroscopy (RS)
Spectroscopic studies were performed using an N-TEGRA Spectra platform (NT-MDT, Moscow, Russia). The vibrations of the molecules were excited using 532 nm wavelength. The exposure time was 10 s. Measurements were performed at ten different points of each sample.
3. Results
3.1. Results of the Graphite Measurements
The first step of the material characterization included determination of the crystallite diameter (La), crystalline height (Lc) and interplanar distance (d002).
As shown in Table 2, the analysed graphites are characterized by similar interplanar distances. Moreover, the crystallites have a large diameter (50–82 nm), which is desirable for subsequent synthetic processes. The crystalline height is from 20 to 29 nm, and the interplanar distance is in the range from 0.3362 to 0.3379 nm.
Table 2.
The structural parameters of the graphites determined using the XRD method, nm.
| Graphite | La | Lc | d002 |
|---|---|---|---|
| scale (S) | 59 | 29 | 0.3379 |
| flake (F) | 82 | 20 | 0.3362 |
| synthetic (E) | 50 | 26 | 0.3369 |
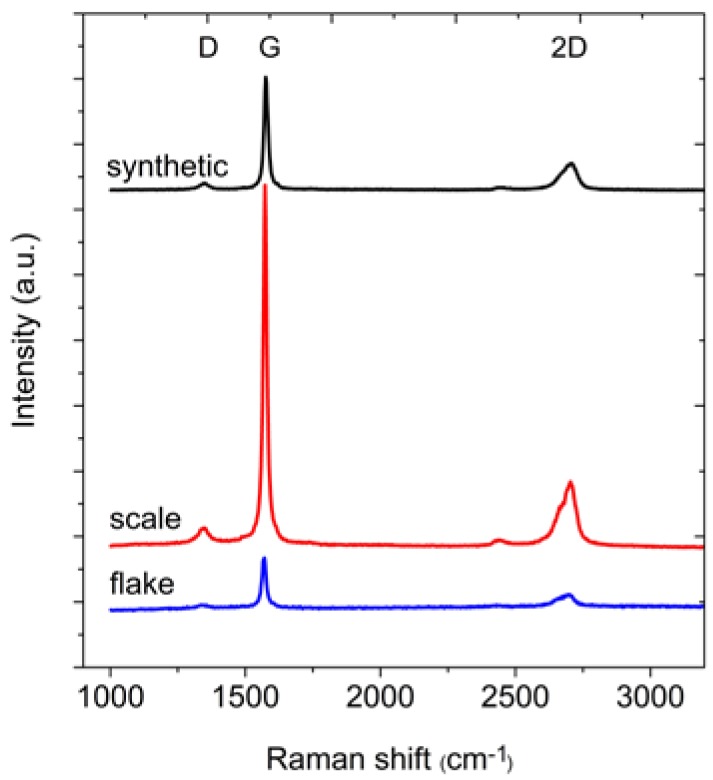
In the next step of the measurements, the Raman spectra of the graphite samples were collected (Figure 3). From these spectra, the position and full width at half maximum (FWHM) of the D, G and 2D bands were determined (Table 3). The differences between the positions of the individual bands are small. The FWHM of the G peak is in the range 16–19 cm−1, and this small value indicates that the peak originates from the stretching vibrations of carbon bonds in the rings of the sp2 hybridized graphene layers. Meanwhile, the FWHM of the D band is in the range from 38 to 52 cm−1. This large FWHM means that the structure is perturbed, for example by the presence of heteroatoms or point dislocations in the structure (this band should not be observed in the case of perfect graphite [30]). However, the structures of all graphites are quite well-ordered, as confirmed by the AG/AD ratio (where A represents the area under the peak). The area under the G peak is approximately 7–8 times larger than the area under the D peak. For all analysed graphites, the intensity of the G peak is much larger than the intensity of the D peak.
Figure 3.
Raman spectra of the graphites.
Table 3.
FWHM values and position of the D, G and 2D bands in the Raman spectra.
| Graphite | D Band | G Band | 2D Band | AG/AD |
|---|---|---|---|---|
| FWHM/Position of the Band (cm−1) | ||||
| scale (S) | 52/1346 | 16/1573 | 67/2695 | 7.44 |
| flake (F) | 43/1344 | 19/1569 | 102/2685 | 7.55 |
| synthetic (E) | 38/1347 | 17/1576 | 65/2699 | 8.36 |
3.2. Results of the Graphite Oxide Measurements
The first stage of measurements of the graphite oxides included analysis using the XRD method. Based on the obtained data (Table 4), the largest interplanar distances (d001) are obtained for the GROFs. The smallest crystallite diameter, La, is observed for the graphite oxides obtained from synthetic graphite, regardless of the oxidation method. The crystalline height (Lc) is similar for all the GROs.
Table 4.
The structural parameters of the graphite oxides determined using the XRD method, nm.
