Abstract
Introduction:
Retinal ganglion cell (RGC) degeneration was histopathologically proved previously in Alzheimer's disease (AD) patients. In this study, we aimed to determine RGC degeneration in vivo using optical coherence tomography (OCT) in AD.
Methods:
Twenty-one mild-to-moderate AD patients and 25 cognitively healthy age-matched controls were enrolled in this case–control prospective study. All participants underwent OCT examination to assess peripapillary retinal nerve fiber layer (RNFL) thickness, macular volume, and thickness.
Results:
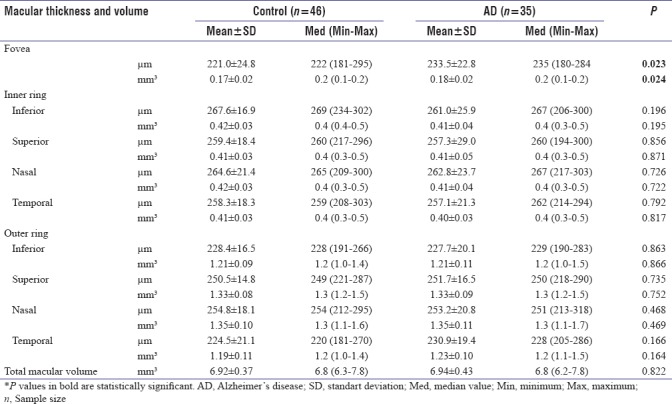
Foveal thickness and volume were significantly higher in AD patients than controls (P = 0.023 and P = 0.024, respectively). Compared to controls, peripapillary RNFL and other macular region measurements of AD patients were not statistically different (for all P > 0.05).
Discussion:
Increased foveal thickness and volume can be associated with the pathological changes in the early stages of degeneration These results differ from previous studies, but still confirm retinal degeneration in AD.
Conclusion:
With further OCT studies on large populations, OCT will be in clinical use for early diagnosis of AD.
Keywords: Alzheimer's disease, biomarker, optical coherence tomography, retinal nerve fiber layer
INTRODUCTION
Dementia is a clinical situation characterized by progressive deterioration in more than one cognitive function as a result of central nervous system damage.[1] The impairment in cognitive functions affects daily life by decline in memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgment.[1] In the population older than 65, Alzheimer's disease (AD) is the most common cause of dementia.[2] Definitive diagnosis of AD is based on histopathologic hallmarks, including amyloid plaques and neurofibrillary tangles in specific neuroanatomical distribution.[3] For diagnosis of probable AD, the criteria were defined by the American Psychiatric Association.[1] The National Institute on Aging–Alzheimer's Association also indicated the importance of biomarkers for diagnosis.[4]
Visual impairments in a broad range were reported previously in AD. Pathologies in visual system described in previous studies include difficulty in maintaining fixation,[5,6] deterioration in visuospatial function,[6] depth perception,[7] saccadic and smooth pursuit eye movements,[8,9,10] impairment in color vision[11] and spatial contrast sensitivity,[11] inferiorly located visual field deficits,[12] delayed P2 latency in flash visual evoked potential,[13] delayed latency and decreased amplitude in pattern electroretinogram.[14] Formerly visual symptoms were believed to be associated with histopathological changes in cerebral cortex, particularly visual association cortex.[5,15] However, postmortem histopathological studies on optic nerve and retina of AD patients showed retinal ganglion cell (RGC) degeneration (especially M cells).[16,17,18,19]
Retinal alterations due to RGC degeneration can also be a biomarker for AD diagnosis. Optical coherence tomography (OCT) is a noninvasive method for evaluating retinal changes. In this study, we aimed to show RGC degeneration in vivo in AD patients via OCT.
METHODS
Subjects
Thirty-five eyes of 21 AD patients and 46 eyes of 25 cognitively healthy age-matched controls examined in Ophthalmology Department, Faculty of Medicine, Uludag University between December 2011 and May 2015 were enrolled. All AD patients were diagnosed by Neurology Department, Faculty of Medicine, Uludag University, considering Diagnostic and Statistical Manual of Mental Disorders-V criteria.[1] AD patients with mild-to-moderate cognitive disorders (mini-mental state examination score between 10 and 24) were included in AD group. Alzheimer disease patients with severe dementia and other causes of dementia (such as cerebrovascular diseases, metabolic and psychiatric disorders) were excluded. Controls did not have a medical history of neurological disorder, and their mini-mental state examination score was above 25. Patients with medical history of systemic arterial hypertension and diabetes mellitus were excluded in both groups.
