Abstract
LIGHT (homologous to lymphotoxins, exhibiting inducible expression, and competing with herpes simplex virus (HSV) glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) has been involved in various autoimmune and inflammatory disorders. LIGHT induces the expression of interleukin-8 (IL-8), which is up-regulated in chronic spontaneous urticaria (CSU). To determine circulating soluble LIGHT concentration and its relationship with IL-8 concentration in patients with CSU. Concentrations of LIGHT, IL-8, and C-reactive protein (CRP) were determined in plasma or serum of CSU patients by an enzyme-linked immunosorbent assay. LIGHT plasma concentration was significantly higher in moderate–severe CSU patients as compared with the healthy subjects, but not with mild CSU patients. There were significant correlations between increased LIGHT and IL-8 concentrations, but not with increased CRP in CSU patients. Enhanced plasma concentrations of soluble LIGHT and its association with IL-8 concentration suggest the role of LIGHT in systemic inflammatory activation in CSU patients. We hypothesize that LIGHT-mediated immune–inflammatory response plays a role in severe phenotypes of the disease.
Keywords: chronic spontaneous urticaria, IL-8, LIGHT/TNFSF14
Introduction
Chronic spontaneous urticaria (CSU) is a chronic inflammatory disease associated with autoimmune phenomena and systemic inflammatory response.1,2
The tumor necrosis factor superfamily member 14 (TNFSF14), also known as LIGHT (homologous to lymphotoxins, exhibiting inducible expression, and competing with herpes simplex virus (HSV) glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes) has been involved in various autoimmune and inflammatory disorders. LIGHT is known to regulate T cell immune responses and has pro-inflammatory activity.3–5 Circulating soluble LIGHT has been reported as a potential biomarker of autoimmune and inflammatory diseases, including atopic dermatitis and sclerodermia,6,7 but its association with the pathogenesis of CSU has not been clarified. In addition, it has been demonstrated that LIGHT induces the expression of various cytokines that are involved in urticarial inflammation, including interleukin-8 (IL-8),5 which is up-regulated in CSU patients.8 Therefore, in this study, we determined plasma LIGHT concentration and its associations with circulating IL-8 and acute phase response in CSU patients.
Materials and methods
In total, 52 active CSU patients (14 men and 38 women; median age: 38 years, range: 24–50) and 21 sex-, age-, and body mass index (BMI; <30)-matched healthy subjects were enrolled in the study.
Each patient underwent tests, including the autologous serum skin test (ASST) and consultations, which were described previously.9 The study comprised 28 mild CSU patients and 24 moderate–severe CSU patients graded during 4 days as follows: mild—0 to 8 urticaria activity score (UAS), moderate—9 to 16 UAS, and severe—17 to 24 UAS.9
H1 antihistamines and immunosuppressants were withdrawn at least 4 days and 8 weeks, respectively, before blood sampling.
The Ethics Committee of the Medical University of Silesia approved the study, and all subjects gave informed, signed consent to participate in the study.
Blood collection
Fasting blood samples were collected in a tube with anticoagulants. Plasma samples were frozen at −85°C until the tests were performed.
Assay of C-reactive protein (CRP)
Serum CRP concentrations were measured automatically using a Roche/Hitachi cobas c system. Normal lab ranges (<5.0 mg/L).
Assay of IL-8
Plasma IL-8 concentrations were measured using a Quantikine Human CXCL8/IL-8 ELISA kit (R&D Systems, MN, USA). The coefficients of variation for intra- and inter-assays were below 8% and 10%, respectively.
Assay of LIGHT/TNFSF14
Concentrations of LIGHT in plasma were measured by enzyme-linked immunosorbent assay (ELISA) method using a commercially available kit (Quantikine Human LIGHT/TNFSF14 ELISA kit; R&D Systems, MN, USA). The coefficients of variation for intra- and inter-assays were below 6% and 8%, respectively.
