Abstract
Background:
Many patients who receive chronic hemodialysis have a limited life expectancy comparable to that of patients with metastatic cancer. However, patterns of home palliative care use among patients receiving hemodialysis are unknown.
Objectives:
We aimed to undertake a current-state analysis to inform measurement and quality improvement in palliative service use in Ontario.
Methods:
We conducted a descriptive study of outcomes and home palliative care use by Ontario residents maintained on chronic dialysis using multiple provincial healthcare datasets. The period of study was the final year of life, for those died between January 2010 and December 2014.
Results:
We identified 9611 patients meeting inclusion criteria. At death, patients were (median [Q1, Q3] or %): 75 (66, 82) years old, on dialysis for 3.0 (1.0-6.0) years, 41% were women, 65% had diabetes, 29.6% had dementia, and 13.9% had high-impact neoplasms, and 19.9% had discontinued dialysis within 30 days of death. During the last year of life, 13.1% received ⩾1 home palliative services. Compared with patients who had no palliative services, those who received home palliative care visits had fewer emergency department and intensive care unit visits in the last 30 days of life, more deaths at home (17.1 vs 1.4%), and a lower frequency of deaths with an associated intensive care unit stay (8.1 vs 37.8%).
Conclusions:
Only a small proportion of patients receiving dialysis in Ontario received support through the home palliative care system. There appears to be an opportunity to improve palliative care support in parallel with dialysis care, which may improve patient, family, and health-system outcomes.
Keywords: palliative care, end-of-life care, health service utilization, home care, chronic dialysis, integrated care
Abrégé
Contexte:
L’espérance de vie de bon nombre de patients traités par hémodialyse chronique se compare à celle des patients atteints d’un cancer métastatique. Cependant, les tendances d’utilisation des soins palliatifs à domicile chez les patients hémodialysés sont encore peu connues.
Objectif de l’étude:
Nous souhaitions faire une analyse de l’état actuel des choses afin d’éclairer sur la mesure et l’amélioration de la qualité des soins palliatifs en Ontario.
Méthodologie:
Nous avons mené une étude descriptive des issues pour les patients et de l’utilisation des soins palliatifs à domicile chez les patients hémodialysés en Ontario. Plusieurs ensembles de données provinciales en soins de santé ont été employés pour procéder à l’analyse. La dernière année de vie des patients décédés entre janvier 2010 et décembre 2014 a constitué la période étudiée.
Résultats:
Les patients satisfaisant les critères d’inclusion étaient au nombre de 9 611. La cohorte était constituée à 41 % de femmes. Au moment du décès, l’âge médian (Q1; Q3) des patients était de 75 ans (66; 82 ans) et la médiane de la durée des traitements d’hémodialyse était de trois ans en moyenne (1,0; 6,0 ans). Parmi les comorbidités recensées au décès, 65 % des patients étaient aussi diabétiques, environ un tiers (29,3 %) étaient atteints de démence et 13,9 % présentaient des néoplasmes. Dans les 30 jours précédant leur décès, 19,9 % des patients avaient cessé leurs traitements de dialyse. Au cours de la dernière année de vie, seulement 13,1 % des patients de la cohorte avaient reçu au moins un service de soins palliatifs à domicile. Lorsque comparés aux patients n’ayant reçu aucun service en soins palliatifs, ils se sont moins souvent présentés aux urgences et ont moins souvent séjourné dans les unités de soins intensifs. De plus, une plus grande proportion des patients ayant reçu des soins palliatifs sont décédés à domicile, soit 17,1 % contre 1,4 % des patients n’ayant reçu aucun service en soins palliatifs. Enfin, le taux de mortalité associé à un séjour aux soins intensifs s’est avéré bien inférieur chez les patients qui avaient reçu des soins palliatifs, soit 8,1 % contre 37,8 % pour les patients n’ayant reçu aucun service de soins palliatifs.
Conclusion:
En Ontario, une très faible proportion des patients hémodialysés a reçu du soutien par l’entremise du système de soins palliatifs à domicile au cours de la période étudiée. Il semble donc y avoir une possibilité d’améliorer l’offre de soins palliatifs parallèlement aux traitements de dialyse; et ceci pourrait avoir une incidence positive sur les patients et leurs proches, de même que sur le système de santé.
What was known before
Patterns of palliative service use and associated outcomes among Canadians on chronic dialysis were previously unknown.
What this adds
In this study, we found very low overall rates of home palliative service use among Ontarians on chronic dialysis. Patients who died while on chronic dialysis used acute inpatient, emergency, and intensive care services. Measures reported in this study can be used as a baseline against which to track future improvements in palliative and end-of-life care provided to chronic dialysis patients.
