Abstract
Background:
One of the most common problems in edentulous patients is the low stability of lower dentures. The most effective method to overcome this problem is implant-supported overdentures. After placing an implant, for the process of osseointegration to be complete and successful, it is better that patients do not use their denture for few months. This may be nonconvenient for patient because they are unable to speak and eat properly. The aim of this study was to evaluate the effect of low level laser (LLL) and light-emitting diode (LED) photobiomodulation on implant stability as well as their effect on interleukin-1 beta (IL-1β) and prostaglandin E2 (PGE2) biomarkers around implant in lower anterior region (over denture).
Materials and Methods:
In this clinical trial, 36 implants were placed in fully edentulous mandibles (12 people per person - three implants in areas of midline and canine). Each of the implants was randomly placed in one of three groups of laser, LED, and control. LLL (power of 50 mw and the amount of 20 J/cm2 for each implant) and LED with dose (20 mw/cm2) were irradiated on the day of surgery (zero), 3, 7, 10, and 14 days. The stability of implants was measured on the day of surgery and weeks 3, 4, and 8 after surgery with Periotest. The inflammatory biomarkers of IL-1β and PGE2 were also collected from gingival crevicular fluid around implants in 4 and 8 weeks. The collected data were analyzed by ANOVA statistical tests.pvalue<0.05 considered significant.
Results:
The amounts of Periotest significantly increased 3rd week after surgery in the control group (P < 0.001). However, the laser group and LED group were associated with minimal changes, which indicates lower stability of implant in 3rd week in control group but no changes in stability of test groups (laser and LED). Laser and LED had no effect on the level of IL-1β and PGE2 in 4 and 8 weeks.
Conclusion:
The use of LLL or LED has a positive effect on the stability of the implants 3 weeks after surgery.
Key Words: Dental implants, interleukin, laser, overdentures, prostaglandin E2
INTRODUCTION
After surgical phase of implant placement, it usually needs few months before prosthetic loading. This period is for the “Osseointegration process” to be completed. In this regard, it is recommended to patients not to wear any temporary prostheses, which may interfere with the process of osseointegration. This transitional period of edentulism causes nutritional and verbal problems, which are unpleasant and unacceptable for many patients. In this regard, immediate loading of dental implants has been recommended; however, the success of this method is subject to certain conditions. One of the main issues for immediate loading is some measures to improve bone to implant contact (BIC) in early healing periods.
The use of light-emitting diode photobiomodulation (LED PBM) light source and low-level laser (LLL) has been suggested with the aim to accelerate the osseointegration and BIC.[1,2,3,4,5]
The mechanism of action of LED and LLL is based on energy photon irradiation by light in the far red to near-infrared (NIR) spectrum (630–1000 nm) using low energy lasers or LEDs. In general, all cell metabolisms are controlled through the redox state of the cell. Cellular response to the energy of photons stimulated by light varies according to the redox quality. The response is low in normal or near the normal states, while the response is higher in cases of transfer to tendency toward the reduction. Normal cells show lower reaction to the energy of photons stimulated by light, which is of the advantages of these photons. In this method, the photons' energy is absorbed by the cells and stimulates them, which is called “PBM”. According to a review of the literature, LLL and LED application could have additional benefits on bone healing process following implant therapy.[6,7,8,9,10,11] When an implant is inserted into the bone, blood cells come into contact with the implant surface, and blood monocytes secrete cytokines, growth factor, and prostaglandins.[12] These mediators, especially interleukin-1 beta (IL-1β)[13,14,15,16,17,18] and prostaglandin E2 (PGE2),[19,20,21,22] can be detected in the peri-implant crevicular fluid, and therefore, this fluid might be useful for evaluating the phases of healing.
The aim of the present study was to evaluate the effect of LLL and LED PBM on implant stability (measured by Periotest), acceleration of osseointegration process, and their possible effects on BIC. The possible effects of LED and LLL on IL-1β and PGE2 biomarkers around implant were also evaluated in patients with implants placed in the lower anterior region (over denture).
