Abstract
New paradigm in cancer pathogenesis revealed that microenvironmental conditions significantly contribute to cancer. Hence, Warburg stated that cancer is a metabolic disease. Chlorogenic acid (CGA) is a polyphenol that is found abundantly in coffee. This compound has proven ability in ameliorating some metabolic diseases through various pathways. This article will elaborate the potency of CGA as a chemosensitizer in suppressing tumor growth through a metabolic pathway. AMPK pathway is the main cell metabolic pathway that is activated by CGA in some studies. Moreover, CGA inhibited EGFR/PI3K/mTOR, HIF, VEGF pathways and MAPK/ERK pathway that may suppress tumor cell growth. Furthermore, CGA induced intracellular DNA damage and topoisomerase I- and II-DNA complexes formation that plays a key role in apoptosis. Conclusively, based on the ability of CGA in activate and inhibit some important pathways in cancer metabolism, it may act as a chemosensitizing agent leading to cancer growth suppression.
Keywords: chlorogenic acid, cancer, Warburg effect
Cancer is a multifactorial disease that has been hypothesized to be caused by genetic mutation. New paradigm in cancer pathogenesis suggested that cancer is not solely caused by genetic mutation. Moreover, cell microenvironment has a significant contribution in cancer growth.1 The new paradigm that considered cancer as a metabolic disease was stated by Warburg 30 years ago.2 This paradigm explicated that cancer is a transition from mitochondrial respiration into aerobe fermentation.3 This imperative transition contributes to phenotype changes from epithelial cells to mesenchymal cells. Furthermore, cancer proliferation takes advantage of lactate produced by the glycolysis process to invade its surrounding normal stromal cells.4 The lactate acidity contributes to cytotoxicity on its surrounding cells compared with that of the cancer cells.5 Some recent studies revealed that cancer stem cells demonstrate very different characteristics compared with those of normal cells. This metabolic difference contributes to driving the pluripotent cells to cancer stem cells.6 This phenomenon is known as metabolic reprogram.2 Thus, recent cancer therapy approaches focus on inhibiting the metabolic reprogram.
Chlorogenic acid (CGA) is a well-known polyphenol that is abundantly present in coffee.7 Many studies showed that CGA contributes to modulating the metabolic features of type 2 diabetes and obesity through some pathways such as AMPK pathway.8 Moreover, this substance has a potential effect on suppressing growth of cancer cells mainly through inhibiting cancer metabolic features. This article will elaborate the potential of CGA as a chemosensitizing chemotherapy agent to suppress tumor growth.
Metabolic Features of Cancer Cells
Warburg effect is a significant mechanism of metabolic changes in cancer cells.3 Cellular metabolism needs 3 basic features to divide: generating ATP rapidly to sustain energy status, increase biosynthesis of macromolecules, and an appropriate cellular redox status for tight maintenance.2 However, cancer cells express metabolic alteration on 4 major macromolecular metabolisms (carbohydrate, proteins, lipid, and nucleic acids) to enhance energy biosynthesis and redox reaction.5 They tend to preform aerobic glycolysis that produce more ATP compared with that of oxidative phosphorylation in mitochondria during the adaptation process to stress and microenvironmental changes, especially hypoxia, pH changes, and nutrition deprivation.9 These mechanisms are driven by PI3K, hypoxia-inducible factor (HIF), p53, MYC, AMP-activated protein kinase (AMPK), and liver kinase B1 (LKB1) pathways.10,11 This adaptation capability is affected by mutation of oncogenesis and tumor suppressor gene. Thus, tumor cells may skip metabolism checkpoints to maintain their growth and proliferation. Among all those pathways, PI3K and AMPK pathways have significant contribution in determining the metabolic profile of cancer cells.12