Introduction
Accessible and high-quality palliative care remains a Canadian national priority. Since the recognition of suboptimal palliative care capacity 2 decades ago,1 policy reforms and, ultimately, the Canadian Federal Health Accord of 2003 led to major federal and provincial investments. These were aimed at shifting end-of-life care from acute care hospitals to preferred settings, by enhancing system capacity and improving the coordination of care.2,3 In Ontario, efforts to bolster the palliative care system have largely focused on cancer patients, who have historically constituted 85% of palliative care referrals.4 The establishment of Cancer Care Ontario in 1997 led to a formal End-of-Life Care Strategy and the creation of various structures and collaborations that have translated into better outcomes and less resource use.4 In 2011, the Declaration of Partnership and Commitment to Action was developed and articulated a shared vision of palliative care in Ontario that was disease agnostic.5
End-stage kidney disease (ESKD) is a debilitating condition with limited life expectancy and symptom burden that rivals that of many cancers.6 Recognizing the progressive nature of kidney disease, and the high burden of concomitant frailty and comorbidity in the ESKD population, all aspects of palliative care planning and delivery have become a focus of attention in the kidney community.
The Ontario Renal Network (ORN) oversees the funding and quality of kidney care services for patients in Ontario with chronic kidney disease (CKD). Over 11 000 Ontario residents receive some form of dialysis care. Through engagement of patients and other stakeholders, the ORN has developed a 5-year strategic plan, published in the 2015-2019 Ontario Renal Plan (ORP), to improve access to palliative care services; enhance kidney health-system integration with primary care, home, and community services; and improve provider competency in facilitating patient education and shared decision-making.7 To guide quality improvement initiatives and obtain baseline measures against which to track progress, we undertook a current-state analysis of home palliative care (hPC) service use and outcomes in Ontario’s dialysis population.
Methods
Study Design and Setting
We conducted a retrospective descriptive cohort study using linked healthcare databases held at the Institute for Clinical Evaluative Sciences (ICES) in Ontario. Ontario residents receive universal healthcare with most encounters captured in administrative data. Funded palliative services range from home care (nurse, physician, personal support worker, or multidisciplinary teams), palliative care clinics, hospice care facilities, and inpatient palliative consultative services. Given the central role of home services in Ontario’s palliative care strategies, we restricted our analyses to administrative codes representing hPC services. This study was approved by the research ethics board at Sunnybrook Health Sciences Centre (Toronto, Canada). Manuscript preparation adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Appendix A).8
We used an integrated knowledge translation approach to develop our research protocol and interpret our findings with the Ontario Renal Network Palliative Care Priority Panel—the advisory group that oversees the provincial renal palliative care strategy and includes palliative care physicians, the full spectrum of healthcare provider stakeholders, administrators, patients, and families.
Data Sources
We ascertained baseline characteristics and outcomes using a variety of linked databases (details below and Appendix B). We obtained death date and other vital statistics from the Registered Persons Database. We identified individuals on chronic dialysis using the Canadian Organ Replacement Register (CORR) and the Ontario Renal Reporting System (ORRS). We captured nurse and physician hPC codes in the Ontario Health Insurance Plan (OHIP), Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), National Ambulatory Care Reporting System (NACRS), Home Care Database (HCD), Continuing Care Reporting System (CCRS), Resident Assessment Instrument (RAI)–Contact Assessment (CA), and RAI–Home Care (HC). All databases were linked using unique encoded identifiers and analyzed at ICES.
Population
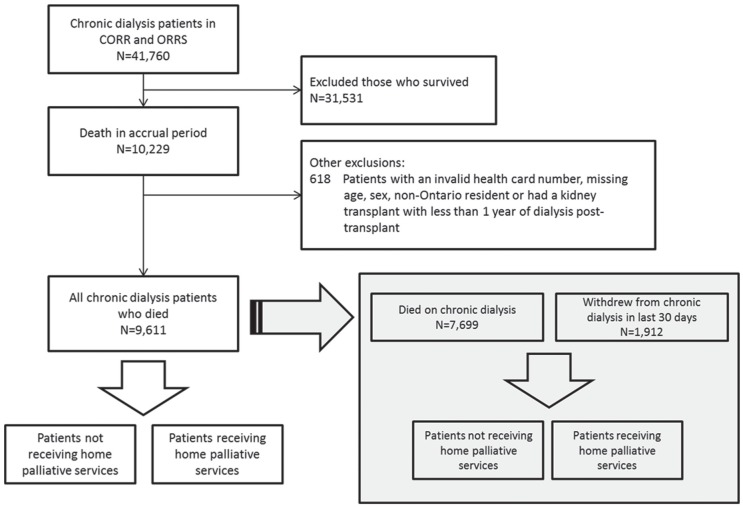
We identified chronic dialysis patients who died between January 1, 2010, and December 31, 2014, in CORR and ORRS, and used their death date as the index date. We looked back 365 days from this index date to determine their trajectory of care in the last year of life. Patients who had received a kidney transplant required a minimum survival on dialysis of at least one year after transplant failure before they could enter the cohort. We reported outcomes separately for patients who received and did not receive hPC services in the last year of life. We also identified a subgroup of patients who electively discontinued dialysis within 30 days of death (Figure 1). Home palliative care service use was defined as receiving at least one administrative code from OHIP, HCD, RAI-CA, or RAI-HC at any time in the last year of life (Appendix C). We counted the number of days that a patient had received at least one palliative care code to define a palliative visit (ie, multiple codes on the same day were counted as a single encounter). We did not count hospital or clinic-based palliative care visits in the main analysis. We ascertained home deaths using OHIP codes.
