Abstract
The beneficial effects of exercise training (EX) on cardiac pathology are well recognized. Previously, we found that the effects of EX on cardiac dysfunction in mice critically depend on the underlying etiology. EX exerted beneficial effects after myocardial infarction (MI); however, cardiac pathology following pressure overload produced by transverse aortic constriction (TAC) was aggravated by EX. In the presented study, we investigated whether the contrasting effects of EX on cardiac dysfunction can be explained by an etiology-specific response of endothelial nitric oxide (NO) synthase (eNOS) to EX, which divergently affects the balance between nitric oxide and superoxide. For this purpose, mice were exposed to eight weeks of voluntary wheel running or sedentary housing (SED), immediately after sham, MI, or TAC surgery. Left ventricular (LV) function was assessed using echocardiography and hemodynamic measurements. EX ameliorated LV dysfunction and remodeling after MI, but not following TAC, in which EX even aggravated fibrosis. Strikingly, EX attenuated superoxide levels after MI, but exacerbated NOS-dependent superoxide levels following TAC. Similarly, elevated eNOS S-glutathionylation and eNOS monomerization, which were observed in both MI and TAC, were corrected by EX in MI, but aggravated by EX after TAC. Additionally, EX reduced antioxidant activity in TAC, while it was maintained following EX in MI. In conclusion, the present study shows that EX mitigates cardiac dysfunction after MI, likely by attenuating eNOS uncoupling-mediated oxidative stress, whereas EX tends to aggravate cardiac dysfunction following TAC, likely due to exacerbating eNOS-mediated oxidative stress.
Keywords: exercise, myocardial infarction, aortic stenosis, oxidative stress, nitric oxide synthase
1. Introduction
During the last decade, heart failure (HF) has developed into a major public-health burden worldwide [1,2]. Consequently, the development of novel strategies for the treatment of HF becomes increasingly important. In addition to optimal pharmacotherapy, physical exercise training (EX) is increasingly implemented in the rehabilitation process of HF patients. The beneficial effects of EX on exercise capacity and cardiovascular performance are well established [3], and translate into improvements in quality of life, as well as reductions in HF-related hospitalizations and mortality [3,4,5,6,7].
An important player in the many protective effects of exercise is endothelial nitric oxide (NO) synthase (eNOS) [8,9,10]. In the presence of its co-factors, eNOS transfers electrons from reduced nicotinamide adenine dinucleotide phosphate (NADPH), via the flavin adenine dinucleotide and flavin mononucleotide to the heme site, where electrons are utilized to reduce and activate oxygen, and to oxidize L-arginine to L-citrulline and NO [11]. EX increases shear stress and mechanical stretch, which stimulate eNOS activity and increase NO production [9,12]. NO has various beneficial effects on cardiovascular function, including vasodilation, the inhibition of platelet aggregation and adhesion, the inhibition of leukocytes and vascular inflammation, the stimulation of angiogenesis, the proliferation of vascular smooth muscle cells, and the activation of endothelial progenitor cells [13]. In addition to the stimulation of eNOS, EX beneficially influences the cardiac nitroso–redox balance by activating reactive oxygen species (ROS) scavenger enzymes such as superoxide dismutases (SODs) [14,15]. Accordingly, we and others have demonstrated that EX improves survival and attenuates LV dysfunction, oxidative stress, and fibrosis in a mouse model of myocardial infarction (MI) [16,17,18,19], and have additionally demonstrated that full eNOS expression is required for these beneficial EX effects after MI [20].
In contrast to experimental [18] and clinical [21] evidence demonstrating beneficial effects of EX after MI, patients with obstruction of the left ventricular (LV) outflow tract, are advised to perform only mild exercise or to refrain from physical strain [22,23]. In line with these guidelines, we previously found that EX failed to exert a beneficial effect, and even tended to aggravated cardiac dysfunction and remodeling in mice suffering from chronic pressure overload produced by transverse aortic constriction (TAC) [24].
The mechanism underlying these contrasting effects of EX in different cardiac pathologies remains unresolved, but could involve divergent effects of EX on eNOS. Under pathological conditions, eNOS can uncouple and convert from an NO− to a superoxide (O2−)-producing enzyme. Several mechanisms have been proposed to induce eNOS uncoupling, including eNOS monomerization [13] and S-glutathionylation of eNOS [25]. Uncoupled eNOS is still capable of transferring electrons, but the electrons are diverted to oxygen rather than to L-arginine, resulting in the production of O2−, and subsequently, further eNOS uncoupling [13].
In light of these considerations, we hypothesized that EX influences the nitroso–redox balance and eNOS in a pathology-specific manner so that EX can either stimulate NO or eNOS-mediated superoxide production, depending on the underlying cardiac pathology. To test our hypothesis, we investigated how EX affects eNOS and the nitroso–redox balance in two distinct but clinically highly relevant cardiac pathologies by studying the effects of EX in mice with either MI or TAC.