PI3K pathway is the main pathway in cell glycolysis. This pathway activates the AKT1 pathway that augments the expression and translocation of membrane glucose transporter, the phosphorylation of glycolysis key enzyme, inhibits Forkhead Box Family O (FOXO) increasing glycolytic capacity,13 and activates ectonucleoside triphosphate diphosphorylation that support protein glycosylation in the endoplasmic reticulum.11 Moreover, this pathway activates mTOR, which plays a key role in metabolism integration, especially protein and lipid biosynthesis during nutrition and energy deprivation.14 Furthermore, it activates HIF-1 during hypoxia. HIF-1 is a transcription factor that plays a role in augmenting glucose transporter gene transcription.15 Furthermore, it activates pyruvate dehydrogenase reducing pyruvate flow in the tricarboxylic acid cycle.16 Hence, oxidative phosphorylation in mitochondria and oxygen consumption declines. Moreover, HIF-1 can be activated by mutated tumor suppressor protein such as VHL,17 succinate dehydrogenase, and fumarate dehydrogenase.18
AMPK pathway is the main regulator in energy status sensor, metabolism checkpoint, proliferation inhibitor, and has a pleiotropic role in metabolic stress. The AMPK pathway can be activated through some mechanism:
ATP–ADP ratio difference in hypoxia, nutrition deprivation condition.19 Moreover, some chemical substances from plants or drugs can activate the liver kinase B1 (LKB1)–AMPK pathway.20
Ob-Rb and ADRA1A receptors are activated by leptin. Furthermore, these receptors activate the CaMKK-AMPK pathway.19
AdipoR receptor is activated by adiponectin.21 Furthermore, this receptor activates the CaMKKI-AMPK pathway.
LKB1 is one of tumor suppressor genes. Cancer cells experience mutation in LKB1 and AdipoR receptors. Hence, it cannot recognize the adiponectin signal that activates AMPK. AMPK inhibition will enhance glycolysis that supports cells’ ability to proliferate in abnormal environment conditions. Glycolysis upsurge is a result of AMPK failure in inhibiting glycogenic gene expression, inhibiting the gluconeogenesis program, and inhibiting the synthesis of fatty acid, cholesterol, and glycogen.22
Cancer incidence is frequently associated with hypoadiponectinemia, and insulin resistance results in hyperinsulinemia.23 Moreover, many cancers often overexpress AdipoR1/R2. In fact, the mechanism of this overexpression remains obscure. Metabolic compensatory response to hypoadiponectinemia is mostly considered as the reason of this phenomenon. Moreover, the AdipoR1/R2 downstream signaling pathway activation will affect the likelihood of tumorigenesis.24
MAPK Pathway in Cancer Cell
Mitogen-activated protein kinases (MAPKs) are a kinase protein family that has a main role in proliferation, gene expression, differentiation, mitosis, cell motility, cell metabolism, apoptosis, and embryogenesis.25 MAPK consists of 3 subfamilies: extracelullar signal-regulated kinase (ERK; ERK 1 and ERK 2), c-Jun N terminal kinase (JNK; JNK1 and JNK 2), and p38-MAP.26 MAPK is activated by MAP kinase kinase (MKK or MAP2K) or MAP kinase kinase kinase (MEKK or MAP2 K). MAPK needs at least 2 similar MAP2Ks and some MAP3Ks. ERK 1/2 is a pathway that has a role in regulating cell cycle such as meiosis, mitosis, and cell differentiation.27 Moreover, this pathway has a role in DNA repair, anti-apoptosis, and pro-apoptosis.27 Furthermore, this pathway is activated by some extracellular stimulus such as growth factor, cytokines, virus infection, G protein ligand, and carsinogen.28 ERK 1/2 activation through extracellular stimulus activates Ras protein, Rat, and MKK 1/2.29 ERK 1/2 is activated continuously in cancer cells because mutation of Ras protein to proto-oncogen Ras escalates cancer and tumor cell proliferation.
Chlorogenic Acid
Chemical Structure of Chlorogenic Acid
Chlorogenic acid is polyphenol that possess many advantages in health. This substance is mostly found in green coffee bean.30 Moreover, this substance is an ester that consist of cinnamic acid and quinic acid.7 Furthermore, it has hydroxyl axis chain in the first and third carbons and equatorial hydroxyl in the fourth and fifth carbons, known as 5-O-caffeoylquinic acid (5-CQA) or 3-caffeoylquinic acid (3-CQA). 5-CQA is the most abundant form of CGA (see Figure 1).31
Figure 1.