Figure 1.
Cohort flow diagram.
Note. CORR = Canadian Organ Replacement Register; ORRS = Ontario Renal Reporting System.
Variables
We ascertained demographic information at the time of death. We looked back to 1981 in CORR (inception) to identify complete dialysis history. We defined dialysis discontinuation using ORRS codes for patients marked as having withdrawn from dialysis, counting only those who died within 30 days of dialysis discontinuation to limit misclassification where dialysis was stopped due to kidney recovery.
We captured comorbidities within 2 years of death using OHIP, CIHI-DAD, and NACRS. We used the Johns Hopkins Adjusted Clinical Group (ACG) system to score comorbidity.9 The ACG estimates an individual’s expected service use. The International Classification of Diseases, Ninth Revision (ICD-9) and The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes are categorized into 32 groups, called Ambulatory Diagnostic Groups (ADGs), then further reduced to 12 “Collapsed ADGs” or Collapsed Aggregated Diagnosis Groups (CADGs).10
A panel of content experts prioritized candidate outcomes based on importance and feasibility, given available data. These outcomes were (1) emergency department (ED) visits, (2) intensive care unit (ICU) visits, (3) time to death (starting at dialysis initiation), and (4) place of death. Place of death was coded as ICU, acute care hospital, or ED (counted together because most patients that died in hospital had a corresponding ED visit), long-term care, complex continuing care, home, or unattended death/unknown. If a patient had an admission to ICU, hospital, and Emergency Room (ER), they were considered to have had a hospital death with an ICU stay. We coded patients as having died at home only if they had an associated OHIP code for pronouncement of death at home.
Statistical Analyses
We reported baseline characteristics as frequencies and proportions, medians and interquartile ranges (IQRs) as appropriate. We compared patient characteristics using standardized differences across categories of hPC service use and considered a difference of ⩾10% significant. We reported outcomes as counts and proportions, and medians with corresponding first/third quartiles for continuous variables. We used SAS software, version 9.4 (SAS Institute Inc, Cary, North Carolina) for statistical analyses.
Results
Patient Characteristics
We identified 9611 chronic dialysis patients (Figure 1) who died between 2010 and 2014. Sociodemographic characteristics, dialysis modalities, and comorbidities did not differ by year of death (data not shown). The characteristics of the entire cohort are presented in Table 1. At the time of death, the median age in the overall cohort was 75.0 years, 40.6% were women, and 11.5% resided in rural locations. Comorbid conditions included high rates of diabetes (65.0%), ischemic heart disease (55.3%), congestive heart failure (55.5%), and dementia (29.6%). Patients had received dialysis for a median of 3.0 years prior to death, and 4.0% had a history of transplant, with a median time from transplant to reinitiation of chronic dialysis being 13.0 years (IQR: 13). A total of 912 (19.9%) discontinued dialysis in the last 30 days of life.
Table 1.
Patient Characteristics at Time of Death in Overall 2010 to 2014 Cohort, As Well As Stratified by Patients Receiving/Not Receiving at Least One Home Care Palliative Care Visit in the Last Year of Life With Associate Standardized Differences.
| Characteristic | Total |
At least one home care palliative service in last 365 days of life |
No home care palliative service in last 365 days of life |
Standardized differencea |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | % | |
| Total chronic dialysis patients | 9611 (100.0) | 1258 (13.1) | 8353 (87.0) | — |
| Age | ||||
| Median (25th-75th percentiles) | 75.0 (66.0-82.0) | 77.0 (68.0-83.0) | 75.0 (65.0-82.0) | 14.3 |
| Women | 3898 (40.6) | 503 (40.0) | 3395 (40.6) | 1.3 |
| Rural residenceb | 1101 (11.5) | 182 (14.5) | 919 (11.0) | 10.4 |
| Income quintilesc | ||||
| 1 (lowest) | 2369 (24.7) | 268 (21.3) | 2101 (25.2) | 9.1 |
| 2 | 2137 (22.2) | 238 (18.9) | 1899 (22.7) | 9.4 |
| 3 | 1823 (19.0) | 247 (19.6) | 1576 (18.9) | 2.0 |