MATERIALS AND METHODS
This randomized controlled clinical trial was conducted by “Split Mouth” method on patients referred to the Periodontics unit, School of Dentistry, Isfahan Islamic Azad University (Khorasgan Branch). The inclusion criteria were Type II or III bone quality based on Lekholm and Zarb classification, sufficient amount of bone (no need for augmentation), at least 6 months passed of any tooth extraction, good oral hygiene, and nonsmoker. The exclusion criteria were systemic diseases including diabetes, cardiovascular diseases, hormonal disorders, blood disorders, history of jaundice, kidney failure, asthma, allergies, cancer, weakened immune system, pregnancy and lactation, history of using bisphosphonates, history of radiotherapy of head and neck in past 2 years, history of chemotherapy, and administration of immunosuppressive agents.
Three DIO implants (invasive fungal infections-tissue level) with resorbable blast media surface, made in Korea, with the same diameter and length were placed in the mandible (one in the midline and the other two at the left and right canine teeth positions). Using coin drawing, one implant was considered as control and other two were exposed to LED or laser radiation, respectively.
The LLL (diode laser doctor smile 810 nm, Italy) was radiated in the relevant group on the days of surgery (zero), 3, 7, 10, and 14 with the power of 50 mw and the amount of 20 J/cm2 for each implant by gently pressing on the buccal mucosa of the implant inside the mouth. Irradiation index information are as follows: 50 mW = 0.05W W = J/cm2 × s 0.05 = 20/S S = 400 according to this formula for each of 20 J/cm2 with 50 mW, we apply 400 s. Laser irradiation applied from buccal aspect. Time: 400 s, power density 50 mW, laser diameter: 1 cm2 (fiber 400 and apply low level head with 1 cm2 surface according). Beam shape around laser type was a continuous wave.
The LED PBM radiation (OsseopulseTM AR 300, Biolux Research, Vancouver, British Columbia, Canada) was carried out at a dose of 20 mw/cm2 on the days of surgery (zero), 3, 7, 10, and 14, each time for 20 min, on the skin of the other implants outside of the mouth (During LED radiation, the other two implants were protected by aluminum foil covering them from inside the mouth). The LED devise type and information are as follows: the LED device (Osseopuise TMAR 300, Biolux Research Vancouver, British Columbia, Canada) at the wavelength of 626 nm in the NIR region was applied for 20 min over the surgical area after suturing the flap. The treatment parameters were as follows: treatment array area: 4.80 cm2, average intensity: 38.5 mW/cm2, total power: 185 mW, total energy: 222 J, and average density: 46.2 J/cm2.
The led device had eight emitters with a power of 23.125 mW each. The LED device consisted of a headset that was adjusted on the patient face. A 3-point contact on the ears and nose was provided for device stability.
The third implant was considered as control. The stability of the implants at the day of surgery, 3, 4, and 8 weeks after surgery was evaluated with Periotest Device (Gulden-Medizinteknik, Bensheim an der Bergstrasse, Germany). For any implant, three Periotest measurements were performed at each time, and the mean was recorded.
The gingival crevicular fluid samples were also collected at weeks 4 and 8 by placing a paper point inside the peri-implant sulcus for 30 s. Then, the samples were transferred into the tubes containing storage solution (neutral tris-buffered saline) and sent to the laboratory inside ice bag on the same day. The levels of IL-1β and PGE2 markers were measured by ELISA method and Thermofisher Kit.
Data analysis was done using the SPSS version 22 software (SPSS Inc. Chicago, IL, USA). The ANOVA test was used for quantitative variables within a group (implant stability and levels of inflammatory markers), while the repeated measures were applied for intergroup quantitative variables. P < 0.05 considered significant
RESULTS
Table 1 and Figure 1 show that the mean of Periotest values significantly increased in the control group in the 3rd week compared to the surgery time, i.e., lowest implant stability (P < 0.001), while they were associated with minimal changes in the laser and LED PBM groups.
Table 1.
