Abstract
Background: Food and feed supplements containing microorganisms with probiotic potential are of increasing interest due to their healthy promoting effect on human and animals. Their mechanism of action is still unknown. Using a microarray approach, the aim of this study was to investigate the differences in genome-wide gene expression induced by a mixture of three Lactobacillus strains (L. rhamnosus, L. plantarum, and L. paracasei) in intestinal porcine epithelial cells (IPEC-1) and to identify the genes and pathways involved in intestinal barrier functions. Methods: Undifferentiated IPEC-1 cells seeded at a density of 2.0 × 105/mL in 24-wells culture plates were cultivated at 37 °C and 5% CO2 until they reached confluence (2–3 days). Confluent cells monolayer were then cultivated with 1 mL of fresh lactobacilli (LB) mixture suspension prepared for a concentration of approximately 3.3 × 107 CFU/mL for each strain (1 × 108 CFU/mL in total) for 3 h and analyzed by microarray using Gene Spring GX v.11.5. Results: The functional analysis showed that 1811 of the genes modulated by LB treatment are involved in signaling (95% up-regulation, 121 genes with a fold change higher than 10). The most enhanced expression was registered for AXIN2 (axis inhibition protein 2-AXIN2) gene (13.93 Fc, p = 0.043), a negative regulator of β-catenin with a key role in human cancer. LB affected the cellular proliferation by increasing 10 times (Fc) the NF1 gene encoding for the neurofibromin protein, a tumor suppressor that prevent cells from uncontrolled proliferation. The induction of genes like serpin peptidase inhibitor, clade A member 3 (SERPINA 3), interleukin-20 (IL-20), oncostatin M (OSM), granulocyte-macrophage colony-stimulating factor (GM-CSF), and the suppression of chemokine (C-X-C motif) ligand 2/macrophage inflammatory protein 2-alpha (CXCL-2/MIP-2), regulator of G-protein signaling 2 (RGS2), and of pro-inflammatory interleukin-18 (IL-18) genes highlights the protective role of lactobacilli in epithelial barrier function against inflammation and in the activation of immune response. Conclusion: Gene overexpression was the predominant effect produced by lactobacilli treatment in IPEC-1 cells, genes related to signaling pathways being the most affected. The protective role of lactobacilli in epithelial barrier function against inflammation and in the activation of immune response was also noticed.
Keywords: intestinal porcine epithelial cells (IPEC-1), lactobacilli, microarray, genome, IPA analysis
1. Introduction
Under normal conditions, the structure and functional integrity of the intestinal cell layer forms a physical and immunological barrier that prevents the access of foreign antigens including pathogens, food proteins, and toxins into the underlying tissues [1,2,3]. In this regard, intestinal epithelial cells are considered the “watchdogs” of the immune system [4]. They constitutively produce several cytokines and chemokines including transforming growth factor β (TGF-β) and interleukins (IL-1α, IL-6) which are crucial for the recruitment and activation of neutrophils, macrophages, dendritic cells, T and B cells, mucosal homeostasis, and cell growth [4,5]. Other cytokines such as interleukin-8 (IL-8), interleukin-1 β (IL-1β), and tumor necrosis factor-α (TNF-α) are also synthetized by normal epithelial cells and are markedly upregulated in response to microbial infection [6], where they contribute actively to the initiation of inflammatory cascade in the intestine.
Intestinal epithelial cells also have digestive and absorptive functions including the secretion of water and electrolytes to maintain the viscosity of the luminal content [7,8]. The digestive changes during the weaning period in which the mammal has to adapt to the solid diets and to withstand to pathogen challenge are stressful and interfere with intestinal development and function [9,10]. In the pig for example, post weaning anorexia alters gut integrity, one of the major aetiologic factors in gut associated disorders by increasing mucosal permeability, disturbance in nutrient and ion transport, and stimulation of inflammation at epithelium level [10,11]. In the last decade, many studies have tested probiotics for ameliorating inflammation and gastrointestinal disease symptoms and for decreasing pathogen load in animal [12]. It has been shown that probiotic bacteria reinforce and maintain the intestinal barrier by interaction with the cellular junctional complex (either adherence junction or tight junction) proteins and by up-regulating their expression [13]. Lactobacilli are among the most well-known and safest probiotics with significant beneficial effects on gut health [1,14,15,16]. They contribute to the maturation and modulation of the immune system by enhancing cytokines production, and inhibiting pathogenic bacterial adhesion and activity by lactic acid secretion [17]. Many studies report on the efficiency of lactobacilli in preventing the intestinal diseases, the ability to restore the normal microbiota and the prevention of inflammation [18,19]. These authors [18,19] showed that four distinct Lactobacillus spp. (L. acidophilus, L. gasseri, L. fermentum, and L. rhamnosus) reinforce the epithelial barrier via their effect on the expression and functionality of adherence junction protein E-cadherin and the influence is species specific [18]. Also, L. sobrius could inhibit the Enterotoxigenic Escherichia coli (ETEC) pathogen internalization by acting on tight junction and cytoskeleton protein [19], while L. amylovorus, a novel lactobacillus isolated from unweaned pigs and its cell free supernatant, counteract the ETEC action by suppressing the activation of the different steps of TLR4 signaling and the over-production of inflammatory cytokines IL-8 and IL-1β in pig explants and intestinal Caco-2 cell challenge by ETEC pathogen [20]. Conversely, experimental probiotics (Lactobacillus and Bifidobacterium) either reduced or did not affect the production of cytokines (IL-6, IL-12p70, and TNF-α) in epithelial and dendritic cell co-culture [21]. However, the majority of studies with lactobacilli has been focused on individual strains and their capacity to modulate the immune host response and to limit pathogen invasion [12,22] and very few investigated the effect of mixtures of different probiotic strains and their mechanism of action in pig. However, evidence showed that bacteria mixture can strengthen the effect of individual strains that might last longer.
Using a DNA microarray this study brings information on the differences in genome-wide gene expression induced by a mix of three Lactobacillus strains (L. rhamnosus, L. plantarum, and L. paracasei) in unchallenged intestinal porcine epithelial cells (IPEC-1) cultivated under normal functional conditions. Since probiotics are used as supplement not only for treatment but also for prevention it is important to know their effect in non-pathological challenged conditions in order to discover their mechanism of action and to increase their applications. The most genomic studies were oriented on the cross-talk between intestinal epithelial cells and probiotic microorganisms in response to pathogen bacteria.
The modulation of specific genes and pathways involved in intestinal barrier functions was also analyzed. Gene expression profile has proven to be a powerful tool in studying the molecular cross-talk between intestinal epithelial cells and microorganisms, probiotic bacteria included. Pig is a relevant human model due to its similarity to human’s genome and immune response to different environmental agents.
2. Results
2.1. Microarray Screening
Porcine (S. Scrofa) V2 Genome microarray was used to identify the differences in genes expression in IPEC-1 cells treated or not with a mixture of three lactobacilli for 3 h. The mixture of lactobacilli used in this study contained L. plantarum (ATCC 8014, isolated from horse feces), strain included in the EFSA list of safe substances (QPS) with EFSA recognized probiotic properties, as well as L. paracasei (ATCC 335, isolated from food) and L. rhamnosus (IBNA02, isolated from food), whose probiotic potential was described in the literature. Data analysis was performed using GeneSpring GX Version 12.6.1 Software analysis. A total number of 13,950 genes were found to be differentially expressed when compared to control genes activation (over expression) being the predominant effect produced by LB treatment (12,678 up-regulated and 1272 down-regulated) considering a fold change of 2 and a p-value ≤ 0.05.
