Abstract
Chlorella has great potential as a bio-factory for production of value-added compounds. To produce the desired chemicals more efficiently in Chlorella, genetic tools for modification of Chlorella need to be developed, especially an endogenous promoter. In this study, the promoter of photosystem I protein D (psaD) from Chlorella vulgaris UTEX395 was identified. Computational analysis revealed the presence of several putative cis-acting elements, including a potential core element, and light-responsive or stress-responsive elements. Gene expression analysis in heterologous expression system in Chlamydomonas reinhardtii and Nicotiana benthamiana showed that CvpsaD promoter can be used to drive the expression of genes. Functional analysis of this promoter suggested that the initiator element (Inr) is important for its function (i.e., TATA-less promoter) and that an additional factor (e.g., downstream of the transcriptional start site) might be needed for light response. We have shown that the CvpsaD promoter is functional, but not sufficiently strong, both in microalgae and higher plant.
Keywords: Chlorella vulgaris, native promoter, photosystem I protein D (psaD), heterologous expression
1. Introduction
To date, microalgae are being commercially used to produce food and animal feed additives, as an ingredient in cosmetics, or as value-added compounds, such as pigments, therapeutic proteins, and fatty acids [1,2,3]. Owing to the recent developments in microalgae transformation techniques, microalgae now have much broader industrial applications; use of transgenic microalgae for production of medicines and biofuels has already demonstrated this [4]. However, the production of the biochemical is still insufficient. Therefore, to improve the industrial potential of microalgae, genetic modifications for enhancement of their physiological properties and optimization of production systems of commercial strains are being conducted by many research groups [5]. The well-known unicellular freshwater green microalga, Chlorella vulgaris, is widely cultured and commercialized because of its ability to grow rapidly and economically in both autotrophic and heterotrophic media [6,7]. Although a method for developing transgenic C. vulgaris has already been reported [8], the methods cannot be applied to all fields because of its low efficiency or non-reproducible results. Therefore, to use this promising industrial species more effectively, there is a need to develop reliable and convenient tools for genetic modification [9].
Several methods, such as protoplast transformation [10,11], Agrobacterium-mediated transformation [12,13], electroporation [14,15], and particle bombardment [14,16] have been developed to transform Chlorella. However, some drawbacks are still associated with these methods as inefficient or unstable transformants are produced. The main reasons for the formation of such transformants are as follows: (I) the lack of a high efficiency transfer technique for introduction of a foreign gene into the genome [5], (II) the lack of an appropriate expression system for foreign genes [17,18,19,20], (III) the difference in codon usage between the expression host and the introduced foreign gene [21,22,23].
Chlamydomonas reinhardtii, a green microalga, has long been used as a model strain for molecular biology and genetic experiments using its own native promoters. Several native promoters have been identified that are known to regulate well-characterized and highly expressed genes, such as heat shock protein 70A (HSP70A), Rubisco small subunit (RBCS2), or photosystem I protein D (psaD) [24,25,26]. Moreover, in an attempt to increase the expression rate, a chimeric promoter was developed that contains the HSP70A promoter region fused upstream of the RBCS2 promoter (AR promoter), thereby, leading to increased transcription [25,27]. Recently, some research groups suggested the use of synthetic algal promoters (saps) that are designed based on the characteristics of strong promoter motifs [28,29]. While the use of endogenous promoters is almost optimized in the model algal strain, only heterologous promoters from the plant system, such as the 35S promoter, ubiquitin promoter, and NOS promoter have been used for Chlorella [10,11,12,15,16]. Therefore, research on promoters derived from Chlorella is still in its early stage, and further advancements are necessary.
In general, gene expression is positively correlated with promoter strength. Therefore, the promoter of a gene encoding a highly expressed protein in vivo is considered to be a strong promoter. psaD gene encodes an abundant protein of the photosystem I reaction center subunit II in photosynthetic organisms [30,31,32]. psaD gene is a nuclear gene although psaD protein is located on the stromal side in the chloroplast. The psaD promoter has been used to drive efficient gene expression in Chlamydomonas [26,32]. Since this gene is commonly found in photosynthetic organisms, it is also found in Chlorella species.
In this study, we found the photosystem I protein D (psaD) gene from the whole genome sequence (BioProject: PRJNA278897) of Chlorella vulgaris UTEX395 and predicted its promoter sequence in the 5′ upstream region of psaD. The function of this CvpsaD promoter was confirmed through the expression of aphVIII or luciferase in Chlamydomonas reinhardtii (microalgae) and also the expression of green fluorescent protein (GFP)-fused β-glucuronidase (GUS) in Nicotiana benthamiana (higher plant). This is the first report about the use of an endogenous promoter of Chlorella vulgaris for transgene expression in other organisms.
2. Results and Discussion
2.1. Isolation and Computational Analysis of CvpsaD Gene and the 5′ Upstream Region
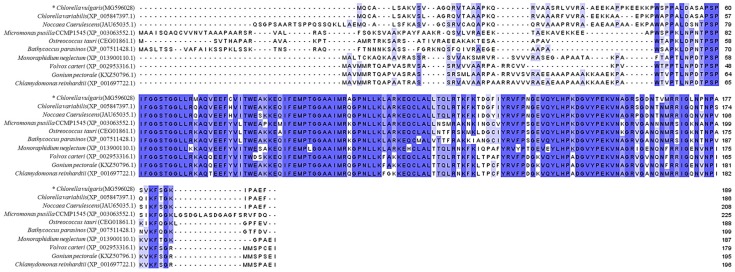
Chlorella vulgaris UTEX395 pasD (MG596028) gene was predicted by the protein basic local alignment search tool (BLASTP) program using the amino acid sequence of Chlamydomonas reinhardtii psaD as the query, and computational analysis was performed using the NEW GENESCAN Web server. Putative psaD sequence of C. vulgaris was subjected to BLASTP analysis for a sequence homology search against the National Center for Biotechnology Information (NCBI) database. The search results indicated that the conserved domain of CvpsaD has a very high similarity with those of other species. Thus, BLASTP analysis and alignment of the conserved domain (psaD superfamily, PLN00041) of psaD family suggested that the predicted sequence is indeed psaD (Figure 1).
Figure 1.
Alignment of amino acid sequence of photosystem I protein D (psaD) using the protein basic local alignment search tool (BLASTP) program against the National Center for Biotechnology Information (NCBI) database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The asterisk indicates the psaD sequence of C. vulgaris UTEX395 used for this study.