The mean periotest values at the time of surgery and weeks 3, 4, and 8
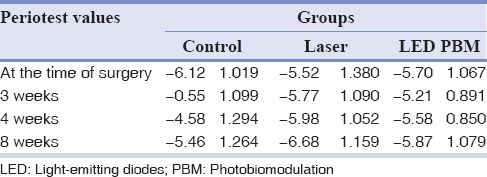
Figure 1.
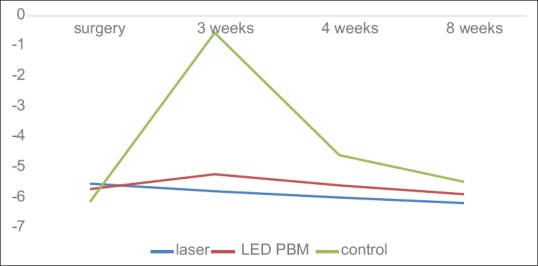
The mean of implant stability rate at four times (periotest).
Furthermore, according to Figure 1 and Table 1, the stability of the implantis on the rise from week 3 onward in all three groups, and this stability was always greater in the laser group than the other two groups. Comparing LED PBM group with control group, higher stability was reported on LED PBM group.
The IL1β and PGE2 values were compared in the 1st and 2nd month after surgery separately for laser, LED PBM, and control groups. First, Kolmogorov–Smirnov test was performed to examine the normality of data distribution in the 1st month, based on which, their normality was not rejected. Then, using the ANOVA method for repeated data, the three methods were compared, according to which, as shown in Table 2, no significant differences were found between the methods (P = 0.0337). With such a result, there is no need to make pair-wise comparisons, and the conclusion is perfect. However, those comparisons were made, and thus, the accuracy of the results was confirmed as well. According to Table 2, the IL1β values result in the 2nd month were also similar in all three groups.
Table 2.
Interleukin-1 beta and prostaglandin E2 values in the 1st and 2nd months
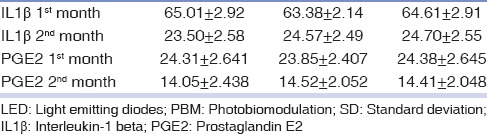
Comparing calculations of IL-1β and PGE2 in the 1st month with the 2nd month, using ANOVA repeated measures with two methods and time variables, the data were analyzed simultaneously, which showed no significant difference between methods in the total of the two times (P = 0.765), but there was a significant difference between the two time periods (P < 0.001). In other words, the amount of IL1β in the 2nd month decreased in all groups compared to the 1st month, which represents a reduction of inflammatory process over the time. Meanwhile, there was no interaction between the methods and materials (P = 0124). This suggests that the changes in these three methods were the same at both times, occurring in the normal range (10–45 ng/ml).
DISCUSSION
The results of this study showed that application of LLL and LED PBM had positive effects on stability of implants placed on mandibular anterior region in subjects with fully edentulous lower jaw. Based on (S. Roghondro, M. Wood, T. D. Taylor) curve, there is a significant reduction of implant stability between 3rd and 4th week after implant placement. This usual reduction of stability during 3rd and 4th week after implant placement was not observed in implants treated with laser and LED PBM.
The possible effect of laser is mainly on cellular level. It is known that light wavelength stimulates cellular energy metabolism by the activation of mitochondrial respiratory chain components. The light absorbed by cytochrome c oxidase, a photoacceptor molecule in the mitochondria, alters the cellular mechanism and causes an increased adenosine triphosphate production that results in improved wound healing in ischemic and wounded tissues.[23]
The results of this study on the stability of the implant are consistent with the studies by Lopes et al.,[24] Mayer et al.,[25] and Maluf et al.[26] However, Lopez and Mayer conducted an animal study using the Osstell device. Maluf also did study on animal using the reverse torque method.