2. Results
2.1. Exercise and Survival
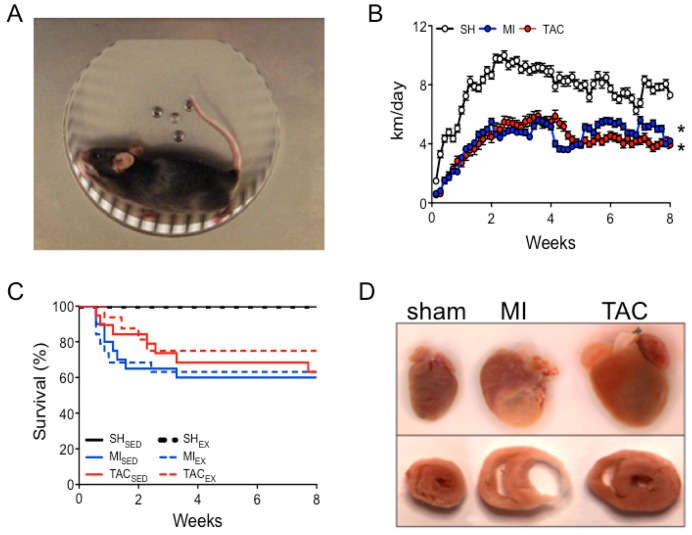
All mice in the EX groups began running voluntarily on the first day after surgery (Figure 1A,B). The total eight-week distance was lower in TACEX (237 ± 57 km) and MIEX (245 ± 47 km) than in sham (SH)EX mice (435 ± 60 km; Figure 1B). In the sedentary housing (SED) group, MISED and TACSED had survival rates of 62% and 63%, respectively, while EX did not affect mortality in any of the groups (Figure 1C).
Figure 1.
Experimental models, daily running distance, and survival. (A) Voluntary wheel running; (B) daily running distance of sham (SH), myocardial infarction (MI), and transverse aortic constriction (TAC) mice; (C) Kaplan–Meier survival curve for all groups; (D) post-mortem examples of hearts and cardiac cross sections of sham mice and mice with an MI or TAC. Total number of animals entering the study in voluntary running (EX) and sedentary housing (SED) groups: SHSED (n = 12), SHEX (n = 22), MISED (n = 21), MIEX (n = 19), TACSED (n = 19), and TACEX (n = 16). * p < 0.05 total running distance vs. total running distance of sham mice.
2.2. LV Remodeling and Dysfunction
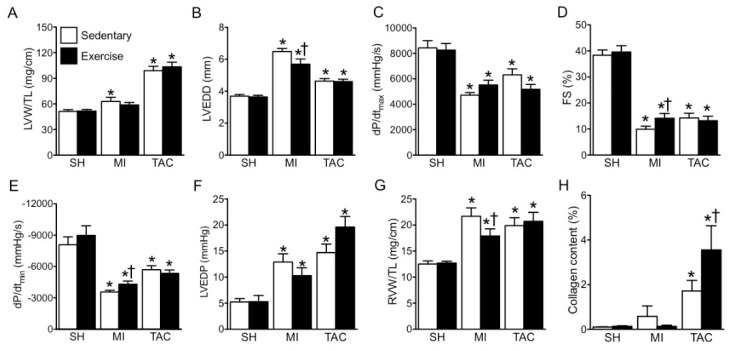
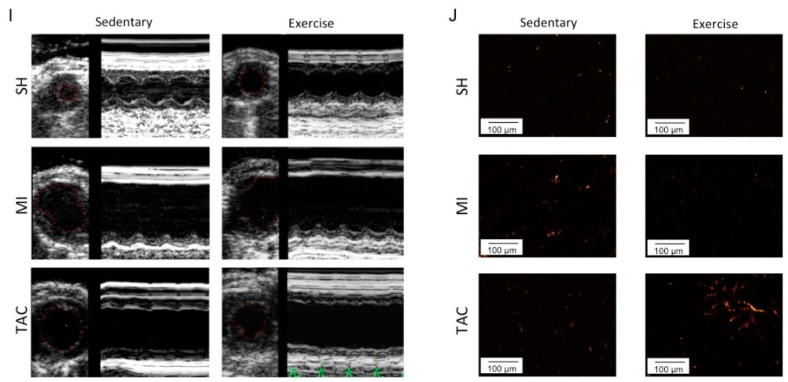
MI and TAC both increased relative LV weight and lumen diameter (Figure 1D and Figure 2A,B), decreased LV systolic dysfunction, as indicated by LV dP/dtmax and fractional shortening (FS), as well as early (LV dP/dtmin) and late LV diastolic dysfunction (LV end diastolic pressure (LVEDP)) (Figure 2C–F,I). Furthermore, in both MI and TAC, cardiac backward failure was evidenced by an increase in relative right ventricular (RV) weight (Figure 2G), while marked LV fibrosis was observed in TAC (Figure 2H,J). EX improved LV remodeling and dysfunction after MI. In contrast, EX failed to ameliorate, and even tended to aggravate LV remodeling and dysfunction after TAC (Figure 2).
Figure 2.
Exercise (EX) mitigates cardiac remodeling and dysfunction following myocardial infarction (MI), but not after transverse aortic constriction (TAC). (A) Left ventricle (LV) weight (LVW) per tibia length (TL); (B) LV end diastolic lumen diameter (LVEDD); (C) maximum rate of rise in LV pressure (dP/dtmax); (D) fractional shortening (FS); (E) maximum rate of fall in LV pressure (dP/dtmin); (F) LV end diastolic pressure (LVEDP); (G) right ventricle weight (RVW) per TL; (H) collagen content; (I) representative LV short axis and M-Mode echo images; (J) representative images of collagen staining. * p < 0.05 vs. corresponding sham, † p < 0.05 vs. corresponding sedentary. n = 12 in all groups.
