Abstract
Background:
Sodium hypochlorite (5.25% NaOCl) and silver nanoparticles (70 ug/ml AgNPs) have a broad spectrum of antimicrobial efficacy for disinfecting gutta percha (GP) point, so this study was conducted to analyze the assay surface topography of GP when disinfected with AgNPs and 5.25% of NaOCl using atomic force microscopy (AFM).
Materials and Methods:
In this in vitro study a total of thirty cones were taken. The samples were divided into three treatment groups: Group I and II with 70 μg/ml AgNPs and 5.25% NaOCl. The time duration was 1 min. Untreated GP points served as control group. After treatment of 1 min for each solution, the samples were positioned in the AFM. For comparison, the root mean square (RMS) was used to investigate the structure of the GP points. Unpaired t-test and ANOVA test were used. The differences among the groups were tested by Tukey's honestly significant difference test and were considered significant when P < 0.05.
Results:
5.25% NaOCl created RMS value of 202.48 nm at 1 min as compared to 70 μg/ml of AgNPs and control which produced RMS value of 44.48 nm and 24.1 nm, respectively (<0.0001).
Conclusion:
The study showed irregularity in the surface of GP with NaOCl and lesser deterioration with AgNPs which could affect the postoperative prognosis. In this study, it was found that NaOCl causes 10 times more surface topography deterioration of GP when compared to AgNPs at 700 times lesser concentration.
Key Words: Atomic force microscopy, gutta-percha, nanoparticles, sodium hypochlorite, surface topography
INTRODUCTION
Microbial colonies are found in huge numbers in root canals and are present inside the canal variably, so it is an absolute requisition not only to remove microbial contamination, but also to make sure that the damage to the dentinal portion of root remains minimal.[1,2,3]
Although there are a plethora of disinfecting agents in the market, still the most common disinfecting agent used endodontically is NaOCl and that too with the concentration of 5.25%; NaOCl has antimicrobial effect because of the emission of hydroxyl ion and chlorite ion, but because of its higher pH, it also causes surface and deep alteration of various materials.[3,4,5,6,7]
Nanoparticles are being used in various methods either embedded in a material and used or being used in metallic complex compound. Silver nanoparticle is one of the materials which has come out to be quite an effective antimicrobial agent and also have a broad-spectrum effect. In the present study, the size of the silver nanoparticle was 9.3 nm 23.1 nm. Shape of AgNPs used in the present study was truncated triangular which was found to provide better bactericidal action which corroborates with earlier studies.[8,9] Truncated triangular silver nanoplates with a (111) lattice plane as the basal plane displayed the strongest biocidal action, compared with spherical and rod-shaped nanoparticles with Ag (in the form of AgNO3). It is proposed that nanoscale size and the presence of a (111) plane combine to promote this biocidal property.
This competency of silver nanoparticles has allowed researcher to look for its diversity in various other aspects or fields. Studies have shown that silver and nanoparticles in combination is excellent in removing microbes from root canals.[9]
Today, the atomic force microscopy (AFM) is the most commonly used scanning probe technique for material characterization. Major advantages of AFM include a combination of high resolution in three dimensions (3D), the sample does not have to be conductive, and there is no requirement for operation within a vacuum. There is an AFM mode called contact mode imaging (CMI) which is employed for the determination of surface topography. AFM hardware consists of a sharp tip mounted at the end of a microscopic cantilever, which systematically probes a surface.[10,11,12,13]
Gutta percha (GP) is used in dentistry since many decades and its sterilization is very important for the better prognosis of the treatment. GP sterilization is done with NaOCl, but it is quite evident in literatures that NaOCl also disrupts the polymeric chains of GP quite drastically and irreversibly which not only increases the risk of microleakage, but also can create the niche for microbial re-growth.[13]
None of the studies are available which show the topographical effect of silver nanoparticles on GP and its comparison with 5.25% NaOCl. Owing to the nodus which NaOCl causes has urged the scientists to look forward to new antimicrobial agents having dominance but also have minimal side effects on the material or tissue itself.
The desideratum of this study is to analyze and assay the effect of 5.25% of NaOCl and 70 ug/ml (0.007%) silver nanoparticles on the surface topography of GP using AFM.