All AD patients and controls underwent complete ophthalmologic examination including assessment of visual acuity, refractive error, intraocular pressure, anterior segment biomicroscopy, and dilated fundus examination. All participants had a corrected visual acuity of >0.5 with a refractive error between ±3 spheric diopters. Eyes with glaucoma, senile macular degeneration, retinal vascular diseases, and media opacity such as cataract and corneal disorders that prevented fundus and OCT examination were excluded.
The research followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Uludag University.
Optical coherence tomography examination
OCT is a noninvasive method allowing cross-sectional imaging of retina. In this study, peripapillary retinal nerve fiber layer (RNFL) thickness, macular thickness (MT), and macular volume (MV) were assessed in both AD patients and controls using Stratus OCT Model 3000 (Carl Zeiss Meditec Inc., Dublin, California, USA). For RNFL measurements, “fast RNFL thickness (3.4)” scan protocol was used. Three circular scan with a 3.4 mm diameter centering optic nerve head was used for calculating the average, per quadrant (superior, inferior, nasal, and temporal), each clock position RNFL thickness in microns (μm) [Figure 1].
Figure 1.
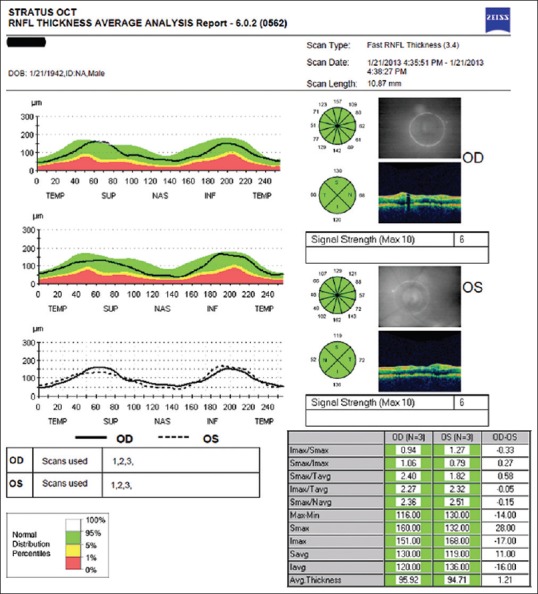
Optical coherence tomography output of peripapillary retinal nerve fiber layer thickness measurement of a patient with Alzheimer's disease. Circular scan with a 3, 4 mm diameter centering optic nerve head can be seen in fundus image for both eyes. Average retinal nerve fiber layer thicknesses in quadrants (superior, inferior, nasal, and temporal) and clock hours are shown on diagrams
MT and MV were measured using “MT map” protocol. This protocol consists of six consecutive radial line scans each centering on fovea and containing 512 A-scans [Figure 2a]. “Retinal thickness/volume tabular analysis” program was used for the evaluation of 3072 A-scans (512 × 6). The data verified from analysis program were displayed on a map divided into 9 Early Treatment Diabetic Retinopathy Study (ETDRS) macular fields [Figure 2]. This map contained three concentric circles with a diameter of 1 mm, 3 mm, and 6 mm, respectively. Central 1 mm circle corresponded to foveal region [Figure 2c]. Inner and outer rings were divided into four quadrants (superior, inferior, nasal, and temporal) forming nine macular fields of ETDRS with central circle [Figure 2].
Figure 2.