2.3. Superoxide Production
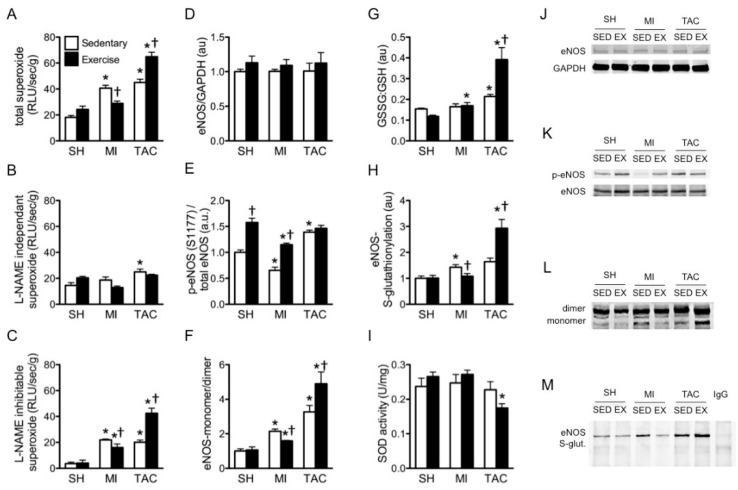
Having established that the effects of exercise on LV function and remodeling were etiology-dependent, we sought to determine the potential role of etiology-dependent superoxide generation in the effects of exercise. In LV myocardium of the sedentary groups, MI and TAC both increased superoxide generation compared to sham-operated animals (Figure 3A). This increase was abolished in MI and markedly reduced in TAC by NOS inhibition with NG-nitro-L-arginine methyl ester (L-NAME; Figure 3B). Interestingly, EX inhibited superoxide production in MI, but significantly increased superoxide levels following TAC (Figure 3A). Similarly, L-NAME-inhibitable, and therefore, NOS-dependent superoxide generation was blunted by EX following MI, but markedly aggravated by EX following TAC (Figure 3C). These findings suggest a direct functional relation between NOS and the contrasting effects of EX in myocardial superoxide production.
Figure 3.
Assessment of exercise (EX) effects on superoxide, endothelial nitric oxide synthase (eNOS), and glutathionylation after sham (SH), myocardial infarction (MI), and transverse aortic constriction (TAC). (A) Lucigenin-enhanced chemiluminescence superoxide production is expressed as relative light unit (RLU) per second per gram protein; (B) evaluation of superoxide production with NG-nitro-L-arginine methyl ester (L-NAME), presented as L-NAME-independent superoxide; (C) subtraction of L-NAME-independent superoxide from total superoxide, presented as L-NAME-inhibitable superoxide; (D) eNOS protein expression; (E) phosphorylated eNOS (p-eNOS) serine (Ser)1177 protein expression; (F) eNOS monomer–dimer ratio; (G) oxidized glutathion (GSSG) and reduced glutathion (GSH) ratio; (H) S-glutathionylation of eNOS; (I) superoxide dismutase (SOD) activity; (J) representative western blot of eNOS protein expression; (K) representative western blot of p-eNOS serine (Ser)1177 protein expression; (L) representative eNOS monomer dimer blot; (I) representative co-immunoprecipitation of eNOS S-glutathionylation with unspecific mouse immunoglobulin G (IgG) antibody as a negative control. p-eNOS: phosphorylated eNOS; eNOS S-glut: eNOS S-glutathionylation. * p < 0.05 vs. corresponding sham, † p < 0.05 vs. corresponding sedentary. n = 3–8 in all groups.
2.4. Total eNOS and Phosphorylated eNOS (p-eNOS) Protein Expression
To investigate whether the divergent effects of EX in MI and TAC could be explained by eNOS expression or phosphorylation levels, we examined the effect of EX on eNOS protein expression and eNOS serine (Ser)1177 phosphorylation. There was no difference in total cardiac eNOS protein expression between sham, MI, and TAC groups either with or without EX (Figure 3D,J). In contrast, p-eNOS Ser1177 levels were reduced after MI, but were increased after TAC. EX significantly increased the expression of p-eNOS Ser1177 in sham and MI groups, but did not further change p-eNOS Ser1177 levels in TAC mice (Figure 3E,K).
2.5. eNOS Uncoupling and eNOS S-Glutathionylation
Since eNOS uncoupling is a potential inducer of eNOS-mediated superoxide formation, we subsequently determined the eNOS monomer–dimer ratio and the level of eNOS S-glutathionylation. The eNOS monomer–dimer ratio was increased after both MI and TAC, and reduced by EX in MI, but further elevated by EX in TAC mice (Figure 3F,L).
The oxidized glutathione disulfide (GSSG) to reduced glutathione (GSH) ratio was not affected by MI, but was elevated after TAC. Additionally, the increase in superoxide levels in TACEX mice was accompanied by a further increase in the GSSG:GSH ratio (Figure 3G). Moreover, while S-glutathionylation of eNOS was modestly elevated in MI and TAC mice, this increase in eNOS S-glutathionylation was normalized by EX in MI, but aggravated in exercised TAC mice (Figure 3H,M).
Taken together, eNOS S-glutathionylation and eNOS monomerization data indicate that eNOS uncoupling is normalized by EX following MI, but aggravated by EX in the presence of TAC.