| d001 | La | Lc | |
|---|---|---|---|
| GROS-A | 0.7795 | 32 | 4 |
| GROF-A | 0.7926 | 31 | 5 |
| GROE-A | 0.7687 | 23 | 5 |
| GROS-B | 0.7983 | 30 | 4 |
| GROF-B | 0.8040 | 30 | 3 |
| GROE-B | 0.7939 | 25 | 4 |
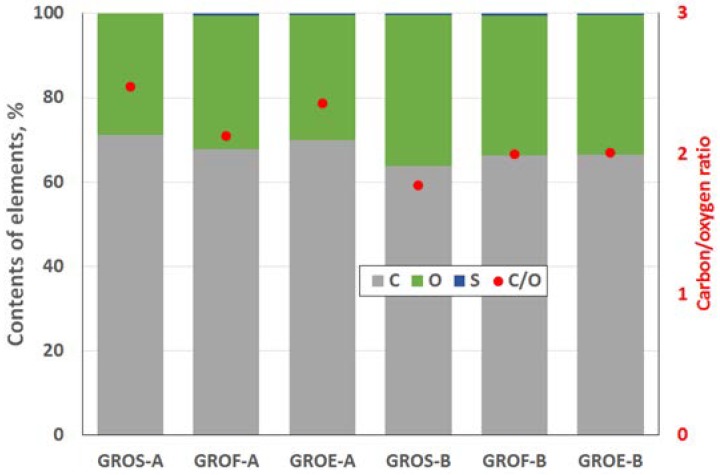
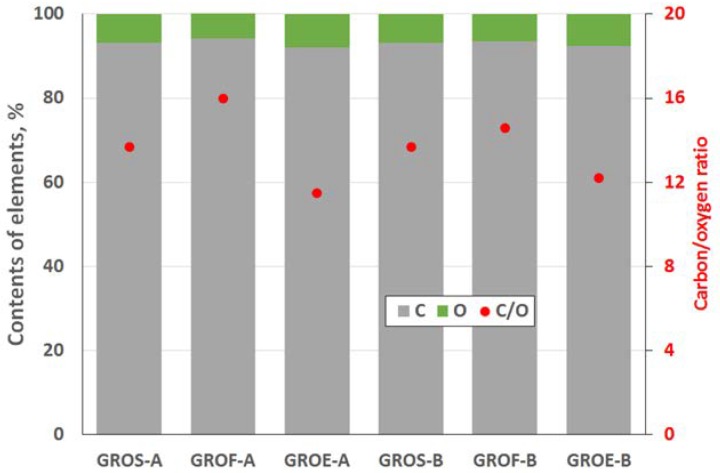
The GROs were also analysed using the XPS method (Figure 4 and Figure 5). All the graphite oxides are composed mainly of carbon and oxygen. The amount of carbon (>63%) in the GROs is much smaller than that in the graphites, regardless of the graphite type. The amount of oxygen is above 28%. The C/O ratio determined for the GROs oxidized by method A is larger than that for the GROs oxidized by method B. This result indicates that the B method was more effective for introducing oxygen into the graphite structure. The content of sulphur is low—below 0.5 at %.
Figure 4.
Elemental compositions of the graphite oxides determined by the XPS method, at %.
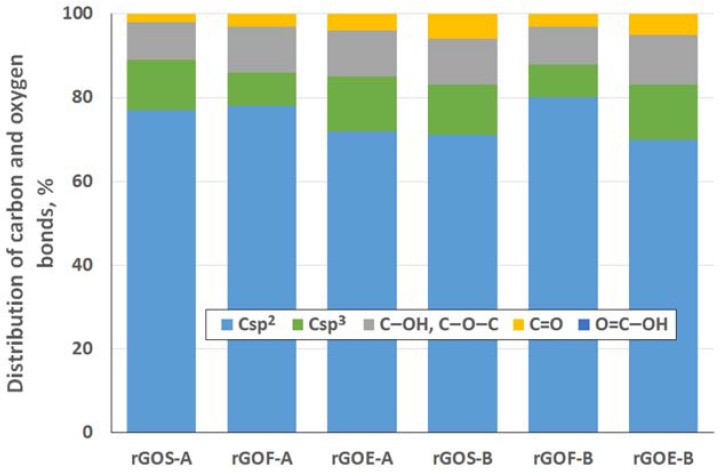
Figure 5.
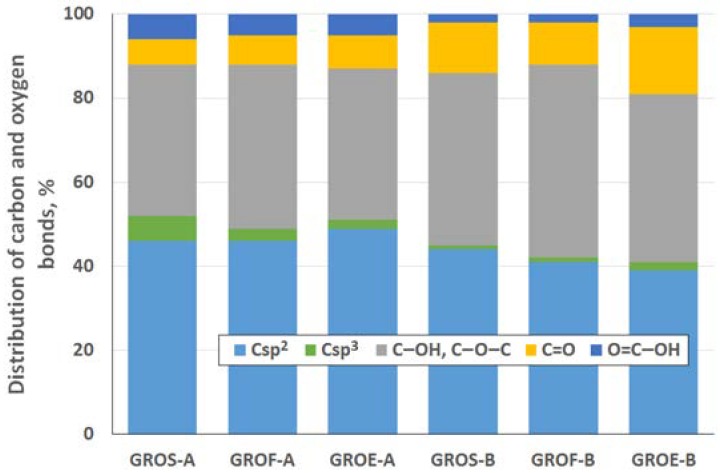
Distribution of carbon and oxygen bonds (in graphite oxide) determined by XPS, at %.
To determine the distribution of carbon and oxygen bonds, the C 1s XPS spectra of the graphite oxides were deconvoluted into five components. After deconvolution, two peaks appear at energies (284.5 ± 0.1 eV and 285.4 ± 0.2 eV) that correspond to the sp2 and sp3 bonds of carbon. Moreover, peaks corresponding to carbon bonded to oxygen dominate the spectra of the GROs: three peaks (286.5 ± 0.3 eV, 287.6 ± 0.2 eV and 288.9 ± 0.3 eV) that correspond to hydroxyl/epoxy (C–OH, C–O–C), carbonyl/quinone (C=O) and carboxyl (O=C–OH) groups are identified. Based on the obtained data, the hydroxyl and epoxy groups are the most abundant. Smaller amounts of carbonyl and quinone groups are present, and the carboxyl group content is the smallest (more carbonyl and quinone groups and fewer carboxyl groups are present in the GROs oxidized by method B). Furthermore, the lowest degree of graphite oxidation is observed for the GROSs.