Statistical analysis
The results were presented as the median and mean values, quartile range, and standard deviation. Normal data distribution was measured using the Shapiro–Wilk test. The data of the CSU patients and the controls as well as those of the mild and moderate–severe CSU groups were compared using Mann–Whitney U test. Spearman’s rank correlation test was used for correlations. The P value < 0.05 was considered statistically significant. Analyses were performed with Statistica (StatSoft, Cracow, Poland).
Results
Plasma LIGHT concentrations
No significant differences were observed in LIGHT concentration between the controls and CSU patients (median: 54.77 and 78.07 pg/mL, P > 0.05; Figure 1).
Figure 1.
LIGHT concentration in plasma of chronic spontaneous urticaria (CSU) patients and in healthy subjects. CSU versus controls, P > 0.05; moderate–severe CSU versus controls, P < 0.05; moderate–severe versus mild CSU, P > 0.05.
LIGHT concentration was significantly higher in moderate–severe CSU patients as compared with the controls, but not with mild disease activity (median: 106.63 vs 54.77 vs 50.94 pg/mL, P < 0.05 and P > 0.05, respectively; Figure 1).
There were no significant differences in LIGHT concentrations between CSU patients with positive and negative ASST responses, selected according to the similar UAS (median: 57.55 vs 97.92 pg/mL, P > 0.05).
Plasma IL-8 concentrations
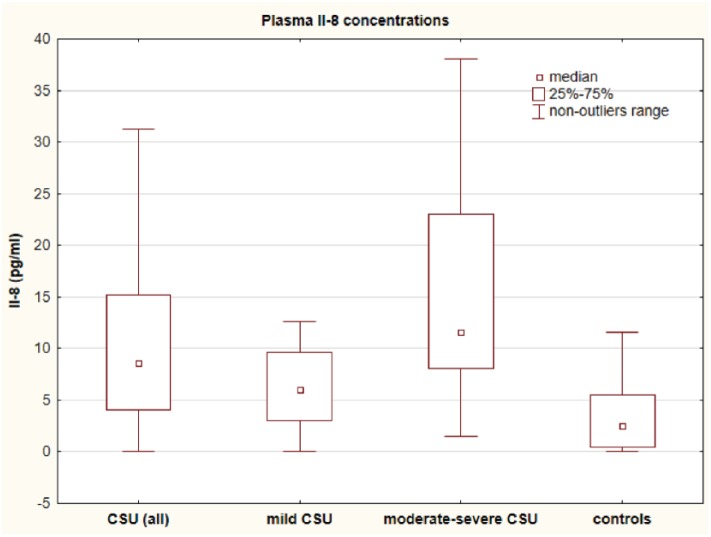
Concentrations of IL-8 were significantly higher in the CSU group versus the controls (median: 8.58 vs 2.5 pg/mL; P < 0.001; Figure 2).
Figure 2.
IL-8 concentration in plasma of chronic spontaneous urticaria (CSU) patients and in healthy subjects. CSU (all) and moderate–severe CSU versus controls, P < 0.001; mild CSU versus moderate–severe CSU versus controls, P < 0.01 and P < 0.05, respectively.
IL-8 concentrations were significantly higher in moderate–severe and mild CSU groups than in the controls (median: 11.61 vs 6.06 vs 2.5 pg/mL, P < 0.01 and P < 0.001, respectively). The cytokine concentrations in patients with mild CSU were significantly higher than those in the controls (6.06 vs 2.5 pg/mL, P < 0.05; Figure 2).
We did not observe any significant difference in IL-8 concentrations between CSU patients with positive and negative ASST responses, selected according to the similar UAS (median: 8.33 vs 8.58 pg/mL, P > 0.05).