| 4 | 1771 (18.4) | 255 (20.3) | 1516 (18.2) | 5.4 |
| 5 (highest) | 1511 (15.7) | 250 (19.9) | 1261 (15.1) | 12.6 |
| Johns Hopkins Expanded Diagnosis Clusters | ||||
| Cerebrovascular disease | 2538 (26.4) | 293 (23.3) | 2245 (26.9) | 8.3 |
| Congestive heart failure | 5331 (55.5) | 637 (50.6) | 4694 (56.2) | 11.2 |
| Dementia & delirium | 2848 (29.6) | 360 (28.6) | 2488 (29.8) | 2.6 |
| Ischemic heart disease | 5319 (55.3) | 620 (49.3) | 4699 (56.3) | 14.0 |
| High impact malignant neoplasmsd | 1337 (13.9) | 500 (39.8) | 837 (10.0) | 73.2 |
| Low impact malignant neoplasmsd | 1284 (13.4) | 357 (28.4) | 927 (11.1) | 44.5 |
| Peripheral vascular disease | 2394 (24.9) | 244 (19.4) | 2150 (25.7) | 15.2 |
| Diabetes | 6242 (65.0) | 721 (57.3) | 5521 (66.1) | 18.1 |
| John Hopkins Adjusted Clinical Group comorbidity score | ||||
| Median (25th-75th percentiles) | 14.0 (12.0-16.0) | 15.0 (12.0-17.0) | 14.0 (11.0-16.0) | 18.6 |
| Comorbidities 2 years from deathe: | ||||
| Immobilityf | 75 (0.8) | 12 (1.0) | 63 (0.8) | 2.2 |
| Lower leg amputation | 694 (7.2) | 54 (4.3) | 640 (7.7) | 14.2 |
| Myocardial infarction | 2239 (23.3) | 191 (15.2) | 2048 (24.5) | 23.6 |
| Gastrointestinal hemorrhage | 1000 (10.4) | 127 (10.1) | 873 (10.5) | 1.2 |
| Time on chronic dialysis (years) | ||||
| Median (25th-75th percentiles) | 3.0 (1.0-6.0) | 2.0 (1.0-5.0) | 3.0 (1.0-6.0) | 13.6 |
| Cause of end-stage renal disease | ||||
| Diabetes | 1581 (16.5) | 119 (9.5) | 1462 (17.5) | 23.7 |
| Vascular disease | 981 (10.2) | 131 (10.4) | 850 (10.2) | 0.8 |
| Glomerulonephritis | 425 (4.4) | 48 (3.8) | 377 (4.5) | 3.5 |
| Other | 266 (2.8) | 43 (3.4) | 223 (2.7) | 4.4 |
| Polycystic kidney disease | 120 (1.3) | 15 (1.2) | 105 (1.3) | 0.3 |
| Obstruction | 95 (1.0) | 11 (1.6) | 84 (1.0) | 1.4 |
| Missing | 6113 (63.6) | 886 (70.4) | 5227 (62.6) | 16.7 |
| Initial dialysis modality | ||||
| In-centre hemodialysis | 7151 (74.4) | 949 (75.4) | 6202 (74.3) | 2.7 |
| Peritoneal dialysis | ⩾806 (⩾8.4)g | ⩾77 (⩾6.1)g | 729 (8.7) | 10.0h |
| Home hemodialysis | ⩽17 (⩽0.2)g | ⩽5 (⩽0.4)g | 12 (0.1) | 0.5h |
| Missing | 1637 (17.0) | 227 (18.0) | 1410 (16.9) | 3.1 |
| Final dialysis modality | ||||
| In-centre hemodialysis | 7597 (79.0) | 983 (78.1) | 6614 (79.2) | 2.5 |
| Peritoneal dialysis | 477 (5.0) | 81 (6.4) | 396 (4.7) | 7.4 |
| Home hemodialysis | 49 (0.5) | 10 (0.8) | 39 (0.5) | 4.1 |
| Missing | 1344 (14.0) | 170 (13.5) | 1174 (14.1) | 1.6 |
| Kidney transplant recipient | 381 (4.0) | 47 (3.7) | 334 (4.0) | 1.4 |
Note. EDCs = Johns Hopkins Extended Diagnosis ClustersTM; CIHI-DAD = Canadian Institute for Health Information Discharge Abstract Database; ACG = Adjusted Clinical Group®; ESRD = end-stage renal disease; LTC = long-term care.
Standardized differences (SD) are less sensitive to sample size than traditional hypothesis tests. They provide a measure of the difference between groups divided by the pooled SD; a value greater than 10% is interpreted as a meaningful difference between groups. Some cell values were suppressed for the purposes of privacy and confidentiality.
Rural was defined as population < 10 000.
Income was categorized into fifths of average neighborhood income on death date. Income Quintile category 3 includes the missing values.
Malignant neoplasm categories were defined using Johns Hopkins Expanded Diagnosis Clusters (EDC), which are based on proprietary ICD codes.
Comorbidities were assessed by administrative database codes or by EDC in the previous 2 years from death
Immobility was defined using ICD-10 codes in CIHI-DAD: “R263,” “R2681,” “Z740.”
Number have been altered to suppress small cells.
Standardized difference has been changed to accommodate suppressed cells.
Home Palliative Care Service Use in the Final Year of Life
Among the 9611 chronic dialysis patients who died during the study period, 1258 (13.1%) received at least 1 hPC service in the last year of life. Compared with those who received no hPC, hPC recipients had fewer comorbidities, with the exception of concurrent malignancy (Tables 1 and 2). Among the 7699 patients who did not discontinue dialysis, 742 (9.6%) received hPC services in the last year of life. In contrast, 516 (27.0%) of those who discontinued dialysis received hPC services.
Table 2.