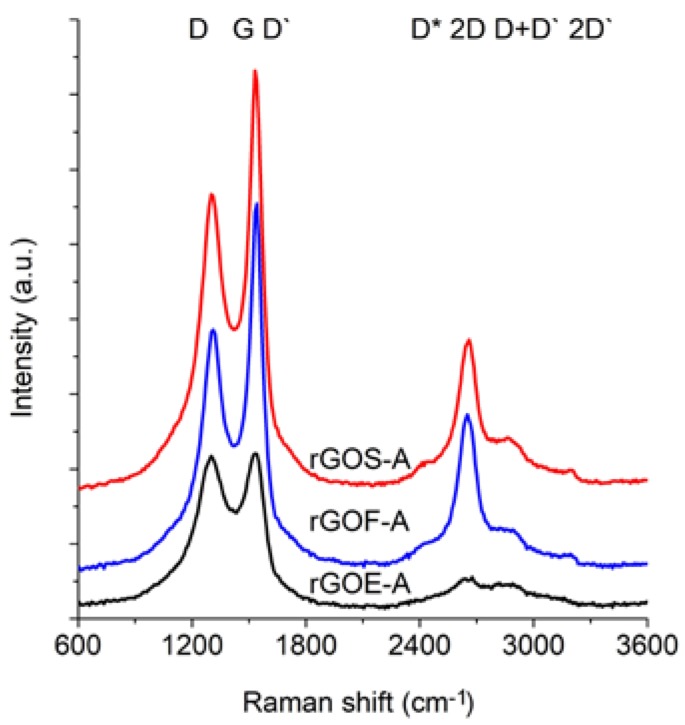
All of the experiments whose results were described above (XRD and XPS) were performed to obtain information about the tested graphites and graphite oxides. This knowledge was helpful for interpreting the data obtained from Raman Spectroscopy (RS). The RS method enables much more detailed analysis of the changes related the introduction of oxygen atoms into the graphite structure, thus increasing the understanding of the unstructured phase. Figure 6 and Figure 7 show the Raman spectra of the GROs oxidized by methods A and B, respectively. All spectra were deconvoluted according to the method in reference [28] (for further analysis, the corresponding values were obtained from the deconvoluted spectra). The obtained data (FWHM and position of the peaks) are shown in Table 5, and the results of the calculations (ID/IG, I2D/IG, and I2D/ID+D’) are shown in Figure 8.
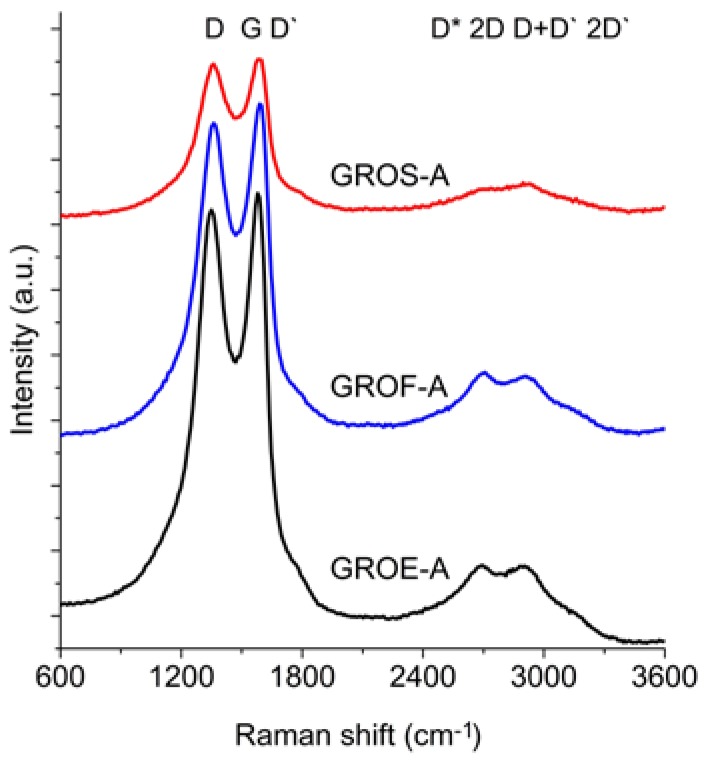
Figure 6.
Raman spectra of graphite oxide (oxidation method A).
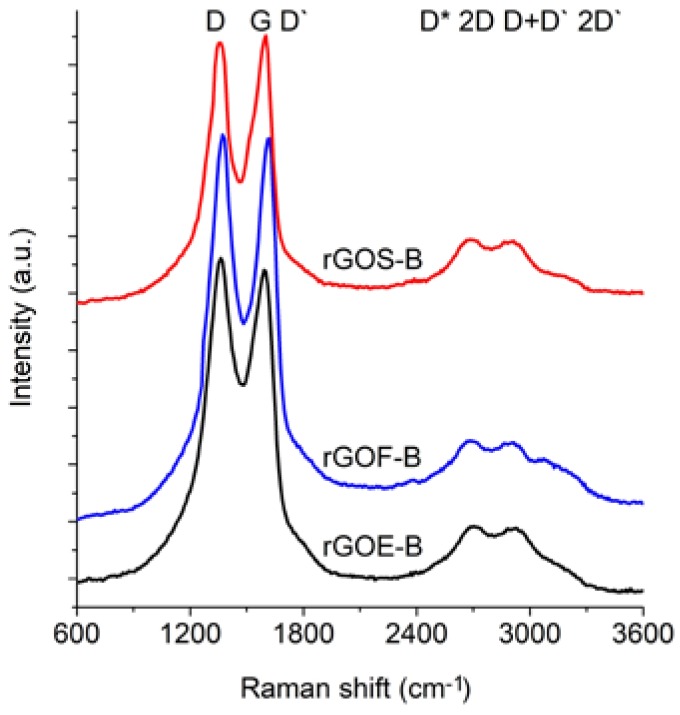
Figure 7.
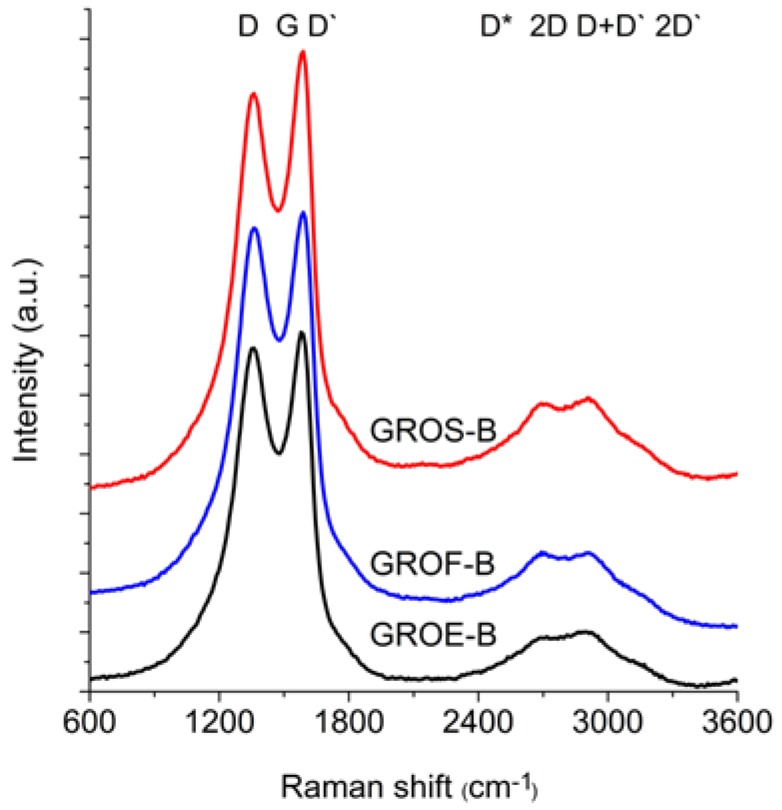
Raman spectra of graphite oxide (oxidation method B).