2.6. Superoxide Dismutase (SOD) Activity
To investigate whether divergent effects of EX on eNOS-mediated superoxide generation additionally involved altered antioxidant activity, we studied the activity of SOD. Total SOD activity was not changed after MI or TAC compared to sham-operated animals. Interestingly, EX did not alter SOD activity in sham and MI groups, but significantly decreased SOD activity in TAC mice (Figure 3I).
3. Discussion
In the presented study, we tested the hypothesis that eNOS-derived NO and superoxide play a key role in the divergent effects of EX on cardiac remodeling and dysfunction following MI versus chronic pressure overload produced by TAC. The major findings were that (i) eight weeks of voluntary EX after MI decreased superoxide production, whereas EX following TAC increased superoxide production; (ii) these divergent responses were principally explained by EX-induced reduction of eNOS-mediated myocardial superoxide levels after MI, as opposed to an EX-induced increase in eNOS-mediated superoxide production in TAC; (iii) accordingly, EX reduced eNOS uncoupling, evidenced by reductions in myocardial eNOS S-glutathionylation and eNOS monomerization after MI, whereas EX aggravated TAC-induced eNOS uncoupling evidenced by increased S-glutathionylation and eNOS monomerization after TAC (Figure 4).
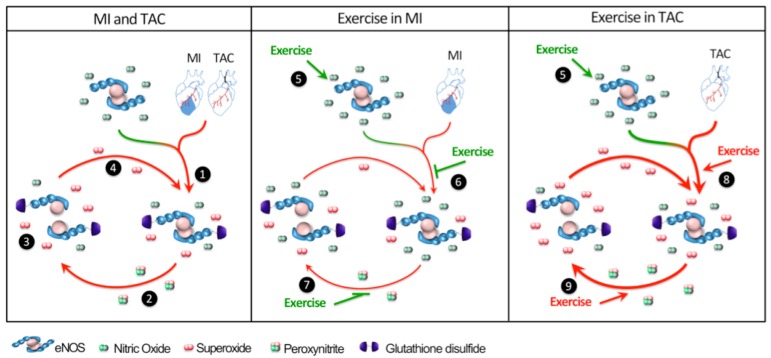
Figure 4.
Divergent effects of exercise (EX) on endothelial nitric oxide synthase (eNOS) regulation after myocardial infarction (MI) and transverse aortic constriction (TAC). MI and TAC induce eNOS S-glutathionylation (1), resulting in eNOS uncoupling and eNOS-mediated superoxide production (2). The consequently formed peroxynitrite further uncouples eNOS by inducing eNOS monomerization and more eNOS-mediated superoxide production (3), which further uncouples eNOS (4). EX stimulates eNOS (5). Beneficial effects of EX following MI: EX diminishes eNOS S-glutathionylation (6), as well as eNOS monomerization (7). Detrimental effects of EX following TAC: EX aggravates eNOS S-glutathionylation (8), thus inducing more eNOS monomerization (9), resulting in further elevation of superoxide production.
EX is known to produce multiple cardiac changes, including physiological cardiac hypertrophy, increased SOD activity, and reduced oxidative stress [26]. However, the EX levels of healthy individuals are not easily achieved by patients with severe cardiac disease, which is why we subjected our mice to a voluntary wheel-running protocol. This likely explains why our sham-operated animals demonstrated only trends toward the cardiac adaptations reported for intense EX training. Still, the EX protocol did produce skeletal muscle adaptations as evidenced by increased citrate synthase activity in skeletal muscle [17]. Moreover, the mild-to-moderate EX levels performed in our study significantly affected the diseased heart by stimulating cardiac performance after MI, while aggravating the nitroso–redox imbalance in the heart following TAC. In the current study, the beneficial effects of eight weeks of EX on cardiac function were not accompanied by improved survival. This might be partly explained by the fact that mortality in C57Bl6 MI mice is largely caused by cardiac rupture [27], and EX started after the onset of MI does not influence this phenomenon [28]. To study the long-term effects of EX on survival after MI and TAC, it would be interesting to study exercising MI and TAC mice for a longer period of time.
One of the key components of the beneficial effects of EX in cardiovascular disease is improved endothelial function through eNOS [12,29]. Accordingly, previous studies from our laboratory showed that the beneficial effects of EX following MI require full eNOS expression [20]. In line with this observation, eNOS overexpression was shown to improve cardiac function after MI [30,31]. In the present study, we found that exercise after MI attenuated eNOS uncoupling, which explains the reduction of eNOS-dependent superoxide production after EX in MI.