The results of this study are inconsistent with the clinical trial of García-Morales et al.[27] García-Morales et al. showed that laser has no positive effect on the stability of the implant in the 3rd week compared with the control group. Perhaps, the reasons for the differences in results are due to the settings of the laser dose. In García-Morales et al.'s[27] study, the 830 nm laser with a power of 86 mw was radiated pointwise, 0.25 J at each spot for 3 s at 20 points around the implant area. The protocol is suggested for soft tissue wound healing, while in the process of stabilization, bone repair and osseointegration are considered. In this study, the 810 nm diode laser with a power of 50 mw and dosage of 20 J/cm was used. The reason for choosing this dose was based on the results of two separate animal histological studies conducted by Mayer et al.[6] as well as Gomes et al.[7] In addition, in Garcia study, the laser radiation was pointwise and in contact with the soft tissue, while in this study, contact was at a surface (an area of 1 cm2) by putting gentle pressure on the soft tissue. The reason for gentle pressure at the time of laser irradiation is due to the fact that the diode laser absorption in hemoglobin is high, and the hemoglobin in the blood vessels of soft tissue prevents the laser reaching to the bone, using gentle pressure, through temporary closing of vessels, will remove or reduce the hemoglobin in the blood vessels of soft tissues.
Considering the impact of LED on the implant stability, the results of this study are consistent with Gokmenoglu et al.'s studies.[1]
Regarding the inflammatory factors, there was a significant reduction of both PGE2 and IL1B after 2 months. This is natural phenomenon due to the reduction of inflammatory processes while healing is completed. However, LLL and LED had no impact on the level of these inflammatory markers at different time interval compare to control group. The results of this study are consistent with the studies by de Jesus et al.[28] and Gokmenoglu et al.,[1] which indicates that the laser radiation and LED have no effect on the inflammatory factors. Based on the studies by Yaghobee et al.[29] and Aboyoussef et al.,[30] the effect of laser on these factors may be considered effective in real infectious situations such as peri-implantitis and not natural healing phenomenon of inflammation.
CONCLUSION
LLL had a positive effect on the stability of implants at 3–4-week time after implant placement.
Financial support and sponsorship
This project was sponsored by research committee of Islamic azad university Isfahan (khorasgan) Branch.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Gokmenoglu C, Ozmeric N, Erguder I, Elgun S. The effect of light-emitting diode photobiomodulation on implant stability and biochemical markers in peri-implant crevicular fluid. Photomed Laser Surg. 2014;32:138–45. doi: 10.1089/pho.2012.3473. [DOI] [PubMed] [Google Scholar]
- 2.Freddo AL, Hauser EB, de Castro VV, Noritomi PY, de Almeida AS, de Oliveira MG, et al. Finite element analysis of masticatory stress on neoformed bone tissue after distraction osteogenesis and low-level laser therapy: A pilot study. Photomed Laser Surg. 2014;32:429–36. doi: 10.1089/pho.2013.3671. [DOI] [PubMed] [Google Scholar]
- 3.Massotti FP, Gomes FV, Mayer L, de Oliveira MG, Baraldi CE, Ponzoni D, et al. Histomorphometric assessment of the influence of low-level laser therapy on peri-implant tissue healing in the rabbit mandible. Photomed Laser Surg. 2015;33:123–8. doi: 10.1089/pho.2014.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares LG, Magalhães EB, Magalhães CA, Ferreira CF, Marques AM, Pinheiro AL, et al. New bone formation around implants inserted on autologous and xenografts irradiated or not with IR laser light: A histomorphometric study in rabbits. Braz Dent J. 2013;24:218–23. doi: 10.1590/0103-6440201302186. [DOI] [PubMed] [Google Scholar]