MATERIALS AND METHODS
Materials
GP cones – 30 cones were taken of size 80 (Dentsply)
Concentration of silver nanoparticles – 70 ug/ml (Banaras Hindu University, Varanasi, Uttar Pradesh, India)
5.25% NaOCl (HiMedia)
AFM (MANIT, Bhopal, Madhya Pradesh, India).
Methods
In this in vitro study a total of thirty cones were taken. The samples were divided into three treatment groups
Synthesis of silver nanoparticles
Silver nanoparticles were synthesized by inert gas condensation method by metal evaporation in inert atmosphere. Silver wires (99.9% purity) were placed in the molybdenum boat. Argon gas (99.99% purity) was passed inside the chamber through a moisture collector at a pressure of 13.33 Pa. Electric current to the boat was supplied which makes silver to evaporate. There was a disc shutter over the boat, which was opened when supersaturation conditions were achieved. The particles formed in the gas phase were allowed to lodge at the surface of a stainless steel flat surface cooled by flowing liquid nitrogen.
Synthesized nanoparticles were collected by sweeping them off the stainless steel plate with a Teflon scrapper. The collected silver nanoparticles were characterized by X-Ray diffraction and transmission electron microscopy (TEM) for approximation of crystalline structure, mean size, and architecture. For TEM dissection, dilute suspensions of nanoparticles in pure acetone were processed by ultrasonication.[14]
Samples
Thirty GP cones taken from the same batch were randomly selected for the study. It was ascertained that all samples used were before expiration date. GP points were sectioned 3 mm from their tip and attached to a glass base with rapid-setting cyanoacrylate glue. Following these procedures, the samples were divided into two treatment groups: Group I – GP point immersed in 70 ug/ml (0.007%) silver nanoparticles and Group II – GP point immersed in 5.25% NaOCl. Untreated GP points served as control group. After treatment time of 1 min for each solution, the samples were positioned in the AFM. Fresh aliquots (5 mL) of 5.25% NaOCl or silver nanoparticles were used for each period of immersion. After the immersion, the samples were thoroughly rinsed with 5 mL of nanopure water, and the region around the point was then dried with filter paper.[15]
Analyses with atomic force microscopy
AFM images of the GP points were recorded in the contact mode on a NT-MDT, NEXT AFM (NT-MDT, Zelenograd, Moscow, Russia) under ambient conditions. Typical AFM probes for contact mode CSG10 series having resonant frequency of 8–39 kHz and force constant of 0.01–0.5 N/m (curvature radius <20 nm) mounted on cantilevers (225 um) were used. Scanned areas (16 um/s) were perfect squares (4 um × 4 um) in which a weak force was applied (<1 nN). CMI was simultaneously obtained during scanning procedures to investigate topography of specimens. AFM images (500 × 500 lines) were acquired with NOVA PX 3.1.0 rev 1312 (Zelenograd, Moscow, Russia) software for analyzing and processing of images of surfaces obtained by AFM s. Nova PX software contains predefined settings for fast configuration of the NEXT operation. Panoramic optical view allows collection of high-resolution, large-scale images of the sample.
For the purpose of comparison, the root mean square (RMS) was used to investigate the structure of the GP points. All statistical analyses were performed with StatView for Windows 7.0 software (SAS Institute, Cary, NC, USA). Mean and standard error of the mean values of the RMS parameters achieved from CMI were calculated. The differences among the groups were tested by Tukey's honestly significant difference test and were considered significant when P < 0.05. SPSS statistics is a software package used for statistical analysis. SPSS stands for statistical package for the social sciences. SPSS is acquired by IBM which is a Chicago, Illinois (US) based company in the year 2009. The SPSS version 19 (2010) was used for the data calculation in this study.
RESULTS
AFM images showed that untreated GP showed minimum topographical alterations at 24.1 ± 24 nm as compared to the treated GP cones.