It is known that divergent cardiac pathologies activate distinct pathological signaling pathways that result in specific disease characteristics [32]. Consequently, measures that prove to be protective of the diseased heart are not necessarily equally beneficial in all cardiac etiologies. Accordingly, although the benefit of EX is clearly established in most forms of cardiac disease [7], patients with moderate-to-severe aortic stenosis are recommended to avoid competitive sports to reduce the risk of sudden death [22,23]. We recently observed that EX not only failed to ameliorate, but even tended to aggravate cardiac dysfunction in chronic pressure overload produced by TAC [24]. In the present study, we found that this lack of benefit from EX in TAC is accompanied by EX-induced worsening of eNOS uncoupling (evidenced by eNOS S-glutathionylation and eNOS monomerization), as well as EX-induced reduction of SOD activity, leading to increased eNOS-mediated oxidative stress. Even though we did not demonstrate a direct causal relationship between elevated ROS and aggravated cardiac dysfunction following TAC and EX, the data strongly suggest that elevated eNOS-mediated ROS levels importantly contribute to the worsening of cardiac performance when TAC is combined with EX. Likely, the fixed stenosis in TAC counteracts an important part of the beneficial effects of eNOS since it precludes the reduction in LV afterload resulting from eNOS-mediated systemic vasodilation. Indeed, beneficial effects of EX were described in hypertension, where stimulation of eNOS is able to reduce cardiac afterload [33,34]. Moreover, the fixed stenosis amplifies the EX-induced increase in LV systolic pressure, and hence, LV afterload during each exercise bout [35]. A meta-analysis performed by Haykowsky et al. [36] suggests this may adversely affect the LV response to EX by showing that dynamic EX, which results in modest increases in LV systolic pressure, had beneficial effects on ejection fraction in heart-failure patients, whereas static EX, which is known to result in more marked elevations in LV systolic pressure, failed to improve ejection fraction [11]. Apparently, the EX-induced increase in LV systolic pressure during EX in TAC exacerbated the TAC-induced cardiac pathology, and further aggravated detrimental eNOS uncoupling and concomitant oxidative stress. Treatment of TAC mice with phosphodiesterase type 5 (PDE5) inhibitors was previously shown to improve cardiac pathological hypertrophy [37]. Therefore, it could be interesting to explore whether treatment of EX TAC mice with NO donors might mitigate the detrimental effects of exercise and TAC on eNOS uncoupling and consequent ROS production. However, this should be the topic of future studies.
Clinical guidelines recommend at least 30 min of physical activity on most, if not all, days of the week to reduce risk factors for chronic disease [38,39]. In cardiovascular disease patients, EX not only improves exercise performance and quality of life, but also reduces morbidity and mortality [3,5,6,7]. Notwithstanding these clear benefits of EX, EX restriction is recommended for patients with aortic stenosis [22]. However, no beneficial effects of EX restriction were observed in a large cohort study involving more than 500 patients suffering from congenital aortic stenosis [40]. Thus, the potential benefits or hazards of EX in patients with aortic stenosis remain under debate, and recommendations appear to be based on pathophysiological considerations, rather than on hard clinical evidence [7]. Our study shows that, unlike after MI, EX may aggravate cardiac pathology in the presence of an aortic stenosis because of an etiology-dependent effect of EX on the nitroso–redox balance. We demonstrate that the balance between cardiac NO and superoxide is differentially affected by EX in diverse etiologies and pivotal in the mechanisms of both beneficial and detrimental effects of EX.
In conclusion, the presented study demonstrates that the balance between cardiac NO and superoxide plays a pivotal role in determining both the beneficial and detrimental effects of EX. Thus, the underlying pathology determines whether eNOS tilts the nitroso–redox balance toward either a beneficial or a detrimental influence. This observation may be important for therapies aiming to restore the nitroso–redox balance in patients with heart failure due to different etiologies, and may aid in improving disease-mechanism-specific interventions.
4. Materials and Methods
All experiments were performed in accordance with and following approval by the Animal Research Committee of the Erasmus MC University of Rotterdam (27 June 2006 nr: 109-06-12 EUR976). A total of 99 C57Bl/6 mice 12–20-week-old mice entered the study: sham sedentary (SHSED; n = 12), sham exercise (SHEX; n = 12), MI sedentary (MISED; n = 21), MI exercise (MIEX; n = 19), TAC sedentary (TACSED; n = 19), and TAC exercise (TACEX; n = 16). Males and females were equally distributed between groups.
4.1. Animal Experiments
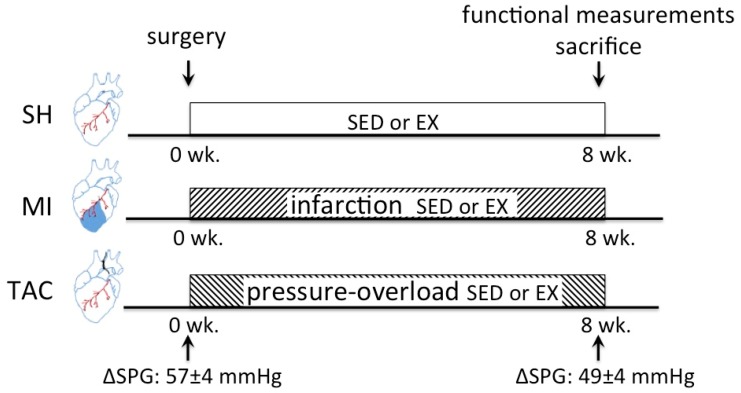
All mice were sedated with 4% isoflurane, intubated, and connected to a pressure-controlled ventilator (SAR-830/P; CWE, Ardmore, PA, USA). Anesthesia was maintained using 2.5% isoflurane, while body temperature was kept at 37 °C, and buprenorphine was administered (50 μg/kg; intraperitoneally (i.p.)) for postsurgical analgesia. Sham, MI, and TAC surgery were performed as previously described [24,41]. After recovery from surgery, all exercise groups were exposed to eight weeks of voluntary wheel running (Figure 1B). The timeline of SH, MI, and TAC experiments is visualized in Figure 5.