Table 5.
FWHM values and position of the bands in the Raman spectra of the graphite oxides.
| G Band | D Band | D’ Band | D* Band | 2D Band | D+D’ Band | 2D’ Band | |
|---|---|---|---|---|---|---|---|
| FWHM/Position of the Band (cm−1) | |||||||
| GROS-A | 114/1563 | 214/1361 | 64/1609 | 133/2558 | 226/2700 | 251/2905 | 130/3118 |
| GROF-A | 130/1563 | 223/1360 | 65/1607 | 150/2524 | 229/2697 | 274/2910 | 124/3105 |
| GROE-A | 125/1553 | 219/1350 | 66/1598 | 110/2546 | 237/2692 | 256/2901 | 99/3094 |
| GROS-B | 120/1566 | 217/1364 | 61/1606 | 131/2556 | 229/2703 | 247/2901 | 126/3114 |
| GROF-B | 133/1568 | 226/1363 | 62/1603 | 148/2522 | 232/2700 | 270/2906 | 120/3101 |
| GROE-B | 128/1556 | 222/1353 | 63/1595 | 108/2544 | 240/2695 | 252/2897 | 95/3090 |
Figure 8.
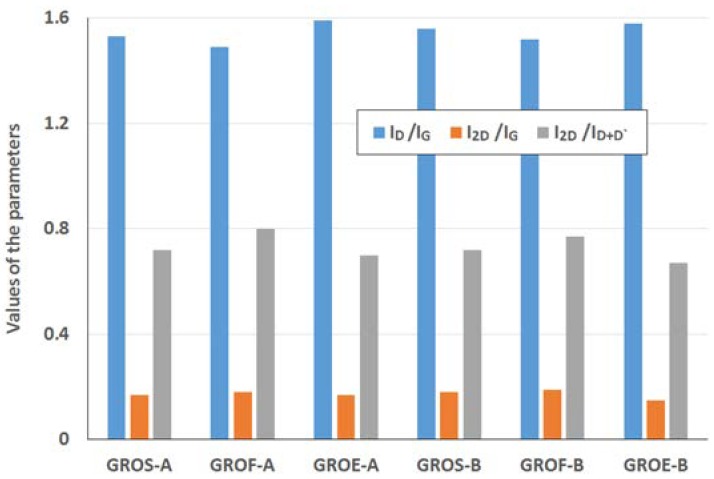
Average values of the parameters ID/IG, I2D/IG, and I2D/ID+D’ obtained from the Raman spectra of the graphite oxides.
The spectra of GRO are characterized by intense D and G bands and a wide 2D band. Such an intense D band indicates the formation of defects in the structures. The increase in the intensity of the D band in relation to that of the G band indicates an increase in the amount of the disordered phase in the GROs. Unlike in the Raman spectra of the graphites, for the GROs, the D band is more intense than the G band, which is related to the formation of sp3 hybridized bonds as a result of the oxidation of graphite. Both the D and G bands of the GROs are wider than the D and G bands in the Raman spectra of graphite. Moreover, the G band of GRO is shifted towards lower wavenumbers, which confirms the presence of defects in the graphene layers.
The presence of oxygen atoms causes both an increase in the interplanar distance and changes in the characteristics of the vibrations in the material’s lattice. For this reason, the Raman spectra of oxidized graphites are characterized by more intense D peaks in comparison to the D peaks of the unoxidized graphites. If the degree of oxidation is larger, the intensity of this peak is also larger [20,31].
The FWHM values of the D and G bands are larger for the GROs than for the graphites. The wide G bands in the GROs are accompanied by a D’ band, the intensity of which is proportional to the number of defects. The FWHM of the D’ peak is in the range from 61 to 66 cm−1. A D’ band is observed in the spectra of graphene materials obtained from the oxidation or direct exfoliation of graphites and subsequent reduction of the GROs, which confirms the formation of structural defects [20,22,23,31,32]. Nevertheless, many researchers regard the wide G band in GROs as a single peak (not a sum of G and D’ peaks) [20].
The 2D band is less intense than the D band. Based on the position, shape and intensity of the 2D band obtained from deconvolution of the Raman spectrum into components (Lorentz curves), the number of graphene layers can be determined [33,34,35]. In the case of graphene monolayers, the 2D band occurs as a single peak near 2675 cm−1 [33]. In contrast, the 2D band of two graphene layers consists of four peaks: D*, 2D, D+D’ and 2D’. In this case, the D+D’ peak is more intense than the others. An increase in the number of layers reduces the intensities of the D* and 2D peaks and shifts the components towards higher wavenumbers.
In the spectra of the analysed GROs, four peaks: D* (2522–2558 cm−1), 2D (2695–2703 cm−1), D+D’ (2897–2910 cm−1) and 2D’ (3090–3118 cm−1), are obtained after deconvolution. The FWHM of the 2D peak is approximately 230 cm−1, which is much larger than that of the 2D peak of graphene (approximately 30 cm−1) [30]. The shape of the spectra and the observation of D*, 2D, D+D’ and 2D’ peaks indicate that the GROs consist of two or more graphene layers in an aggregate. Moreover, the higher intensity of the 2D band in relation to that of the G band confirms the presence of more defects in the structure. Please note that when the material is composed of two or more layers in an aggregate, the 2D band consists of two peaks, 2D1 and 2D2 [36] (marked according to the earlier designation as 2D and D+D’). In the case of the examined GROs, both the 2D and D+D’ peaks are observed.
