Abstract
In this study, hepatocellular carcinoma (HCC) mouse xenograft model, MTT assay, colony formation, nuclear staining, and Annexin-V/PI staining assays were used to evaluate the effect of Qingjie Fuzheng granules (QFG) on cell proliferation and apoptosis of HCC cell in vivo and in vitro. Furthermore, Western blotting was performed to detect the expression of Fas, FasL, Bcl-2, Bax, and the activation of caspase-3/-8/-9. The results showed that QFG reduced tumor weight (P < .05) but had no effect on body weight gain in HCC mice in vivo. QFG significantly reduced HCC cell viability and attenuated cell proliferation in a dose-dependent manner (P < .05). QFG increased the expression of Fas, FasL, and Bax (P < .05). QFG downregulated the expression of Bcl-2 and promoted the activation of caspase-8, -9, and -3 (P < .05). These results suggested that QFG possessed anticancer effects, and the mechanisms of action may involve the death receptor pathway and mitochondrion-dependent pathway-mediated apoptosis.
Keywords: Qingjie Fuzheng granules, hepatocellular carcinoma, xenograft, growth, apoptosis
Hepatocellular carcinoma (HCC) has a high prevalence worldwide.1,2 The disease has aggressive characteristics and high mortality rates following symptom onset, particularly jaundice and/or ascites.3,4 High recurrence rate and liver failure are attributable to poor prognosis.5 During late diagnosis, such as in the symptomatic phase, life expectancy is approximately 1 month, and available treatments are limited and ineffective.3,4 Consequently, novel and effective therapeutic approaches need to be developed. Considering the cytotoxicity of traditional chemotherapeutic and radiotherapeutic treatments,6 alternative treatments with more selective cancer cell targeting and fewer side effects are required.
Apoptosis, a natural process that causes death of abnormal and unneeded cells in animals, is crucial for development and tissue homeostasis. Perturbed apoptosis regulation is a major causative factor for cancer pathogenesis, including HCC.7–9 In 1972, Kerret and colleagues10 defined apoptosis as a form of programmed cell death that depends on adenosine triphosphate and is distinct from necrotic cell death.8,9 Many studies have implicated the role of apoptosis in the pathogeneses of multiple diseases in humans and animals.11–15 In development of anticancer drugs, some of the main approaches used are to develop drugs that inhibit excessive growth of tumor cells or promote apoptosis in tumor cells. There are 2 major signaling pathways during the initiation of apoptosis: extrinsic (death receptor) apoptotic pathway and intrinsic (mitochondrial) apoptotic pathway. Also, there are lots of important protein contained in those 2 pathway, such as Bax, Bcl-2, and the cysteine aspartic acid-containing protease (caspase) family. The caspase family plays an important role during the initiation of apoptosis. In the caspase family, caspase-8 and caspase-9 are categorized as initiators, and caspase-3 is categorized as an effector. Caspase activity is initiated by cleavage and leads to activation of substrate molecules downstream.16 Caspase-8 is typically involved in the death receptor pathway, caspase-9 is usually involved in the mitochondria-dependent apoptotic pathway, and caspase-3 is involved in both pathways. The death receptor pathway is activated by binding of tumor necrosis factor family extracellular protein death ligands, such as Fas ligand (FasL), to receptors, and these ligands interact with Fas. The death receptor and mitochondria-dependent apoptotic pathways are the most widely known pathways involved in apoptosis.
Qingjie Fuzheng granule (QFG) is a Chinese medicinal formula comprising a 4-herb mixture: Hedyotis diffusa Willd, malt, Astragalus, and Scutellaria barbata D. Don. The mixture confers anti-inflammatory, antioxidative, and antibacterial properties; enhances immunity; and promotes digestion. It is widely used as an alternative medicine for the clinical treatment of various cancers, including liver cancer. However, the underlying mechanism and molecular signaling pathways involved in QFG activity remain unclear. In this study, many modern experimental technologies were used to determine the anticancer activity of QFG.