Figure 5.
Timeline of SH, MI, and TAC experiments. wk., week; SED, sedentary; EX, exercise; ΔSPG, systolic pressure gradient. (ΔSPGs are historical data from van Deel et al. [24]).
4.2. Cardiac Function and Geometry Measurements
Eight weeks after sham, MI, or TAC surgery, all mice were re-anesthetized and ventilated, and body temperature was kept at 37 °C while M-mode LV echocardiography was performed using an Aloka SSD 4000 echo device (Aloka; Tokyo, Japan). Following echocardiography, a pressure catheter (Millar Instrument; Houston, TX, USA) was inserted into the LV via the carotid artery, and LV pressure (LVP) was measured. LV dP/dtmax and LV dP/dtmin were later calculated using MatLab [24]. Subsequently, the heart was excised and the LV, right ventricle (RV), and lung weight, as well as tibia length, were measured. LV tissue was rapidly snap-frozen after extraction, and stored for further molecular analysis, or embedded in paraffin for histological analysis. In MI animals, all analyses were performed in remote, viable, LV myocardium.
4.3. Histology
Paraffin-embedded LV tissue of six mice per group was serially sectioned (4 µm). Collagen content was measured after Picrosirius red staining under polarized light, and analyzed with a quantitative image-analysis system (Clemex Technologies, Longueuil, QC J4G 1T5, Canada).
4.4. Detection of Superoxide Production
Superoxide generation in homogenized snap-frozen LV tissue (4–6 samples per group) was evaluated by lucigenin-enhanced chemiluminescence using a luminometer (Luminoskan Ascent, Thermo Fisher Scientific, Waltham, MA, USA)) as previously described [42]. Briefly, LV tissue was homogenized in Krebs-Hepes buffer, dark-adapted lucigenin (final concentration 5 µM) was added to the homogenates, and basal chemiluminescence was measured after reaching equilibrium. Subsequently, NOS-dependent superoxide production was measured by repeating the measurements after adding the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME; 1 mM), to the homogenates. Light emission was recorded, and was expressed as relative light units (RLU)/sec/g. Each experiment was performed in duplicate.
4.5. Total and Phosphorylated eNOS Protein Analysis
Total eNOS and p-eNOS protein levels were determined in LV homogenates as previously described [43], and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Novus Biologicals, Littleton, CO, USA)).
Subsequent to SDS-PAGE, the proteins were transferred to nitrocellulose membranes, and the blots were probed with primary anti-eNOS (1:500, BD Transduction Laboratory, San Jose, CA, USA), anti-p-eNOS (1:1000 Cell Signaling, Danvers, MA, USA), anti-GAPDH (1:10,000, Imgenex), and secondary rabbit anti-mouse immunoglobulin G (IgG) antibody conjugated with horseradish peroxidase (HRP; 1:1000, Santa Cruz Biotechnology, Dallas, TX, USA). All blots were analyzed using the Odyssey system (LI-COR, Lincoln, NE 68504, USA).
4.6. Quantification of GSSG and GSH
The oxidized glutathione disulfide (GSSG) to reduced glutathione (GSH) ratio was assessed using a glutathione assay kit (Cayman Chemical, Ann Arbor, MI, USA). LV tissue was homogenized in cold 2-(N-morpholino) ethanesulfonic acid (MES) buffer, before being centrifuged at 10,000× g for 15 min at 4 °C. Samples were deproteinated using metaphosporic acid and triethanolamine (Sigma-Aldrich, Saint Lois, MI, USA), and total GSH was immediately measured at 405 nm at five-minute intervals for 30 min. GSSG was measured independently, using 2-vinylpiridine (Sigma-Aldrich).
4.7. eNOS S-Glutathionylation
To determine S-glutathionylation of eNOS, an eNOS pull-down assay was performed with a protein G-conjugated (Novex) anti-eNOS antibody (Santa Cruz). Subsequently, immunoblotting was performed using an anti-glutathione monoclonal antibody (ViroGen, Watertown, MA, USA). To confirm the detection of eNOS S-glutathionylation, control samples were treated with dithiothreitol, which removes the glutathionylation modification of eNOS.
4.8. eNOS Monomer–Dimer Ratio
The eNOS monomer–dimer ratio was determined using low-temperature SDS-PAGE (LT-PAGE) as previously described [43]. Briefly, total proteins were incubated in 5× Laemmli buffer without 2-mercaptoethanol, and the gels and buffers were equilibrated at 4 °C before electrophoresis, during which the buffer tank was placed in an ice bath to maintain the temperature of the gel. After probing with the anti-eNOS antibody (BD Transduction Laboratory), immunoreactive bands were visualized and analyzed with the Odyssey system (LI-COR).
4.9. SOD Activity
To evaluate the effects of EX on the SOD activity, intracellular SOD activity (cytosolic (SOD1) and mitochondrial (SOD2)) activity was measured using a commercially available kit (Cayman chemical).
4.10. Statistical Analysis
Data are expressed as means ± standard error of the mean (SEM). Statistical significance was assessed using a two-way ANOVA, followed by post-hoc testing with Newman–Keuls test. Total running distances were compared with one-way ANOVA, followed by Newman–Keuls post-hoc test. A value of p < 0.05 was considered as statistically significant (two-tailed).
