Abstract
Background:
Parkinson's disease (PD) is one of the most common neurodegenerative movement disorders and its incidence is increasing worldwide along with population aging. Previous clinical and histologic studies suggest that the neurodegenerative process, which affects the brain, may also affect the retina of PD patients.
Objective:
The objective of this study was to determine the thickness changes of retina nerve fibers and macular volume with optical coherence tomography (OCT) in PD patients.
Materials and Methods:
The spectral domain OCT was used to assess the thickness of retinal nerve fiber layer (RNFL) and macular volume from 34 PD patients and 50 healthy age-matched controls.
Results:
Compared with healthy age-matched controls, the RNFL thickness of PD patients was much thinner (P < 0.05) in all retinal quadrants, with temporal thinning being more than nasal thinning. Macular volumes were diminished in both perifoveal and outer macular regions in all sectors (P < 0.05) with preserved foveal volume. The degree of tissue loss corroborated with the severity of disease as objectively assessed by standardized rating scales (UPDRS).
Conclusion:
There is generalized retinal nerve degeneration in patients of PD and the degree of loss correlated with the severity and duration of disease.
Keywords: Macular volume, optical coherence tomography, Parkinson's disease, retinal nerve fiber layer
INTRODUCTION
Parkinsonism refers to the bradykinetic group of movement disorders, the prototype of which is idiopathic Parkinson's disease (IPD). It was first described in 1817 by James Parkinson with works by Charcot, Carlsson et al., and Ehringer and Hornykiewicz further defining the clinical characteristics and establishing the central role of dopamine depletion in the pathogenesis of the disease.[1,2,3,4,5,6,7] Such dopaminergic cell loss is seen most prominently in the basal ganglia but also in other parts of the brain that cause many of the nonmotor deficits as well as parts of the visual apparatus including certain layers of the retina, parts of the visual pathway, and layers of the occipital cortex. The loss in the retina is reflected as reduced thickness of the retinal nerve fiber layer (RNFL) which can be measured by optical coherence tomography (OCT).
OCT is a noninvasive technology, which acquires cross-sectional images of retinal structures allowing neural fundus integrity assessment and quantifying structural axonal damage by measuring OCT peripapillary RNFL (PRNFL) that allows an indirect estimation of retinal ganglion cell (RGC) layer impairment or directly by estimating macular thickness measurements, since 30%–35% of the retina thickness in macular area is composed by the RGCs and their fibers.[8,9,10] The purpose of this cross-sectional study was to evaluate the OCT findings in an Indian cohort of PD patients and try to corroborate patterns of RNFL thinning or macular volume loss as demonstrated in previous studies from other parts of the world. Depending on the findings from this study, a future long-term prospective study may be planned in a select group of high-risk patients (those with familial PD, isolated hyposmia, or isolated rapid eye movement sleep behavior disorder) who have not yet developed any clinical motor manifestations of PD with the purpose of finding if retinal nerve fiber changes predate clinical disease.
MATERIALS AND METHODS
A cross-sectional, observational case study was conducted over a period of 1½ years from January 2013 to June 2014 in the Department of Neurology at Medical College Hospital, Kolkata, after obtaining clearance from the Institutional Ethics Committee. Patients were selected from the Neurology OPD at MCH and suitable age-matched, healthy consenting controls were included from among friends and relatives. Only those cases of Parkinsonism which satisfied the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria (UKPDS Brain Bank Criteria) were diagnosed as IPD and included in the study. Owing to resource constraints, secondary Parkinsonian disorders were not included in the study.
Inclusion criteria
Age between 40 and 80 years
Ability to understand the instructions given
Only those providing written informed consent
Fulfillment of the UKPDS Brain Bank Criteria for the diagnosis of PD.
Exclusion criteria
Presence of diabetes mellitus
Glaucoma, intraocular pressure (IOP) >21 mmHg, history of surgery for glaucoma, or patients on antiglaucoma treatment
Media opacity sufficient to preclude optical imaging by OCT
History of optic neuritis or history of sudden loss of vision in either eye
History of multiple sclerosis or other demyelinating CNS disease
History of serious head injury, meningitis or encephalitis, or radiological evidence of basal ganglia infarct even in the absence of a clear-cut history of stroke
History of HIV infection.