Comparison of Home Palliative Care Service Measures in the Last Year of Life and Outcomes Among Ontario Residents Who Died on or Discontinued Chronic Dialysis.
| Overall cohort |
Overall cohort by palliative care status (n = 9611) |
Patients who died while receiving dialysis (n = 7699) |
Patients who discontinued dialysis within the last 30 days of life (n = 1912) |
||||
|---|---|---|---|---|---|---|---|
| Both palliative and nonpalliative | Not receiving palliative care in the last 365 days | Receiving palliative care in the last 365 days | Not receiving palliative care in the last 365 days | Receiving palliative care in the last 365 days | Not receiving palliative care in the last 365 days | Receiving palliative care in the last 365 days | |
| Number of patients | 9611 | 8353 | 1258 | 6957 | 742 | 1396 | 516 |
| ED visits | |||||||
| In last 14 days | 4239 (44.1) | 3776 (45.2) | 463 (36.8) | 3329 (47.9) | 317 (42.7) | 447 (32.0) | 146 (28.3) |
| In last 30 days | 5771 (60.0) | 5084 (60.9) | 687 (54.6) | 4326 (62.2) | 423 (57.0) | 758 (54.3) | 264 (51.2) |
| ICU admissions | |||||||
| In last 14 days | 3503 (36.4) | 3349 (40.1) | 154 (12.2) | 2957 (42.5) | 105 (14.2) | 392 (28.1) | 49 (9.5) |
| In last 30 days | 3687 (38.4) | 3500 (41.9) | 187 (14.9) | 3075 (44.2) | 123 (16.6) | 425 (30.4) | 64 (12.4) |
| Survival time, years | |||||||
| Median (25th-75th percentiles) | 2.7 (0.9, 5.6) | 2.8 (0.9, 5.8) | 2.0 (0.8, 4.7) | 2.8 (1.0, 5.8) | 1.9 (0.8, 4.4) | 2.9 (0.8, 5.7) | 2.4 (0.8, 5.2) |
| Location of death | |||||||
| ICU | 3256 (33.9) | 3154 (37.8) | 102 (8.1) | 2826 (40.6) | 78 (10.5) | 328 (23.5) | 24 (4.7) |
| Acute care hospital or EDa | 3484 (36.3) | 3094 (37.0) | 390 (31.0) | 2503 (36.0) | 272 (36.7) | 591 (42.3) | 118 (22.9) |
| LTC | 592 (6.2) | 567 (6.8) | 25 (2.0) | 359 (5.2) | 15 (2.0) | 208 (14.9) | 10 (1.9) |
| CCC | 750 (7.8) | 640 (7.7) | 110 (8.7) | 427 (6.1) | 57 (7.7) | 213 (15.3) | 53 (10.3) |
| Home | 328 (3.4) | 113 (1.4) | 215 (17.1) | 101 (1.5) | 101 (13.6) | 12 (0.9) | 114 (22.1) |
| Other/unknown | 1201 (12.5) | 785 (9.4) | 416 (33.1) | 741 (10.7) | 219 (29.5) | 44 (3.2) | 197 (38.2) |
Note. ED = emergency department; ICU = intensive care unit; LTC = long-term care; CCC = complex continuing care. All values expressed as n (%) unless otherwise specified. Survival time is defined as the number of years between the first-ever ESKD service date and death date; presented values are median (25th, 75th percentile).
Acute care hospital and ED visits were combined to avoid small cells, and for the purposes of ensuring privacy and confidentiality.
Of those who received hPC services, only 382 (30.3%) had one or more visits in the period 31 to 365 days prior to death, while the majority (n = 1036, 82.4%) had visit(s) only in the last 30 days of life. Among those who received an hPC visit, most received a single such visit (n = 685, 54.4% of those receiving hPC) often within the last 30 days of life (n = 876, 69.6%). A total of 564 (44.8%) of these patients received their first visit in the 7 days prior to death.
ED Visits
The proportion of all dialysis patients who died and had ⩾1 ED visit in the last 14 and 30 days of life was 44.1% and 60.0%, respectively (Table 2). Patients had fewer ED visits in the last year of life if they had hPC services.
ICU Admissions
Patients who received hPC services had fewer admissions to ICU in the last days of life (12.2 and 14.9% within 14 and 30 days, respectively), compared with patients who did not receive hPC (40.1 and 41.9% within 14 and 30 days, respectively).
Time to Death
The median (IQR) time on dialysis before death was 2.7 (4.7) years for all patients (n = 9611) (Table 2). Patients who had received hPC services had a marginally shorter time on dialysis (median: 2.0 years; IQR: 3.9) than those who did not have any palliative care visits (median: 2.8 years; IQR: 4.9). Similar results were seen for those who discontinued dialysis. The median time to death from discontinuation of dialysis was 4 days (IQR: 6).
Location of Death
In the overall cohort, 70.2% of patients died in the ED, acute care hospital, or ICU. Patients who received one or more hPC visits (vs none) were more likely to die at home (17.1 vs 1.4%). Patients who received hPC in the last year had a higher rate of deaths in unclassified locations (33.1%) compared with those who received no hPC (9.4%).
Sensitivity Analyses
We repeated the main analysis after restricting the observation window for hPC services to the final 30 days of life. All results were qualitatively similar in direction and magnitude as in the main analysis in which we captured hPC service use in the last 365 days of life. In addition, we analyzed the data using inpatient palliative care service visits (DAD and OHIP). Results were similar for most comparisons. The details of these analyses are available upon request.