2.2. Functional Classification of Differentially Expressed Genes
Genes that were found to be significantly modulated by LB treatment were then subjected to a cluster analysis and classified into nine functional categories and pathways: signaling, cell signaling, proliferation, transcription factors, growth factors, cytokines, interleukins, immune response, and inflammatory response (Table 1). Within these clusters, the gene over-expression was seen to be the most significant effect of LB. The functional analysis showed that 1811 of the genes modulated by LB treatment are involved in signaling. From these, 1735 genes were up-regulated, 121 of them had a fold change greater than 4; the most enhanced expression was registered for AXIN2 (axis inhibition protein 2-AXIN2) gene (13.93 Fc, p = 0.043). Seventy-six out of the 1811 genes were found to be down-regulated, expression of RGS2 and OR1L8 gene being the most suppressed: −6.67 Fc, p = 0.017 and −5.26 Fc, p = 0.259, respectively (Table 2).
Table 1.
Functional classification of differentially expressed genes under lactobacilli mixture treatment.
| Function Name | LB Treatment | ||||
|---|---|---|---|---|---|
| Up-Regulated | Down-Regulated | Total Found Genes | |||
| No. | % | No. | % | No. | |
| Signaling | 1735 | 95.8 | 76 | 4.2 | 1811 |
| Cell signaling | 132 | 96.4 | 5 | 3.7 | 137 |
| Proliferation | 632 | 91.3 | 60 | 8.7 | 692 |
| Transcription factors | 597 | 90.9 | 60 | 9.1 | 657 |
| Growth factors | 398 | 96.8 | 13 | 3.2 | 411 |
| Cytokines | 224 | 95.7 | 10 | 4.3 | 234 |
| Interleukin | 186 | 93.0 | 14 | 7.0 | 200 |
| Immune response | 110 | 92.4 | 9 | 7.6 | 119 |
| Inflammatory response | 205 | 94.0 | 13 | 6.0 | 218 |
Table 2.
List of the most strongly upregulated or downregulated genes involved in signaling in IPEC-cells.
| Gene ID | Gene Symbol | Gene Description | Fold Change | Regulation |
|---|---|---|---|---|
| Signaling | ||||
| GACC01000361 | nf1 | neurofibromin 1 (NF1), transcript variant 1 | 10.20 | up |
| AK349266 | fuz | fuzzy homolog (Drosophila) (FUZ), transcript variant 1 | 10.63 | up |
| - | - | - | 10.70 | up |
| XM-001928433 | or2m3 | olfactory receptor, family 2, subfamily M, member 3 (OR2M3) ectonucleoside triphosphate | 11.63 | up |
| AK345382 | entpd1 | diphosphohydrolase 1 (ENTPD1), transcript variant 1 | 12.13 | up |
| - | - | - | 13.45 | up |
| XM-003482962 | axin2 | axin 2 | 13.93 | up |
| AB530146 | rgs2 | regulator of G-protein signaling 2, 24 kDa | −6.67 | down |
| XM-001925049 | or1l8 | olfactory receptor, family 1, subfamily L, member 8 | −5.26 | down |
| - | - | - | −4.17 | down |
| NM-001001861 | cxcl2 | chemokine (C-X-C motif) ligand 2 | −4.00 | down |
| NM-214376 | areg | amphiregulin | −3.85 | down |
| NM-214376 | areg | amphiregulin | −3.85 | down |
| AY609724 | tcf21 | transcription factor 21 | −3.70 | down |
The cluster of genes related to proliferation also encountered a high number of differentially expressed genes (692 genes): of these, 632/692 were up-regulated (with 59/632 exceeding a fold change of 4, e.g., NF1, 10.20 Fc) and 60/692 were down-regulated (AREG, -3.85 Fc, IL1α, -3.33 Fc, Table 3). Following this, was the cluster relating to transcription factors genes with 657 modulated genes, of which 597 were over expressed (the most up-regulated being, for instance, TSHZ2, 12.21 Fc, p = 0.058, NF1, 10.20 Fc, p = 0.133 and EMX1, 9.00 Fc, respectively) and 60 were suppressed (PKNOX2, −8.33 Fc, p = 0.015, Table 4).
Table 3.
List of the most strongly upregulated or downregulated genes involved in proliferation in IPEC-cells.
| Gene ID | Gene Symbol | Gene Description | Fold Change | Regulation |
|---|---|---|---|---|
| Proliferation | ||||
| GACC01000361 | nf1 | neurofibromin 1 (NF1), transcript variant 1 | 10.20 | up |
| - | - | - | 10.70 | up |
| - | - | - | 13.45 | up |
| XM-003482962 | axin2 | axin 2 | 13.93 | up |
| NM-214376 | areg | amphiregulin | −3.85 | down |
| - | - | - | −3.45 | down |
| NM-214029 | il1α | interleukin 1, alpha (IL1α) | −3.33 | down |
| AY610314 | ube2v2 | ubiquitin-conjugating enzyme E2 variant 2 | −3.33 | down |
Table 4.
List of the most strongly upregulated or downregulated genes associated with transcription factors and inflammatory response in IPEC-cells.
| Gene ID | Gene Symbol | Gene Description | Fold Change | Regulation |
|---|---|---|---|---|
| Transcription Factors | ||||
| XM-003125031 | emx1 | empty spiracles homeobox 1 (EMX1) | 9.00 | up |
| GACC01000361 | nf1 | neurofibromin 1 (NF1), transcript variant 1 | 10.20 | up |
| AK347929 | tshz2 | teashirt zinc finger homeobox 2 (TSHZ2), transcript variant 1 | 12.21 | up |
| XM-003361490 | pknox2 | PBX/knotted 1 homeobox 2 | −8.33 | down |
| AY609724 | tcf21 | ref|Homo sapiens transcription factor 21 (TCF21), transcript variant 2 | −3.70 | down |
| Inflammatory Response | ||||
| XM-003131278 | prkcα | protein kinase C, alpha | 5.13 | up |
| - | - | - | 5.58 | up |
| AK396677 | pla2g7 | phospholipase A2, group VII (platelet-activating factor acetylhydrolase, plasma) | 5.82 | up |
| XM-001929161 | osm | oncostatin M | 6.19 | up |
| XM-003130465 | il20 | interleukin 20 | 6.23 | up |
| AY669080 | bmp2 | bone morphogenetic protein 2 | 6.87 | up |
| AK232615 | serpina3 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | 9.85 | up |
| AK345252 | cxcl2 | chemokine (C-X-C motif) ligand 2 | −6.25 | down |
| XM-003129107 | cxcl2 | chemokine (C-X-C motif) ligand 2 | −6.25 | down |
| XM-003126166 | cxcl2 | chemokine (C-X-C motif) ligand 2 | −4.00 | down |
| NM-001001861 | cxcl2 | chemokine (C-X-C motif) ligand 2 | −3.45 | down |
| - | - | - | −3.45 | down |
| AY577905 | cxcl2 | chemokine (C-X-C motif) ligand 2 | −3.45 | down |
| NM_214029 | il1α | interleukin 1, alpha | −3.33 | down |
LB treatment also enhanced the expression of genes encoding for growth factors. This category encountered 411 modified genes of which 398 genes were up-regulated (HSPB1, 7.67 Fc and PHLPP1, 7.89 Fc for example) and 13 genes were down-regulated (IL1α, −3.36 Fc).