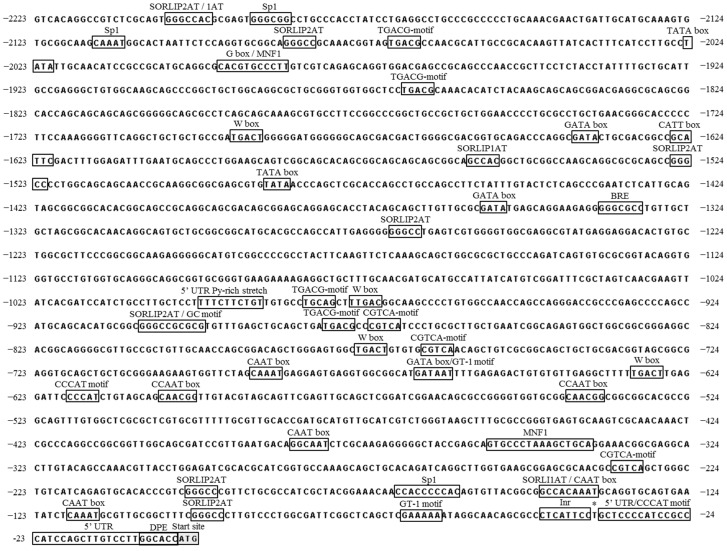
To determine the putative cis-acting elements, the 2.2 kb putative promoter sequence upstream of the CvpsaD protein-coding sequence CDS was analyzed using PLACE and PlantCARE databases. Additionally, the Promoter 2.0 Prediction Server was used to predict the transcription start site (TSS), and 5′-rapid amplification of complementary DNA ends (5′-RACE) was performed to determine the 5′ untranslated region (5′-UTR) sequence. The TSS site was found to be at −36 bp by 5′-RACE (Figure 2), whereas the predicted TSS site by Promoter 2.0 Prediction Server was at −1200 bp. The difference in position between the actual TSS and the predicted TSS site was remarkable. Thus, TSS analysis through computational techniques was not effective in the Chlorella promoter study. The TATA-box, the core element, which is typically located 30~40 bp upstream of the TSS site in eukaryotes, was identified at two positions by PlantCARE, but they were quite far away (−1480 bp and −2020 bp) from the TSS. Therefore, TATA-box might not be the core element in the CvpsaD promoter. In plants, TATA-less promoters are commonly found in the photosynthetic genes, such as photosystem I subunits [33]. The initiator element (Inr) consensus sequence, YYANWYY, is one of the alternative core elements found in TATA-less promoters [34]. The sequence-based structural features of CvpsaD promoter are similar to those of psaDb promoter which is a TATA-/Inr+ promoter in Nicotiana sylvestris [33]. Their Inr has the same consensus sequence as CTCAYTYY. Therefore, instead of the TATA-box, Inr, located at −38 bp, could play an important role in in mediating the functions of the CvpsaD promoter. Moreover, it is expected that a downstream promoter element (DPE, −6 bp) will act as a core element with Inr in the CvpsaD promoter [33,35]. BRE, another core element, was found at −1330 bp. This element is located immediately upstream of the TATA-box; thus, it is a non-critical factor in TATA-less promoters, like the psaDb and CvpsaD promoters. The distal sequence may contain additional regulatory elements, often with a weaker influence than the core element. The CAAT box, which is a common cis-acting element in promoters, was found at −80 bp, −100 bp, −340 bp, and −650 bp from TSS. This motif is normally located 60–100 bp upstream of the TSS and about 150 bp upstream of the TATA box. The 5′ UTR Py-rich stretch motif is also located in the −950 bp upstream region. This 5′ UTR motif can provide high transcription levels without the need for other upstream cis-acting elements [36,37]. Two CCCAT motifs [28], which are putative core elements isolated from synthetic promoters, were observed at −600 bp and +13 bp from TSS. Although these three elements are not key elements, they are located close to the TSS and will have a greater effect on the CvpsaD promoter than the TATA-box. Additionally, as shown in Figure 2 and Table 1, the CvpsaD promoter contains several light-responsive elements, such as CATT box, G-box [38], GATA box [39], MNF1 motif [24], SORLIP1AT, SORLIP2AT [29,40,41], and the Sp1 motif [42], which are found in higher plants. These light-responsive elements are widely distributed throughout the promoter of CvpsaD. Also, there is a report that the Cppsy promoter of Chlorella protothecoide which has MNF1, Sp1 motif regulated by light [43]. Thus, the CvpsaD promoter may respond to light. Simultaneously, other elements associated with stress-responses, like the GT-1 motif [44], W box [45], CCAAT box [46] CGTCA-motif, and TGACG-motif [47], which are found in plants, were also observed in the promoter of CvpsaD. These elements are known to be related by methyl jasmonate (MeJA), but the direct association of the psaD gene by MeJA has not been reported.
Figure 2.
Nucleotide sequence of the CvpsaD promoter and its predicted motifs. The start codon (ATG) is highlighted in a gray box and the putative transcriptional start site (TSS) is indicated by an asterisk. The putative cis-acting elements are boxed and labeled.
Table 1.
List of putative cis-acting elements in the psaD promoter in Chlorella vulgaris.