Subgroup Analysis
Among the 1912 patients who discontinued dialysis, 516 (27.0%) received hPC in the final 365 days of life. Compared with those who had no hPC, those who received hPC had similar rates of ED visits, but lower rates of ICU admission (30.4 vs 12.4%) and fewer deaths in ICU (23.5 vs 4.7%) or acute care hospital/ED (42.3 vs 22.9).
Discussion
We report a current-state analysis of hPC use among Ontario residents maintained on chronic dialysis. We found that hPC services are infrequently used in this population and that, in most cases, used only within the last days of life.
Our findings suggest that at present, there is infrequent and late collaboration with home-based palliative care teams. We found, much like other studies,11-14 that palliative services were mostly used in end-of-life situations. Even when death is anticipated, patients most often do not appear to receive palliative care. In our cohort, only 27% of patients who discontinued dialysis received home palliative services in the last year of life. In their series, Couchoud et al found that among patients who discontinued dialysis while in hospital, 90% were not admitted to designated palliative care beds.15 While various patient, hospital, and system-level barriers may limit patients’ access to palliative services, provider attitudes and practices are likely major determinants of palliative care use. In a questionnaire-based study of European nephrologists, only 10% reported involving palliative care services when they started discussions around dialysis discontinuation.14 In a recent survey of Ontario renal providers (nurses, physicians and social workers), the provincial mean score on a subscale measuring providers’ propensity to engage community palliative services was only 2.5 out of 5.0, suggesting room for improvement (unpublished data, Ontario Renal Network).
A large proportion of patients in our cohort had ED and ICU visits during their final days of life. This is consistent with the high rate of cardiovascular and infection-related death in the dialysis-treated population.16 Patients who received hPC had fewer ED and ICU visits compared with those who did not. However, in this descriptive study, we did not adjust for confounders that might have accounted for these differences. Interestingly, patients who received hPC had a higher rate of high-impact neoplasia compared with those who did not (39.8 vs 10.0%), suggesting that they may have accessed hPC through the cancer care system.
Developing disease-agnostic palliative care services is a priority in Ontario. These services are intended to extend beyond end-of-life care and provide multidisciplinary symptom and existential support to individuals with life-limiting, progressive, and burdensome disease. When optimally used, palliative care is introduced early in the disease trajectory and is provided in conjunction with disease modifying treatments.17-19 As the disease progresses along the trajectory, the focus of care shifts, gradually, from disease-modification to symptom management and focuses largely on the individual patient’s experience. ESKD is well recognized as a serious and life-limiting illness with a high symptom burden. Treatments often centre around dialysis care, with until recently, little focus on symptom management or the patient experience. We observed that, in Ontario, palliative care was not being used in this way, with most hPC recipients having only 1 hPC visit. This finding suggests it is unlikely patients have sufficient time to establish a trusting relationship with the palliative care team with, likely, a higher reliance on care provided in the acute care setting. We also found a high rate of deaths occurring in hospital, often in association with an ICU stay. Although it is not possible from these data to tease out those deaths that were unexpected from those potentially anticipated, it is likely that a substantial proportion of these patients had predicable chronic disease progression and may have benefitted from hPC services at an earlier stage of their disease trajectory.
Our study has recognized limitations. Our findings may not be generalizable outside of Ontario. We used administrative data, which did not allow for analysis of patient and family experience, quality and appropriateness of care, and the specific types of services provided. We did not analyze referral sources (eg, primary care versus nephrology). Death location was unconfirmed in up to 38% of patients, as it could only be ascertained through an OHIP claim for pronouncement of death at home, or if the death occurred in an institution. However, it is very likely that most undocumented deaths occurred in the home setting; hence, home deaths likely occurred in 16% for the overall cohort and 50% for patients who had one or more hPC encounter, which is in keeping with patients’ preferences as captured in surveys.20
These limitations notwithstanding, our findings identify opportunities for improvement and provide baseline measures for gauging the success of future efforts. In a comprehensive provincial strategy, we hope to improve patients’ access to and experience of care by introducing interventions including formalized goals of care assessments, patient and provider education, standardized care pathways, and team-based approaches and by improving collaboration with primary care.21 Developing these interventions in partnership with patients and families, partner agencies,5 and the primary care community will be critical to the success of the provincial renal palliative care strategy.
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES) Western site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine & Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). This project was conducted with members of the provincial ICES Kidney, Dialysis and Transplantation Research Program (www.ices.on.ca/kdt), at the ICES Western facility, which receives programmatic grant funding from the Canadian Institutes of Health Research (CIHR) in collaboration with the ORN. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information. The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, CIHR, or the MOHLTC is intended or should be inferred. Dr. Amit Garg is supported by the Dr. Adam Linton Chair in Kidney Health Analytics. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Appendix
Appendix A.