The GROs obtained by method B are characterized by equal or lower I2D/ID+D’ ratios relative to those of the GROs prepared by method A. This difference suggests that more defects are present in the aggregates of the GROs prepared by method B. The largest ID/IG ratio and the smallest I2D/IG and I2D/ID+D’ ratios are observed for the oxides obtained from synthetic graphite, which may indicate that synthetic graphite is the most susceptible to oxidation and exfoliation.
3.3. Results of the Reduced Graphene Oxide Measurements
All reduced graphene oxides were examined by the same methods as those employed for the graphite oxides, and XRD measurements were performed first. The structural parameters of the rGOs are shown in Table 6. Following thermal exfoliation and reduction, the (001) band completely disappears from the diffraction patterns of the rGOs. Simultaneously, a (00X) band appears due to the partial removal of oxygen groups. Moreover, a more intense (002) is observed, which indicates the progressive reconstruction of the crystalline structure. The oxidation of graphite by method B can be concluded to lead to a larger separation of graphene layers (larger values of d00X and d002) as a consequence of the greater degree of exfoliation that occurs during high-temperature processing. The values calculated on the basis of the 002 band are in the range (0.3373–0.3393 nm) for method A and in the range (0.3421–0.3440 nm) for method B. The largest values of the interplanar distances (d00X) are observed for the rGOF materials, while the smallest ones are observed for rGOE. The maintenance of a large distance between graphene layers may be related to the presence of significant amounts of oxygen in the rGOs and a large degree of defect formation in the graphene layers. The crystallite sizes of the rGOs differ depending on the type of graphite used and the oxidation method. The smallest values of Lc and La are obtained for rGOs fabricated from synthetic graphite. Moreover, analysis of the obtained parameters reveals that higher values of Lc and La are obtained for the rGO-A series independent of the type of graphite. This result suggests that a lower degree of exfoliation occurs for the GROs obtained by method A. The resulting rGOs (method A) are characterized by a lower susceptibility to high-temperature exfoliation (larger number of layers).
Table 6.
The structural parameters of the reduced graphene oxides determined using the XRD method, nm.
| d00X | d002 | Lc | La | N | |
|---|---|---|---|---|---|
| rGOS-A | 0.4031 | 0.3393 | 8 | 21 | 25 |
| rGOF-A | 0.4040 | 0.3383 | 9 | 25 | 28 |
| rGOE-A | 0.3981 | 0.3373 | 7 | 18 | 22 |
| rGOS-B | 0.4072 | 0.3421 | 4 | 10 | 11 |
| rGOF-B | 0.4151 | 0.3421 | 4 | 11 | 11 |
| rGOE-B | 0.4055 | 0.3440 | 3 | 8 | 8 |
Based on the data obtained using the XPS method (Figure 9), a larger percentage of carbon and smaller percentage of oxygen are observed in the rGOs relative to those of the GROs, in which the amount of oxygen decreased 4–5 times. The degree of reduction of the rGO surface, represented by the C/O ratio, depends on the type of graphite used and increases in the order rGOE < rGOS < rGOF.
Figure 9.
Surface elemental composition of the reduced graphene oxides determined by the XPS.
As was done for the GROs, the distribution of carbon and oxygen bonds was determined for rGO using the XPS method. Analysis of the XPS C 1s spectra after deconvolution (data presented in Figure 10) confirms that the highest degree of reduction of the rGOs is obtained for the samples prepared from flake graphite. The highest percentage of sp2 hybridized carbon (78% for A method and 80% for B method) occurs for these samples, and their percentage of sp3 hybridized carbon is the smallest among all the rGOs. Meanwhile, the largest amount of sp3 hybridized carbon in the rGOs is obtained for the samples prepared from synthetic graphite. As a reminder, the lowest degree of reduction, expressed as the C/O ratio, also occurs in the rGOEs.
Figure 10.
Distribution of carbon and oxygen bonds (reduced graphene oxide) determined by XPS, %.
Deconvolution of the C 1s spectra shows that the rGOs mainly contain hydroxyl and epoxy groups. The carbon in the carbonyl and quinone groups accounts for 2% to 6% of the total content, and only a trace content of carboxyl carbons is observed. Following thermal exfoliation/reduction to produce rGO, the amounts of hydroxyl and epoxy groups decreases almost four times (Figure 10) compared to those in GRO (Figure 5). Furthermore, fewer carbonyl and quinone groups are present in the structures of the rGOs.
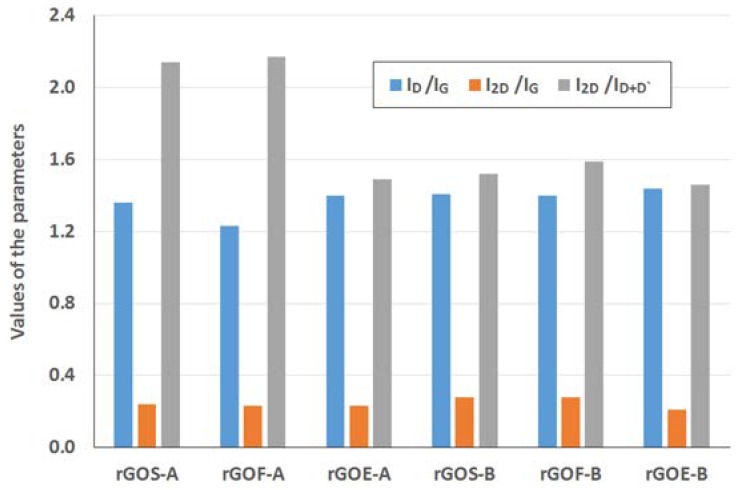
The Raman spectra of the reduced graphene oxides obtained by methods A and B are presented in Figure 11 and Figure 12, respectively. The position and the FWHM of the peaks are shown in Table 7 while the average values of the parameters: ID/IG, I2D/IG, and I2D/ID+D’ are shown in Figure 13. These spectra exhibit the same bands as those of the GROs. However, the G bands shift towards lower wavenumbers compared to the G bands in the Raman spectra of the GROs. This difference is associated with the lower content of oxygen groups in the rGOs and the regeneration of the graphite structure. At the same time, the intensity of the D band increases, which indicates the formation of new defects in the structures during the reduction process.