In IPEC-1 cells, LB mixture modulated a lower number (between 100–200) of genes encoding for cytokines, interleukins, immune and inflammatory response and of these most were up-regulated (cytokines 224 genes, interleukins 186 genes, immune response 119 genes, and inflammatory response 205 genes). LB treatment up-regulated the expression of SERPINA-3 (9.85-Fc), IL-20 (6.23 Fc), OSM (6.19 Fc), GM-CSF (6.06 Fc) and suppressed that of CXCL-2 (MIP-2, −6.25 Fc) and RGS2 (−6.67 Fc) genes and of IL-18 (−3.45 Fc) pro-inflammatory cytokine (Table 4 and Table 5).
Table 5.
List of the most strongly upregulated or downregulated genes involved in immune response in IPEC-cells.
| Gene ID | Gene Symbol | Gene Description | Fold Change | Regulation |
|---|---|---|---|---|
| Immune Response | ||||
| NM-001102680 | cd1d | CD1d molecule (CD1D) | 5.21 | up |
| AY506573 | pbd-2 | beta-defensin 2 mRNA, complete cds | 5.24 | up |
| D21074 | csf2 | GM-CSF for granulocyte-macrophage colony-stimulating factor, complete cds | 6.06 | up |
| XM-003129107 | loc100525528 | hypothetical protein LOC100525528 (LOC100525528) | −6.25 | down |
| NM-001001861 | cxcl2 | chemokine (C-X-C motif) ligand 2 (CXCL2) | −4.00 | down |
| U68701 | Il-18 | interleukin-18, complete cds | −3.45 | down |
| AY577905 | cxcl2 | CXCL2, complete cds | −3.45 | down |
| NM-214029 | Il-1a | interleukin 1, alpha (IL1α) | −3.33 | down |
2.3. Real-Time Quantitative Real Time Validation
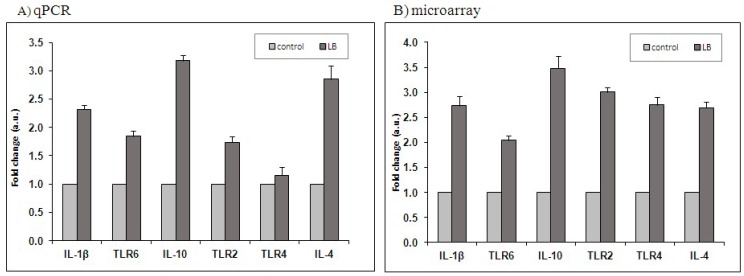
Quantitative Real Time (qPCR) analysis for the expression of 6 genes (IL-1β, TLR-6, IL-10, TLR-2, TLR-4, IL-4) was used to validate the microarray results. The expression levels of selected genes showed a similar pattern (up-regulation) either in microarray (IL-1β, 2.73 Fc; TLR-6, 2.04 Fc; TLR-2, 3.01 Fc; TLR-4, 2.75 Fc; IL-10, 3.48 Fc; IL-4, 2.69 Fc) or in qPCR (IL-1β, 2.32 Fc; TLR-6, 1.84 Fc; TLR-2, 1.74 Fc; IL-10, 3.18 Fc; IL-4, 2.85 Fc) analysis (Figure 1). These results reveal a good correlation among the microarray and qRT-PCR data, being able to eliminate all the variability that can affect the quantification of the gene expression level.
Figure 1.
Validation of microarray results by real-time RT-PCR. Gene expression of six selected genes obtained by qPCR (A) was compared with that obtained by microarray (B). Each experiment was repeated four times ( ) lactobacilli mixture versus (
) lactobacilli mixture versus ( ) Control cells); n = 4.
) Control cells); n = 4.
2.4. Ingenuity Pathway Analyses
The results from Table 6, Table 7, Table 8 and Table 9 were obtained by using Ingenuity Pathway Analysis (IPA) and emphases that in IPEC-1 cells lactobacilli treatment could modulate to a different extent many genes involved in important canonical pathways, biological functions, and networks. Top canonical pathways such as Wnt/β-catenin and molecular mechanism of cancer (Table 6) as well as cellular functions like, cellular growth and proliferation, cell development, cell death and survival (598, 572 and 478 focus transcripts, Table 7 and Table 9) were modulated. IPA analyses demonstrated also the association of lactobacilli treatment with important diseases states and biological function such as Gastrointestinal Diseases (761 molecules), cancer (849 molecules), and inflammatory response (362 molecules), as shown in Table 8.
Table 6.
Top gene Canonical Pathways associated with Lactobacilli treatment—Ingenuity Pathway Analysis (IPA).
| Name | LB Treatment | |
|---|---|---|
| p-Value | Ratio | |
| Role of Macrophages, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | 9.33 × 10−21 | 56/309 (0.181) |
| Molecular mechanism of cancer | 4.29 × 10−20 | 61/374 (0.163) |
| Wnt/β-catenin | 1.32 × 10−18 | 39/169 (0.231) |
| Human embryonic Stem Cell Pluripotency | 1.33 × 10−18 | 36/143 (0.252) |
Table 7.
Top gene molecular and cellular functions associated with lactobacilli treatment—IPA analysis.
| Name | LB Treatment | |
|---|---|---|
| p-Value | Molecules | |
| Cellular growth and proliferation | 2.79 × 10−20–1.53 × 10−124 | 598 |
| Cellular Development | 2.79 × 10−20–1.13 × 10−117 | 572 |
| Gene Expression | 1.83 × 10−33–1.33 × 10−117 | 439 |
| Cellular Movement | 3.35 × 10−20–2.04 × 10−95 | 392 |
| Cell death and Survival | 4.95 × 10−20–5.24 × 10−86 | 478 |
Table 8.
Top gene Diseases and Bio Function associated with lactobacilli treatment—IPA analysis.
| Name | LB Treatment | |
|---|---|---|
| p-Value | Molecules | |
| Cancer | 2.41 × 10−20–1.11 × 10−58 | 849 |
| Organism Injury and Abnormalities | 2.41 × 10−20–1.11 × 10−58 | 860 |
| Gastrointestinal Diseases | 2.38 × 10−20–1.53 × 10−42 | 761 |
| Developmental Disorder | 3.43 × 10−21–5.85 × 10−42 | 282 |
| Inflammatory Response | 5.00 × 10−33–2.08 × 10−43 | 362 |
Table 9.
Top gene networks associated with lactobacilli treatment—IPA analysis.
| Associated Network Function | Score |
|---|---|
| Cell Signaling Cell-to-Cell Signaling and Interaction, Cell Cycle | 52 |
| Gene Expression, Cellular Development, Digestive System Development and Function | 41 |
| Gene Expression, Skeletal and Muscular Disorders, Skeletal and Muscular System Development and Function | 37 |
| Cellular Movement, Hematological System Development and Function, Immune Trafficking | 37 |
| Gene Expression, Hematological System Development and Function, Tissue Morphology | 37 |
Several relevant biological networks, Cell-to-Cell Signaling and Interaction, (52 focus molecules), Cellular Development, Digestive System, (41 focus molecules), and cellular movement, (37 focus molecules) were also modulated by lactobacilli mixture (Table 9).