STROBE Criteria.
| Item No. | Recommendation | Reported | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | Title Page |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | Abstract | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | Introduction |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | Introduction (last paragraph) |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | Methods |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | Methods |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | Methods |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | NA | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | Methods |
| Data sources/ Measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | Methods |
| Bias | 9 | Describe any efforts to address potential sources of bias | NA |
| Study size | 10 | Explain how the study size was arrived at | Methods |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the anayses. If applicable, describe which groupings were chosen and why | Methods |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | Methods |
| (b) Describe any methods used to examine subgroups and interactions | Methods | ||
| (c) Explain how missing data were addressed | |||
| (d) If applicable, explain how loss to follow-up was addressed | NA | ||
| (e) Describe any sensitivity analyses | NA | ||
| Results | |||
| Participants | 13 | (a) Report numbers of individuals at each stage of study—eg, numbers potentially eligible, examined for eligibilty, confirmed eligible, included in the study, completing follow-up, and analysed | Figure 1 and Results |
| (b) Give reasons for nonparticipation at each stage | Figure 1 | ||
| (c) Consider use of a flow diagram | Figure 1 | ||
| Descriptive data | 14 | (a) Give charchteristics of study participants (eg, demographic, clinical, social) and information on exposures and potential confounders | Results (Patient Characteristis) |
| (b) Indicate number of participants with missin data for each variable of interest | NA | ||
| (c) Summarize follow-up time (eg, average and total amount) | NA | ||
| Outcome data | 15 | Report numbers of outcome events or summary measures over time | Results and Table 1 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Results and Table 1 |
| (b) Report category boundaries when continuous variables were categorized | Results and Table 1 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | NA | ||
| Other analyses | 17 | Report other analyses done—eg,analyses of subgroups and interactions, and sensitivity analyses | Results and Table 1 |
| Discussion | |||
| Key results | 18 | Summarize key results with reference to study objectives | Discussion |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuus both direction and magnitude of any potential bias | Discussion |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | Discussion |
| Generalizability | 21 | Discuss the gerneralisability (external validity) of the study results | Discussion |
| Other Information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | Funding |
Note. STROBE = Strengthening the Reporting of Observational Studies in Epidemiology.
Appendix B.
Description of Databases.
| Type of health services | Database | Description |
|---|---|---|
| Kidney care | ||
| Chronic dialysis | Canadian Organ Replacement Register | Registry tracking the long-term trends of vital organ transplantation, donations, and dialysis activities. |
| Ontario Renal Reporting System | Database of predialysis, acute dialysis, and chronic dialysis patients in Ontario. | |
| Acute care | ||
| Hospital inpatient | Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) | Patient-level administrative, clinical, and demographic data on hospital discharges from all acute facilities in Ontario |
| Emergency department | National Ambulatory Care Reporting System (NACRS) | Patient demographics and clinical, administrative, and service-specific data related to emergency department visits |
| Physician billings | Ontario Health Insurance Plan (OHIP) Claims Database | Claims data paid for by the Ontario Health Insurance Plan for approximately 98% of physicians in Ontario who claim under OHIP. |
| Continuing care | ||
| Long-term care | Continuing Care Reporting System (CCRS) | The CCRS is a database of clinical and demographic information on residents receiving facility based long-term care in Ontario. Contains information on over 600 publicly funded residential care homes with 24-hour nursing care |
| Complex continuing care | CCRS | The CCRS also provides data on hospitalized patients who are deemed to be in a nonacute, alternate level of care, receiving continuing care services. |
| Home care | Home Care Database (HCD) | Data from the Ontario Association of Community Care Access Centres, responsible for providing publicly funded home care. Client-level data for all of those receiving home care services from these facilities. |
| Resident Assessment Instrument (RAI)–Home Care (HC) | Person-centered assessment system used in home and community-based settings typically among those receiving formal care of supportive services. Dataset captures an individual’s functioning and quality of life | |
| RAI-Contact Assessment (CA) | A short screening assessment completed at the time of intake into home care services. | |
Appendix C.
Palliative Care Codes by Administrative Database.
| Type | Database | Code |
|---|---|---|
| Acute care services | CIHI-DAD | PATSERV = 58, Main patient service, palliative care |
| OHIP | C945, Special palliative care consult hospital inpatient | |
| C882, Terminal care in-hospital general practitioner/family practitioner | ||
| C982, Palliative care | ||
| K023, Palliative care support individual care 1/2 h or major part | ||
| Home care services | RAI-CA | B2c = 1, Referral to initiate or continue palliative services = Yes |
| B4 = 12, Expected residential/living status during service provision = Hospice facility/palliative care unit | ||
| RAI-HC | P2S = 1 or 2, Special Treatments, Therapies, Programs—Hospice Care= Scheduled, full adherence as prescribed OR Scheduled, partial adherence | |
| CC3f = 1, Understanding of Goals of Care—Palliative Care = Yes | ||
| OHIP | B966, Travel Premium—Palliative Care Home Visit | |
| B998, Special visit palliative care home, days or evenings | ||
| C997, Special visit palliative care home, days or evenings (starting Oct 2009) | ||
| G511, Telephone management of palliative care at home | ||
| HCD: Clients | SRC_admission = 95, Service Care goals at time of submission for open admission= End of Life (In-Home) | |
| Service_RPC = 95, Service Recipient Code associated with the care delivery event = End of Life (In-Home) | ||
| SRC_discharge = 95, Service Care goals (service receipt code) at time of discharge = End of Life (In-Home) |
Note. CIHI-DAD = Canadian Institute for Health Information Discharge Abstract Database; OHIP = Ontario Health Insurance Plan; RAI-CA = Resident Assessment Instrument–Contact Assessment; RAI-HC = Resident Assessment Instrument–Home Care; HCD = Home Care Database.