Similar to previous observations [24], we did not observe an influence of sex on the effects of TAC and MI and/or EX on the responses of survival and LV hypertrophy or dysfunction. Consequently, we pooled male and female mice for final analysis.
Acknowledgments
We gratefully acknowledge the expert technical assistance by Laurens A.J. Blonden.
Author Contributions
E.D.v.D., Y.O., M.C.d.W., and M.d.B. performed the experiments; E.D.v.D., Y.O., M.C.d.W., and M.d.B. analyzed the data; E.D.v.D. and Y.O. interpreted the results of the experiments; E.D.v.D. and Y.O. prepared the figures; E.D.v.D., Y.O. and D.J.D. drafted the manuscript; M.d.B. and M.C.d.W. edited and revised the manuscript; E.D.v.D., Y.O., M.C.d.W., M.d.B., and D.J.D. approved the final version of the manuscript.
Funding
This work was supported by the Cardiovascular Research Initiative CVON-ARENA (to D.J.D.) and Dutch Heart Foundation (2007B024 to D.J.D.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., de Ferranti S., Despres J.P., Fullerton H.J., Howard V.J., et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosy A.P., Fonarow G.C., Butler J., Chioncel O., Greene S.J., Vaduganathan M., Nodari S., Lam C.S., Sato N., Shah A.N., et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Downing J., Balady G.J. The role of exercise training in heart failure. J. Am. Coll. Cardiol. 2011;58:561–569. doi: 10.1016/j.jacc.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher G.F., Balady G., Blair S.N., Blumenthal J., Caspersen C., Chaitman B., Epstein S., Sivarajan Froelicher E.S., Froelicher V.F., Pina I.L., et al. Statement on exercise: Benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1996;94:857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor C.M., Whellan D.J., Lee K.L., Keteyian S.J., Cooper L.S., Ellis S.J., Leifer E.S., Kraus W.E., Kitzman D.W., Blumenthal J.A., et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies E.J., Moxham T., Rees K., Singh S., Coats A.J., Ebrahim S., Lough F., Taylor R.S. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur. J. Heart Fail. 2010;12:706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gielen S., Laughlin M.H., O’Conner C., Duncker D.J. Exercise training in patients with heart disease: Review of beneficial effects and clinical recommendations. Prog. Cardiovasc. Dis. 2015;57:347–355. doi: 10.1016/j.pcad.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Farah C., Kleindienst A., Bolea G., Meyer G., Gayrard S., Geny B., Obert P., Cazorla O., Tanguy S., Reboul C. Exercise-induced cardioprotection: A role for eNOS uncoupling and NO metabolites. Basic Res. Cardiol. 2013;108:389. doi: 10.1007/s00395-013-0389-2. [DOI] [PubMed] [Google Scholar]
- 9.Powers S.K., Smuder A.J., Kavazis A.N., Quindry J.C. Mechanisms of exercise-induced cardioprotection. Physiology. 2014;29:27–38. doi: 10.1152/physiol.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann N., Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126:2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughlin M.H., Davis M.J., Secher N.H., van Lieshout J.J., Arce-Esquivel A.A., Simmons G.H., Bender S.B., Padilla J., Bache R.J., Merkus D., et al. Peripheral circulation. Compr. Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 12.Balligand J.L., Feron O., Dessy C. eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 13.Forstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008;44:142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Linke A., Adams V., Schulze P.C., Erbs S., Gielen S., Fiehn E., Mobius-Winkler S., Schubert A., Schuler G., Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 16.Bito V., de Waard M.C., Biesmans L., Lenaerts I., Ozdemir S., van Deel E., Abdel-Mottaleb Y., Driesen R., Holemans P., Duncker D.J., et al. Early exercise training after myocardial infarction prevents contractile but not electrical remodelling or hypertrophy. Cardiovasc. Res. 2010;86:72–81. doi: 10.1093/cvr/cvp381. [DOI] [PubMed] [Google Scholar]
- 17.De Waard M.C., van der Velden J., Bito V., Ozdemir S., Biesmans L., Boontje N.M., Dekkers D.H., Schoonderwoerd K., Schuurbiers H.C., de Crom R., et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ. Res. 2007;100:1079–1088. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 18.Duncker D.J., van Deel E.D., de Waard M.C., de Boer M., Merkus D., van der Velden J. Exercise training in adverse cardiac remodeling. Pflugers Arch. 2014;466:1079–1091. doi: 10.1007/s00424-014-1464-8. [DOI] [PubMed] [Google Scholar]
- 19.Rolim N., Skardal K., Hoydal M., Sousa M.M., Malmo V., Kaurstad G., Ingul C.B., Hansen H.E., Alves M.N., Thuen M., et al. Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction. Basic. Res. Cardiol. 2015;110:44. doi: 10.1007/s00395-015-0502-9. [DOI] [PubMed] [Google Scholar]