For staging the severity of disease and rating, the various clinical parameters (cognitive and behavioral, activities of daily living, motor manifestations, and complications), the Unified PD Rating Scale (UPDRS) developed by Fahn et al. was used.[11] Where possible, we obtained the UPDRS score upon diagnosis of PD in a new patient before initiation of therapy, while for patients already on drug therapy with no prior UPDRS scores available, we had to rely on the “off scores” for the “motor examination” section. However, obtaining reliable “off scores” was not possible for 6 patients who were either on long-acting preparations and were not willing to change over to short-acting drugs or could not be kept off drugs for several hours due to severe symptoms in the “off periods.” The other sections were scored for every patient including the Schwab and England Activities of Daily Living Scale. The total UPDRS score, representing the sum total of scores in items 1 through 42, was calculated. However, in few cases where the motor subscale could not be scored, a valid total UPDRS score was not possible. Scores in each section and the total score were used for statistical analysis.
The radiological workup consisted of at least one noncontrast magnetic resonance imaging of brain. Biochemical workup included at least evaluation of the fasting and 2 h postprandial blood glucose (FBS and PPBS), serum electrolytes, urea and creatinine, thyroid function test, and serum Vitamin B12 levels.
Patients who passed the radiological and biochemical screen in view of the inclusion/exclusion criteria were subjected to ophthalmological examination including clinical ophthalmoscopy, applanation tonometry (for IOP measurement), and visual-evoked potential measurement to rule out exclusion criteria as well as collect data pertaining to the eyes. Where there was any suspicion of glaucoma (including suggestive or definite family history), automated perimetry was done even if IOP was normal to rule out glaucoma with confidence since it may mimic OCT findings.
After this, all the participants underwent OCT scanning of both eyes. Spectral domain OCT (SD-OCT) was performed using Heidelberg Engineering Spectralis HRA + OCT Rev 1.5.2.0 machine at the Regional Institute of Ophthalmology, Kolkata. The OCT machine obtained separate reflexes of the polarized single mode light from various layers of the retina starting from the inner limiting membrane to the retinal pigment epithelial layer, analysis of which gave the thickness of the different retinal layers. The standard definition of the inner and outer retinal layers which has been clearly stated by Hajee et al.[12] and is clearly delineated by the standard color coding in any modern OCT machine was used for this study. This study focused on the inner retinal layer which reflected the nerve fiber changes which are of interest.
The PRNFL thickness was studied in the temporal superior (TS), nasal superior (NS), temporal (T), nasal (N), temporal inferior (TI), and nasal inferior (NI) quadrants. The average global thickness (G) was extrapolated from the formula G= (TS + NS + 2T + 2N + TI + NI)/8. These data were compared with similar parameters recorded from the eyes of age-matched healthy controls. The average RNFL thickness on the temporal and nasal sides was calculated from the following formulae.
Temporal side average RNFL thickness (Tavg) = (TS + [2 × T] + TI) divided by 4
Nasal side average RNFL thickness (Navg) = (NS + [2 × N] + NI) divided by 4
Two further calculations were made for each eye.
Temporal-nasal difference (TND) = Tavg-Navg
Temporal-nasal ratio (TNR) = Tavg/Navg.
The macular thickness was also studied in three concentric circles of 1 mm (central macula), 3 mm, and 6 mm called M1, M3, and M6, respectively. The outer two circles were further divided into four sectors such as superior (S), inferior (I), temporal (T), and nasal (N) by diagonal lines. The volume of each of these parts was calculated by multiplying the sectoral area with the average macular thickness in that sector. Total macular volume was also determined as the sum total of all such measurements.
The clinical and OCT data were then compared among cases and controls to look for any predictable patterns and also any definite relationship with disease stage or severity. The numerical data (parametric and nonparametric) were subjected to appropriate statistical analysis using MedCalc, a standard statistical software accepted for biomedical research. For each statistical analysis, as per convention, the result was considered statistically significant only if a P < 0.05 was obtained. The central tendencies, mean, and median, along with variance, standard deviation, and standard error of the mean were calculated for various parameters under study for each group, and the F-ratio was used to compare the standard deviation of the two samples. The difference between mean values of a particular observation (e.g. PRNFL thickness in the temporal sector) between test and control groups were analyzed using the “independent sample t-test” if the F-ratio was close to 1 and P < 0.05 (implying that SD between the two groups was not significantly different). Conversely, when the F-ratio showed a significant difference of SD between the groups under comparison, Welch test was used. One-way ANOVA was used to study the influence of categories on a continuous variable (e.g., Modified Hoehn and Yahr Stage of PD on macular volume). Then, the F-ratio and P values are calculated. Again, an F-ratio >1 with a P < 0.05 shows that the influence of that parameter on the variable under study was significant. Pearson's rank correlation and Kruskal–Wallis were used to test the correlation between clinical and OCT findings for nominal and ordinal data respectively.