Figure 11.
Raman spectra of the reduced graphene oxides obtained by thermal reduction of graphite oxide (oxidation method A).
Figure 12.
Raman spectra of the reduced graphene oxides obtained by thermal reduction of graphite oxide (oxidation method B).
Table 7.
FWHM values and position of the bands in the Raman spectra of the reduced graphite oxides.
| G Band | D Band | D’ Band | D* Band | 2D Band | D+D’ Band | 2D’ Band | |
|---|---|---|---|---|---|---|---|
| FWHM/Position of the Band (cm−1) | |||||||
| rGOS-A | 109/1533 | 198/1349 | 62/1598 | 132/2511 | 202/2646 | 232/2825 | 162/3048 |
| rGOF-A | 125/1543 | 217/1353 | 65/1600 | 156/2526 | 214/2657 | 251/2850 | 174/3065 |
| rGOE-A | 119/1550 | 215/1358 | 68/1607 | 143/2540 | 250/2666 | 256/2867 | 165/3098 |
| rGOS-B | 118/1546 | 198/1334 | 61/1594 | 151/2516 | 239/2622 | 244/2801 | 156/3014 |
| rGOF-B | 122/1558 | 211/1349 | 62/1599 | 178/2527 | 242/2645 | 258/2816 | 150/3031 |
| rGOE-B | 120/1550 | 202/1353 | 63/1607 | 168/2540 | 255/2665 | 256/2837 | 140/3040 |
Figure 13.
Average values of the parameters ID/IG, I2D/IG, and I2D/ID+D’ obtained from the Raman spectra of the graphite oxides.
Reduced graphene oxides are made of graphene layers with residual oxygen groups attached to the planes and edges. The obtained materials exhibit intense G band and D bands, which confirms the presence of defects in the graphene layers. The FWHM values of the D and G peaks of the rGOs are smaller than those of the GROs, which indicates the presence of fewer defects in the structure (associated with a lower oxygen content). In the spectra of the rGOs, the intense G band is accompanied by a D’ band, the intensity of which is proportional to the amount of defects in the structure. The FWHM of this band is ~61–68 cm−1, which is comparable to the corresponding FWHM values of the GROs (~61–66 cm−1), thereby confirming that both the reduction and oxidation process contribute to the formation of defects in the graphene layers.
The rGO-Bs are characterized by D* and 2D bands with larger FWHM values compared to those of the rGO-As, which indicates that these samples have fewer graphene layers in their aggregates (as a reminder: the average number of layers determined using the XRD method is 22–28 for the rGO-As and 8–11 for the rGO-Bs). Simultaneously, the FWHM values obtained for the rGO-Bs are larger than those obtained for the GRO-Bs. This result indicates that the aggregates of the rGOs contain fewer graphene layers than the aggregates of the GROs (also, the intensities of these bands are weaker).
The intensity of the D peak is lower for the rGO-A samples than the rGO-B samples, confirming that these materials contain fewer defects. This feature further results in lower ID/IG ratios. The higher ID/IG ratios obtained for the rGO-B samples confirm that despite thermal reduction of these structures, more defects and imperfections are present in these samples than in the rGO-A structures. This difference can be explained by the higher oxygen content in the rGO-Bs, which is related to larger distances between the layers and a greater degree of exfoliation. The ID/IG values determined for the rGOs depend on the type of graphite used and increase in the order: flake (F) < scale (S) < synthetic (E) graphite (the lowest ID/IG values are obtained for the rGOs prepared from flake graphite, which indicates that these samples contain the fewest defects). This trend can be related to the size of the graphene layers (La) determined using the XRD method, in which the opposite trend is observed (synthetic < scale < flake graphite). Based on the measurements performed, smaller graphite crystallites lead to larger amounts of defects in the obtained rGO structures. This relationship was also confirmed in the papers [1,37], where the authors determined that graphene oxides containing the fewest defects were obtained from graphite precursors with the largest crystallites.
Analysis of the Raman spectra of the rGOs shows that the intensity of the 2D band is much lower than that of the G band. The I2D/IG ratio is related to the number of graphene layers, and larger values of this ratio are observed for the rGOs than for the corresponding GROs. The smallest values are obtained for rGOE-B, which indicates that this material has the fewest layers in its aggregates. Due to the type of graphite used, the I2D/ID+D’ ratio is the smallest for the GRO-Es. Please note that these materials are characterized by the smallest quantity of sp2 hybridized carbon and the largest quantity of sp3 hybridized carbon (determined by XPS measurements), indicating a high degree of destruction of the graphene layers. Furthermore, the I2D/ID+D’ ratios are the smallest in this case. Conversely, the rGO-Fs are characterized by the largest quantity of sp2 hybridized carbon and the smallest quantity of sp3 hybridized carbon, which indicates the presence of fewer defects in these graphene structures.
4. Discussion
From comparison of the different oxidation methods, method B is determined to be more effective for introducing oxygen into the graphite structure (more carbonyl and quinone groups and fewer carboxyl groups are present). Furthermore, comparison of the type of precursor (graphite) used shows that the synthetic graphite (E) is the least susceptible to oxidation. This conclusion is confirmed by the results of RS. The largest ID/IG ratio and the smallest I2D/IG and I2D/ID+D’ ratios are obtained for the oxides prepared from synthetic graphite (E), which indicates that this graphite is the most susceptible to oxidation and exfoliation. In the case of the oxides obtained from flake graphite (F), the smallest ID/IG ratio and the largest I2D/IG and I2D/ID+D’ ratios are obtained. Based on the Raman spectra, an increase in the D band is observed for the GROs in comparison to that of the unoxidized graphite. This increase is related to the formation of sp3 hybridized bonds as a result of the oxidation of graphite. The presence of oxygen atoms causes both an increase in the interplanar distance and changes in the characteristics of the vibrations of the material’s lattice.
Please note that rapid degradation of the oxygen groups occurs during thermal exfoliation and reduction of GRO. This degradation is accompanied by an increase in pressure due to the evolution of CO2, CO and H2O, which leads to a weakening of the van der Waals forces between graphene planes, and as a consequence, individual graphene sheets are obtained. In such a process, complete elimination of oxygen from the graphite structure cannot be achieved, and the obtained product may contain even several percent of oxygen depending on the degree of reduction.