- 20.De Waard M.C., van Haperen R., Soullie T., Tempel D., de Crom R., Duncker D.J. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J. Mol. Cell. Cardiol. 2010;48:1041–1049. doi: 10.1016/j.yjmcc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Haykowsky M., Scott J., Esch B., Schopflocher D., Myers J., Paterson I., Warburton D., Jones L., Clark A.M. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: Start early and go longer for greatest exercise benefits on remodeling. Trials. 2011;12:92. doi: 10.1186/1745-6215-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonow R.O., Cheitlin M.D., Crawford M.H., Douglas P.S. Task Force 3: Valvular heart disease. J. Am. Coll. Cardiol. 2005;45:1334–1340. doi: 10.1016/j.jacc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Rome J.J. Exercise restriction to prevent sudden death in congenital aortic stenosis: Whom are we treating? J. Am. Coll. Cardiol. 2010;56:1947–1948. doi: 10.1016/j.jacc.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Van Deel E.D., de Boer M., Kuster D.W., Boontje N.M., Holemans P., Sipido K.R., van der Velden J., Duncker D.J. Exercise training does not improve cardiac function in compensated or decompensated left ventricular hypertrophy induced by aortic stenosis. J. Mol. Cell. Cardiol. 2011;50:1017–1025. doi: 10.1016/j.yjmcc.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Chen C.A., Wang T.Y., Varadharaj S., Reyes L.A., Hemann C., Talukder M.A., Chen Y.R., Druhan L.J., Zweier J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gielen S., Schuler G., Adams V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation. 2010;122:1221–1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- 27.Van den Borne S.W., van de Schans V.A., Strzelecka A.E., Vervoort-Peters H.T., Lijnen P.M., Cleutjens J.P., Smits J.F., Daemen M.J., Janssen B.J., Blankesteijn W.M. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc. Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 28.De Waard M.C., Duncker D.J. Prior exercise improves survival, infarct healing, and left ventricular function after myocardial infarction. J. Appl. Physiol. 2009;107:928–936. doi: 10.1152/japplphysiol.91281.2008. [DOI] [PubMed] [Google Scholar]
- 29.Yang L., Jia Z., Yang L., Zhu M., Zhang J., Liu J., Wu P., Tian W., Li J., Qi Z., et al. Exercise Protects against Chronic beta-Adrenergic Remodeling of the Heart by Activation of Endothelial Nitric Oxide Synthase. PLoS ONE. 2014;9:e96892. doi: 10.1371/journal.pone.0096892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones S.P., Greer J.J., van Haperen R., Duncker D.J., de Crom R., Lefer D.J. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc. Natl. Acad. Sci. USA. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Waard M.C., van der Velden J., Boontje N.M., Dekkers D.H., van Haperen R., Kuster D.W., Lamers J.M., de Crom R., Duncker D.J. Detrimental effect of combined exercise training and eNOS overexpression on cardiac function after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1513–H1523. doi: 10.1152/ajpheart.00485.2008. [DOI] [PubMed] [Google Scholar]
- 32.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell. Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 33.Graham D.A., Rush J.W. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J. Appl. Physiol. 2004;96:2088–2096. doi: 10.1152/japplphysiol.01252.2003. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes T., Nakamuta J.S., Magalhaes F.C., Roque F.R., Lavini-Ramos C., Schettert I.T., Coelho V., Krieger J.E., Oliveira E.M. Exercise training restores the endothelial progenitor cells number and function in hypertension: Implications for angiogenesis. J. Hypertens. 2012;30:2133–2143. doi: 10.1097/HJH.0b013e3283588d46. [DOI] [PubMed] [Google Scholar]
- 35.Duncker D.J., Ishibashi Y., Bache R.J. Effect of treadmill exercise on transmural distribution of blood flow in hypertrophied left ventricle. Am. J. Physiol. 1998;275:H1274–H1282. doi: 10.1152/ajpheart.1998.275.4.H1274. [DOI] [PubMed] [Google Scholar]
- 36.Haykowsky M.J., Liang Y., Pechter D., Jones L.W., McAlister F.A., Clark A.M. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J. Am. Coll. Cardiol. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 37.Hsu S., Nagayama T., Koitabashi N., Zhang M., Zhou L., Bedja D., Gabrielson K.L., Molkentin J.D., Kass D.A., Takimoto E. Phosphodiesterase 5 inhibition blocks pressure overload-induced cardiac hypertrophy independent of the calcineurin pathway. Cardiovasc. Res. 2009;81:301–309. doi: 10.1093/cvr/cvn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A., Macera C.A., Heath G.W., Thompson P.D., Bauman A., et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 39.Steg P.G., James S.K., Atar D., Badano L.P., Blomstrom-Lundqvist C., Borger M.A., Di Mario C., Dickstein K., Ducrocq G., Fernandez-Aviles F., et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Eur. Heart J. 2012;33:2569–2619. doi: 10.1016/j.rec.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Brown D.W., Dipilato A.E., Chong E.C., Gauvreau K., McElhinney D.B., Colan S.D., Lock J.E. Sudden unexpected death after balloon valvuloplasty for congenital aortic stenosis. J. Am. Coll. Cardiol. 2010;56:1939–1946. doi: 10.1016/j.jacc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 41.Van Deel E., Ridwan Y., van Vliet J.N., Belenkov S., Essers J. In Vivo Quantitative Assessment of Myocardial Structure, Function, Perfusion and Viability Using Cardiac Micro-computed Tomography. J. Vis. Exp. 2016:53603. doi: 10.3791/53603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y.M., Guzik T.J., Zhang Y.H., Zhang M.H., Kattach H., Ratnatunga C., Pillai R., Channon K.M., Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 43.Moens A.L., Champion H.C., Claeys M.J., Tavazzi B., Kaminski P.M., Wolin M.S., Borgonjon D.J., Van Nassauw L., Haile A., Zviman M., et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117:1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]