RESULTS
Of a total of 204 patients with primary presenting features consistent with parkinsonism, 66 were eventually found to fit into the criteria for PD. Among them, 32 were excluded from the final study based on our exclusion criteria and a final of 34 patients or 68 eyes were included for clinical and OCT analysis. Fifty out of 139 screened controls were also similarly examined giving us 100 age-matched “control” eyes.
Among the study subjects, 14 patients (9 males and 5 females) were newly diagnosed as having PD at our clinic and were treatment naive. The remaining twenty patients had been on dopamine replacement therapy for a variable period of time. In this study population, 19 subjects (55.9%) were males and 15 (44.1%) were females, while in the control group, there were 27 males (54%) and 23 females (46%), respectively. The mean age of the study group was 64.882 years while that of the control group was 67.260 years. There was no significant age difference (P = 0.16) or sex difference (P = 0.52) between our study subjects of PD and the controls.
As explained in the methods section, reliable-off scoring for UPDRS-II was not possible for six patients, hence UPDRS-T could not be calculated for them. The other subscale scores were analyzed for all patients. The mean duration of tremor which was taken as the clinical starting point of motor symptoms in Parkinsonism (as most patients tend to remember that rather than the subtle nonmotor symptoms preceding it) was 53.03 months, while bradykinesia was present for a mean duration of 51.58 months. The UPDRS subscales were analyzed and the mean scores obtained in subgroups I through IV were 5.273, 17.182, 19.750, and 3.576, respectively. The UPDRS-T score which is a sum total of Scores I-IV was calculated separately and found to be 42.93. The average Modified Hoehn and Yahr Stage (UPDRS V) score was 2.818, while the Schwab and England ADL Score (UPDRS VI) was 69.697. Both eyes of our 34 study subjects (68 eyes in total) and 50 control subjects (100 eyes) were subjected to OCT analysis as mentioned in the methodology. The thickness of the inner retina in the peripapillary area (nerve fiber layer), macula and posterior pole, and macular volume were studied [Table 1].
Table 1.
Global and sector.wise comparison of retinal nerve fiber thickness and macular volume among Parkinson’s disease patients and control
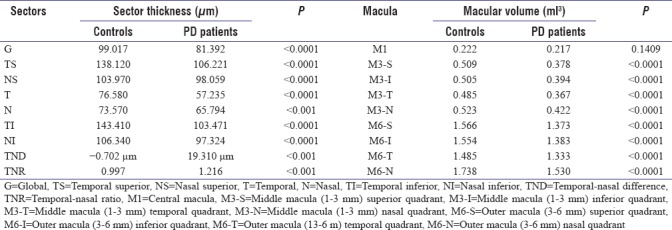
Table 1 shows that there was a statistically significant global reduction in the PRNFLT in PD patients compared to age-matched controls. Significant thinning was found in each of the six sectors of peripapillary retina. The influence of UPDRS clinical scores on the global RNFL thickness was compared using ANOVA along with Levene's test for equality of variances and Student Newman Keuls' test for all pair-wise comparisons (if ANOVA was positive). No significant relation between UPDRS subscales I, II, and IV as well as UPDRS-T and the averaged RNFL thickness was found. The UPDRS-II scale that measures difficulty in performing daily activities, however, had a significant relation to RNFL thinning. RNFL thinning was most influenced by the modified Hoehn and Yahr Stage (UPDRS-V), the Schwab and England ADL Scale (UPDRS-VI), and the duration of bradykinesia and tremor. Thus, the severity of RNFL thinning paralleled the clinical severity of and duration of parkinsonism in these patients [Table 2].
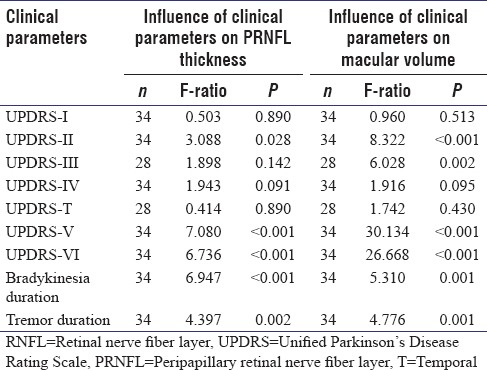
Table 2.
Comparison of clinical parameters with retinal nerve fiber layer thickness and macular volume by one-way ANOVA
Furthermore, the RNFL thickness on the temporal side (TI, T, and TS combined) was more than that on the nasal side (NI, N, and NS combined) in control eyes. However, more severe thinning was noted on the temporal side than on the nasal side in PD patients. To see if this difference was statistically significant, the average RNFL thickness on the temporal and nasal sides was calculated from the following formulae.