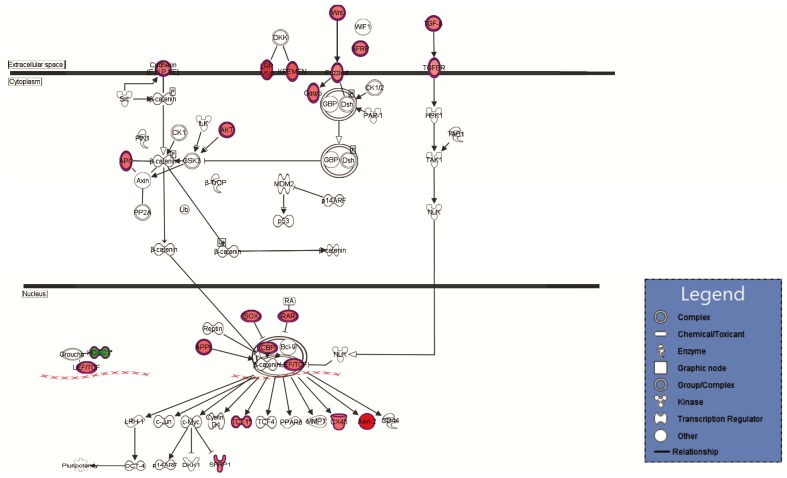
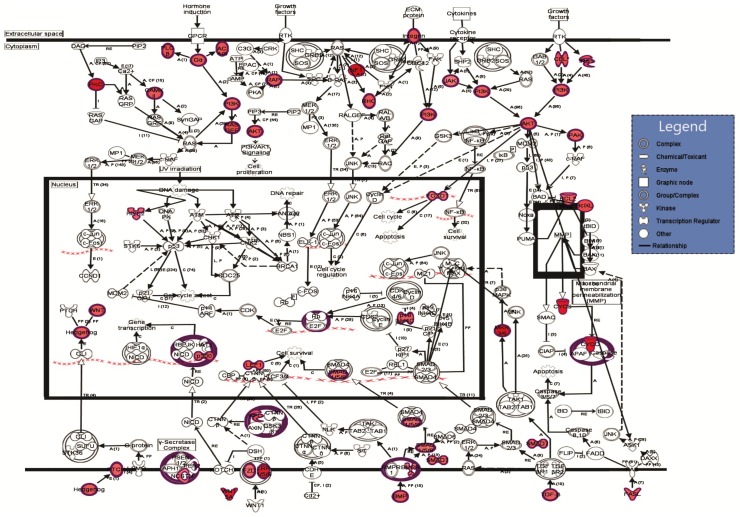
The networks from Figure 2 and Figure 3 showed a high number of predicted molecular interactions between the regulated genes indicating an up-regulation tendency (red color) in both cytoplasm and nucleus after the lactobacilli treatment.
Figure 2.
The predicted molecular connections between the differentially regulated genes in the extracellular space, cytoplasm, and nucleus of IPEC-1 cells. Information about the regulation of genes is included in the figure: the red color gradient from dark to light shows the degree of genes up-regulated in lactobacilli treated IPEC cells versus control cells; the green color shows the down-regulated in lactobacilli treated IPEC cells versus control cells; arrow ( ) = direct relationship; (
) = direct relationship; ( ) = inhibition.
) = inhibition.
Figure 3.
The predicted molecular connections between the differentially regulated genes in the extracellular space, cytoplasm, and nucleus of IPEC-1 cells under the LB mixture action. Information about the regulation of genes is included in the figure: the red color gradient from dark to light shows the degree of genes up-regulated in lactobacilli treated IPEC cells versus control cells. Nodes were used to connect the regulated genes; grey lines show inconsistent or no predicted effects; ( ) = direct relationship; (
) = direct relationship; ( ) = inhibition; n = 4.
) = inhibition; n = 4.
3. Discussion
The normal functionality of the intestinal epithelial cells that cover the intestinal villi is essential for nutrient absorption [23]. Changes in the diet can affect its activity. Probiotic bacteria are often administered in order to confer health benefits to the intestinal epithelium such as cell proliferation and survival, prevention of epithelial injury, and improvement of epithelial barrier and immune function [24]. Probiotic applications are used therapeutically to prevent and treat gastrointestinal diseases including inflammatory bowel diseases, diarrhoea, gastroenteritis, irritable bowel syndrome, etc. [25,26], and a number of studies have elucidated some aspects of the mechanism involved in their effects.
Using a genome microarray analysis, we showed in this study that the exposure to lactobacilli mixture distinctly modulated a high number of genes encoding for different processes. The three Lactobacilli sp. were chosen as they are part of a probiotic bacterial product currently under development in the Institute of Biology and Animal Nutrition (IBNA) biotechnology laboratory, which has proved healthy benefits and excellent efficiency in reducing the gastrointestinal infections in pig as well as in other farm animals in experimental trails. A preliminary in vivo study of our team using the mixture reported a beneficial effect on both humoral (increase in IgG concentration) as well as on cellular immune response (decrease in cytokine TNF-α and increase in chemokine IL-8) [27]. Up-regulation of gene expression was the most prominent effect (with 15 genes’ expression being up-regulated by more than 10-fold). The genes failing within the signaling functional cluster were the most affected by lactobacilli mixture, followed by proliferation and transcription factors. A recent microarray study in neonatal gnobiotic pig using Lactobacillus rhamnosus and Lactobacillus acidophilus showed a similar alteration of canonical pathways involving genes associated with cell signaling and cellular growth and proliferation [11]. Likewise, in the study herein the IPA top canonical pathway analyses revealed that exposure to lactobacilli mixture significantly affected the Wnt/β-catenin signaling and Molecular mechanisms of cancer pathways (p = 1.32 × 10−18 and 4.29 × 10−20 respectively). Wnt/β-catenin signaling is an important pathway involved in development, cellular proliferation, and differentiation [28]. In the gastrointestinal tract Wnt/β-catenin pathway maintains the self-renewal capacity of epithelial stem cells and reestablishes the architecture of crypts after mucosal injury [29]. Microarray results of Hummel et al. [18] indicated that lactobacilli improved epithelial barrier function of T84 cells via enhancement of β-catenin phosphorylation, a subunit of the cadherin protein complex and an intracellular signal transducer in the Wnt signaling pathway with a key role in the creation and maintenance of epithelial cell layers and barrier through the regulation of cell growth and adhesion [30]. Mutation or overexpression of β-catenin is associated with many cancers, colorectal carcinoma among them [31]. It has been demonstrated that β-catenin is clearly involved in human cancer [32] together with other downstream components of Wnt pathway, adenomatous polyposis coli (APC), Dishevelled (Dsh), and the serine/threonine kinase GSK-3beta (GSK-3β) intracellular signaling molecules [32]. For example, inactivation of APC gene or activation of β-catenin mutation was found in more than 90% of all tumors; a hyper-activation of Wnt/β-catenin pathway leading to pathological transformation of gastrointestinal epithelium has been observed in the absence of a negative feedback mechanism that involve the up-regulation of AXIN1 or AXIN2 genes, negative regulators of β-catenin [29,33,34]. A former study [35,36] demonstrated that the overexpression of AXIN2 induced a dramatic reduction of β-catenin level in human colorectal cancer cell line SW480. In this regard, a recent study of Du et al. (2016) demonstrated that genistein-27, a derivative of isoflavone genistein was able to inhibit the proliferation of human colorectal cancer cells by reducing β-catenin nuclear localization and by increasing the expression of AXIN2 and APC (adenomatous polyposis coli) [37]. In the study herein, the microarray results showed that incubation of IPEC cells with lactobacilli mixture enhanced in a distinct manner the expression of AXIN2 (13.92 Fc).