Materials and Methods
Materials and Reagents
Dulbecco’s modified Eagle’s medium (DMEM; C11960500BT), Roswell Park Memorial Institute (RPMI)-1640 medium (C11875500BT), trypsin-EDTA (C1816869), penicillin-streptomycin (sv30010), and fetal bovine serum (FBS; C10099141) were obtained from Life Technologies Corporation (Grand Island, NY). Phosphate-buffered saline (PBS; SH30256.01B) was bought from Hyclone (Waltham, MA). Cell culture consumables bicinchoninic acid (BCA) protein assay kit and RIPA cell lysis buffer (Pierce, 89900) were obtained from Thermo Fisher Scientific, Inc (Waltham, MA). Rabbit polyclonal antibodies for Fas (bs-0215R) and FasL (bs-0216R) were purchased from Biosynthesis Biotechnology (Beijing, China). Rabbit polyclonal antibodies for caspase-3 (ab44976), caspase-8 (ab194145), as well as caspase-9 (ab202068) were provided by Abcam (Burlingame, CA). Rabbit polyclonal antibodies for cleaved caspase-3 (#9662) as well as cleaved caspase-8 (#4790), rabbit monoclonal antibodies against β-actin (#4967), rabbit monoclonal antibodies against B-cell leukemia/lymphoma (Bcl-2; #4223), and rabbit monoclonal antibodies against Bcl-2 associated X (Bax; #5023) were provided by Cell Signaling Technology (Beverly, MA). Mouse monoclonal antibodies for cleaved caspase-9 (#9508) were provided by Cell Signaling Technology. Goat anti-mouse IgG secondary antibody HRP-conjugated (#L3032) and goat anti-rabbit IgG secondary antibody HRP-conjugated (#L3012) were provided by Signalway Antibody (College Park, MD). Annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit (KGA108) was obtained from KeyGen Biotech (Nanjing, China). The compound 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; #0290022) was obtained from Sigma-Aldrich, Merck KGaA (Darmstadt, Germany). Dimethyl sulfoxide (DMSO; 3072418.0) Hoechst 33258 staining assay kit (c0003) was acquired from Beyotime (Shanghai, China). Immobilon-NC membrane (HATF00010) was purchased from Millipore (Burlington, MA). Formaldehyde (HX056) was obtained from Sinopharm Group Co Ltd (Shanghai China). Cell culture consumables were both obtained from Nest (Wuxi, China).
Preparation of QFG
QFG was provided by the Academy of Pharmacy of Fujian University of Traditional Chinese Medicine (Fuzhou, Fujian, China) and were dissolved in to a concentration of 200 mg/mL and stored at −20°C.
Cell Culture and Detection of Cell Viability
Human HCC cells (SK-Hep-1 and Bel-7402) and mouse HCC cells (H22) were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, China). The genetic backgrounds were confirmed by the company. The SK-Hep-1, Bel-7402, and H22 cells were grown in DMEM and RPMI-1640 medium, respectively, supplemented with 10% FBS, 1% penicillin-streptomycin, and 5% CO2 in a 37°C humidified atmosphere. MTT assay was used to detect cell viability according to the manufacture’s instruction.
Animals and In Vivo Mice Xenograft Study
Forty-five six-week-old male BALB/c mice, which weighed about 20 to 22 g, were obtained from Shanghai SLAC Laboratory Animal Co, Ltd (Shanghai, China) and supported in a specific pathogen-free controlled environment. A total of 1.5 × 106 of H22 cells were harvested, mixed with PBS and Matrigel (1:1), then the cells were subcutaneously injected in the right flank area of each mouse. After 4 days of xenograft implantation, mice were randomized into 3 groups (n = 15) and given intragastric administration of 0.75 g/kg of QFG, 1.5 g/kg of QFG, or saline daily. After 7 days of administration, the mice were anaesthetized with pelltobarbitalum natricum, and the tumor tissue was removed and weighed. The experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, China).
Observation of Cell Density Changes and Colony Formation
After treatment with different concentrations of QFG (0.5, 1, and 1.5 mg/mL), cell density was observed by a phase-contrast microscope (Leica, Solms, Germany). Colony formation assay was conducted as described previously.17
Detection of Apoptosis
Cell apoptosis was detected by Hoechst 33258 and Annexin V–FITC/propidium iodide (PI) staining assays. In Hoechst 33258 staining assay, the treated cells were fixed in 4% paraformaldehyde for 10 minutes. The fixation solution was then discarded, and the cells were rinsed 3 times in 1× PBS. After discarding the buffer, the cells were incubated in 100 μL of Hoechst 33258 solution at room temperature in the dark. After removal of the Hoechst 33258 solution, the stained cells were rinsed 3 times in 1× PBS and supplemented with fresh 1× PBS. The stained cells were observed under an inverted fluorescence microscope (Leica DMI4000B; Leica Camera AG, Solms, Germany). Then the annexin V–FITC/PI staining assay was done as described previously.18
Western Blot Analysis
Total proteins of treated cells were subjected to Western blots as described previously.18
Statistical Analysis
Statistical analysis was performed by using SPSS software (version 17.0) for Windows (SPSS, Inc, Chicago, IL) and 1-way analysis of variance. Data are expressed as the mean ± standard deviation. P < .05 was considered as indicating statistical significance.