Analysis of the data obtained from XPS measurements reveals that the degree of reduction (represented as the C/O ratio) changes in the order rGOE < rGOS < rGOF.
The ID/IG values, which indicate the content of defects in the structure, of the rGOs increase in the order rGOF < rGOG < rGOE. This trend is related to the size of the graphene layers (La), which exhibits the opposite trend (rGOE < rGOS < rGOF).
As we have proven, both the type of graphite used and the method of oxidation have a fundamental influence on the properties of the resulting rGOs. Further research on the prepared materials will include studies of their electrical properties. The sensitivity of selected parameters to the surrounding environment will also be tested (the rGOs will be applied as sensor layers in gas sensing devices).
5. Conclusions
This work involved various advanced research methods that enable deeper analysis of the changes related to the incorporation of oxygen atoms into graphite oxides and reduced graphene oxides.
The research facilitated the development of preliminary criteria for the selection of a graphite precursor for the preparation of reduced graphene oxides with the desired textural and structural parameters. The reduced graphene oxides obtained from flake graphites, which had the largest crystallite diameter among the analysed graphites, were characterized by the highest degree of reduction and the fewest defects. Moreover, in each investigated case, large crystallite dimensions in the graphite precursor corresponded to large crystallite dimensions in the obtained reduced graphene oxide (regardless of the oxidation method).
This work also showed that the oxidation method has a large influence on the studied parameters of the obtained materials. The research allowed to indicate which of three graphite precursors is the most susceptible to oxidation and what structural and textural parameters will graphene materials have after exfoliation/reduction process. Graphite oxides obtained by method B (modified Tour’s method) were characterized by higher content of oxygen groups in comparison to method A (Hummers’ method). The flake graphite, characterized by the largest diameter of crystallites, proved to be the most suitable material for the preparation of graphene materials. Reduced graphene oxides obtained from flake graphite are characterized by a greater reduction degree and less amounts of defect (compared to reduced graphene oxides obtained from scale and synthetic graphite).
Author Contributions
Concepts and assumption, R.M., S.D., T.P., G.G.; Research, R.M., S.D., T.P., M.C., G.G.; Writing the article, R.M., S.D., T.P., M.C., G.G.
Funding
This work was partially financed by Statutory Research for Department of Optoelectronics; Silesian University of Technology (05/040/BK_18/0023); the Polish Ministry of Science and Higher Education for Institute for Chemical Processing of Coal (11.15.024) and for the Faculty of Chemistry of Wrocław University of Science and Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.El-Khodary S.A., El-Enany G.M., El-Okr M., Ibrahim M. Preparation and characterization of microwave reduced graphite oxide for high-performance supercapacitors. Electrochim. Acta. 2014;150:269–278. doi: 10.1016/j.electacta.2014.10.134. [DOI] [Google Scholar]
- 2.Muhammad Hafiz S., Ritikos R., Whitcher T.J., Razib N., Bien D.C.S., Chanlek N., Nakajima H., Saisopa T., Songsiriritthigul P., Huang N.M., et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B Chem. 2014;193:692–700. doi: 10.1016/j.snb.2013.12.017. [DOI] [Google Scholar]
- 3.Kumar S., Baruah B., Kumar A. Tunable degree of oxidation through variation of H2O2 concentration and its effect on structural, optical and supercapacitive properties of graphene oxide powders synthesized using improved method. Mater. Today Commun. 2017;13:26–35. doi: 10.1016/j.mtcomm.2017.08.007. [DOI] [Google Scholar]
- 4.Drewniak S., Muzyka R., Stolarczyk A., Pustelny T., Kotyczka-Morańska M., Setkiewicz M. Studies of reduced graphene oxide and graphite oxide in the aspect of their possible application in gas sensors. Sensors. 2016;16:103. doi: 10.3390/s16010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma N., Sharma V., Jain Y., Kumari M., Gupta R., Sharma S.K., Sachdev K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017;376:1–5. doi: 10.1002/masy.201700006. [DOI] [Google Scholar]
- 6.Drewniak S., Pustelny T., Muzyka R., Konieczny G., Kałużyński P. The effect of oxidation and reduction processes of graphite on physicochemical properties of graphite oxide and reduced graphene oxide. Photonics Lett. Pol. 2014;6:130–132. doi: 10.4302/plp.2014.4.06. [DOI] [Google Scholar]
- 7.Wei M., Qiao L., Zhang H., Karakalos S., Ma K., Fu Z., Swihart M.T., Wu G. Engineering reduced graphene oxides with enhanced electrochemical properties through multiple-step reductions. Electrochim. Acta. 2017;258:735–743. doi: 10.1016/j.electacta.2017.11.120. [DOI] [Google Scholar]
- 8.Chua C.K., Sofer Z., Pumera M. Graphite oxides: Effects of permanganate and chlorate oxidants on the oxygen composition. Chem. Eur. J. 2012;18:13453–13459. doi: 10.1002/chem.201202320. [DOI] [PubMed] [Google Scholar]
- 9.Pei S., Cheng H.-M. The reduction of graphene oxide. Carbon. 2012;50:3210–3228. doi: 10.1016/j.carbon.2011.11.010. [DOI] [Google Scholar]
- 10.Stankovich S., Dikin D.A., Piner R.D., Kohlhaas K.A., Kleinhammes A., Jia Y., Wu Y., Nguyen S.T., Ruoff R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45:1558–1565. doi: 10.1016/j.carbon.2007.02.034. [DOI] [Google Scholar]