The study of Taherian-Esfahani et al., 2016 [38] found that the expression of secreted frizzled related protein 2 (SFRP2) gene, another antagonist of Wnt pathway was increased in HT-29 cells after L. rhamnosus GG treatment and in HeLa cells after the treatment with supernatant of L. crispatus and L. rhamnosus GG culture. The IPA top network from Figure 2 also illustrates that APC, SFRP, AXIN2, and other key components involved in Wnt signaling transduction system were positively regulated whereas histone deacetylase (HDAC) gene was negatively regulated. The HDAC3 expression decreased after a dietary treatment with a broccoli sprout extract supplement [39]. Many studies showed that the uses of lactobacilli are promising tools against the uncontrolled proliferation occurred in cancer [40]. Supernatants and lactobacilli extract reduced cell proliferation, cell migration, invasion, and increased apoptosis of colorectal tumor cell CaCO-2 and HT-29 [40,41,42]. The results of our wide-genome study (Table 2, Figure 3) indicated a 10-fold increase of NF1 gene expression encoding for neurofibromin protein which act as a tumor suppressor that prevent cells from growing and dividing too rapidly or in an uncontrolled way. NF1 is a negative regulator of the ras signal transduction pathway that stimulates cell division and growth. Patients with NF1 mutation are predisposed to benign as well as malignant tumors like neurofibroma and malignant peripheral nerve-sheath tumors [43]. It is believed that neurofibromatosis type 1, associated with an increased risk of gastrointestinal stromal tumors (GISTs) is caused by functionally biallelic losses of the tumor suppressor gene, NF1 [43,44].
Probiotics have often been investigated for their protective effect against colitis and other pro-inflammatory gastrointestinal diseases. The recent study of Kumar et al. [11] reported that a probiotic mixture (VSL#3, manufactured in India by Sun Pharmaceutical Ind. Ltd.) reduced basal levels of pro-inflammatory cytokines and chemokines like monocyte chemotactic protein-2 (MCP-2), chemokine (C-X-C motif) ligand 2 (CXCL2) and improved epithelial barrier function in mucin deficient (Muc2−/−) animals or DSS-induced colitis. Likewise, assessment of anti-inflammatory activities of lactic acid bacteria in porcine intestinal epithelial (PIE) cells challenged with heat-killed enterotoxigenic E. coli showed a significant down-regulation in the levels of pro-inflammatory interleukins (IL-8, IL-6), and chemokines (MCP-1) [45]. Our study shows that when the three lactobacilli strains acted together the expression of genes associated with “inflammatory response” functional category was modified. For example, a strong down regulation (p < 0.002) of pro-inflammatory CXCL2 (MCP-1) chemokine was found (−6.25 Fc); the IPA Top Diseases and Bio Function analysis showed also a significant modulation of 362 molecules involved in inflammatory response. This reduction effect is most likely to be due to the regulation of RGS2 gene expression. In the present study, RGS2 gene expression was strongly down-regulated (−6.67 Fc) in the intestinal cells incubated with lactobacilli mixture. The RGS2 gene encodes for the RGS2 protein (regulator of G-protein signaling 2 protein), which is known to have putative tumor suppression function by inhibiting the proliferation and migration of cancer cells through the acceleration of GTP-ase activity and controlling the alpha subunits of G proteins [46]. In accordance with this observation, Boelte et al. (2011) [47] reported that genetic deletion or inactivation of RGS2 results in a significant reduction of tumor growth and of MCP-1 in a Rgs2−/− tumor myeloid derived suppressor cells (MDSCs) mouse model. While searching for molecular mediators responsible for tumor derived MDSCs, they found an increased level of RGS2 in tumors. When MCP-1 was blocked with a neutralizing antibody it resulted in diminished tumor metastases. These findings suggest that the expression of RGS is different in various types of cancers [46].Our results have confirmed also that administration of lactobacilli could have implications beyond the treatment of cancer, bowel diseases, etc. The interest in using probiotic bacteria against intestinal inflammatory response has been increased lately. The up-regulation of genes like SERPINA 3 (by 9.85-fold), IL-20 (by 6.23-fold), OSM (by 6.19-fold), and the down-regulation of the previously mentioned CXCL-2 which regulates cell proliferation and differentiation during inflammation highlight the protective role of lactobacilli in epidermal barrier function against inflammation.
Findings have shown that probiotics like lactobacilli could be associated with beneficial modulation of immune response. In our study, for instance, the three lactobacilli mixture enhanced expression of GM-CSF (granulocyte-macrophage colony-stimulating factor gene) and PBD-2 (beta-defensin 2) and suppressed expression of IL-18 pro-inflammatory cytokine genes. Indeed, recent studies showed that probiotics, lactobacilli among them activate both natural and acquired immune responses by induction of a broad array of cytokines (IL-1β, IL-10, IL-8, MIP-1α, MIP-1β, and GM-CSF) as well as by suppression of others (i.g. IL-1α, TNF-α) [48,49,50].
The extrapolation of pig genes to human resulted in an average overlapping yield of approximately 60% compared to 3.64 when applying to other methods.
4. Materials and Methods
4.1. IPEC-1 Cell Culture
The Intestinal Porcine Epithelial Cell line-1 (IPEC-1) was used in this study as previously described [51]. Briefly, cells were grown at 37 °C, under 5% CO2 atmosphere incubation in complete Dulbecco’s Modified Eagle Medium DMEM/F-12 medium (Sigma, Saint Louis, MO, USA), supplemented with antibiotics, 5% foetal bovine serum (Sigma), 2 mM l-glutamine, 15 mM Hepes (Sigma), epidermal growth factor, 5 µg/L (Sigma), insulin (10 µg/mL), transferrin (5 µg/mL), and sodium selenite (5 ng/mL) (ITS Premix, Sigma).
4.2. Lactobacilli (LB) Preparation
Three lactic acid bacteria, Lactobacillus plantarum (ATCC8014, isolated from horse feces), Lactobacillus paracasei (ATCC335, isolated from food; provided by from National Research Institute Cantacusino, Bucharest, Romania, Cantacusino Collection, ID12353 and ID13239), and Lactobacillus rhamnosus (IBNA collection, IBNA02, isolated from food) kept in glycerol were cultured individually in anaerobic plastic tubs in DeMan Rogosa and Sharpe (MRS, Sigma) medium (1:10), overnight at 37 °C under anaerobic conditions as described by [19]. The following day the culture was diluted 1:15 in fresh MRS and cultivated another 3–4 h until the OD600 reached values of about 1 ± 0.1, which corresponded to 1.0 × 109 CFU/mL for L. plantarum, 2.9 × 108 CFU/mL for L. paracasei and 3.2 × 108 CFU/mL for L. rhamnosus. Bacterial viability was tested by CFU counts after agar plating of bacterial serial dilution. Lactic bacteria were harvested by centrifugation at 4000 rpm for 10 min at 4 °C, and resuspended in antibiotic free DMEM/F-12 medium (Sigma). The lactobacilli mixture suspension was prepared in IPEC cell culture medium for a concentration of approximately 0.5 × 108 CFU/mL for each strain (1.5 × 108 CFU/mL in total) and added to the epithelial cells.