Footnotes
Ethics Approval and Consent to Participate: This project has been approved by the Research Ethics Board at Sunnybrook Health Sciences Centre, Toronto, Canada.
Consent for Publication: As this work included secondary use of administrative health care data we do not require consent here.
Availability of Data and Materials: The data set from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the data set publicly available, access can be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Ontario Renal Network and the Ontario Ministry of Health and Long-Term Care funded this work.
References
- 1. Carstairs S. Of life and death: final report of the special senate committee on euthanasia and assisted suicide. In: Senate of Canada - Chapter III: Palliative Care, Canada: 35th Parliament, 1st Session; 1995. Accessed at: https://sencanada.ca/content/sen/committee/351/euth/rep/lad-tc-e.htm
- 2. Ministry of Health and Long-Term Care. McGuinty Government Improving End-of-Life Care: Strategy Will Enhance Services in Homes and Hospices. Toronto, Ontario, Canada: MOHLTC; 2005. [Google Scholar]
- 3. Kirby M. The health of Canadians—the federal role. Final report of the standing senate committee on social affairs, science and technology. In: 37th Parliament, 2nd Session ed Government of Canada Publications; 2002. [Google Scholar]
- 4. Dudgeon D, Vaitonis V, Seow H, King S, Angus H, Sawka C. Ontario, Canada: using networks to integrate palliative care province-wide. J Pain Symptom Manage. 2007;33:640-644. [DOI] [PubMed] [Google Scholar]
- 5. Advancing High Quality, High Value Palliative Care in Ontario: This Declaration of Partnership and Commitment to Action. Date unknown. http://health.gov.on.ca/en/public/programs/ltc/docs/palliative%20care_report.pdf. Accessed December, 2011.
- 6. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20:631-636. [DOI] [PubMed] [Google Scholar]
- 7. Ontario Renal Network. The Ontario Renal Plan. Date unknown. http://wwwrenalnetworkonca/common/pages/UserFileaspx?fileId=333923. Accessed 2013.
- 8. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805-835. [DOI] [PubMed] [Google Scholar]
- 9. Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452-472. [DOI] [PubMed] [Google Scholar]
- 10. The John Hopkins University Bloomberg School of Public Health HSRDC. The Johns Hopkins ACG® Case-Mix System Version 11.0 Release Notes. In: Weiner J, ed. The Johns Hopkins; 2014. https://www.healthpartners.com/ucm/groups/public/@hp/@public/documents/documents/cntrb_035024.pdf
- 11. Redahan L, Brady B, Smyth A, Higgins S, Wall C. The use of palliative care services amongst end-stage kidney disease patients in an Irish tertiary referral centre. Clin Kidney J. 2013;6:604-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishikawa H, Kida M, Sakamoto J. “Palliative hemodialysis” in the context of end-of-life care for dialysis patients. Ther Apher Dial. 2014;18:212-213. [DOI] [PubMed] [Google Scholar]
- 13. Teruel JL, Rexach L, Burguera V, et al. Home palliative care for patients with advanced chronic kidney disease: preliminary results. Healthcare (Basel). 2015;3:1064-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Biesen W, van de, Luijtgaarden MW, Brown EA, et al. Nephrologists’ perceptions regarding dialysis withdrawal and palliative care in Europe: lessons from a European Renal Best Practice survey. Nephrol Dial Transplant. 2015;30:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couchoud C, Bello AD, Lobbedez T, et al. Access to and characteristics of palliative care-related hospitalization in the management of end-stage renal disease patients on renal replacement therapy in France. Nephrology (Carlton). 2017;22(8):598-608. [DOI] [PubMed] [Google Scholar]
- 16. Canadian Institute for Health Information. Treatment of End-Stage Organ Failure in Canada, 2003 to 2012—CORR 2014 Annual Report. Canadian Institute for Health Information; 2013. https://secure.cihi.ca/free_products/2016_CORR_Snapshot_EN(web).pdf [Google Scholar]
- 17. Tamura MK, Meier DE. Five policies to promote palliative care for patients with ESRD. Clin J Am Soc Nephrol. 2013;8:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romano TG, Palomba H. Palliative dialysis: a change of perspective. J Clin Med Res. 2014;6:234-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davison SN, Jassal SV. Supportive care: integration of patient-centered kidney care to manage symptoms and geriatric syndromes. Clin J Am Soc Nephrol. 2016;11(10):1882-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson DM, Cohen J, Deliens L, Hewitt JA, Houttekier D. The preferred place of last days: results of a representative population-based public survey. J Palliat Med. 2013;16:502-508. [DOI] [PubMed] [Google Scholar]
- 21. OHTAC End-of-Life Collaborative. Health care for people approaching the end of life: an evidentiary framework. Ont Health Technol Assess Ser. 2014;14:1-45. [PMC free article] [PubMed] [Google Scholar]