- 5.Pereira CL, Sallum EA, Nociti FH, Jr, Moreira RW. The effect of low-intensity laser therapy on bone healing around titanium implants: A histometric study in rabbits. Int J Oral Maxillofac Implants. 2009;24:47–51. [PubMed] [Google Scholar]
- 6.Mayer L, Gomes FV, Carlsson L, Gerhardt-Oliveira M. Histologic and resonance frequency analysis of peri-implant bone healing after low-level laser therapy: An in vivo study. Int J Oral Maxillofac Implants. 2015;30:1028–35. doi: 10.11607/jomi.3382. [DOI] [PubMed] [Google Scholar]
- 7.Gomes FV, Mayer L, Massotti FP, Baraldi CE, Ponzoni D, Webber JB, et al. Low-level laser therapy improves peri-implant bone formation: Resonance frequency, electron microscopy, and stereology findings in a rabbit model. Int J Oral Maxillofac Surg. 2015;44:245–51. doi: 10.1016/j.ijom.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Torkzaban P, Kasraei S, Torabi S, Farhadian M. Low-level laser therapy with 940 nm diode laser on stability of dental implants: A randomized controlled clinical trial. Lasers Med Sci. 2017 Oct 29; doi: 10.1007/s10103-017-2365-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.de Vasconcellos LM, Barbara MA, Rovai ES, de Oliveira França M, Ebrahim ZF, de Vasconcellos LG, et al. Titanium scaffold osteogenesis in healthy and osteoporotic rats is improved by the use of low-level laser therapy (GaAlAs) Lasers Med Sci. 2016;31:899–905. doi: 10.1007/s10103-016-1930-y. [DOI] [PubMed] [Google Scholar]
- 10.Kim JR, Kim SH, Kim IR, Park BS, Kim YD. Low-level laser therapy affects osseointegration in titanium implants: Resonance frequency, removal torque, and histomorphometric analysis in rabbits. J Korean Assoc Oral Maxillofac Surg. 2016;42:2–8. doi: 10.5125/jkaoms.2016.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68:217–69. doi: 10.1111/prd.12080. [DOI] [PubMed] [Google Scholar]
- 12.Soskolne WA, Cohen S, Sennerby L, Wennerberg A, Shapira L. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin Oral Implants Res. 2002;13:86–93. doi: 10.1034/j.1600-0501.2002.130111.x. [DOI] [PubMed] [Google Scholar]
- 13.Khoury SB, Thomas L, Walters JD, Sheridan JF, Leblebicioglu B. Early wound healing following one-stage dental implant placement with and without antibiotic prophylaxis: A pilot study. J Periodontol. 2008;79:1904–12. doi: 10.1902/jop.2008.070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talo Yildirim T, Acun Kaya F, Yokus B, Colak M, Ozdemir Kaya E, Tekin G, et al. Associations of alveolar bone loss and interleukin-1β levels in one- and two-stage surgical procedures: A randomized prospective trial. Acta Odontol Scand. 2017;75:608–15. doi: 10.1080/00016357.2017.1367959. [DOI] [PubMed] [Google Scholar]
- 15.Al Amri MD, Alfadda SA, Labban NY, Alasqah MN, Alshehri FA, Al-Rasheed AS, et al. Comparison of clinical, radiographic, and immunologic inflammatory parameters around crestally and subcrestally placed dental implants: 5-year retrospective results. J Prosthodon. 2017 Sep 28; doi: 10.1111/jopr.12637. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Gürlek Ö, Gümüş P, Nile CJ, Lappin DF, Buduneli N. Biomarkers and bacteria around implants and natural teeth in the same individuals. J Periodontol. 2017;88:752–61. doi: 10.1902/jop.2017.160751. [DOI] [PubMed] [Google Scholar]