4.3. Bacterial Treatment
Undifferentiated IPEC-1 cells were seeded at a density of 2.0 × 105/mL in 24-well cell culture plates (Costar, Corning, NY, USA) and cultivated at 37 °C and 5% CO2 until they reached confluence (2–3 days). Then, the cell culture supernatant was removed and 1 mL of fresh lactobacilli mixture suspension with 1 × 108 CFU/mL prepared as describe above was added to the IPEC-1 monolayer and incubated for 3 h at 37 °C and 5% CO2. After incubation and removal of supernatant the cells were washed with PBS, resuspended in 0.5 mL of Trizol (Sigma) and stored at −80 °C until analyses. The experiments were carried out in four independent replicates.
4.4. Extraction of Total RNA for Microarray
The total RNA from untreated and LB treated IPEC-cells was isolated using a QiagenRNeasy midi kit (QIAGEN GmbH, Hilden, Germany), according to the manufacturer’s recommendations as described by [52] and their quality and integrity was verified by using an Agilent 2100 bioanalyzer and Agilent RNA 6000 nano kit (Agilent Technologies, Santa Clara, CA, USA). The RNA integrity number (RIN) score ranged between 8 and 10. The purified RNA samples were preserved at −80 °C until used.
4.5. Microarray Assay
Agilent SurePrint G3 Custom Gene Expression Microarray 8 × 60K slides (Santa Clara, CA, USA) were used for hybridization, the array consisting in 60 mer oligonucleotide probes with a total number of 45,220 features and 1417 Agilent features control. 200 ng of purified RNAs was tagged using Low Input Quick Amp Labeling Kit, Two-Color (48 rxn, 5190-2306, Santa Clara, CA, USA), that implies reverse transcribing the mRNA in the presence of T7-oligo-dT primer to furnish cDNA and afterwards in vitro transcribing with T7 RNA polymerase in the presence of Cy3-CTP and Cy5-CTP to construct labelled cRNA. The labelled cRNA of the LB-treated and the control specimens from every biological replicate were labelled with Cy-3 and Cy-5 dyes and hybridized in duplicate with dye reversal to the Agilent Whole Genome Porcine (S. Scrofa)- V2 8 × 60K microarray Slides (G2519F-026440, Santa Clara, CA, USA) according to the Agilent manufacturer’s protocol. The slides were washed with Agilent buffer and scanned on Agilent Microarray Scanner G2565BA (Santa Clara, CA, USA) at high and low resolution.
4.6. Statistical Analyses of Microarray Data
The pre-processing, normalization, and differential analysis of data were done as described by [53] by using the Gene-Spring GX version 12.6.1 software (Santa Clara, CA, USA). Smoothing adjustment in the differences of Cy5 intensity was done by using Lowes normalization which removes such variation. Student’s t-test were applied in order to assess the statistical differences in gene expression, the data are presented as log2 value transformed in fold change by applying the function of Power (2, log2-Value).
Genes with fold change of ±2 and a p-value of ≤0.05 were considered statistically significant using Gene-Spring GX version 12.6.1 Software. The genes expression significantly modulated by the treatment was subjected to a cluster analysis based on Pearson coefficient correlation algorithm and classified into nine functional categories and pathways (signaling, cell signaling, proliferation, transcription factors, growth factors, cytokines, interleukins, immune response, and inflammatory response) to assess the global effect on biological processes. All the transcript sequences of Sus scrofa were extrapolated to their human counterparts using Homology Based Annotation retrieved from NCBI Database (available online: www.ncbi.nlm.nih.gov) and BLAST [46]. Ingenuity Pathway Analysis (IPA; available online: http://www.ingenuity.com) was applied in order to evaluate the impact of lactobacilli on human health. A network interaction between the genes with an altered expression level is also presented in order to better understand the LB mode of action in the context of functional clusters and pathway.
4.7. Validation of Gene Expression by Quantitative Real-Time PCR (RT-qPCR)
For the validation of microarray analysis, the expression levels of five randomly selected genes (IL-1β, TLR6, TLR4, TRL2, IL-10) were measured by real time RT-qPCR in all samples used for microarray analysis as described by [51]. Primer pairs used for validation are listed in Table 10.
Table 10.
Oligonucleotide Polymerase chain reaction (PCR) primers.
| Gene | Accesion No. | Primer Source | Primer Sequence (5′→3′) | Orientation | Tm (°C) | Amplicon Lenght (bp) | References |
|---|---|---|---|---|---|---|---|
| IL-1β | NM_214055 | Pig | ATGCTGAAGGCTCTCCACCTC | forward | 62 | 89 | [54] |
| TTGTTGCTATCATCTCCTTGCAC | reverse | 59 | |||||
| TLR2 | NM_213761.1 | Pig | TCACTTGTCTAACTTATCATCCTCTTG | forward | 59 | 162 | [55] |
| TCAGCGAAGGTGTCATTATTGC | reverse | 59 | |||||
| TLR4 | NM_001113039.1 | Pig | GCCATCGCTGCTAACATCATC | forward | 60 | 108 | [55] |
| CTCATACTCAAAGATACACCATCGG | reverse | 59 | |||||
| TLR6 | NM_213760.1 | Pig | AACCTACTGTCATAAGCCTTCATTC | forward | 59 | 95 | [55] |
| GTCTACCACAAATTCACTTTCTTCAG | reverse | 59 | |||||
| IL-10 | NM_214041.1 | Pig | GGCCCAGTGAAGAGTTTCTTTC | forward | 54 | 55 | [56] |
| CAACAAGTCGCCCATCTGGT | reverse | 51 | |||||
| IL-4 | NM_214123.1 | Pig | CAACCCTGGTCTGCTTACTG | Forward | 52 | 173 | [57] |
| CTTCTCCGTCGTGTTCTCTG | reversed | 52 | |||||
| Cyclophilin A | NM_214353.1 | Pig | CCCACCGTCTTCTTCGACAT | forward | 54 | 92 | [58] |
| TCTGCTGTCTTTGGAACTTTGTCT | reverse | 55 | |||||
| β-actina | NM_213978.1 | Pig | GGACTTCGAGCAGGAGATGG | forward | 60 | 230 | [59] |
| GCACCGTGTTTGCGTAGAGG | reverse | 62 |
5. Conclusions
Gene overexpreession was the predominant effect produced by lactobacilli treatment in IPEC-1 cells, genes related to signaling pathways being the most affected (95% up-regulation, 121 genes with a fold change higher than 10). lactobacilli mixture significantly affected the Wnt/β-catenin signaling by up-regulating AXIN2 gene (13.93-fold), a negative regulator of β-catenin, which plays a key role in human cancer as well as the cellular proliferation by increasing 10 times (Fc) the NF1 gene encoding for the neurofibromin protein, a tumour suppressor that prevent cells from uncontrolled proliferation. The induction of genes like SERPINA 3, IL-20, OSM, and GM-CSF, and the suppression of CXCL-2 (MIP-2), RGS2 genes, and pro-inflammatory IL-18 cytokine highlight the protective role of lactobacilli in epithelial barrier function against inflammation and in the activation of immune response. However, further studies are needed to confirm these results and to understand the in vivo underlying mechanism of lactobacilli action.
Acknowledgement
This work was supported by funds from the National Research Project PNII-PCCA-102/2011-2016 granted by the Romanian Ministry of Research and Technology. The authors thank Catarina Gosh and Patricia Adamo for proofreading this manuscript.