Results
QFG Inhibited the Tumor Growth in HCC Xenograft Mice
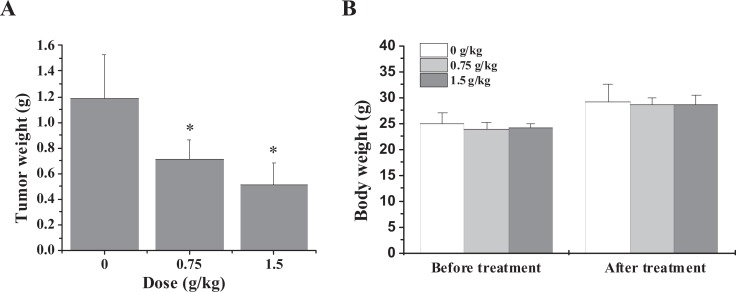
The inhibitory effect of QFG in vivo was evaluated by measuring tumor weight in HCC xenograft mice, while the body weight gain was used to evaluate the adverse effect of QFG. As shown in Figure 1A, the tumor weight of the control group is 1.187 ± 0.34 g and the tumor weights of the treatment groups are 0.716 ± 0.75 and 0.511 ± 1. G, respectively. So administration of QFG markedly decreased tumor weight, compared with control. However, QFG treatment had no effect on the body weight gain in experimental animals (Figure 1B). These data suggest that QFG can inhibit HCC tumor growth in vivo, without apparent signs of toxicity.
Figure 1.
Effect of QFG on tumor growth in HCC xenograft mice. Tumor weight (A) and body weight (B) were measured at the end of the experiment. Data shown are averages with SD (error bars) from 15 individual mice in each group. *P < .05.
QFG Inhibited Proliferation of SK-Hep-1 and Bel-7402 Cells
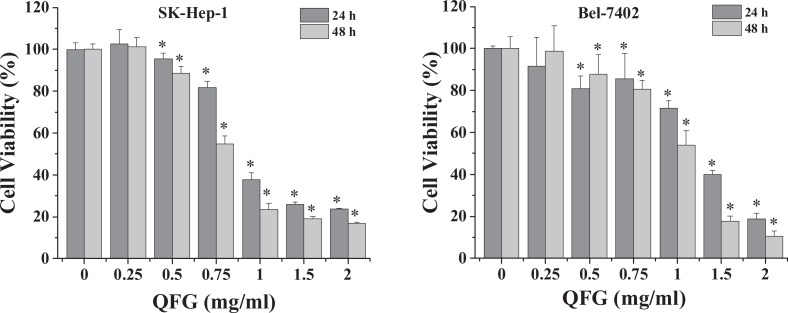
The effect of QFG on SK-Hep-1 and Bel-7402 cell viability was assessed by MTT assay. Compared with the viability of the control cells, the viabilities of SK-Hep-1 and Bel-7402 cells were reduced by treatment with 0.5 to 2.0 mg/mL of QFG for 24 hours by 5% to 76% and 14% to 83%, respectively, and for 48 hours by 8% to 81% and 2% to 89%, respectively (P < .05; Figure 2). After treatment with QFG at different concentrations for 24 and 48 hours, the 2 cell lines showed estimated half maximal inhibitory concentration (IC50) values of 1.09 and 0.838 mg/mL (SK-Hep-1 cells) and 1.207 and 1.036 mg/mL (Bel-7402 cells), respectively. These results suggested that cell viability was reduced dose dependently by QFG treatment.
Figure 2.
Effect of QFG on the viabilities of SK-Hep-1 and Bel-7402 cells. Cells were treated with QFG at different concentrations for 24 and 48 hours. The cell viability was measured using MTT assay. Data were normalized to the viability of control cells (100%). Data are expressed as mean ± SD of at least 3 independent experiments. *P < .05.
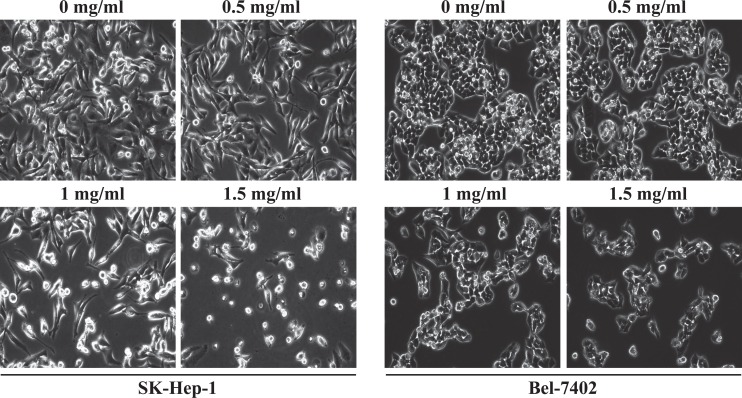
Untreated SK-Hep-1 and Bel-7402 cells appeared as densely packed and disorganized multilayers (Figure 3). However, after incubation for 24 hours with various concentrations of QFG, many cells were round, shrunken, and detached from adjacent cells adhering to the plate or floating in the media. Furthermore, cell density was decreased. In addition, following 24 hours of exposure to QFG, the monolayers were less confluent than the untreated controls. Taken together, QFG decreased the density and increased the damage to both SK-Hep-1 and Bel-7402 cell lines.