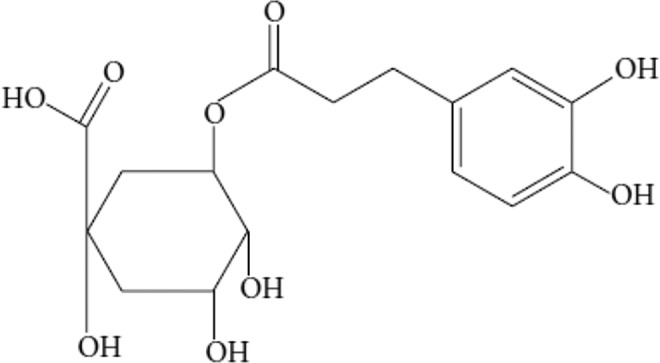
Chemical structure of chlorogenic acid.32
Chlorogenic Acid Bioavailability
Around 70% of CGA is absorbed in small intestine and colon. CGA is relatively stable in saliva and gastric acid. At the first digestion some CGA is absorbed in gastric and the remaining will be absorbed in jejunum and ileum.33–35 Furthermore, it will enter portal circulation that is directly connected with hepatic artery to undergo enzymatic reaction.36 CGA absorption is facilitated through passive paracellular diffusion, facilitating transportation. In appropriate pH CGA holds 2 hydroxyl ions that come from quinic acid and succinic acid residues.37 Moreover, these ions bind to transporter in intestine known as sodium glucose cotransporter 1 (SGLT 1) and sodium dicarboxylate cotransporter 1 (SDCT 1). Furthermore, the absorption process of CGA in the intestine is affected by normal flora cleaving CGA cinnamic acid to caffeic acid and ferulic acid. These substances can be absorbed and metabolized through reduction, demethylation, dehydroxylation, and isomerization. Based on pharmacokinetic analysis most circulating CGA is eliminated quickly from the circulatory system with half-time of 0.3 to 1.9 hours and Tmax of 0.6 to 1 hour. Furthermore, about 120.2 μmol or 29.2% of ingested CGA is excreted in urine 24 hours after consumption.37–40
Potential Mechanism of Chlorogenic Acid as Anticancer Agent
CGA Effects on Metabolic Diseases
Many studies have reported CGA as an antidiabetic and antilipidemic. A study by Murase et al in humans suggested metabolism increase and fatty acid oxidation increase after injection of CGA. A study of the effects of CGA in experimental animals that had induction of high-fat diet revealed that CGA therapy significantly reduced triglyceride levels in the mice liver,41 improved insulin resistance,42 and inhibited adipogenesis, TLR4-mediated pro-inflammatory pathway, and stimulation of GLUT4 translocation.43 These effects were mediated through AMPK activation. CGA worked in ameliorating metabolic disease via increasing the level of adiponectin. Another study by Jin et al suggested that CGA administration significantly increase the protein expression of adiponectin receptors in late diabetic db/db mice.44 After 12 weeks of CGA administration, the expression of adiponectin receptor-1 (ADPNR-1) in liver and adiponectin receptor-2 (ADPNR-2) in skeletal muscle was significantly higher than that of the control group. Moreover, AMPK phosphorylation was significantly higher in both liver and skeletal tissue compared with that of the control group. This study suggested that CGA administration not only increases the level of adiponectin but also the expression of the receptor both in the liver and skeletal tissue. These findings may potentiate a hypothesis that CGA has some abilities as an anticancer agent through cancer metabolism, growth, and proliferation inhibition.