- 17.Bielemann AM, Marcello-Machado RM, Leite FR, Martinho FC, Chagas-Júnior OL, Antoninha Del Bel Cury A, et al. Comparison between inflammation-related markers in peri-implant crevicular fluid and clinical parameters during osseointegration in edentulous jaws. Clin Oral Investig. 2017 Jul 14; doi: 10.1007/s00784-017-2169-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Pettersson M, Kelk P, Belibasakis GN, Bylund D, Molin Thorén M, Johansson A, et al. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J Periodontal Res. 2017;52:21–32. doi: 10.1111/jre.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkan EA, Tüter G, Parlar A, Yücel A, Kurtiş B. Evaluation of peri-implant crevicular fluid prostaglandin E2 levels in augmented extraction sockets by different biomaterials. Acta Odontol Scand. 2016;74:532–8. doi: 10.1080/00016357.2016.1214979. [DOI] [PubMed] [Google Scholar]
- 20.Manfredi E, Lumetti S, Rivara F, Toffoli A, Calciolari E, Cacchioli A, et al. Role of prostaglandin E2 in the modulation of wnt canonical signaling in cells on microstructured titanium surfaces. J Appl Biomater Funct Mater. 2016;14:e181–8. doi: 10.5301/jabfm.5000267. [DOI] [PubMed] [Google Scholar]
- 21.Basegmez C, Yalcin S, Yalcin F, Ersanli S, Mijiritsky E. Evaluation of periimplant crevicular fluid prostaglandin E2 and matrix metalloproteinase-8 levels from health to periimplant disease status: A prospective study. Implant Dent. 2012;21:306–10. doi: 10.1097/ID.0b013e3182588408. [DOI] [PubMed] [Google Scholar]
- 22.Venza M, Visalli M, Lo Giudice G, Cicciù M, Passi P, Teti D, et al. Changes in inflammatory mediators in peri-implant fluid after implant insertion. J Periodontol. 2009;80:297–306. doi: 10.1902/jop.2009.080411. [DOI] [PubMed] [Google Scholar]
- 23.Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–67. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Lopes CB, Pinheiro AL, Sathaiah S, Da Silva NS, Salgado MA. Infrared laser photobiomodulation (lambda 830 nm) on bone tissue around dental implants: A Raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed Laser Surg. 2007;25:96–101. doi: 10.1089/pho.2006.2030. [DOI] [PubMed] [Google Scholar]
- 25.Mayer L, Gomes FV, de Oliveira MG, de Moraes JF, Carlsson L. Peri-implant osseointegration after low-level laser therapy: Micro-computed tomography and resonance frequency analysis in an animal model. Lasers Med Sci. 2016;31:1789–95. doi: 10.1007/s10103-016-2051-3. [DOI] [PubMed] [Google Scholar]
- 26.Maluf AP, Maluf RP, Brito Cda R, França FM, de Brito RB., Jr Mechanical evaluation of the influence of low-level laser therapy in secondary stability of implants in mice shinbones. Lasers Med Sci. 2010;25:693–8. doi: 10.1007/s10103-010-0778-9. [DOI] [PubMed] [Google Scholar]
- 27.García-Morales JM, Tortamano-Neto P, Todescan FF, de Andrade JC, Jr, Marotti J, Zezell DM. Stability of dental implants after irradiation with an 830-nm low-level laser: A double-blind randomized clinical study. Lasers Med Sci. 2012;27:703–11. doi: 10.1007/s10103-011-0948-4. [DOI] [PubMed] [Google Scholar]
- 28.de Jesus JF, Spadacci-Morena DD, dos Anjos Rabelo ND, Pinfildi CE, Fukuda TY, Plapler H, et al. Low-level laser therapy in IL-1β, COX-2, and PGE2 modulation in partially injured achilles tendon. Lasers Med Sci. 2015;30:153–8. doi: 10.1007/s10103-014-1636-y. [DOI] [PubMed] [Google Scholar]
- 29.Yaghobee S, Khorsand A, Rasouli Ghohroudi AA, Sanjari K, Kadkhodazadeh M. Assessment of interleukin-1beta and interleukin-6 in the crevicular fluid around healthy implants, implants with peri-implantitis, and healthy teeth: A cross-sectional study. J Korean Assoc Oral Maxillofac Surg. 2014;40:220–4. doi: 10.5125/jkaoms.2014.40.5.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aboyoussef H, Carter C, Jandinski JJ, Panagakos FS. Detection of prostaglandin E2 and matrix metalloproteinases in implant crevicular fluid. Int J Oral Maxillofac Implants. 1998;13:689–96. [PubMed] [Google Scholar]