Figure 3.
Effect of QFG on the densities of SK-Hep-1 and Bel-7402 cells. Cells were treated with QFG at various concentrations for 24 hours. Density changes were observed using a phase-contrast microscope. Photographs were captured at 200× magnification. Images are representative of 3 independent experiments.
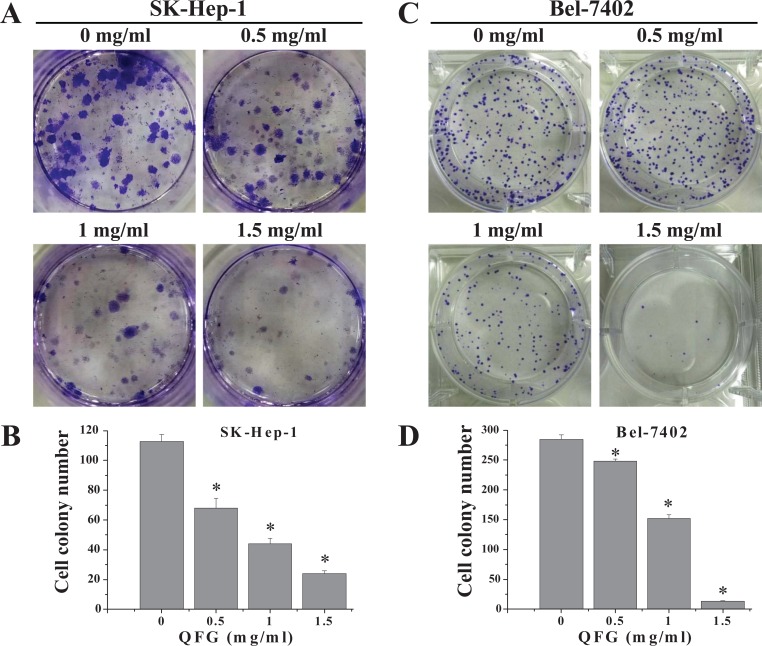
To confirm these results, a colony formation assay was performed to determine the effect of QFG on SK-Hep-1 and Bel-7402 cell survival. Treatment with 0.5, 1, and 1.5 mg/mL of QFG for 24 hours reduced colony formation in SK-Hep-1 (Figure 4A) and Bel-7402 (Figure 4C) cells in a dose-dependent manner from 112 ± 4.73 (control) to 68 ± 6.66, 44 ± 3.61, and 24 ± 2.00 and from 285 ± 7.02 (control) to 248 ± 4.01, 152 ± 6.01, and 13 ± 1.12, respectively (P < .05). These data suggested that QFG inhibited the proliferation of SK-Hep-1 and Bel-7402 cells.
Figure 4.
Effect of QFG on the colony formation abilities of SK-Hep-1 and Bel-7402 cells. The detection of SK-Hep-1 (A) and Bel-7402 (C) cells’ colony formation abilities after treatment with QFG for 24 hours at indicated concentrations. Colony numbers of SK-Hep-1 (B) and Bel-7402 (D) cells are expressed as mean ± SD of at least 3 independent experiments. *P < .05.
QFG Induced Apoptosis of SK-Hep-1 and Bel-7402 Cells
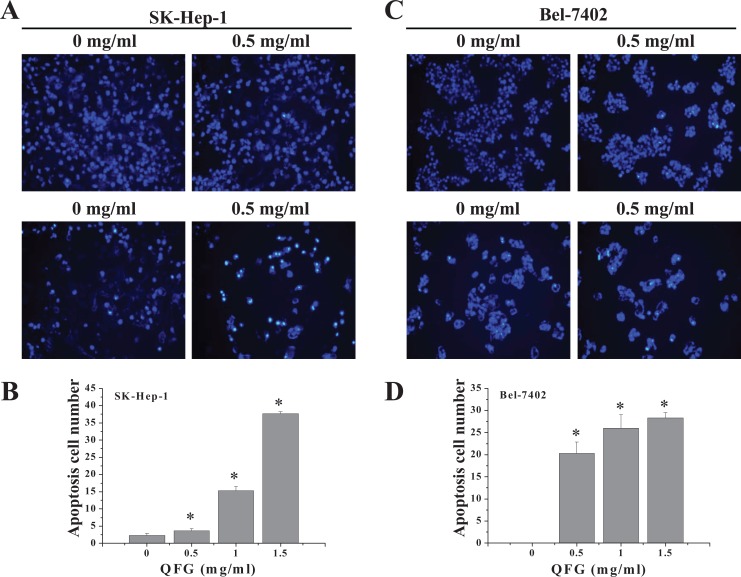
SK-Hep-1 and Bel-7402 cells were stained with Hoechst 33258 to examine nuclear morphological changes in response to QFG treatment and visualized by fluorescence microscopy (Figure 5). Faintly stained cells were observed in the control group, which indicated typical living cells. In contrast, the QFG-treated groups (0.5, 1, and 1.5 mg/mL) presented gradually increasing degrees of apoptosis with evidence of improved brightness. In addition, treatment increased the number of apoptotic cells. After treatment with QFG at 0.5, 1, and 1.5 mg/mL for 24 hours, the number of apoptotic SK-Hep-1 and Bel-7402 cells increased from 2.33 ± 0.577 (control) to 3.67 ± 0.578, 15.33 ± 1.15, and 37.67 ± 0.58 and from 0 ± 0 (control) to 20.33 ± 2.52, 26.12 ± 3.00, and 28.33 ± 1.15, respectively (P < .05; Figure 5A and C). These results suggested that QFG exhibited significant apoptosis-inducing activity against SK-Hep-1 and Bel-7402 cells in a dose-dependent manner.