CGA Inhibits HIF-1α/AKT Pathway
Aberrant in EGFR/PI3K/mTOR pathway, HIF and VEGF expression induced most cancer. Hypoxia is the primary stimulus for HIF-1α upregulation.16 Moreover, epidermal growth factor receptor (EGFR) and PI3K pathway contribute to HIF-1α upregulation.5 Thus, the components of this pathway becomes the primary target in cancer therapy. Park et al suggested that CGAs downregulate the HIF-1α/AKT pathway to inhibit hypoxia-induced angiogenesis in HUVEC cells.45 CGA suppressed VEGF-induced angiogenesis in vivo via AKT activation blocking. Moreover, CGA-treated cells showed a downregulation of VEGF expression and secretion compared with that of hypoxia alone. In addition, CGA exposure (2 μM or 10 μM) decreased transcriptional activity of HIF-1.46 However, this study used CGA only a as single agent. Thus, another study that combines CGA with other targeted inhibitors is warranted.
5-FU and CGA Combination Inactivated MAPK/ERK in Hepatocellular Carcinoma Cells
Cell proliferation is mainly driven by mitogen activated protein kinase signaling pathway. Disturbance in the regulation of this pathway will lead to carcinogenesis and contribute to cancer drug resistance.29 Yan et al revealed that the combination of CGA and 5-FU inhibited MAPK/ERK activation via ROS overproduction.47 Combination of 250 μmol/L CGA and 20 μmol/L 5-FU promoted a prominent production of ROS in HepG2 and Hep3B cells. Consequently, combination of CGA and 5-FU significantly inhibited ERK1/2 phosphorylation in HepG2 and Hep3B cells. On the other hand, either 5-FU or CGA alone resulted in no significant change. It can be concluded that ROS overproduction due to CGA administration led to HCC cells’ sensitization to 5-FU treatment by suppressing ERK activation. Consequently, this mechanism enhanced 5-FU-induced inhibition of HCC cells’ proliferation.
CGA May Work as Metformin in Inhibiting Tumor Growth via Activation of AMPK Pathway
Many studies have suggested that metformin might ameliorate drug resistant in breast cancer chemotherapy.48 Moreover, many studies revealed that metformin has a direct antiproliferative effect through AMPK activation.49 CGA is a substance that works in the AMPK pathway to ameliorate metabolic condition of patients with type 2 diabetes mellitus and obesity.32 CGA induced AMPK activation via promoting the rise of CAMKKβ expression in HepG2 hepatoma cells.8 Moreover, this study suggested that CGA administration induced an increase in the intracellular Ca2+concentration measured by Fluo-4.50 This study suggested that CGA might have a beneficial effect on cancer therapy by affecting the upstream kinases that mediates AMPK phosphorylation.
Some studies suggested that CGA treatment increase the level of adiponectin.44 Adiponectin is a cytokine from adipocyte that is known to play a role in cancer.24 Adiponectin acts as a key activator of the AMPK pathway. Some studies suggested that lower expression of adiponectin correlates with increased risk of breast, endometrial, colon, prostate, hepatic, renal cell, and lung cancers.23
CGA Induces Cellular DNA Damage and Formation of Topoisomerase I- and II-DNA Complexes
Topoisomerase inhibitor, known as cancer killer drug, works by inducing topoisomerase-mediated DNA damage.51 Topo I and topo II are key players of DNA fragmentation during apoptosis.52 A study conducted by Burgos-Morón et al suggested that CGA formed topo-DNA complexes and induced cellular DNA damage.53 In fact, CGA induced significant levels of topo-DNA complexes after 24 hours of exposure in cells. Flow cytometry assay showed a high percentage of late apoptotic cells after 24 hours of exposure to CGA. This action mediated through the generation of hydrogen peroxide.
Chlorogenic acid as a polyphenol has a wide range of pharmacokinetic profiles such as cancer prevention, carcinogenic, and therapeutic potential on cancer cells depending on the concentration, dose, and duration of exposure.31,54 Burgos-Morón et al suggested that CGA induced DNA damage and formed topoisomerase-DNA complexes at concentrations of 0.5 to 5 mM.53
Conclusion
Based on the ability of CGA in activating and inhibiting some important pathways in cancer metabolism, it may act as chemosensitizing agent leading to cancer growth suppression.