Author Contributions
Conceptualization, I.T.; Investigation, D.E.M., C.B., G.C.P., I.S., D.C.V. and L.L.P.; Visualization, I.B.N.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kahlert S., Junnikkala S., Renner L., Hynonen U., Hartig R., Nossol C., Barta-Boszormenyi A., Danicke S., Souffrant W.B., Palva A., et al. Physiological concentration of exogenous lactate reduces antimycin a triggered oxidative stress in intestinal epithelial cell line IPEC-1 and IPEC-J2 in vitro. PLoS ONE. 2016;11:e0153135. doi: 10.1371/journal.pone.0153135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taranu I., Marin D.E., Pistol G.C., Motiu M., Pelinescu D. Induction of pro-inflammatory gene expression by Escherichia coli and mycotoxin zearalenone contamination and protection by a Lactobacillus mixture in porcine IPEC-1 cells. Toxicon. 2015;97:53–63. doi: 10.1016/j.toxicon.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Pinton P., Braicu C., Nougayrede J.P., Laffitte J., Taranu I., Oswald I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010;140:1956–1962. doi: 10.3945/jn.110.123919. [DOI] [PubMed] [Google Scholar]
- 4.Pie S., Lalles J.P., Blazy F., Laffitte J., Seve B., Oswald I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- 5.Maresca M., Fantini J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon. 2010;56:282–294. doi: 10.1016/j.toxicon.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Pie S., Awati A., Vida S., Falluel I., Williams B.A., Oswald I.P. Effects of added fermentable carbohydrates in the diet on intestinal proinflammatory cytokine-specific mRNA content in weaning piglets. J. Anim. Sci. 2007;85:673–683. doi: 10.2527/jas.2006-535. [DOI] [PubMed] [Google Scholar]
- 7.Boudry G., Guerin S., Henri Malbert C. Effect of an abrupt switch from a milk-based to a fibre-based diet on gastric emptying rates in pigs: Difference between origins of fibre. Br. J. Nutr. 2004;92:913–920. doi: 10.1079/BJN20041271. [DOI] [PubMed] [Google Scholar]
- 8.Boudry G., Peron V., Le Huerou-Luron I., Lalles J.P., Seve B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- 9.Lalles J.P., Bosi P., Smidt H., Stokes C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- 10.Montagne L., Boudry G., Favier C., Le Huerou-Luron I., Lalles J.P., Seve B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007;97:45–57. doi: 10.1017/S000711450720580X. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A., Vlasova A.N., Liu Z., Chattha K.S., Kandasamy S., Esseili M., Zhang X., Rajashekara G., Saif L.J. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut Microbes. 2014;5:152–164. doi: 10.4161/gmic.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi H., Albarracin L., Sato N., Kanmani P., Kober A.K., Ikeda-Ohtsubo W., Suda Y., Nochi T., Aso H., Makino S., et al. Modulation of porcine intestinal epitheliocytes immunetranscriptome response by Lactobacillus jensenii TL2937. Benef. Microbes. 2016;7:769–782. doi: 10.3920/BM2016.0095. [DOI] [PubMed] [Google Scholar]
- 13.Karimi S., Ahl D., Vagesjo E., Holm L., Phillipson M., Jonsson H., Roos S. In vivo and in vitro detection of luminescent and fluorescent Lactobacillus reuteri and application of red fluorescent mCherry for assessing plasmid persistence. PLoS ONE. 2016;11:e0151969. doi: 10.1371/journal.pone.0151969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roselli M., Finamore A., Hynonen U., Palva A., Mengheri E. Differential protection by cell wall components of Lactobacillus amylovorus DSM 16698Tagainst alterations of membrane barrier and NF-kB activation induced by enterotoxigenic F4+ Escherichia coli on intestinal cells. BMC Microbiol. 2016;16:226. doi: 10.1186/s12866-016-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoda K., Miyazawa K., Hosoda M., Hiramatsu M., Yan F., He F. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. Eur. J. Nutr. 2014;53:105–115. doi: 10.1007/s00394-013-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finamore A., Roselli M., Britti M.S., Merendino N., Mengheri E. Lactobacillus rhamnosus GG and Bifidobacterium animalis MB5 induce intestinal but not systemic antigen-specific hyporesponsiveness in ovalbumin-immunized rats. J. Nutr. 2012;142:375–381. doi: 10.3945/jn.111.148924. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y., Li T., Li S., Jiang Z., Yang Y., Huang J., Liu Z., Sun H. Achieving high yield of lactic acid for antimicrobial characterization in cephalosporin-resistant lactobacillus by the co-expression of the phosphofructokinase and glucokinase. J. Microbiol. Biotechnol. 2016;26:1148–1161. doi: 10.4014/jmb.1601.01043. [DOI] [PubMed] [Google Scholar]
- 18.Hummel S., Veltman K., Cichon C., Sonnenborn U., Schmidt M.A. Differential targeting of the E-Cadherin/beta-Catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl. Environ. Microbiol. 2012;78:1140–1147. doi: 10.1128/AEM.06983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roselli M., Finamore A., Britti M.S., Konstantinov S.R., Smidt H., de Vos W.M., Mengheri E. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J. Nutr. 2007;137:2709–2716. doi: 10.1093/jn/137.12.2709. [DOI] [PubMed] [Google Scholar]
- 20.Finamore A., Roselli M., Imbinto A., Seeboth J., Oswald I.P., Mengheri E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PLoS ONE. 2014;9:e94891. doi: 10.1371/journal.pone.0094891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.Y., Park M.S., Ji G.E. Probiotic modulation of dendritic cells co-cultured with intestinal epithelial cells. World J. Gastroenterol. 2012;18:1308–1318. doi: 10.3748/wjg.v18.i12.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulder I.E., Schmidt B., Stokes C.R., Lewis M., Bailey M., Aminov R.I., Prosser J.I., Gill B.P., Pluske J.R., Mayer C.D., et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79. doi: 10.1186/1741-7007-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonecova Z., Toth S., Ciccocioppo R., Rodrigo L., Kruzliak P., Nemcova R. Influence of dietary supplementation with flaxseed and lactobacilli on the mucosal morphology and proliferative cell rate in the jejunal mucosa of piglets after weaning. Int. J. Exp. Pathol. 2015;96:163–171. doi: 10.1111/iep.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seth A., Yan F., Polk D.B., Rao R.K. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liv. Physiol. 2008;294:G1060–G1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding S.V., Fraser K.G., Wykes L.J. Probiotics stimulate liver and plasma protein synthesis in piglets with dextran sulfate-induced colitis and macronutrient restriction. J. Nutr. 2008;138:2129–2135. doi: 10.3945/jn.108.090019. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z., Cao L., Zhou Y., Wang S., Zhou L. Analysis of the duodenal microbiotas of weaned piglet fed with epidermal growth factor-expressed Saccharomyces cerevisiae. BMC Microbiol. 2016;16:166. doi: 10.1186/s12866-016-0783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marin D.E., Taranu I., Motiu M., Manda G. Effect of Lactobacillus feed supplement in deoxynivalenol intoxicated piglets. Arch. Zootech. 2010;13:12–22. [Google Scholar]