Figure 5.
Effect of QFG on the morphologies of SK-Hep-1 and Bel-7402 cells. Apoptosis in SK-Hep-1 (A) and Bel-7402 (C) cells exposed to different concentrations of QFG for 24 hours was detected by Hoechst 33258 staining and visualized under a fluorescence microscope (200× magnification). The rate of apoptosis of SK-Hep-1 (B) and Bel-7402 (D) cells is expressed as mean ± SD of at least 3 independent experiments. *P < .05.
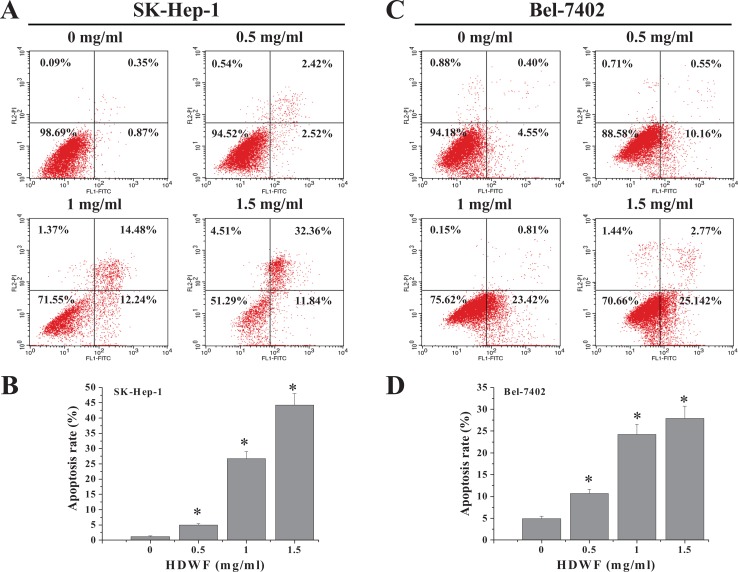
To confirm these results, we assessed the effects of QFG on SK-Hep-1 and Bel-7402 cell viabilities by performing an annexin V-FITC/PI staining flow cytometry assay. The different quadrants in Figure 6 show living cells (lower left), early apoptotic cells (lower right), late apoptotic cells (upper right), and mechanically damaged cells (upper left). The percentages of SK-Hep-1 cells undergoing apoptosis after treatment with 0.5, 1, and 1.5 mg/mL QFG (including early and late apoptotic cells) were 4.94 ± 0.50%, 26.72 ± 2.24%, and 44.2 ± 3.80% (control, 1.22 ± 0.25%; Figure 6A); and those of Bel-7402 cells were 10.71 ± 0.90%, 24.23 ± 2.32%, and 27.91 ± 2.83%, respectively (control, 4.95 ± 0.50%; Figure 6C). Taken together, QFG exhibited significant apoptosis-inducing activity against SK-Hep-1 and Bel-7402 cells in a dose- and time-dependent manner.
Figure 6.
Effect of QFG on the apoptosis of SK-Hep-1 and Bel-7402 cells. SK-Hep-1 (A) and Bel-7402 (C) cells were treated with QFG at various concentrations for 24 hours and stained with annexin V–FITC/PI, followed by flow cytometry analysis. Quantification of FACS analysis of each group is expressed as mean ± SD of 3 independent experiments in SK-Hep-1 (B) and Bel-7402 (D) cells. *P < .05.