3D images were obtained from AFM (composite images). Mean values of RMS for CMI profiles are shown in Tables 1–3. The values are expressed in nm (1:1000 um). 5.25% NaOCl produced higher RMS at 1 min than to both 70 ug/ml of silver nanoparticles and control group. NaOCl created RMS value of 202.48 nm at 1 min as compared to 70 ug/ml of AgNPs which produced RMS value of 44.48 nm as shown in Figures 1 and 2. Where as the control group has got the RMS value of 18.83 nm. as shown in Figure 3. Composite figure presenting all the RMS value in Figure 4. Comparative evaluation was done between Group I and Group II, Group I and control group, and Group II and control group, all showed highly significant difference (P < 0.0001) [Tables 1–3]. ANOVA test of variance was used to compare between all the groups and again highly significant result was observed with P < 0.0001 [Table 4].
Table 1.
Mean value of RMS between Group 1 and Group 2

Table 3.
Mean Value of RMS between Control group and Group 2

Figure 1.
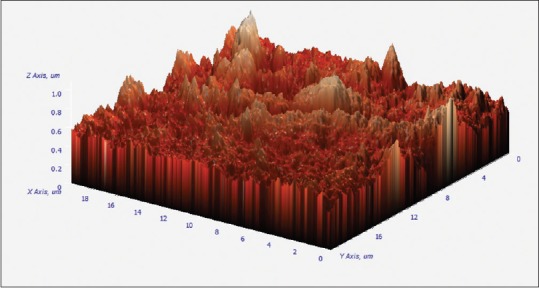
Atomic force microscopy image showing topographical alteration of 5.25% NaOCl for one minute.
Figure 2.
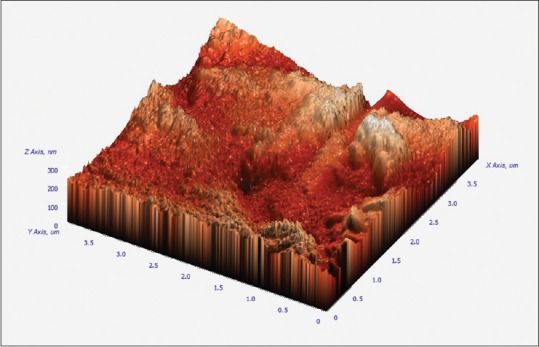
Atomic force microscopy image showing topographical alteration of 70ug/ml AgNPs for one minute.
Figure 3.
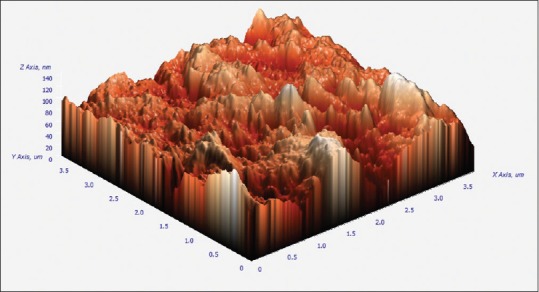
Atomic force microscopy image showing topographical alteration of control group for one minute.
Figure 4.
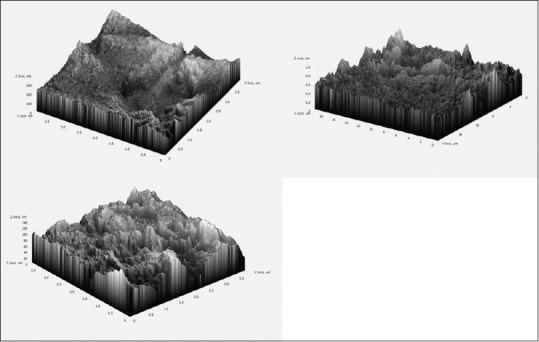
Composite image of topographical alterations on gutta percha observed in Atomic force microscope.
Table 4.
Mean value of RMS between all groups combined
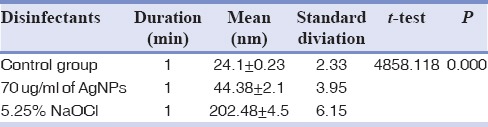
Table 2.
Mean value of RMS between Control group and Group 1

The differences between RMS values were tested by unpaired t-test and ANOVA test. There was significant difference between all groups and the statistical analyses were performed.