- 28.Kikuchi A. Modulation of Wnt signaling by Axin and Axil. Cytokine Growth Factor Rev. 1999;10:255–265. doi: 10.1016/S1359-6101(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 29.Krausova M., Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell. Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Brembeck F.H., Rosario M., Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Morin P.J. Beta-catenin signaling and cancer. BioEssays N. Rev. Mol. Cell. Dev. Biol. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Sakanaka C., Sun T.Q., Williams L.T. New steps in the Wnt/beta-catenin signal transduction pathway. Recent Prog. Horm. Res. 2000;55:225–236. [PubMed] [Google Scholar]
- 33.Owczarek D., Rodacki T., Domagala-Rodacka R., Cibor D., Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016;22:895–905. doi: 10.3748/wjg.v22.i3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamorro M.N., Schwartz D.R., Vonica A., Brivanlou A.H., Cho K.R., Varmus H.E. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura T., Hamada F., Ishidate T., Anai K., Kawahara K., Toyoshima K., Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3β and APC and reduces the beta-catenin level. Genes Cells Devot. Mol. Cell. Mech. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 36.Ng S.C., Hart A.L., Kamm M.A., Stagg A.J., Knight S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 37.Du Q., Wang Y., Liu C., Wang H., Fan H., Li Y., Wang J., Zhang X., Lu J., Ji H., et al. Chemopreventive activity of GEN-27, a genistein derivative, in colitis-associated cancer is mediated by p65-CDX2-beta-catenin axis. Oncotarget. 2016;7:17870–17884. doi: 10.18632/oncotarget.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taherian-Esfahani Z., Abedin-Do A., Nouri Z., Mirfakhraie R., Ghafouri-Fard S., Motevaseli E. Lactobacilli differentially modulate mTOR and Wnt/beta-catenin pathways in different cancer cell lines. Iran. J. Cancer Prev. 2016;9:e5369. doi: 10.17795/ijcp-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajendran P., Dashwood W.M., Li L., Kang Y., Kim E., Johnson G., Fischer K.A., Lohr C.V., Williams D.E., Ho E., et al. Nrf2 status affects tumor growth, HDAC3 gene promoter associations, and the response to sulforaphane in the colon. Clin. Epigenet. 2015;7:102. doi: 10.1186/s13148-015-0132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeghi-Aliabadi H., Mohammadi F., Fazeli H., Mirlohi M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran. J. Basic Med. Sci. 2014;17:815–819. [PMC free article] [PubMed] [Google Scholar]
- 41.Soltan Dallal M.M., Mojarrad M., Baghbani F., Raoofian R., Mardaneh J., Salehipour Z. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2) Arch. Iran. Med. 2015;18:167–172. [PubMed] [Google Scholar]
- 42.Azam R., Ghafouri-Fard S., Tabrizi M., Modarressi M.H., Ebrahimzadeh-Vesal R., Daneshvar M., Mobasheri M.B., Motevaseli E. Lactobacillus acidophilus and Lactobacillus crispatus culture supernatants downregulate expression of cancer-testis genes in the MDA-MB-231 cell line. Asian Pac. J. Cancer Prev. APJCP. 2014;15:4255–4259. doi: 10.7314/APJCP.2014.15.10.4255. [DOI] [PubMed] [Google Scholar]
- 43.Nishida T., Tsujimoto M., Takahashi T., Hirota S., Blay J.Y., Wataya-Kaneda M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J. Gastroenterol. 2016;51:571–578. doi: 10.1007/s00535-015-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan D., Liang P., Xiao H. Neurofibromatosis type 1 associated with pheochromocytoma and gastrointestinal stromal tumors: A case report and literature review. Oncol. Lett. 2016;12:637–643. doi: 10.3892/ol.2016.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomosada Y., Villena J., Murata K., Chiba E., Shimazu T., Aso H., Iwabuchi N., Xiao J.Z., Saito T., Kitazawa H. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE. 2013;8:e59259. doi: 10.1371/journal.pone.0059259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethakorn N., Dulin N.O. RGS expression in cancer: Oncomining the cancer microarray data. J. Recept. Signal Transduct. Res. 2013;33:166–171. doi: 10.3109/10799893.2013.773450. [DOI] [PubMed] [Google Scholar]
- 47.Boelte K.C., Gordy L.E., Joyce S., Thompson M.A., Yang L., Lin P.C. Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1. PLoS ONE. 2011;6:e18534. doi: 10.1371/journal.pone.0018534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong H., Rowland I., Yaqoob P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012;108:459–470. doi: 10.1017/S0007114511005824. [DOI] [PubMed] [Google Scholar]
- 49.Bahrami B., Macfarlane S., Macfarlane G.T. Induction of cytokine formation by human intestinal bacteria in gut epithelial cell lines. J. Appl. Microbiol. 2011;110:353–363. doi: 10.1111/j.1365-2672.2010.04889.x. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Cano F.J., Dong H., Yaqoob P. In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: Two probiotic strains isolated from human breast milk. Immunobiology. 2010;215:996–1004. doi: 10.1016/j.imbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Taranu I., Braicu C., Marin D.E., Pistol G.C., Motiu M., Balacescu L., Beridan Neagoe I., Burlacu R. Exposure to zearalenone mycotoxin alters in vitro porcine intestinal epithelial cells by differential gene expression. Toxicol. Lett. 2015;232:310–325. doi: 10.1016/j.toxlet.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Pistol G.C., Gras M.A., Marin D.E., Israel-Roming F., Stancu M., Taranu I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/NF-κB signalling molecules in pigs. Br. J. Nutr. 2014;111:452–464. doi: 10.1017/S0007114513002675. [DOI] [PubMed] [Google Scholar]
- 53.Braicu C., Cojocneanu-Petric R., Jurj A., Gulei D., Taranu I., Gras A.M., Marin D.E., Berindan-Neagoe I. Microarray based gene expression analysis of Sus Scrofa duodenum exposed to zearalenone: Significance to human health. BMC Genom. 2016;17:646. doi: 10.1186/s12864-016-2984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Royaee A.R., Husmann R.J., Dawson H.D., Calzada-Nova G., Schnitzlein W.M., Zuckermann F.A., Lunney J.K. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet. Immunol. Immunopathol. 2004;102:199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arce C., Ramirez-Boo M., Lucena C., Garrido J.J. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 and IPI-2I) in response to LPS from Salmonella typhimurium. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:161–174. doi: 10.1016/j.cimid.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Grenier B., Bracarense A.P., Schwartz H.E., Trumel C., Cossalter A.M., Schatzmayr G., Kolf-Clauw M., Moll W.D., Oswald I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012;83:1465–1473. doi: 10.1016/j.bcp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Meurens F., Berri M., Auray G., Melo S., Levast B., Virlogeux-Payant I., Chevaleyre C., Gerdts V., Salmon H. Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet. Res. 2009;40:5. doi: 10.1051/vetres:2008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devriendt B., Gallois M., Verdonck F., Wache Y., Bimczok D., Oswald I.P., Goddeeris B.M., Cox E. The food contaminant fumonisin B1 reduces the maturation of porcine CD11R1+ intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 2009;40:40. doi: 10.1051/vetres/2009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meadus W.J., MacInnis R., Dugan M.E. Prolonged dietary treatment with conjugated linoleic acid stimulates porcine muscle peroxisome proliferator activated receptor gamma and glutamine-fructose aminotransferase gene expression in vivo. J. Mol. Endocrinol. 2002;28:79–86. doi: 10.1677/jme.0.0280079. [DOI] [PubMed] [Google Scholar]