Future Challenges
Despite the advantages of CGA as chemosensitizer, there are many challenges on its mode of administration. Researchers are still developing this potential agent since CGA has low oral bioavailability. Monteiro et al33 measured the level of CGA in plasma and urine and revealed that the level of CGA in plasma was low. Moreover, they revealed that the level of CGA in urine was also low. They suggested that oral administration of CGA may not achieve the desired effect on cancer cells. Hence, there should be other technologies to deliver CGA to the target.
A study by Park et al45 successfully developed CGA-AuNPS as a novel green synthesis method for gold nanoparticles. This agent had successfully enhanced anti-inflammatory effects on an NF-Kb-mediated inflammatory network compared with that of CGA only. A study reported by Del Rio et al33 suggested that CGA was mainly absorbed in the small intestine. Moreover, CGA is fragmented into at least 3 main chemistry structure, 5CQA, 4CQA, and 3CQA, in the small intestine. Furthermore, Renouf et al55 reported that colon and microflora have a primary role in CGA absorption and metabolism. Thus, dihydrophenolic acid can be detected in plasma after 8 hours. More research is required that explores the role of each of the CGA chemical compounds to inhibit the growth, proliferation, and metastasis of cancer cells and verify the different and specific functions of each compound.
Acknowledgments
We acknowledge Brawijaya Cardiovascular Research Center, Brawijaya University for the support during preparation of the article.
Author Contributions: Mifetika Lukitasari: Literature search, literature review, and manuscript preparation.
Dwi Adi Nugroho: Literature search, literature review, and manuscript preparation.
Nashi Widodo: Consultant, literature review.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Cardiovascular Research Group, Faculty of Medicine, Brawijaya University.
Ethical Approval: Ethical approval was not required for this study.
References
- 1. Tsai MJ, Chang WA, Huang MS, Kuo PL. Tumor microenvironment: a new treatment target for cancer. ISRN Biochem. 2014;2014:351959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menendez J, Joven J, Cufí S, et al. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle. 2013;12:1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. [DOI] [PubMed] [Google Scholar]
- 4. Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. [DOI] [PubMed] [Google Scholar]
- 5. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. [DOI] [PubMed] [Google Scholar]
- 6. Menendez JA. Metabolic control of cancer cell stemness: lessons from iPS cells. Cell Cycle. 2015;14:3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clifford MN. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–1043. [Google Scholar]
- 8. Ong KW, Hsu A, Tan BKH. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol. 2013;85:1341–1351. [DOI] [PubMed] [Google Scholar]
- 9. Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011;1807:552–561. [DOI] [PubMed] [Google Scholar]
- 10. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. [DOI] [PubMed] [Google Scholar]
- 11. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51 doi:10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes (Lond). 2008;32(suppl 4):S36–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khatri S, Yepiskoposyan H, Gallo CA, Tandon P, Plas DR. FOXO3a regulates glycolysis via transcriptional control of tumor suppressor TSC1. J Biol Chem. 2010;285:15960–15965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kenerson HL, Subramanian S, McIntyre R, Kazami M, Yeung RS. Livers with constitutive mTORC1 activity resist steatosis independent of feedback suppression of Akt. PLoS One. 2015;10:e0117000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayashi M, Sakata M, Takeda T, et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1 under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. [DOI] [PubMed] [Google Scholar]
- 16. Kim J, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. [DOI] [PubMed] [Google Scholar]
- 17. Haase VH. The VHL tumor suppressor: master regulator of HIF. Curr Pharm Des. 2009;15:3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. [DOI] [PubMed] [Google Scholar]
- 19. Hardie DG. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Trans. 2011;39:1–13. [DOI] [PubMed] [Google Scholar]
- 20. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. [DOI] [PubMed] [Google Scholar]
- 22. Kishton RJ, Barnes CE, Nichols AG, et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 2016;23:649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–931. [DOI] [PubMed] [Google Scholar]