DISCUSSION
Due to a strong oxidizing effect 5.25% NaOCl causes extreme topographic alterations in the cones which in turn cause deterioration and also there is some crystal formation on GP. Moreover, crystal formation on the surface of GP cones has been identified after rapid sterilization with 2.5% and 5.25% NaOCl.[13]
Disintegration of GP points includes certain extent of surface discrepancy. It causes extensive irregularities which can create cracks or breach in uniformity between the GP cone and the root canal wall, hence increasing the plausibility of leakage.[11] Resin sealers when coated in canals fill the crevasse that was created after disinfecting the GP with 5.25% NaOCl. Once set, the resin shrinks leaving behind empty spaces which may increase the risk of leakage.[12]
Topographical alterations may be observed on GP points when these are kept immersed into disinfection may compromise the standardization of the points, making difficult their adaptation to root canal walls because of the disintegration of polymer chains.[15,16] NaOCl solution influence apical sealing failure because it promotes irreversible changes on GP points, and therefore the disinfection period should not be extended beyond the minimum necessary.[17] Valois et al. conducted a study in which topographical alteration of GP was seen after irrigation with three different concentrations of NaOCl (0.5%, 2.5%, and 5.25%) and arrived at a conclusion that 2.5% and 5.25% NaOCl solutions caused aggressive topographical changes after 1 min when compared to the control (P < 0.05). Conversely, 0.5% NaOCl solution did not cause any alteration on topography or elasticity of GP cone structure but this concentration is not valid for chairside decontamination of GP.[17] The present study is concurrent to the previous studies as this study also shows that 5.25% NaOCl causes maximum alteration in GP in 1 min using AFM, hence can have ominous effect in postoperative prognosis.
AFM is a device with 3D imaging of molecular surfaces of nanometer resolution. and as compared to the SEM, AFM provides a much better job. It provides reliable 3D surface profile and does not even require any special treatment (metal coating), which could lead to irreversible damage.[18] In addition, in contrast to the SEM which requires expensive vacuum environment necessary for normal functioning, an AFM has the ability to operate effectively even in a liquid or air.[18]
Recent advances in nanotechnology have invigorate us to produce pure silver, as nanoparticles, which are more efficient than silver ions.[19] AgNPs are attractive because they are nontoxic to the human body at low concentrations and have a broad spectrum of antibacterial actions.[14] Several studies have reported that the positive charge on the Ag+ ion is crucial for its antimicrobial activity through the electrostatic attraction between the negatively charged cell membrane of the microorganism and the positively charged nanoparticles.[8] AgNPs accumulated in the bacterial membrane disturb the membrane permeability, resulting in cell death.[20]
With regard to cytotoxicity of AgNPs, the biologic effects of AgNPs' exposure to mammalian cells were evaluated. Hela cells were evaluated by being exposed to different concentrations of AgNPs which showed that 80 μg/ml concentration could be harmful for Hela cells.[21,22] Accordingly, Iranian researchers[23] have introduced nanosilver-coated GP, as an attempt to improve the antibacterial effect of GP. The new material, which is standard GP coated with AgNPs, has demonstrated significant effect against Enterococcus faecalis, Staphylococcus aureus, Candida albicans, and Escherichia coli. Considering that this antimicrobial efficacy of silver nanoparticles has already been proven, its effect on the surface topography of GP was never observed. Besides that, Shantiaee et al.[24] tested the biocompatibility by comparing the cytotoxicity of nanosilver-coated GP and normal GP on mouse fibroblasts and found that nanosilver-coated GP presented cytotoxicity similar to normal GP and after 1 week, it reached the lowest level of cytotoxicity among the tested materials.
This study surface architecture of GP was analyzed, assayed, and correlated with 5.25% NaOCl and it was commenced that silver nanoparticles caused far lesser deterioration than NaOCl when observed under AFM (as shown in composite image).
CONCLUSION
It was ascertained in this study that irregularity in the surface of GP is observed when decontamination is done with NaOCl; on the other hand, AgNPs cause far lesser surface deterioration which ultimately aids in postoperative prognosis. From this study, it was found that NaOCl causes five times more surface topography damage of GP when compared to AgNPs and ten times more deterioration when compared to untreated GP. As far as efficacy of AgNPs is concerned, it has been proven that it is equivalent to that of NaOCl in eliminating microorganism because of AgNPs' high spectrum ability. Further study needs to be conducted to investigate surface energy and wetting ability of AgNPs to analyze its compatibility with sealers when used as an irrigating solution.