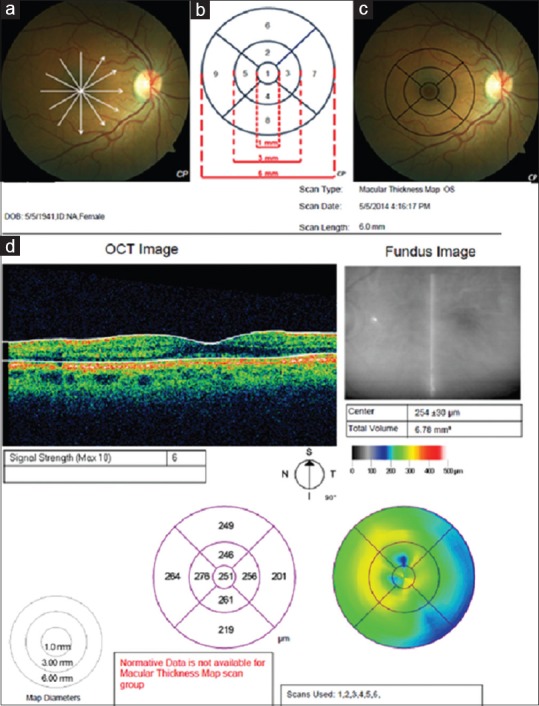
(a) Six consecutive radial line scans each centering on fovea schematized on the fundus photograph of an Alzheimer's disease patient, (b) Nine macular fields of early treatment diabetic retinopathy study, (c) Nine macular fields schematized on the fundus photograph of an Alzheimer's disease patient, (d) Optical coherence tomography output for macular scan of a patient in Alzheimer's disease group. The average thickness of the nine regions and topographic map of retinal thickness are shown on diagrams
Stratus OCT (Carl Zeiss Meditec Inc., Dublin, California, USA) is a conventional time domain OCT. Since it was the only accessible OCT device in our ophthalmology department till the last months of our study, RNFL, MV, and MT were measured in both groups with this device. The RNFL data of two patients in AD group could not obtained due to the difficulty in maintaining fixation during RNFL measurement.
Spectral domain OCT is the latest technology for retinal evaluation. In the recent days of our study, our devices were updated, and we had the opportunity to evaluate another 19 cognitively healthy individuals with both Stratus OCT and Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany); a spectral-domain OCT. Peripapillary RNFL thickness, MT, and MV data of 32 eyes of 19 cognitively healthy individuals obtained from both devices were used to check up the reliability of conventional time-domain OCT and the correlation between the devices.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Mean, standard deviation, median, minimum, maximum, frequency, and ratio values were used for descriptive statistics. The differences between AD and control group were statistically evaluated with Mann–Whitney U-test for nonparametric variables and independent sample t-test for normally distributed variables. To assess whether a correlation existed between time domain and spectral domain OCT Pearson's correlation test was used. P < 0.05 was considered statistically significant.
RESULTS
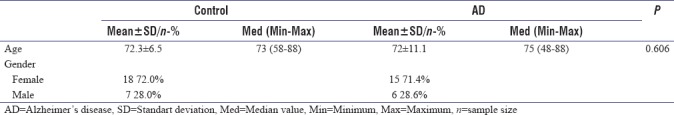
Thirty-five eyes of 21 AD patients (age 72 ± 11.1 years, 15 female/6 male) and 46 eyes of 25 cognitively healthy age-matched controls (age 72.3 ± 6.5 years, 18 female/7 male) were included in the study. There was no statistically significant difference in age and gender between groups. Demographic datas of AD and control groups are shown in Table 1.
Table 1.
Demographic data of groups
Optical coherence tomography
Peripapillary retinal nerve fiber layer thickness
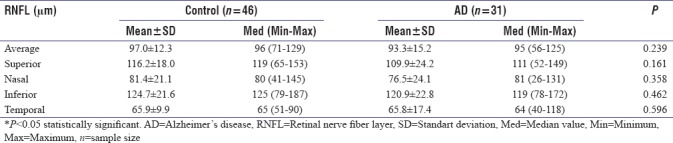
The average RNFL thickness was thinner in AD patients (93.3 ± 15.2 mm) when compared to controls (97 ± 12.3 mm) but not statistically significant (P > 0.05) [Table 2]. The RNFL thickness of AD patients in all quadrants was thinner than controls. However, thinning of RNFL was not statistically significant in none of the quadrants (for all P > 0.05). Peripapillary RNFL thickness measurements are shown in Table 2.
Table 2.
Peripapillary retinal nerve fiber layer thickness (μm) in patients with Alzheimer’s disease and healthy controls
Macular thickness and volume
Foveal thickness (233.5 ± 22.8 mm) and volume (0.18 ± 0.02 mm3) were significantly higher in AD patients than controls (thickness 221 ± 24.8 mm and volume 0.17 ± 0.02 mm 3) (P = 0.023 and P = 0.024, respectively) [Table 3]. No other statistically significant difference was detected between the groups when thickness and volume values of other eight macular regions were compared (P > 0.05). Volume and thickness values of nine regions are shown in Table 3.