- 24. Obeid S, Hebbard L. Role of adiponectin and its receptors in cancer. Cancer Biol Med. 2012;9:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. [DOI] [PubMed] [Google Scholar]
- 26. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. [DOI] [PubMed] [Google Scholar]
- 27. Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. [DOI] [PubMed] [Google Scholar]
- 28. Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–211. [DOI] [PubMed] [Google Scholar]
- 29. Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. [DOI] [PubMed] [Google Scholar]
- 30. Olthof MR, Hollman PC, Zock PL, Katan MB. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am J Clin Nutr. 2001;73:532–538. [DOI] [PubMed] [Google Scholar]
- 31. Maalik A, Bukhari SM, Zaidi A, Shah KH, Khan FA. Chlorogenic acid: a pharmacologically potent molecule. Acta Poloniae Pharmaceutica. 2016;74:851–854. [PubMed] [Google Scholar]
- 32. Meng S, Cao J, Feng Q, Peng J, Hu Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Alternat Med. 2013;2013: 801457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Del Rio D, Stalmach A, Calani L, Crozier A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients. 2010;2:820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr. 2008;138:2309–2315. [DOI] [PubMed] [Google Scholar]
- 35. Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728–733. [DOI] [PubMed] [Google Scholar]
- 36. de Sotillo DVR, Hadley M. Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J Nutr Biochem. 2002;13:717–726. [DOI] [PubMed] [Google Scholar]
- 37. Lafay S, Morand C, Manach C, Besson C, Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br J Nutr. 2006;96:39–46. [DOI] [PubMed] [Google Scholar]
- 38. Farrell TL, Dew TP, Poquet L, Hanson P, Williamson G. Absorption and metabolism of chlorogenic acids in cultured gastric epithelial monolayers. Drug Metab Dispos. 2011;39:2338–2346. [DOI] [PubMed] [Google Scholar]
- 39. Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J Int Med Res. 2007;35:900–908. [DOI] [PubMed] [Google Scholar]
- 40. Mills CE, Tzounis X, Oruna-Concha MJ, Mottram DS, Gibson GR, Spencer JP. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br J Nutr. 2015;113:1220–1227. [DOI] [PubMed] [Google Scholar]
- 41. Cho AS, Jeon SM, Kim MJ, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48:937–943. [DOI] [PubMed] [Google Scholar]
- 42. Ma Y, Gao M, Liu D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm Res. 2015;32:1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song SJ, Choi S, Park T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in mice. Evid Based Complement Alternat Med. 2014;2014: 718379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin S, Chang C, Zhang L, Liu Y, Huang X, Chen Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS One. 2015;10:e0120842 doi:10.1371/journal.pone.0120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park JJ, Hwang SJ, Park JH, Lee HJ. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell Oncol (Dordr). 2015;38:111–118. [DOI] [PubMed] [Google Scholar]
- 46. Miao M, Cao L, Li R, Fang X, Miao Y. Protective effect of chlorogenic acid on the focal cerebral ischemia reperfusion rat models. Saudi Pharm J. 2017;25:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yan Y, Li J, Han J, Hou N, Song Y, Dong L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anticancer Drugs. 2015;26:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hadad SM, Hardie DG, Appleyard V, Thompson AM. Effects of metformin on breast cancer cell proliferation, the AMPK pathway and the cell cycle. Clin Transl Oncol. 2014;16:746–752. [DOI] [PubMed] [Google Scholar]
- 49. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ong KW, Hsu A, Tan BK. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7:e32718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. [DOI] [PubMed] [Google Scholar]
- 52. Deweese JE, Osheroff MA, Osheroff N. DNA topology and topoisomerases: teaching a “knotty” subject. Biochem Mol Biol Educ. 2009;37:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burgos-Morón E, Calderón-Montaño JM, Orta ML, et al. The coffee constituent chlorogenic acid induces cellular DNA damage and formation of topoisomerase I- and II-DNA complexes in cells. J Agric Food Chem. 2012;60:7384–7391. [DOI] [PubMed] [Google Scholar]
- 54. Belkaid A, Currie JC, Desgagnés J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Renouf M, Guy PA, Marmet C, et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol Nutr Food Res 2009; 54: 760–766. [DOI] [PubMed] [Google Scholar]