| No | Name | Sequence | Function |
|---|---|---|---|
| 1 | BRE | GGGCGCC | TFIIB recognition element |
| 2 | CAAT box | GGCAAT CAAT CAAAT |
Common cis-acting element in promoter and enhancer regions |
| 3 | CCAAT box | CAACGG | MYBHv1 binding site |
| 4 | CATT box | GCATTC | Part of light-responsive element |
| 5 | CCCAT motif | CCCAT | Putative core element, sap |
| 6 | CGTCA-motif | CGTCA | MeJA-responsiveness |
| 7 | DPE | GGCACC | Downstream promoter element, requires an Inr |
| 8 | G-box | CACGTG | cis-acting regulatory element involved in light responsiveness |
| 9 | GATA box | GATA | Binding with ASF-2, required for high level, light-regulated, and tissue-specific expression |
| 10 | GT-1 motif | GATAAT GAAAA |
Pathogen- and salt-induced element |
| 11 | Inr | CTCATTCC | Initiator element, core promoter element |
| 12 | MNF1 | GTGCCCTAAAGCTGCA | Light-responsive element |
| 13 | SORLIP1AT/2AT | GCCAC GGGCC |
Light-responsive elements |
| 14 | Sp1 | CCACCC | Light-responsive element |
| 15 | TATA box | TATA | Core promoter element around −30 of transcription start |
| 16 | TGACG-motif | TGACG | MeJA-responsiveness |
| 17 | W box | TGACT TTGAC |
cis-regulatory element, recognized by WRKY transcription factors, stress responsive |
| 18 | 5’ untranslated region (UTR) Py-rich stretch | TTTCTTCTGT | Confers high transcription levels without the need for other upstream cis-acting elements |
2.2. Construction of the CvpsaD Promoter Cassettes and Validation of Its Function in C. reinhardtii
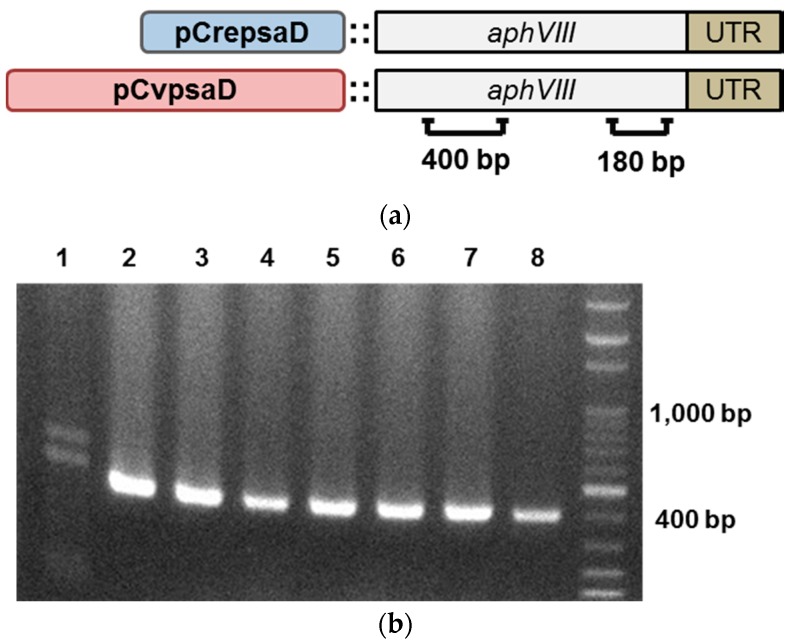
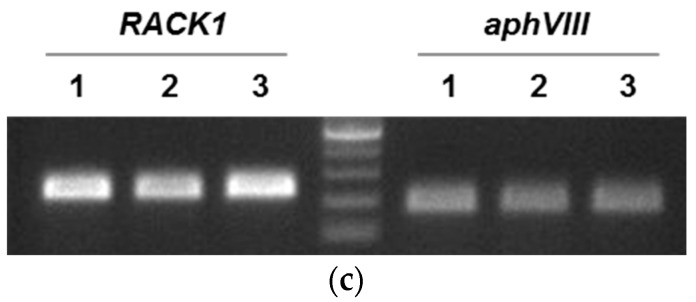
The gene encoding aminoglycoside 3′-phosphotransferase (aphVIII) was chosen as the selective marker gene as it provides stable resistance against paromomycin, a commonly used selection antibiotic for microalgae. For the construction of promoter cassette containing the desired promoter and selective marker, first the aphVIII-3′ UTR fragment was cloned into pChlamy3 plus vector and digested with restriction enzymes (SpeI and KpnI), and then the promoter sequence was cloned upstream of aphVIII-3′ UTR (Figure 3a). To validate the function of CvpsaD promoter, the promoter cassette with aphVIII was transformed into Chlamydomonas. The CrepsaD promoter cassette was used as a positive control. The transformants with paromomycin resistance were selected on agar plates and three different transformants of each promoter cassette were sub-cultured in liquid medium for polymerase chain reaction (PCR) analysis. To confirm that the promoter cassette DNA was introduced into the cells, PCR was performed with specific primers (400 bp product, Figure 3a,b). The expression of aphVIII RNA in transgenic Chlamydomonas was determined by reverse transcription PCR with specific primers (180 bp product, Figure 3a,c). Unless the antibiotic resistance genes introduced into microalgae are expressed sufficiently to resist against antibiotics, transformants will be not produced [48]. Thus, from this result, we found that aphVIII was highly expressed by the CvpsaD promoter, at levels sufficient enough to confer antibiotic resistance in Chlamydomonas.
Figure 3.
Validation of CvpsaD promoter functionality by expression of aphVIII. (a) Vector map of promoter cassette conjugated with resistance gene for verification of functionality. The CrepsaD promoter cassette serves as a positive control in Chlamydomonas. (b) The presence of aphVIII was confirmed in putative transformants by polymerase chain reaction (PCR) analysis with 400 bp primer sets. Lane 1: wild type, Lane 2–4: pCrepsaD::aphVIII, Lane 5–7: pCvpsaD::aphVIII, Lane 8: plasmid positive, (c) RNA expression was detected by RT-PCR using 180 bp primer sets for aphVIII. The RACK1 gene served as the internal control. Lane 1: pCrepsaD::aphVIII, Lane 2–3: pCvpsaD::aphVIII.