Table 3.
Macular thickness (μm) and volume (mm3) in patients with Alzheimer’s disease and healthy controls
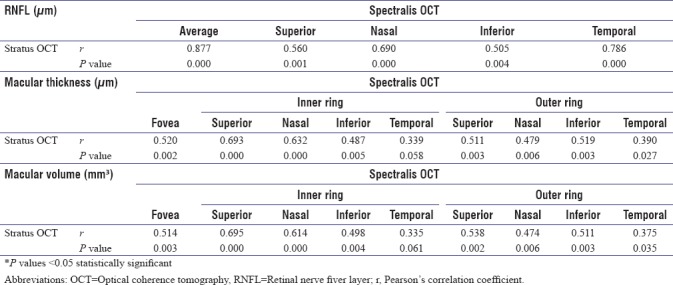
Correlation between time-domain optical coherence tomography and spectral-domain optical coherence tomography
A significant positive correlation was found between Stratus OCT (Carl Zeiss Meditec Inc., Dublin, California, USA) and Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) for both peripapillary and macular measurements. (P < 0.05). Correlations between two devices on peripapillary and macular measurements are shown in Table 4.
Table 4.
Correlation between Stratus and Spectralis OCT on peripapillary and macular measurements
DISCUSSION
In this study, we evaluated peripapillary RNFL thickness, MV, and MT in mild-to-moderate AD patients and compared the results with age-matched controls. Axons of RGCs are not constant with age; there is an age-related loss resulting with reduction in RNFL.[20] But in AD patients, there is more than age-related changes revealed by postmortem histopathological studies as RGC degeneration.[16,17,18,19] In our study, the average and four quadrant peripapillary RNFL thicknesses were reduced in AD patients when compared to controls, but the difference between the groups was not significant. The RNFL thickness results of previous studies vary.[21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] Some OCT studies found that RNFL thinning was restricted to superior quadrant[22,24,31,41] or superior and inferior quadrant.[23,25,30,35,42,43] On the other hand, some studies showed significant reduction only in average RNFL[25,29,34] or in all quadrants.[21,27,28,32,33,38] The variability can be related with the stages of AD patients enrolled in the studies and also the smaller sample size of the studies. In our study, only mild-to-moderate AD patients were included, and average mini-mental test score was 17.4 ± 3.5 (ranged from 12 to 22). Previous analysis with lower mini-mental test scores which means also severe AD patients were enrolled, demonstrated reduction in RNFL thickness in all quadrants.[21,27,28,32,33,38] However, higher mini-mental test scores accompanied reduction only in average and superior quadrant RNFL thicknesses.[22,24,31,41] Previous reports also indicated primarily peripapillary quadrant affected in AD as superior quadrant, differing from glaucoma which primarily affects inferior quadrant.[37] Inferior visual field deficits reported previously in AD patients is consistent with this literature data.[12] Furthermore, it is claimed that the greater amount of amyloid plaque and neurofibrillary tangle accumulation in cuneal gyrus in comparison to the lingual gyrus, are also relevant with predominantly inferior visual field deficits seen in AD patients, by causing retrograde neuronal degeneration.[5] Inferior quadrant was reported to be the following affected peripapillary region after superior quadrant in AD patients in previous studies.[35]
Histopathological studies revealed that approximately 35% of retinal thickness in macula consists of RGCs and axons.[44] We can evaluate the quantity of RGC axons by peripapillary measurements with OCT. OCT can also be useful for evaluating quality and quantity of the neuron bodies with macular measurements. Blank et al.[18] observed 25% reduction of RGCs in foveal/parafoveal retina in AD patients. The loss of RGCs in central retina, which was histopathologically proved, can be displayed by OCT with MT and MV measurements. Previous reports using time domain or spectral domain OCT stated reduced MT and MV, consistent with histopathological studies.[26,27,28,32,33,39,42,43,45] Spectral domain OCT is able to demonstrate the layer of retina involved in degeneration and differs from time domain OCT with this feature. Spectral domain OCT studies on AD patients showed that inner retinal layers (ganglion cell complex) were involved (reduced) in AD, external retinal layers were not affected.[43,45] We carried out our study with Stratus OCT (time domain OCT), so we did not have the chance of evaluating the affected layers due to the limited features of Stratus OCT. Our MT and MV results differ from literature. We found contrarily increased foveal thickness and volume and no other statistically significant change on the other eight macular region. Previously, only Ascaso et al.