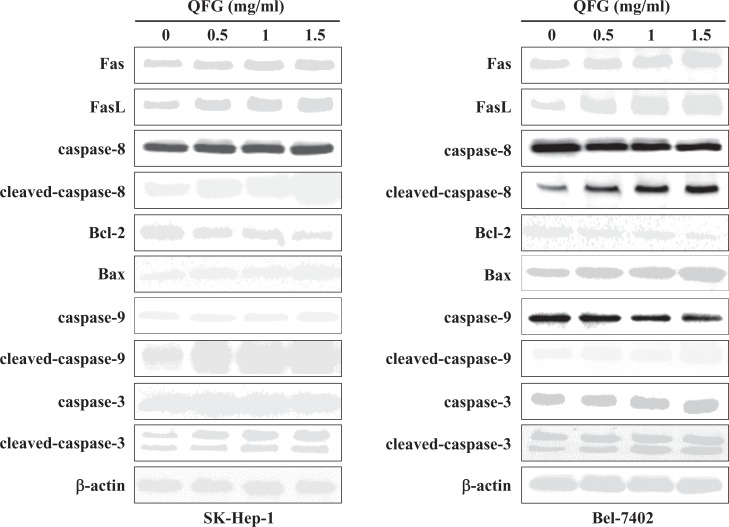
QFG Regulated the Expressions of Fas, FasL, B-Cell Lymphoma (Bcl)-2, and Bcl-2-Associated X (Bax) and Promoted the Activations of Caspase-8, Caspase-9, and Caspase-3 in SK-Hep-1 and Bel-7402 Cells
Caspase-3 is a key apoptosis activator, for which initiation involves 2 major signaling pathways: the death receptor and mitochondria-dependent pathways. FasL, Fas, and caspase-8 activate the death receptor pathway, whereas Bcl-2, Bax, and caspase-9 regulate the mitochondria-dependent pathway. Both pathways can trigger the activation of caspase-3 and eventually induce apoptosis.19 In this study, QFG increased the expressions of Fas, FasL, cleaved caspase-8, Bax, cleaved caspase-9, and cleaved caspase-3, whereas it decreased the expression of Bcl-2 in SK-Hep-1 and Bel-7402 cells (Figure 7). These data suggested that QFG promotes activation of caspase-3 via both the death receptor and mitochondria-dependent pathways, thereby promoting apoptosis in SK-Hep-1 and Bel-7402 cells.
Figure 7.
Effect of QFG on the expressions of Fas, FasL, Bax, Bcl-2, and on the activation of caspase-3, -8, and -9 in SK-Hep-1 and Bel-7402 cells. Cells were treated with QFG at different concentrations for 24 hours. The expressions of Fas, FasL, Bax, Bcl-2, and caspase-3, -8, and -9, as well as cleaved caspase-3, -8, and -9 in SK-Hep-1 and Bel-7402 cells were determined using Western Blotting. β-Actin was used as an internal control. Images are representatives of 3 independent experiments.
Discussion
HCC has an impalpable pathogenesis, rapid progression, poor prognosis, and a high mortality rate, leading to tumor invasiveness, frequent intrahepatic spread, and extrahepatic metastasis as well as frequent recurrence after resection.20 Owing to the lack of effective means of clinical diagnosis at an early stage, only 30% to 40% of patients with HCC may require curative resection, whereas 5% to 9% of patients show up to 69% improvement in 5-year survival.21,22 Thus, novel agents, including natural products, are considered for a more efficient cancer treatment. Traditional Chinese medicine, with its higher safety and long history of pharmacological applications, has attracted great attention in the field of cancer treatment.23,24 Nevertheless, with the rapid growing interest in the use of natural products as medicinal remedies, dietary supplements, and functional foods worldwide, it is now well recognized that the concept of no adverse effect and/or toxicity of natural products is a misnomer. To date, about 6000 plant species mainly from 3 families—Asteraceae (Compositeae), Boraginaceae, and Fabaceae (Liguminosae)—have been identified as potentially hepatotoxic, and a large number of these plant species have been traditionally used as herbal remedies worldwide.25–27 So the safety of natural products appears timely and imperative for global concern. QFG, is a 4-herb Chinese medicinal formula comprising H diffusa Willd, malt, Astragalus, and S barbata D. Don. Previous studies have shown that H diffusa Willd and S barbata D. Don promoted apoptosis and inhibited proliferation and angiogenesis in many human cancer cells, including HCC.26,28 Malt can improve Qi movement and food digestion.29 Astragalus is another important component of numerous traditional Chinese medicinal formulas used to clinically treat various cancers and can delay the occurrence of complications and improve patients’ quality of life.30 However, the mechanism and signaling pathways underlying QFG activity remain unclear.