Serum CRP concentrations
CRP concentrations were significantly higher in CSU patients than in the controls (median: 3.8 vs 0.4 mg/L; P < 0.001).9 Significant differences in CRP concentrations between patients with mild and moderate–severe CSU activity and the controls were observed (median: 1.4 vs 9.8 vs 0.4 mg/L, respectively; P < 0.001), (the data have been published previously).9
There were no significant differences in LIGHT concentrations between CSU patients with positive and negative ASST responses, selected according to the similar UAS (median: 3.9 vs 3.3 mg/L, P > 0.05).
Correlations
LIGHT concentrations were significantly correlated with IL-8, but not with CRP in CSU (R = 0.62, P = 0.000001 and R = 0.22, P = 0.12). LIGHT concentrations were significantly correlated with IL-8 in CSU ASST– and CSU ASST+ patients (R = 0.66, P = 0.00003 and R = 0.61, P = 0.008).
In addition, there were significant correlations between LIGHT and IL-8, but not with CRP in moderate–severe CSU (R = 0.53, P = 0.008 and R = 0.14, P = 0.52).
There was a significant correlation between IL-8 and CRP (R = 0.41, P = 0.002).
There were not any significant correlations between LIGHT and UAS4 (R = 0.17, P = 0.23), but were present between UAS4 and IL-8 and CRP, as described previously.9
The platelet or leukocyte count was not significantly correlated with plasma concentrations of LIGHT in both groups.
Discussion
In our study, plasma concentrations of soluble LIGHT were significantly higher in moderate–severe CSU patients as compared with the controls, but not in patients with mild CSU. It is known that moderate–severe CSU is a difficult-to-treat disease and is associated with increased systemic inflammatory response and autoimmune phenomena. The dysregulation or overexpression of LIGHT plays an important role in the induction and/or persistence of inflammatory response and autoimmunity.3,4 Taken together, it seems that elevated soluble LIGHT concentrations may reflect a persistent chronic inflammatory response that is induced during the moderate–severe activity of CSU.
Previously, we demonstrated that plasma IL-8 concentration is significantly increased in patients with CSU,8 which is in line with the current results. In this study, we observed a significant correlation between LIGHT and IL-8 concentrations in CSU patients, including the ASST– and ASST+ CSU subgroups. There were not significant correlations between LIGHT and UAS4, but between UAS4 and IL-8 and CRP, as described previously.9
Interestingly, it has been demonstrated that LIGHT may induce the expression, production, and release of IL-8.5,10 These results suggest that the triggering of LIGHT in more severe CSU leads to the release of pro-inflammatory mediators, including IL-8. In addition, it has been reported that LIGHT induces the expression of matrix metalloproteinase-9 and pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, interleukin-6 (IL-6).11 Taken together, it seems that LIGHT in CSU may contribute to systemic inflammation, especially in CSU patients with more severe disease activity/severity.
LIGHT as a costimulator plays an important role in the activation/proliferation of T cells3,4 and mast cells in the course of T-cell- or B-cell-dependent inflammatory reactions.12 Dysregulation of LIGHT activity may trigger the abnormal T cell activation, leading to a pathological immune–inflammatory response and autoimmune manifestations.4 LIGHT involvement in inflammatory disorders has also been demonstrated in chronic kidney disease and alkaptonuria.13,14
We speculate that the immune response switches to processes involving LIGHT in severe phenotypes of CSU. The underlying mechanism by which LIGHT mediates urticarial processes and its sources in CSU remains unknown. LIGHT (TNFSF14) is a transmembrane protein expressed and shed from different types of immune–inflammatory cells, including T and B lymphocytes, monocytes/macrophages, granulocytes, spleen cells, and dendritic cells,3–5 which may be involved in CSU. CRP concentrations were significantly higher in CSU patients than in the controls, thus confirming previous observations.15
In conclusion, enhanced plasma concentrations of soluble LIGHT and its association with IL-8 concentration suggest the role of LIGHT in systemic inflammatory activation in CSU patients.
We hypothesize that LIGHT-mediated immune–inflammatory response plays a role in severe phenotypes of the disease.