2.3. Validation of the CvpsaD Promoter Efficiency in C. reinhardtii
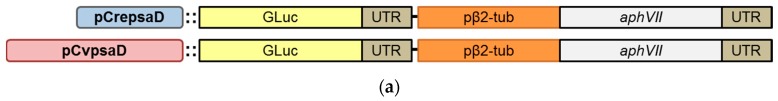
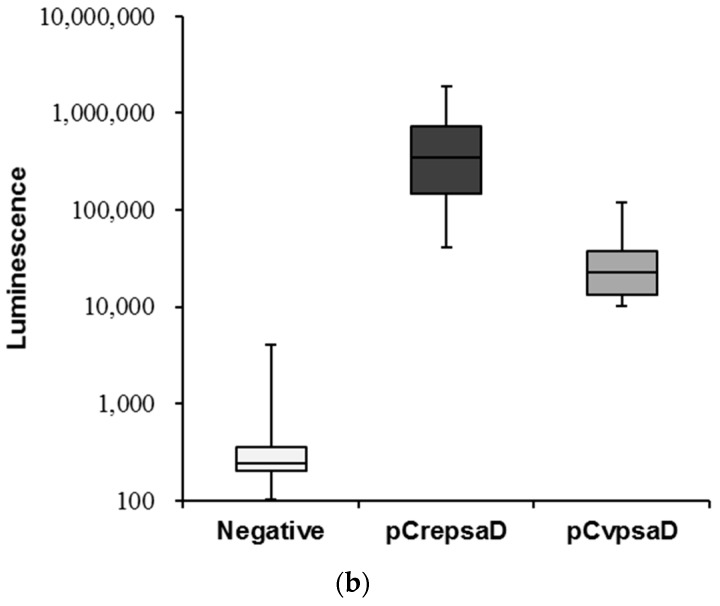
To determine the strength of CvpsaD promoter, a new vector cassettes for Gaussia luciferase (GLuc) expression were made (Figure 4a). The promoter cassette with GLuc was then introduced into Chlamydomonas. Positive transformants harboring GLuc gene were analyzed by PCR. The expression of GLuc in transgenic Chlamydomonas was validated by quantitative real-time PCR (Figure S1). After the expression of GLuc was verified, we compared the strength of CrepsaD promoter, which is one of the strongest promoters in Chlamydomonas, with CvpsaD promoter through luciferase activity assay using 20 randomly selected positive transformants. The luciferase activity operated by CvpsaD promoter was 17.3 times lower than that by CrepsaD promoter. At the same condition, the non-transgenic control showed negligible luciferase expression (Figure 4b). The intensity of the CvpsaD promoter identified through luciferase activity assay was similar to that observed at RNA and protein expression levels, and it was observed to have a lower transformation efficiency as well (Figure S2). In general, heterologous promoters exhibit lower expression efficiency than endogenous promoters. The CvpsaD promoter is heterologous to Chlamydomonas, and therefore the necessary regulatory elements of the CvpsaD promoter may be lacking in Chlamydomonas [17]. Moreover, the sequence of CrepsaD promoter (Figure S3) differs from that of CvpsaD promoter in that there is a TATA box core element near the TSS. Additionally, the CAAT motif, a cis-acting element, is located at about 160 bp upstream of TATA box and 135 bp upstream of TSS. These features observed in the CrepsaD promoter sequence are similar to what is already known. Therefore, in Chlamydomonas, the presence of a core element neighboring the cis-acting elements on the promoter seems to be an important factor in regulating promoter strength.
Figure 4.
Determination of the strength of CvpsaD promoter. (a) Schematic representation of the promoter cassettes conjugated with Gaussia luciferase (GLuc) gene for strength analysis (b) Analysis of luciferase activity by the two promoters.
2.4. Confirmation of the CvpsaD Promoter Functionality by Expression of GFP-GUS Activity in Planta
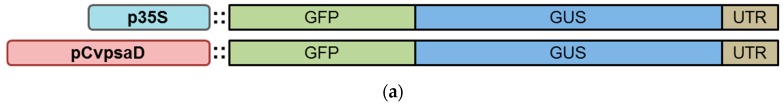
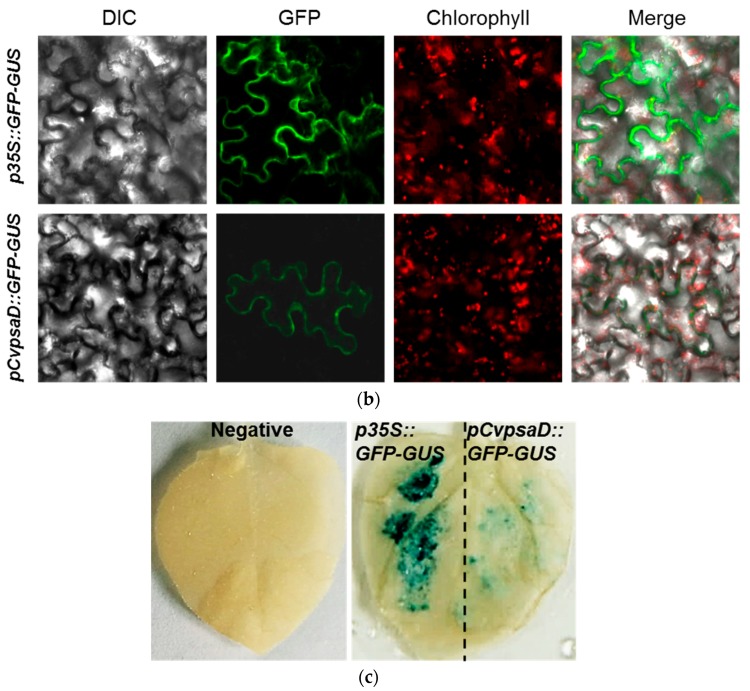
In this experiment, we re-confirmed the function of CvpsaD promoter derived from C. vulgaris by expression in higher plant. We expected the promoter to be able to express the gene in plants, as the CvpsaD sequence is similar to that in Noccaea caerulescens (JAU65035.1) (Figure 1). To evaluate the expression of a gene (GFP-fused GUS reporter system) by the CvpsaD promoter in a higher plant, transient expression system in Nicotiana benthamiana was used. To prepare the GFP-GUS expression cassette, we used pHZM43 vector derived from pGreen. The CvpsaD promoter sequence was cloned into 5′ upstream region of the CDS of GFP-GUS (Figure 5a). The cauliflower mosaic virus (CaMV) 35S promoter cassette was used as a positive control. After vector construction, Agrobacterium harboring the promoter cassette was cultivated for infiltration, Agrobacterium infiltrated N. benthamiana leaves were left for 3 days to allow protein expression. Following that, GFP expression (green) and chlorophyll autofluorescence (red) were detected by fluorescence microscopy and GUS activity was analyzed in the same transgenic leaves (Figure 5b,c). After infiltration, green fluorescence and blue color expressed by pCvpsaD::GFP-GUS were detected in epidermal cell cytosol and leaf tissues (mesophyll cell), although the signal was weaker than that of the positive control. This reflects that the strength of the CvpsaD promoter is not enough high in N. benthamiana. This result is that same as that observed earlier in C. reinhardtii using the pCvpsaD::GLuc cassette (Figure 4). Computational analysis showed that the CaMV 35S promoter has a core element, such as the TATA box, and a cis-acting element, CAAT motif, distributed around the core element (Figure S3). The characteristics of this sequence are similar to those of CrepsaD, as mentioned above. Therefore, this suggests that the absence of a core element in the CvpsaD promoter sequence may be the reason for its weak strength.
Figure 5.