In this study, the animal experiment results showed that QFG can significantly inhibit the tumor growth in HCC xenograft mice. Therefore, we product a series of experiments in vitro to confirm this. In vitro, QFG showed significant anticancer effects in SK-Hep-1 and Bel-7402 cells; this effects mainly through apoptosis induction. The cell apoptosis is mainly identifiable though cell morphological changes, which contains chromatin condensation and membrane blebbing.31 In this study, control cells exhibited a typical polygonal intact shape, while QFG-treated cells became shrunken, exhibited membrane blebbing, or became nonconfluent. Nuclear staining and flow cytometry confirmed these results. Apoptosis initiation involves 2 major signaling pathways: extrinsic (death receptor) and intrinsic (mitochondria-dependent). Increased caspase-3, caspase-8 (death receptor pathway), and caspase-9 (mitochondria-dependent pathway) activities indicated possible apoptotic mechanisms. Thus, QFG-induced apoptosis included both mitochondria-dependent and death receptor pathways.
Upregulation of the extracellular death ligand protein, FasL, and the intracellular death receptor protein, Fas, initiates the death receptor pathway. In SK-Hep-1 and Bel-7402 cells, QFG induced Fas and FasL upregulation. A complex is formed by ligation of Fas by upregulated FasL, which recruits Fas-associated adaptor protein with death domain via interactions with proteins, such as procaspase-8, following caspase-3 activation, causing cell death.19 This study demonstrated that QFG treatment dose-dependently enhanced the expressions of Fas, FasL, caspase-8, caspase-3, cleaved caspase-9, and cleaved caspase-3 in SK-Hep-1 and Bel-7402 cells, indicating that QFG promotes SK-Hep-1 and Bel-7402 cell apoptosis via death receptor pathway.
Mitochondria-dependent apoptotic pathway is regulated by the Bcl-2 family of proteins. Controlled ratio of active antiapoptotic and proapoptotic Bcl-2 family proteins maintains tissue homeostasis. Various cancers commonly show higher Bcl-2/Bax ratios caused by abnormal expression of these proteins. Mitochondrial outer membrane permeabilization is thought to occur via pore formation by proapoptotic Bax-like proteins, which can be inhibited by antiapoptotic Bcl-2-like members. Consequently, Bax/Bcl-2 ratio is a critical factor associated with cell fate. In this study, QFG treatment dose-dependently enhanced Bax protein expression and reduced Bcl-2 protein expression in SK-Hep-1 and Bel-7402 cells. These findings indicated that QFG induced apoptosis by affecting Bax/Bcl-2 ratio at a translational level. A key event in cellular apoptosis is mitochondrial membrane permeabilization accompanied by collapse of the electrochemical gradient across mitochondrial membrane in the mitochondria-dependent apoptotic pathway.32,33 Consequently, numerous caspase activators, such as cytochrome c, which activates caspases by forming a complex with apoptotic protease-activating factor-1 and procaspase-9, which further trigger caspase-9 activation, are released, and the effector caspase-3 is cleaved,34 ultimately inducing apoptosis. In this study, we demonstrated that QFG treatment dose-dependently enhanced the expressions of caspase-9, caspase-3, cleaved caspase-9, and cleaved caspase-3 in SK-Hep-1 and Bel-7402 cells. These results suggest a direct association between mitochondria and QFG-induced apoptosis.
Conclusions
Our findings suggest that inhibitory effects of QFG against HCC tumor and SK-Hep-1 and Bel-7402 carcinoma cells, mediated via death receptor and mitochondria-dependent apoptotic pathways, could be useful.
Footnotes
Author Contributions: Jun Peng and Jiumao Lin designed the research. Pingping Zhong and Hong Yang performed the research. Pingping Zhong, Hong Yang, Shan Lin, and Jiumao Lin analyzed the data. Hong Yang wrote the article. All authors discussed the results and revised the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Project Funding for the Training of Young and Middle-Aged Backbone Personnel of the Fujian Provincial Health and Family Planning Commission (2016-ZQN-67) and by the Scientific Research Foundation of Traditional Chinese Medicine of the Fujian Provincial Health and Family Planning Commission, China (2017FJZYZY203).
Ethical Approval: All animal treatments in this research were performed in accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, China).