Acknowledgments
A.K.-Z. designed the study as well as reviewed the manuscript. A.D.-B. performed the lab and statistical data analysis and wrote the manuscript. J.J. performed data analysis. R.G., A.S.-Z., A.S.-F., and K.B. collected the samples and provided clinical data. All authors read and approved the final manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the Committee for Scientific Research (KNW-1-041/K/6/K); it does not lead to any conflicts of interest regarding the publication of this manuscript.
ORCID iD: Alicja Kasperska-Zając  https://orcid.org/0000-0002-2000-0070
https://orcid.org/0000-0002-2000-0070
References
- 1. Asero R, Tedeschi A, Marzano AV, et al. (2016) Chronic spontaneous urticaria: Immune system, blood coagulation, and more. Expert Review of Clinical Immunology 12: 229–231. [DOI] [PubMed] [Google Scholar]
- 2. Kasperska-Zając A, Grzanka A, Damasiewicz-Bodzek A. (2015) IL-6 signaling in chronic spontaneous urticaria. PLoS ONE 10: e0145751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang J, Fu YX. (2004) The role of LIGHT in T cell-mediated immunity. Immunologic Research 30: 201–214. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Lo JC, Foster A, et al. (2001) The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. The Journal of Clinical Investigation 108: 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim SG, Suk K, Lee WH. (2013) Reverse signaling from LIGHT promotes pro-inflammatory responses in the human monocytic leukemia cell line, THP-1. Cellular Immunology 285: 10–17. [DOI] [PubMed] [Google Scholar]
- 6. Herro R, Antunes Rda S, Aguilera AR, et al. (2015) The tumor necrosis factor superfamily molecule LIGHT promotes keratinocyte activity and skin fibrosis. Journal of Investigative Dermatology 135: 2109–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotani H, Masuda K, Tamagawa-Mineoka R, et al. (2012) Increased plasma LIGHT levels in patients with atopic dermatitis. Clinical and Experimental Immunology 168: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasperska-Zając A, Grzanka A, Damasiewicz-Bodzek A, et al. (2017) Elevated plasma Il-8 concentration is related to severity of systemic inflammation in chronic spontaneous urticaria. Journal of Biological Regulators & Homeostatic Agents 31: 957–961. [PubMed] [Google Scholar]
- 9. Grzanka A, Damasiewicz-Bodzek A, Kasperska-Zając A. (2017) The relationship between circulating concentrations of interleukin 17 and C reactive protein in chronic spontaneous urticaria. Allergy, Asthma, and Clinical Immunology 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Otterdal K, Haukeland JW, Yndestad A, et al. (2015) Increased serum levels of LIGHT/TNFSF14 in nonalcoholic fatty liver disease: Possible role in hepatic inflammation. Clinical and Translational Gastroenterology 6: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim WJ, Kang YJ, Koh EM, et al. (2005) LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology 114: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stopfer P, Männel DN, Hehlgans T. (2004) Lymphotoxin-beta receptor activation by activated T cells induces cytokine release from mouse bone marrow-derived mast cells. Journal of Immunology 172: 7459–7465. [DOI] [PubMed] [Google Scholar]
- 13. Cafiero C, Gigante M, Brunetti G. (2018) Inflammation induces osteoclast differentiation from peripheral mononuclear cells in chronic kidney disease patients: Crosstalk between the immune and bone systems. Nephrology, Dialysis, Transplantation 33: 65–75. [DOI] [PubMed] [Google Scholar]
- 14. Brunetti G, Tummolo A, D’Amato G, et al. (2018) Mechanisms of enhanced osteoclastogenesis in alkaptonuria. The American journal of pathology 188: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 15. Kasperska-Zając A, Damasiewicz-Bodzek A, Bieniek K, et al. (2018) Elevated circulating heat shock protein 70 and its antibody concentrations in chronic spontaneous urticaria. International Journal of Immunopathology and Pharmacology 31: 394632017750440. [DOI] [PMC free article] [PubMed] [Google Scholar]