Evaluation of the CvpsaD promoter function in higher plants. Green fluorescent protein (GFP) analysis and the β-glucuronidase (GUS) histochemical assay were performed on the infiltrated leaves. (a) Schematic representation of promoter cassettes conjugated with the GFP-GUS fusion gene. (b) Green fluorescence in epidermal cells and (c) GUS reporter assay in leaf tissue of N. benthamiana. Transient expression of GFP-GUS three days after infiltration with Agrobacterium harboring GFP-GUS vector.
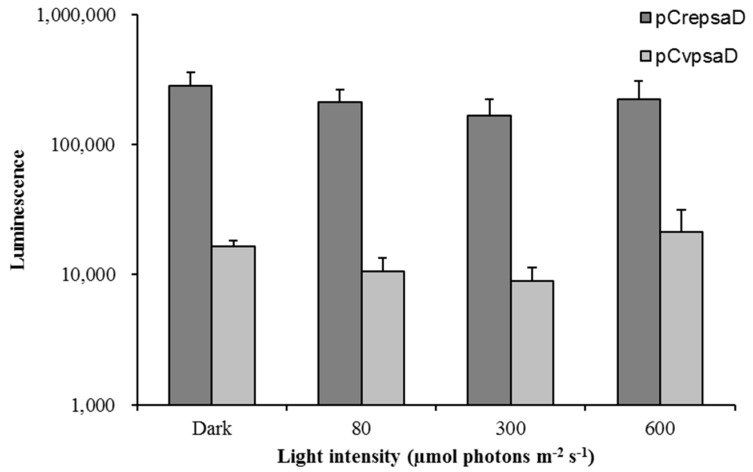
2.5. Light Inducibility of the CvpsaD Promoter under Various Light Conditions
Several light-responsive elements are present on CvpsaD promoter, such as the CATT box, G-box, GATA box, MNF1 motif, SORLIP1AT, SORLIP2AT, and the Sp1 motif (Figure 2). Therefore, in order to determine the light responsiveness of the CvpsaD promoter, we measured luciferase activity under various light conditions (dark to 600 µmol photons m−2 s−1) in C. reinhardtii. The CrepsaD promoter, known as a constitutive promoter [26], was used again as a control in this experiment. In Figure 6, the result shows the difference in luciferase activity affected by the light intensity. Luciferase activity was not significantly increased by high light intensity (300 and 600 µmol photons m−2 s−1). This result suggests that despite the presence of light-responsive elements on the CvpsaD promoter, it is not affected by light. According to a previous report [29,49], the SORLIP motif (GCCAC or GGGCC), known as the overrepresented element near TSS in light-induced genes, plays an important role in determining the reactivity to light. Also, all the other light-responsive elements described above on the CvpsaD promoter are also known to be involved in the light responsiveness of the genes in plants. However, in our experiment, the SORLIP motif, as well as all other light-responsive elements seemed to be ineffective for induction of a light response in C. reinhardtii. In the previous study, it has been reported that psaD gene of spinach is expressed in response to light, but the psaD promoter was insensitive to light [30]. This difference between a gene and promoter response to light can be interpreted as being due to a particular sequence present in the coding region or further downstream [30]. This result shows that a computational analysis using already known motifs cannot be an absolute factor in understanding the function of the new promoter, thus independent and extensive experimental results for each promoter should be carried out.
Figure 6.
Analysis of the inducibility of the CvpsaD promoter under various light conditions. Two different transformants, each harboring one of the promoter cassettes, were used for examination of light response. Luciferase activities were analyzed to determine the light response.
3. Materials and Methods
3.1. Microalgae Cell Culture
Chlorella vulgaris UTEX395 (purchased from UTEX, Culture Collection of Algae, Austin, TX, USA) and Chlamydomonas reinhardtii CC-4349 cw15 mt- (kindly provided by Dr. Jae-Hyeok Lee, University of British Columbia) were maintained photoheterotrophically in Tris-acetate-phosphate (TAP) medium at 25 °C and continuous light (50–70 μmol photons m−2 s−1) conditions on an orbital shaker with shaking at 90 rpm. For selection and maintenance of transgenic cells, TAP medium was fortified with paromomycin (25 µg/mL) or hygromycin-B (25 µg/mL).
3.2. Isolation and Sequence Analysis of Putative CvpsaD Gene
Putative psaD sequence from Chlorella vulgaris was predicted by a BLAST search (TBLASTN), based on the CrepsaD amino acid sequence (Accession: AAL73208.1). A homology analysis of amino acid sequence was conducted using NEW GENESCAN Web server (http://genes.mit.edu/GENSCAN.html) to determine the CDS of CvpsaD. The 570 bp full length complementary DNA (cDNA) of the putative CvpsaD gene was amplified and sequenced using specific primers (Table S1). The alignment of psaD amino acid sequence from various species was performed by NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome) and illustrated using a multiple sequence alignment tool (http://www.jalview.org/). Moreover, 5′ and 3′ RACE was performed for identification of whole mRNA transcript using the 5′/3′ RACE kit (2nd Generation kit, Roche, Germany), according to the manufacturer’s protocol. The location and distribution of putative cis-acting elements in CvpsaD promoter was analyzed using PLACE (https://sogo.dna.affrc.go.jp/cgi-bin/sogo.cgi?lang=en&pj=640&action=page&page=newplace), PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html), and Promoter 2.0 Prediction Server (http://www.cbs.dtu.dk/services/Promoter/).
3.3. Expression Vector Construction
The CvpsaD promoter was conjugated with aphVIII or GLuc vector using restriction enzyme and T4 DNA ligase, according to the protocols of the traditional cloning method. For functionality analysis of the CvpsaD promoter, the aphVIII gene was cloned into pChlamy3 plus vector, which is derived from pChamy3 vector after deleting the AR promoter [29]. The promoter sequence was then sub-cloned upstream of aphVIII::3′ UTR at the SpeI and KpnI restriction sites. The pCrepsaD::aphVIII cassette was created following the same procedure (Figure 3). For analysis of the strength of the CvpsaD promoter, the GLuc cassette was developed by replacing aphVIII with GLuc. aphVIII was removed from the promoter::aphVIII cassette by double digestion with KpnI and NotI, and GLuc was then cloned into the cleaved region by T4 DNA ligase. For Nicotiana transformation, the pHZM43 vector derived from pGreen vector was used. The CvpsaD promoter sequence was cloned upstream of GFP-GUS fusion gene at the KpnI and XhoI restriction sites. All primer sequences are listed in Table S1.