References
- 1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. [DOI] [PubMed] [Google Scholar]
- 2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 5. Hu DH, Yu SM. Association between platelet to lymphocyte ratio (PLR) and overall survival (OS) of hepatocellular carcinoma (HCC): a meta-analysis. Cell Mol Biol (Noisy-le-grand). 2017;63:30–32. [DOI] [PubMed] [Google Scholar]
- 6. Li YL, Zhang J, Min D, Hongyan Z, Lin N, Li QS. Anticancer effects of 1,3-dihydroxy-2-methylanthraquinone and the ethyl acetate fraction of Hedyotis diffusa Willd against HepG2 carcinoma cells mediated via apoptosis. PLoS One. 2016;11:e0151502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. [DOI] [PubMed] [Google Scholar]
- 9. Atia TA. Placental apoptosis in recurrent miscarriage. Kaohsiung J Med Sci. 2017;33:449–452. [DOI] [PubMed] [Google Scholar]
- 10. Takemura G, Kanoh M, Minatoguchi S, Fujiwara H. Cardiomyocyte apoptosis in the failing heart—a critical review from definition and classification of cell death. Int J Cardiol. 2013;167:2373–2386. [DOI] [PubMed] [Google Scholar]
- 11. Arbustini E, Brega A, Narula J. Ultrastructural definition of apoptosis in heart failure. Heart Fail Rev. 2008;13:121–135. [DOI] [PubMed] [Google Scholar]
- 12. Hirsova P, Gores GJ. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bai L, Liu X, Zheng Q, et al. M2-like macrophages in the fibrotic liver protect mice against lethal insults through conferring apoptosis resistance to hepatocytes. Sci Rep. 2017;7:10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuo D, Sun F, Cui J, et al. Alcohol amplifies ketamine-induced apoptosis in primary cultured cortical neurons and PC12 cells through down-regulating CREB-related signaling pathways. Sci Rep. 2017;7:10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–1846. [DOI] [PubMed] [Google Scholar]
- 16. Cillessen SA, Meijer CJ, Notoya M, Ossenkoppele GJ, Oudejans JJ. Molecular targeted therapies for diffuse large B-cell lymphoma based on apoptosis profiles. J Pathol. 2010;220:509–520. [DOI] [PubMed] [Google Scholar]
- 17. Sun GD, Wei LH, Feng JY, Lin J, Peng J. Inhibitory effects of Hedyotis diffusa Willd. on colorectal cancer stem cells. Oncol Lett. 2016;11:3875–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin J, Chen Y, Wei L, et al. Hedyotis diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int J Oncol. 2010;37:1331–1338. [DOI] [PubMed] [Google Scholar]
- 19. Sayers TJ. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol Immunother. 2011;60:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123:1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou J, Yu L, Gao XP, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788. [DOI] [PubMed] [Google Scholar]
- 22. Liu AM, Yao TJ, Wang W, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open. 2012;2:e000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. [DOI] [PubMed] [Google Scholar]
- 24. Zhang MM, Qiao Y, Ang EL, Zhao H. Using natural products for drug discovery: the impact of the genomics era. Expert Opin Drug Discov. 2017;12:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li SL, Lin G, Fu PP, et al. Identification of five hepatotoxic pyrrolizidine alkaloids in a commonly used traditional Chinese medicinal herb, Herba Senecionis scandentis (Qianliguang). Rapid Commun Mass Spectrom. 2008;22:591–602. [DOI] [PubMed] [Google Scholar]
- 26. Gori L, Firenzuoli F. Is black cohosh a hepatotoxic medicinal herb? Forsch Komplementmed. 2007;14:109–110. [DOI] [PubMed] [Google Scholar]
- 27. Manna P, Sinha M, Sil PC. Galactosamine-induced hepatotoxic effect and hepatoprotective role of a protein isolated from the herb Cajanus indicus L in vivo. J Biochem Mol Toxicol. 2007;21:13–23. [DOI] [PubMed] [Google Scholar]
- 28. Chen XZ, Cao ZY, Li JN, et al. Ethyl acetate extract from Jiedu Xiaozheng Yin inhibits the proliferation of human hepatocellular carcinoma cells by suppressing polycomb gene product Bmi1 and Wnt/β-catenin signaling. Oncol Rep. 2014;32:2710–2718. [DOI] [PubMed] [Google Scholar]
- 29. Dianat M, Taghizadeh M, Shahidi F, Razavi S. The flow properties of honey-malt spread. Food Sci Technol Int. 2017;23:415–425. [DOI] [PubMed] [Google Scholar]
- 30. Lin SM, Jiang Y, Chen YJ, Luo L, Doolgindachbaporn S, Yuangsoi B. Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2017;70:40–47. [DOI] [PubMed] [Google Scholar]
- 31. Yaoi X, Lu B, Lü C, Bai Q, Yan D, Xu H. Taraxerol induces cell apoptosis through a mitochondria-mediated pathway in HeLa cells. Cell J. 2017;19:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin-3-gallate -induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003;410:177–185. [DOI] [PubMed] [Google Scholar]
- 33. Lu Z, Cao S, Zhou H, Hua L, Zhang S, Cao J. Mechanism of arctigenin-induced specific cytotoxicity against human hepatocellular carcinoma cell lines: Hep G2 and SMMC7721. PLoS One. 2015;10:e0125727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]