- 11.Drewniak S., Pustelny T., Muzyka R., Plis A. Studies of physicochemical properties of graphite oxide and thermally. Pol. J. Chem. Technol. 2015;17:109–114. doi: 10.1515/pjct-2015-0076. [DOI] [Google Scholar]
- 12.Akhavan O., Ghaderi E., Abouei E., Hatamy S., Ghasemi E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon. 2014;66:395–406. doi: 10.1016/j.carbon.2013.09.015. [DOI] [Google Scholar]
- 13.Diez N., Ś͆liwak A., Gryglewicz S., Grzyb B., Gryglewicz G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015;5:81831–81837. doi: 10.1039/C5RA14461B. [DOI] [Google Scholar]
- 14.Dong L., Yang J., Chhowalla M., Loh K.P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 2017;46:7306–7316. doi: 10.1039/C7CS00485K. [DOI] [PubMed] [Google Scholar]
- 15.Tran M.-H., Jeong H.K. Influence of the Grain Size of Precursor Graphite on the Synthesis of Graphite Oxide. New Phys. Sae Mulli. 2013;63:206–209. doi: 10.3938/NPSM.63.206. [DOI] [Google Scholar]
- 16.Tran M.-H., Yang C.-S., Yang S., Kim I.-J., Jeong H.K. Influence of graphite size on the synthesis and reduction of graphite oxides. Curr. Appl. Phys. 2014;14(Suppl. 1):S74–S79. doi: 10.1016/j.cap.2013.11.038. [DOI] [Google Scholar]
- 17.Muzyka R., Kwoka M., Smędowski Ł., Diez N., Gryglewicz G. Oxidation of graphite by different modified Hummers methods. Xinxing Tan Cailiao/New Carbon Mater. 2017;32:15–20. doi: 10.1016/S1872-5805(17)60102-1. [DOI] [Google Scholar]
- 18.Kubesa O., Horackova V., Moravec Z., Farka Z., Skladal P. Graphene and graphene oxide for biosensing. Monatshefte Chem. 2017;148:1937–1944. doi: 10.1007/s00706-017-2019-4. [DOI] [Google Scholar]
- 19.Ferrari A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007;143:47–57. doi: 10.1016/j.ssc.2007.03.052. [DOI] [Google Scholar]
- 20.Kaniyoor A., Ramaprabhu S. A raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012;2:1–12. doi: 10.1063/1.4756995. [DOI] [Google Scholar]
- 21.Kumar R., Mehta B.R., Bhatnagar M., S R., Mahapatra S., Salkalachen S., Jhawar P. Graphene as a transparent conducting and surface field layer in planar Si solar cells. Nanoscale Res. Lett. 2014;9:1–9. doi: 10.1186/1556-276X-9-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L., Cheng L. Graphite Oxide under High Pressure: A Raman Spectroscopic Study. J. Nanomater. 2013;2013:1–5. doi: 10.1155/2013/731875. [DOI] [Google Scholar]
- 23.Ni Z., Wang Y., Yu T., Shen Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008;1:273–291. doi: 10.1007/s12274-008-8036-1. [DOI] [Google Scholar]
- 24.Beams R., Gustavo Cançado L., Novotny L. Raman characterization of defects and dopants in graphene. J. Phys. Condens. Matter. 2015;27:1–26. doi: 10.1088/0953-8984/27/8/083002. [DOI] [PubMed] [Google Scholar]
- 25.Vecera P., Eigler S., Kolesnik-Gray M., Krstic V., Vierck A., Maultzsch J., Schäfer R.A., Hauke F., Hirsch A. Degree of functionalisation dependence of individual Raman intensities in covalent graphene derivatives. Sci. Rep. 2017;7:1–7. doi: 10.1038/srep45165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Díaz D., López Holgado M., García-Fierro J.L., Velázquez M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C. 2017;121:20489–20497. doi: 10.1021/acs.jpcc.7b06236. [DOI] [Google Scholar]
- 27.Nguyen V.T., Le H.D., Nguyen V.C., Ngo T.T.T., Nguyen X.N., Phan N.M. Synthesis of multi-layer graphene films on copper tape by atmospheric pressure chemical vapor deposition method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013;4:1–5. doi: 10.1088/2043-6262/4/3/035012. [DOI] [Google Scholar]
- 28.Muzyka R., Drewniak S., Pustelny T., Gryglewicz G., Smędowski Ł. RAMAN spectroscopic study of graphite oxide obtained from different graphite precursors. Proc. SPIE. 2016;10034:1–7. doi: 10.1117/12.2244461. [DOI] [Google Scholar]
- 29.Lebiedziewski M., Szudek M. Wyroby z Węgla i Grafitu. WNT; Warszawa, Poland: 1970. [Google Scholar]
- 30.Ferrari A.C., Robertson J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 2000;61:14095–14107. doi: 10.1103/PhysRevB.61.14095. [DOI] [Google Scholar]
- 31.Calizo I., Teweldebrhan D., Bao W., Miao F., Lau C.N., Balandin A.A. Spectroscopic Raman nanometrology of graphene and graphene multilayers on arbitrary substrates. J. Phys. Conf. Ser. 2008;109:1–4. doi: 10.1088/1742-6596/109/1/012008. [DOI] [Google Scholar]
- 32.Ferrari A.C., Meyer J.C., Scardaci V., Casiraghi C., Lazzeri M., Mauri F., Piscanec S., Jiang D., Novoselov K.S., Roth S., et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2008;97:1–4. doi: 10.1103/PhysRevLett.97.187401. [DOI] [PubMed] [Google Scholar]
- 33.Malard L.M., Pimenta M.A., Dresselhaus G., Dresselhaus M.S. Raman spectroscopy in graphene. Phys. Rep. 2009;473:51–87. doi: 10.1016/j.physrep.2009.02.003. [DOI] [Google Scholar]
- 34.Gayathri S., Jayabal P., Kottaisamy M., Ramakrishnan V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014;4:1–12. doi: 10.1063/1.4866595. [DOI] [Google Scholar]
- 35.Toh S.Y., Loh K.S., Kamarudin S.K., Daud W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014;251:422–434. doi: 10.1016/j.cej.2014.04.004. [DOI] [Google Scholar]
- 36.Charlier J.-C., Eklund P.C., Zhu J., Ferrari A.C. Electron And Phonon Properties Of Graphene: Their Relationship With Carbon Nanotubes. Volume 111. Springer; Berlin/Heidelberg, Germany: 2007. pp. 673–709. [Google Scholar]
- 37.Nekahi A., Marashi P.H., Haghshenas D. Transparent conductive thin film of ultra large reduced graphene oxide monolayers. Appl. Surface Sci. 2014;295:59–65. doi: 10.1016/j.apsusc.2014.01.004. [DOI] [Google Scholar]