3.4. Generation of Transgenic Microalgae
To develop transgenic C. reinhardtii, transformation was performed by the glass bead method using the promoter cassette DNA, as previously described [29,50]. C. reinhardtii cells were grown up to mid-log phase in TAP (Tris-Phosphate-Phosphate) medium, and transformation was performed with 1 μg linearized DNA. After transformation, whole cells were spread on TAP agar plates containing antibiotics (25 μg/mL paromomycin or 25 µg/mL hygromycin-B). Antibiotic resistant colonies were selected within 10 days. The transformants were confirmed by colony PCR [41]. Transformation efficiency was analyzed by electroporation. Electroporation was performed using the Bio-Rad Gene Pulser X cell apparatus (Bio-Rad, Hercules, CA, USA) with 40 mM sucrose, following the protocol of GeneArt® Chlamydomonas Engineering Kits (Life Technologies, Camarillo, CA, USA).
3.5. Functional Analysis of the CvpsaD Promoter in Microalgae
3.5.1. Quantitative Real-Time PCR for Analysis of Transgene RNA Expression
The RNA expression of aphVIII or GLuc was determined using quantitative real-time PCR (qPCR) performed by TaKaRa PCR Thermal Cycler Dice (Takara, Shiga, Japan). aphVIII or GLuc were amplified with specific primers (Table S1), and RACK1 gene served as the internal control [51]. Raw data of qPCR was calculated by ΔΔCt method using the software provided by the manufacturer. RNA expression level of gene was normalized to that of RACK1. RNA expression levels for the CrepsaD and CvpsaD promoters were compared.
3.5.2. Western Blot Analysis
Total cells (2.5 × 106 cells) were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and separated on SDS-polyacrylamide gels (12%), and then electro blotted onto a polyvinylidene fluoride (PVDF) membrane using the Semi-Dry Transfer Cell (Bio-Rad, Berkeley, CA, USA). GLuc protein was detected using primary rabbit anti-GLuc antibody (NEB, Beverly, MA, USA) and secondary HRP-conjugated goat anti-rabbit IgG (H + L) antibody (Life Technologies, Carlsbad, CA, USA). GLuc protein was visualized on X-ray film by chemiluminescence using the EPD western reagent (ELPIS-BIOTECH, Daejeon, Korea).
3.5.3. Luciferase Assay
To measure the luciferase activity, the cell density of each transgenic cell culture was so adjusted that optical density (OD)750nm = 0.5 at the mid-log phase. Cells were harvested from 1 mL of cell culture by centrifugation (4000× g for 3 min) and used for the luciferase activity assay. Luciferase activity was measured using the Renilla Luciferase Assay Kit (Promega, Madison, WI, USA), according to manufacturer’s protocol with slight modifications [41]. Cell pellets were resuspended in 100 μL of cell lysis buffer and mixed vigorously by vortexing for 2–3 min. The resuspension was then centrifuged at 13,000 rpm for 5 min at 4 °C. After centrifugation, 90 µL of the supernatant was transferred to a new tube and 10 µL of luciferase substrate was added to it. After mixing the supernatant and substrate, the luminescence was measured immediately using Glo Max™ 20/20 (Promega, Fitchburg, WI, USA). The luciferase assay was repeated three times.
3.6. Analysis of the Function of the CvpsaD Promoter in Plants
N. benthamiana leaves were infiltrated with A. tumefaciens strain GV3101:pMP90 harboring p35S::GFP-GUS or pCvpsaD::GFP-GUS. Transiently expressed proteins were analyzed by fluorescence microscopy and the GUS reporter assay. Green fluorescence and chlorophyll autofluorescence were detected in the infiltrated leaves using Nikon Eclipse Ni-E microscopy with DS-Qi2 camera (Nikon, Tokyo, Japan), 2.5–3 days after infiltration. Fluorescence detection wavelength ranged from 535 ± 50 nm with FITC filter for GFP and from 700 ± 75 nm with Cy5 filter for the chloroplast autofluorescence. The infiltrated leaves were also histochemically assayed for GUS activity [52]. Post-acquisition image processing involved contrast change only.
3.7. Analysis of Light Inducibility of the CvpsaD Promoter
For light inducibility analysis, transgenic cells harboring the pCvpsaD::GLuc cassette were placed under various constant light conditions (dark, 80, 300, and 600 μmol photons m−2 s−1) for 2 h [29]. After the exposure to different light intensities, the cell density was adjusted to equal OD value (OD750nm = 0.5) and cells were then spun down by centrifugation (4000× g for 3 min). Luciferase activity was measured, according to the procedure described above using the Renilla Luciferase Assay Kit (Promega, Fitchburg, WI, USA). Light inducibility analysis was performed with two different transgenic lines.
4. Conclusions
The development of a suitable promoter is one of the basic essentials in the molecular toolbox for genetic modification. In this study, the promoter of photosystem I protein D (psaD) gene was identified from the whole genome sequence of Chlorella vulgaris UTEX395 by computational analysis. And, the function of the new promoter derived from C. vulgaris was verified using model algal strain and higher plant. While its verification directly in Chlorella is still a major challenge, we suggest that the CvpsaD promoter can be used as an effective tool for genetic modification of Chlorella species. Moreover, it should be noted that this is the first report on a promoter derived from C. vulgaris that can be used to drive gene expression in green microalgae, as well as in higher plants. Therefore, CvpsaD promoter investigated in this study could be an efficient tool for the transformation of C. vulgaris through the further optimization of this CvpsaD promoter by several engineering strategies such as the determination of the most effective size of the promoter, a combination with an appropriate terminator or development of fusion promoter.
Acknowledgments
This research was supported by a grant from the Korea CCS R&D Center (KCRC) (NRF-2014M1A8A1049273) funded by the Government of South Korea (Ministry of Science, ICT and Future Planning). This research was also supported by the Basic Core Technology Development Program for the Oceans and the Polar Regions of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT (2015M1A5A1037053).
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-0067/19/7/1969/s1.