[32] reported increase in MT and MV in mild cognitive impairment patients but decreased in AD patients. Our study is the first to demonstrate foveal thickening in AD patients, never reported before. Increased thickness and volume may be associated with the swelling of RGC and Müller cells in the early stages of retinal degeneration. Researchers revealed “balloon-phenotyped” neurons due to amyloid beta and tau-associated neuron degeneration in the brain specimens of AD patients.[46] The degeneration in RGC is similar to the neurons in the brain. Blank et al.[17] demonstrated pale cytoplasm with swollen mitochondria and endoplasmic reticulum, pale nuclei with dispersed chromatin in early stages, but vacuolated cytoplasm and clumped chromatin in late stages of RGC degeneration. In all pathological conditions, Müller cells decrease the expression of Kir 4.1 potassium channels, leading increase in intracellular potassium level.[42,43] This process ends up with increased fluid transport into the Müller cell to maintain osmotic equilibrium and swelling.[47,48] These changes in Müller cells can contribute to increased thickness and volume. Besides, loss of fixation maintenance may be another reason for increased foveal thickness and volume.[5,6] Salobrar-Garcia et al.[39] claimed in their research, in which decrease in macular measurements but no significant change in RNFL detected, that the macular region is the first affected area of the retina in AD due to the arrangement of multilayer bodies of the ganglion cells. Our results are consistent with their hypothesis.
Neuronal degeneration due to the deposition of amyloid beta and hyperphosphorylated tau is the cause of brain damage in AD. Amyloid beta deposition in the retina is the possible reason of the RGC degeneration in AD patients. The histopathological researches on Alzheimer mouse models strengthen the opinion of retinal degeneration, and functional impairment are relevant with amyloid beta deposition.[49,50] Koronyo-Hamaoui[51] and Schön[52] achieved to show amyloid plaques and neurofibrillary tangles in vivo in mice models. Guo and Cordeiro[53] also developed a system named detection of apoptosing retinal cells based on imaging apoptosing RGCs in vivo with confocal scanning laser ophthalmoscopy. With our results, we can claim that OCT is also a method for detecting retinal degeneration in vivo in AD. OCT is a rapid, noninvasive, and in vivo technique for evaluating the retinal changes. Both time domain and spectral domain OCT devices are reliable for detecting retinal alterations in AD patients. The data verified from these devices are correlated but different.[54] That's why data obtained from the same devices should be compared. We made a comparison between two devices by evaluating the data of 19 cognitively healthy individuals obtained from both devices and detected a positive correlation between two devices. In addition, spectral-domain OCT, the latest technology, has some advantages. It is possible to evaluate which layer affected, and also, it is possible to make the evaluation faster than time-domain OCT which may be an important feature for AD patients.
The limitations of this study include the small sample size and the limited features of Stratus OCT for assessing the retinal layers. The incidence of systemic and eye disorders is high in elderly patients. The exclusion of patients with systemic and ocular diseases restricted our sample size. Another reason of small sample size was the application of the AD patients to doctors for diagnosis at advanced stages.
CONCLUSION
Considering the retinal changes in AD, we can present eye as a window to the brain. Our results suggest utilization of OCT by clinicians for early diagnosis of AD may be as a biomarker in the near future. Since the results of the studies are in a big diversity, further studies, especially including larger populations are required, and a normative database for elderly population should be installed for quick assessment of mild cognitive impairment and suspected AD patients.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank Department of Neurology, Uludag University, for their cooperation and patient referral.