Financial support and sponsorship
This study was done for research purpose only with no financial interest or to denigrate other material and also to bring newer material i.e., silver nanoparticles in endodontic usage.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Siqueira JF, Jr, Da Silva CH, Cerqueira M das D, Lopes HP, de Uzeda M. Effectiveness of four chemical solutions in eliminating Bacillus subtilis spores on gutta-percha cones. Endod Dent Traumatol. 1998;14:124–6. doi: 10.1111/j.1600-9657.1998.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang G, Niu A, Peng S, Jiang M, Tu Y, Li M, et al. Formation of novel polymeric nanoparticles. Acc Chem Res. 2001;34:249–56. doi: 10.1021/ar000011x. [DOI] [PubMed] [Google Scholar]
- 4.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J Nanobiotechnology. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 6.Neal AL. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology. 2008;17:362–71. doi: 10.1007/s10646-008-0217-x. [DOI] [PubMed] [Google Scholar]
- 7.Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008;34:1515–20. doi: 10.1016/j.joen.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–20. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jena BP, Horber JK. Atomic Force Microscopy in Cell Biology: Cambridge massachusetts Academic Press. 2002.
- 11.Goldberg F, Massone EJ, Pruskin E, Zmener O. SEM study of surface architecture of gutta-percha cones. Endod Dent Traumatol. 1991;7:15–8. doi: 10.1111/j.1600-9657.1991.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 12.Davidson CL, Van Zeghbroeck L, Feilzer AJ. Destructive stresses in adhesive luting cements. J Dent Res. 1991;70:880–2. doi: 10.1177/00220345910700050301. [DOI] [PubMed] [Google Scholar]
- 13.Pang NS, Jung IY, Bae KS, Baek SH, Lee WC, Kum KY, et al. Effects of short-term chemical disinfection of gutta-percha cones: Identification of affected microbes and alterations in surface texture and physical properties. J Endod. 2007;33:594–8. doi: 10.1016/j.joen.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5:244–9. doi: 10.1166/jnn.2005.034. [DOI] [PubMed] [Google Scholar]
- 15.Valois CR, Silva LP, Azevedo RB, Costa ED., Jr Atomic force microscopy study of gutta-percha cone topography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:250–5. doi: 10.1016/j.tripleo.2004.02.076. [DOI] [PubMed] [Google Scholar]
- 16.Berutti E, Angelini E, Rigolone M, Migliaretti G, Pasqualini D. Influence of sodium hypochlorite on fracture properties and corrosion of protaper rotary instruments. Int Endod J. 2006;39:693–9. doi: 10.1111/j.1365-2591.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- 17.Valois CR, Silva LP, Azevedo RB. Structural effects of sodium hypochlorite solutions on gutta-percha cones: Atomic force microscopy study. J Endod. 2005;31:749–51. doi: 10.1097/01.don.0000158012.01520.e5. [DOI] [PubMed] [Google Scholar]
- 18.Russell P, Batchelor D, Thornton J. SEM and AFM: Complementary Techniques for High Resolution Surface Investigations. DI Digital Instruments. DI Digital Instruments. 2001:1, 10. [Google Scholar]
- 19.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danilczuk M, Lund A, Sadlo J, Yamada H, Michalik J. Conduction electron spin resonance of small silver particles. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:189–91. doi: 10.1016/j.saa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Miura N, Shinohara Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem Biophys Res Commun. 2009;390:733–7. doi: 10.1016/j.bbrc.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Lotfi M, Vosoughhosseini S, Ranjkesh B, Khani S, Saghiri M, Zand V. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr J Biotechnol. 2011;10:6799–803. [Google Scholar]
- 23.Dianat O, Ataie M. Gutta-Percha Coated with Nanosilver Particles. Invention Registered Number: 56019. 2008 [Google Scholar]
- 24.Shantiaee Y, Dianat O, Mohammad Khani H, Akbarzadeh Baghban A. Cytotoxicity comparison of nanosilver coated gutta-percha with Guttaflow and normal gutta-percha on L929 fibroblast with MTT assay. Beheshti Univ Dent J. 2011;29:62–8. [Google Scholar]