Author Contributions
J.K., Z.H., and E.J. conceived and designed the experiments; J.K. and L.L. performed the experiments and analyzed the data; J.K. and E.J. wrote the paper. All authors reviewed the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 2.Stengel D.B., Connan S., Popper Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011;29:483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Leu S., Boussiba S. Advances in the production of high-value products by microalgae. Ind. Biotechnol. 2014;10:169–183. doi: 10.1089/ind.2013.0039. [DOI] [Google Scholar]
- 4.Yu X., Chen L., Zhang W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015;6:56. doi: 10.3389/fmicb.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimpel J.A., Henríquez V., Mayfield S.P. In metabolic engineering of eukaryotic microalgae: Potential and challenges come with great diversity. Front. Microbiol. 2015;6:1376. doi: 10.3389/fmicb.2015.01376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang Y., Sarkany N., Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009;31:1043–1049. doi: 10.1007/s10529-009-9975-7. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Garcia O., Escalante F.M., de-Bashan L.E., Bashan Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011;45:11–36. doi: 10.1016/j.watres.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Yang B., Liu J., Jiang Y., Chen F. Chlorella species as hosts for genetic engineering and expression of heterologous proteins: Progress, challenge and perspective. Biotechnol. J. 2016;11:1244–1261. doi: 10.1002/biot.201500617. [DOI] [PubMed] [Google Scholar]
- 9.Chow K.-C., Tung W. Electrotransformation of Chlorella vulgaris. Plant Cell Rep. 1999;18:778–780. doi: 10.1007/s002990050660. [DOI] [Google Scholar]
- 10.Kim D.-H., Kim Y.T., Cho J.J., Bae J.-H., Hur S.-B., Hwang I., Choi T.-J. Stable integration and functional expression of flounder growth hormone gene in transformed microalga, chlorella ellipsoidea. Mar. Biotechnol. 2002;4:63–73. doi: 10.1007/s1012601-0070-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Wang Y., Zhang Y., Chen X., Zhang P., Ma S. Development of a new method for genetic transformation of the green alga chlorella ellipsoidea. Mol. Biotechnol. 2013;54:211–219. doi: 10.1007/s12033-012-9554-3. [DOI] [PubMed] [Google Scholar]
- 12.Ng S.L., Harikrishna J.A., Bakar F.A., Yeo C.C., San Cha T. Heterologous expression of the streptococcus pneumoniae yoeb and pezt toxin genes is lethal in Chlorella vulgaris. Algal Res. 2016;19:21–29. doi: 10.1016/j.algal.2016.07.011. [DOI] [Google Scholar]
- 13.San Cha T., Yee W., Aziz A. Assessment of factors affecting agrobacterium-mediated genetic transformation of the unicellular green alga, Chlorella vulgaris. World J. Microbiol. Biotechnol. 2012;28:1771–1779. doi: 10.1007/s11274-011-0991-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Sun Z., Gerken H., Huang J., Jiang Y., Chen F. Genetic engineering of the green alga chlorella zofingiensis: A modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl. Microbiol. Biotechnol. 2014;98:5069–5079. doi: 10.1007/s00253-014-5593-y. [DOI] [PubMed] [Google Scholar]
- 15.Run C., Fang L., Fan J., Fan C., Luo Y., Hu Z., Li Y. Stable nuclear transformation of the industrial alga chlorella pyrenoidosa. Algal Res. 2016;17:196–201. doi: 10.1016/j.algal.2016.05.002. [DOI] [Google Scholar]
- 16.Talebi A.F., Tohidfar M., Tabatabaei M., Bagheri A., Mohsenpor M., Mohtashami S.K. Genetic manipulation, a feasible tool to enhance unique characteristic of Chlorella vulgaris as a feedstock for biodiesel production. Mol. Biol. Rep. 2013;40:4421–4428. doi: 10.1007/s11033-013-2532-4. [DOI] [PubMed] [Google Scholar]
- 17.Thanh T., Chi V.T.Q., Omar H., Abdullah M.P., Napis S. Sequence analysis and potentials of the native rbcs promoter in the development of an alternative eukaryotic expression system using green microalga ankistrodesmus convolutus. Int. J. Mol. Sci. 2012;13:2676–2691. doi: 10.3390/ijms13032676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo S., Jeon H., Hwang S., Jin E., Chang K.S. Development of a new constitutive expression system for the transformation of the diatom phaeodactylum tricornutum. Algal Res. 2015;11:50–54. doi: 10.1016/j.algal.2015.05.012. [DOI] [Google Scholar]
- 19.Hallmann A. Algal transgenics and biotechnology. Transgen. Plant J. 2007;1:81–98. [Google Scholar]
- 20.Franklin S., Ngo B., Efuet E., Mayfield S.P. Development of a gfp reporter gene for chlamydomonas reinhardtii chloroplast. Plant J. 2002;30:733–744. doi: 10.1046/j.1365-313X.2002.01319.x. [DOI] [PubMed] [Google Scholar]
- 21.Radakovits R., Jinkerson R.E., Darzins A., Posewitz M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell. 2010;9:486–501. doi: 10.1128/EC.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucho K.-I., Kakoi K., Yamaura M., Iwashita M., Abe M., Uchiumi T. Codon-optimized antibiotic resistance gene improves efficiency of transient transformation in frankia. J. Biosci. 2013;38:713–717. doi: 10.1007/s12038-013-9361-4. [DOI] [PubMed] [Google Scholar]
- 23.Shao N., Bock R. A codon-optimized luciferase from gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga chlamydomonas reinhardtii. Curr. Genet. 2008;53:381–388. doi: 10.1007/s00294-008-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumbreras V., Stevens D.R., Purton S. Efficient foreign gene expression in chlamydomonas reinhardtii mediated by an endogenous intron. Plant J. 1998;14:441–447. doi: 10.1046/j.1365-313X.1998.00145.x. [DOI] [Google Scholar]
- 25.Schroda M., Blöcker D., Beck C.F. The hsp70a promoter as a tool for the improved expression of transgenes in chlamydomonas. Plant J. 2000;21:121–131. doi: 10.1046/j.1365-313x.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A., Falcao V.R., Sayre R.T. Evaluating nuclear transgene expression systems in chlamydomonas reinhardtii. Algal Res. 2013;2:321–332. doi: 10.1016/j.algal.2013.09.002. [DOI] [Google Scholar]
- 27.Wu J., Hu Z., Wang C., Li S., Lei A. Efficient expression of green fluorescent protein (gfp) mediated by a chimeric promoter in chlamydomonas reinhardtii. Chin. J. Oceanol. Limnol. 2008;26:242–247. doi: 10.1007/s00343-008-0242-x. [DOI] [Google Scholar]