REFERENCES
- 1.American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Rockwood K, Stadnyk K. The prevalence of dementia in the elderly: A review. Can J Psychiatry. 1994;39:253–7. doi: 10.1177/070674379403900503. [DOI] [PubMed] [Google Scholar]
- 3.Perl DP. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National İnstitute on Aging-Alzheimer's Association Workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong RA. Alzheimer's disease and the eye. J Optom. 2009;2:103–11. [Google Scholar]
- 6.Armstrong R, Kergoat H. Oculo-visual changes and clinical considerations affecting older patients with dementia. Ophthalmic Physiol Opt. 2015;35:352–76. doi: 10.1111/opo.12220. [DOI] [PubMed] [Google Scholar]
- 7.Mendez MF, Cherrier MM, Meadows RS. Depth perception in Alzheimer's disease. Percept Mot Skills. 1996;83:987–95. doi: 10.2466/pms.1996.83.3.987. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher WA, Sharpe JA. Saccadic eye movement dysfunction in Alzheimer's disease. Ann Neurol. 1986;20:464–71. doi: 10.1002/ana.410200405. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q, Wang T, Su N, Xiao S, Kapoula Z. Specific saccade deficits in patients with Alzheimer's disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age (Dordr) 2013;35:1287–98. doi: 10.1007/s11357-012-9420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaccara G, Gangemi PF, Muscas GC, Paganini M, Pallanti S, Parigi A, et al. Smooth-pursuit eye movements: Alterations in Alzheimer's disease. J Neurol Sci. 1992;112:81–9. doi: 10.1016/0022-510x(92)90136-9. [DOI] [PubMed] [Google Scholar]
- 11.Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, Banks KS, et al. Visual dysfunction in Alzheimer's disease: Relation to normal aging. Ann Neurol. 1991;29:41–52. doi: 10.1002/ana.410290110. [DOI] [PubMed] [Google Scholar]
- 12.Trick GL, Trick LR, Morris P, Wolf M. Visual field loss in senile dementia of the Alzheimer's type. Neurology. 1995;45:68–74. doi: 10.1212/wnl.45.1.68. [DOI] [PubMed] [Google Scholar]
- 13.Wright CE, Harding GF, Orwin A. The flash and pattern VEP as a diagnostic indicator of dementia. Doc Ophthalmol. 1986;62:89–96. doi: 10.1007/BF00140551. [DOI] [PubMed] [Google Scholar]
- 14.Katz B, Rimmer S, Iragui V, Katzman R. Abnormal pattern electroretinogram in Alzheimer's disease: Evidence for retinal ganglion cell degeneration? Ann Neurol. 1989;26:221–5. doi: 10.1002/ana.410260207. [DOI] [PubMed] [Google Scholar]
- 15.McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:621–30. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986;315:485–7. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 17.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer's disease. Brain Res. 1989;501:364–72. doi: 10.1016/0006-8993(89)90653-7. [DOI] [PubMed] [Google Scholar]
- 18.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–84. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 19.Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH, et al. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. 1996;17:385–95. doi: 10.1016/0197-4580(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 20.Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49:4437–43. doi: 10.1167/iovs.08-1753. [DOI] [PubMed] [Google Scholar]
- 21.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F, et al. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin Neurophysiol. 2001;112:1860–7. doi: 10.1016/s1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 22.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer's disease. Invest Ophthalmol Vis Sci. 2007;48:2285–9. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer's disease: Evidence in optical coherence tomography. Neurosci Lett. 2010;480:69–72. doi: 10.1016/j.neulet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Kromer R, Serbecic N, Hausner L, Froelich L, Aboul-Enein F, Beutelspacher SC, et al. Detection of retinal nerve fiber layer defects in alzheimer's disease using SD-OCT. Front Psychiatry. 2014;5:22. doi: 10.3389/fpsyt.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bambo MP, Garcia-Martin E, Pinilla J, Herrero R, Satue M, Otin S, et al. Detection of retinal nerve fiber layer degeneration in patients with Alzheimer's disease using optical coherence tomography: Searching new biomarkers. Acta Ophthalmol. 2014;92:e581–2. doi: 10.1111/aos.12374. [DOI] [PubMed] [Google Scholar]
- 26.Polo V, Garcia-Martin E, Bambo MP, Pinilla J, Larrosa JM, Satue M, et al. Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer's disease. Eye (Lond) 2014;28:680–90. doi: 10.1038/eye.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrosa JM, Garcia-Martin E, Bambo MP, Pinilla J, Polo V, Otin S, et al. Potential new diagnostic tool for Alzheimer's disease using a linear discriminant function for fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:3043–51. doi: 10.1167/iovs.13-13629. [DOI] [PubMed] [Google Scholar]