- 28.Scranton M.A., Ostrand J.T., Georgianna D.R., Lofgren S.M., Li D., Ellis R.C., Carruthers D.N., Dräger A., Masica D.L., Mayfield S.P. Synthetic promoters capable of driving robust nuclear gene expression in the green alga chlamydomonas reinhardtii. Algal Res. 2016;15:135–142. doi: 10.1016/j.algal.2016.02.011. [DOI] [Google Scholar]
- 29.Baek K., Lee Y., Nam O., Park S., Sim S.J., Jin E. Introducing dunaliella lip promoter containing light-inducible motifs improves transgenic expression in chlamydomonas reinhardtii. Biotechnol. J. 2016;11:384–392. doi: 10.1002/biot.201500269. [DOI] [PubMed] [Google Scholar]
- 30.Flieger K., Wicke A., Herrmann R., Oelmüller R. Promoter and leader sequences of the spinach psad and psaf genes direct an opposite light response in tobacco cotyledons: Psad sequences downstream of the atg codon are required for a positive light response. Plant J. 1994;6:359–368. doi: 10.1046/j.1365-313X.1994.06030359.x. [DOI] [PubMed] [Google Scholar]
- 31.Chitnis V.P., Ke A., Chitnis P.R. The psad subunit of photosystem i (mutations in the basic domain reduce the level of psad in the membranes) Plant Physiol. 1997;115:1699–1705. doi: 10.1104/pp.115.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer N., Rochaix J.-D. The flanking regions of psad drive efficient gene expression in the nucleus of the green alga chlamydomonas reinhardtii. Mol. Genet. Genom. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M., Tsunoda T., Obokata J. Photosynthesis nuclear genes generally lack tata-boxes: A tobacco photosystem i gene responds to light through an initiator. Plant J. 2002;29:1–10. doi: 10.1046/j.0960-7412.2001.01188.x. [DOI] [PubMed] [Google Scholar]
- 34.Xi H., Yu Y., Fu Y., Foley J., Halees A., Weng Z. Analysis of overrepresented motifs in human core promoters reveals dual regulatory roles of yy1. Genome Res. 2007;17:798–806. doi: 10.1101/gr.5754707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler J.E., Kadonaga J.T. The rna polymerase ii core promoter: A key component in the regulation of gene expression. Genes Dev. 2002;16:2583–2592. doi: 10.1101/gad.1026202. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Zhang X., Wang Y., Hou H., Qian Y. Characterization of sequence elements from malvastrum yellow vein betasatellite regulating promoter activity and dna replication. Virol. J. 2012;9:234. doi: 10.1186/1743-422X-9-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganguli S., Das S.G., Chakraborty H.J., Gupta S., Datta A. Identification of regulatory sequence signatures in microrna precursors implicated in neurological disorders. Adv. Biosci. Biotechnol. 2013;4:26. doi: 10.4236/abb.2013.45A003. [DOI] [Google Scholar]
- 38.Li T., Gong C., Wang T. The rice light-regulated gene ra68 encodes a novel protein interacting with oxygen-evolving complex psbo mature protein. Plant Mol. Biol. Rep. 2010;28:136. doi: 10.1007/s11105-009-0128-x. [DOI] [Google Scholar]
- 39.Teakle G.R., Manfield I.W., Graham J.F., Gilmartin P.M. Arabidopsis thaliana gata factors: Organisation, expression and dna-binding characteristics. Plant Mol. Biol. 2002;50:43–56. doi: 10.1023/A:1016062325584. [DOI] [PubMed] [Google Scholar]
- 40.Hudson M.E., Quail P.H. Identification of promoter motifs involved in the network of phytochrome a-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol. 2003;133:1605–1616. doi: 10.1104/pp.103.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S., Lee Y., Lee J.H., Jin E. Expression of the high light-inducible dunaliella lip promoter in chlamydomonas reinhardtii. Planta. 2013;238:1147–1156. doi: 10.1007/s00425-013-1955-4. [DOI] [PubMed] [Google Scholar]
- 42.Ahrazem O., Rubio-Moraga A., López R.C., Gómez-Gómez L. The expression of a chromoplast-specific lycopene beta cyclase gene is involved in the high production of saffron’s apocarotenoid precursors. J. Exp. Bot. 2009;61:105–119. doi: 10.1093/jxb/erp283. [DOI] [PubMed] [Google Scholar]
- 43.Li M., Cui Y., Gan Z., Shi C., Shi X. Isolation and analysis of the cppsy gene and promoter from chlorella protothecoides cs-41. Mar. Drugs. 2015;13:6620–6635. doi: 10.3390/md13116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H.C., Kim M.L., Kang Y.H., Jeon J.M., Yoo J.H., Kim M.C., Park C.Y., Jeong J.C., Moon B.C., Lee J.H. Pathogen-and nacl-induced expression of the scam-4 promoter is mediated in part by a gt-1 box that interacts with a gt-1-like transcription factor. Plant Physiol. 2004;135:2150–2161. doi: 10.1104/pp.104.041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rushton P.J., Somssich I.E., Ringler P., Shen Q.J. Wrky transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Wang F., Yu G., Zhang X., Jia C., Qin J., Pan H. Functional analysis of the maize c-repeat/dre motif-binding transcription factor cbf3 promoter in response to abiotic stress. Int. J. Mol. Sci. 2015;16:12131–12146. doi: 10.3390/ijms160612131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu G.-L., Gou W., Han X.-L., Qin C., Zhang L.-X., Abomohra A.E.-F., Ashraf M. Cloning and functional analysis of phosphoethanolamine methyltransferase promoter from maize (Zea mays L.) Int. J. Mol. Sci. 2018;19:191. doi: 10.3390/ijms19010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berthold P., Schmitt R., Mages W. An engineered streptomyces hygroscopicus aph 7 gene mediates dominant resistance against hygromycin b in chlamydomonas reinhardtii. Protist. 2002;153:401–412. doi: 10.1078/14344610260450136. [DOI] [PubMed] [Google Scholar]
- 49.Jiao Y., Ma L., Strickland E., Deng X.W. Conservation and divergence of light-regulated genome expression patterns during seedling development in rice and arabidopsis. Plant Cell. 2005;17:3239–3256. doi: 10.1105/tpc.105.035840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kindle K.L. High-frequency nuclear transformation of chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shtaida N., Khozin-Goldberg I., Solovchenko A., Chekanov K., Didi-Cohen S., Leu S., Cohen Z., Boussiba S. Downregulation of a putative plastid pdc e1α subunit impairs photosynthetic activity and triacylglycerol accumulation in nitrogen-starved photoautotrophic chlamydomonas reinhardtii. J. Exp. Bot. 2014;65:6563–6576. doi: 10.1093/jxb/eru374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jefferson R.A. Assaying chimeric genes in plants: The gus gene fusion system. Plant Mol. Biol. Rep. 1987;5:387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.