- 28.Iseri PK, Altinaş O, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 29.Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2007;420:97–9. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 30.Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin Neurol Neurosurg. 2011;113:523–6. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol. 2013;33:58–61. doi: 10.1097/WNO.0b013e318267fd5f. [DOI] [PubMed] [Google Scholar]
- 32.Ascaso FJ, Cruz N, Modrego PJ, Lopez-Anton R, Santabárbara J, Pascual LF, et al. Retinal alterations in mild cognitive impairment and Alzheimer's disease: An optical coherence tomography study. J Neurol. 2014;261:1522–30. doi: 10.1007/s00415-014-7374-z. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Liu Y, Li X, Bai Q, Liu P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer's disease. Arch Gerontol Geriatr. 2015;60:162–7. doi: 10.1016/j.archger.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Oktem EO, Derle E, Kibaroglu S, Oktem C, Akkoyun I, Can U, et al. The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci. 2015;36:1141–6. doi: 10.1007/s10072-014-2055-3. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Zhang L, Li Z, Zhang X, Wu Y, Yang H, et al. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer's disease. BMC Neurol. 2015;15:14. doi: 10.1186/s12883-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenti DA. Neuroimaging of retinal nerve fiber layer in AD using optical coherence tomography. Neurology. 2007;69:1060. doi: 10.1212/01.wnl.0000280584.64363.83. [DOI] [PubMed] [Google Scholar]
- 37.Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Localized retinal nerve fiber layer thinning in patients with early glaucoma or Alzheimer's disease. Invest Ophthalmol Vis Sci. 2006;47:3379. [Google Scholar]
- 38.He XF, Liu YT, Peng C, Zhang F, Zhuang S, Zhang JS, et al. Optical coherence tomography assessed retinal nerve fiber layer thickness in patients with Alzheimer's disease: A meta-analysis. Int J Ophthalmol. 2012;5:401–5. doi: 10.3980/j.issn.2222-3959.2012.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salobrar-Garcia E, Hoyas I, Leal M, de Hoz R, Rojas B, Ramirez AI, et al. Analysis of retinal peripapillary segmentation in early Alzheimer's disease patients. Biomed Res Int. 2015;2015:636548. doi: 10.1155/2015/636548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson KL, Yeo JM, Waddell B, Cameron JR, Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 2015;1:136–43. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon JY, Yang JH, Han JS, Kim DG. Analysis of the retinal nerve fiber layer thickness in Alzheimer disease and mild cognitive impairment. Korean J Ophthalmol. 2017;31:548–56. doi: 10.3341/kjo.2016.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Haan J, Verbraak FD, Visser PJ, Bouwman FH. Retinal thickness in Alzheimer's disease: A systematic review and meta-analysis. Alzheimers Dement (Amst) 2017;6:162–70. doi: 10.1016/j.dadm.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunha LP, Lopes LC, Costa-Cunha LV, Costa CF, Pires LA, Almeida AL, et al. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer's disease. PLoS One. 2016;11:e0153830. doi: 10.1371/journal.pone.0153830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo L, Duggan J, Cordeiro MF. Alzheimer's disease and retinal neurodegeneration. Curr Alzheimer Res. 2010;7:3–14. doi: 10.2174/156720510790274491. [DOI] [PubMed] [Google Scholar]
- 45.Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer's disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5953–8. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 46.Fujino Y, Delucia MW, Davies P, Dickson DW. Ballooned neurones in the limbic lobe are associated with Alzheimer type pathology and lack diagnostic specificity. Neuropathol Appl Neurobiol. 2004;30:676–82. doi: 10.1111/j.1365-2990.2004.00593.x. [DOI] [PubMed] [Google Scholar]
- 47.Bringmann A, Wiedemann P. Müller glial cells in retinal disease. Ophthalmologica. 2012;227:1–9. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- 48.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A, et al. Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:627–36. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 49.Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49:5136–43. doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez SE, Lumayag S, Kovacs B, Mufson EJ, Xu S. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis Sci. 2009;50:793–800. doi: 10.1167/iovs.08-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(Suppl 1):S204–17. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schön C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7:e53547. doi: 10.1371/journal.pone.0053547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo L, Cordeiro MF. Assessment of neuroprotection in the retina with DARC. Prog Brain Res. 2008;173:437–50. doi: 10.1016/S0079-6123(08)01130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin HJ, Cho BJ. Comparison of retinal nerve fiber layer thickness between Stratus and Spectralis OCT. Korean J Ophthalmol. 2011;25:166–73. doi: 10.3341/kjo.2011.25.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]