Temporal side average RNFL thickness (Tavg) = [TS + (2 × T) + TI] divided by 4
Nasal side average RNFL thickness (Navg) = [NS + (2 × N) + NI] divided by 4
Two further calculations were made for each eye.
iii. TND = Tavg-Navg
iv. TNR = Tavg/Navg.
In our PD study subjects, the average temporal RNFL thickness was less than that on the nasal side by 0. 702 μm. This difference between the two halves was not found to be statistically significant (P = 0.6671). However, as seen in Table 1, when this difference (TND) was compared among our PD study subjects and healthy age-matched controls, the difference in both TND and TNR was found to be statistically significant (P < 0.001).
Table 1 also analyzed the macular volumes among our study subjects and controls. There was a significant loss of macular volume in all sectors in PD patients compared to controls except the central macula which was spared. Furthermore, it was found that there was a significant negative correlation between age and total macular volume in patients of PD, but no such significant correlation was found among the age-matched controls (r = −0.4767). This indicated that significant macular thinning with age, resulting in macular volume loss may be a feature of neurodegenerative diseases and not suffered by all people in general.
Analysis of the UPDRS scores and its correlation with macular volumes [Table 2] showed that the total score as well as the scores in subscales I and IV did not have any significant correlation with loss of macular volume in PD patients. However, the subscale II score (P < 0.001), subscale III “off scores” obtained in 28 out of 34 patients in the OCT study group (P = 0.002), modified Hoehn and Yahr stage of PD i.e. subscale V (P < 0.001), and the Schwab and England ADL scale or UPDRS subscale VI (P < 0.001) all had significant influences on the macular volume. Thus, apart from the motor examination scores, a significant influence on the macular volume was due to the stage of PD and the subscales related to ADL, while the subscales related to mental functions and side effects of treatment had no influence on macular volume. The symptoms' duration for bradykinesia and tremor which were used as surrogates for total disease duration showed significant influence on macular volume too (P = 0.001 for both).
DISCUSSION
The OCT studies showed significant reduction of PRNFL thickness in PD patients compared to age-matched healthy controls. The thinning was significant in all sectors analyzed. However, unlike other neurodegenerative diseases such as AD, the thinning was not uniform, being more on the temporal hemiretina compared to the nasal half. Inzelberg et al. in 2004 found similar RNFL thinning by OCT on ten patients of PD.[13] Similar results were reported later by several other groups including Yavas et al. in 2007.[14] The next year, Altintas et al. reported the results of their research where they examined 17 PD and 11 controls and reported reduced mean RNFL thicknesses in all quadrants except 8 o'clock position, in comparison to control subjects.[15] Kirbas et al. in 2012 studied 42 PD patients and found similar differential hemiretinal thinning of the temporal retina.[16] Moschos et al. in 2011 had corroborative findings in their study on 16 PD patients. However, in addition to temporal hemiretina, they found differential thinning in the inferior quadrant too.[17] However, neither of them could point out any specific explanation for the same.
The central macular (foveal) sparing as seen in our patients could be explained by the fact that the fovea contained almost exclusively the photoreceptor cones without any ganglion cells or their axons there. Hence, in PD, where there is a loss of ganglion cells without any effect on the photoreceptors unlike some other neurodegenerative diseases, fovea is spared.
It might be questioned why a condition like PD, known to involve dopaminergic cells selectively cause retinal cell degeneration. The possible explanation is that dopaminergic cells are present in various parts of the retino-optic pathway including the amacrine cells of the retina, the lateral geniculate body, and parts of the occipital cortex. The amacrine cells, whose dopaminergic output controls the maturation and survival of the RGCs, are involved early in PD, probably before significant damage to the substantia nigra has occurred. The axons of these ganglion cells form the RNFL; hence, loss of these ganglion cells lead to RNFL thinning early on in the natural history of PD. This led Bodis-Wollner et al. in 2008 to stress on diagnosing and treating PD in the precardinal stages of the disease and proposed ophthalmological studies as a reliable modality of such diagnostic evaluation.[18]
We also studied the relation of the OCT findings with the clinical parameters of the patients. Since the clinical scores were discrete, in whole numbers, and basically arbitrary, we considered the scores as categories, rather than variables. Hence, we used ANOVA in addition to Spearman's rank correlation to study the influence of the scores on OCT findings and considered a relation as significant only when both ANOVA and Spearman's coefficient were significant. When the relation of clinical scores to the OCT findings was studied in the patients, some significant differences were found in the study of the peripapillary and the macular regions. The duration of tremor and bradykinesia which were used as surrogates of disease duration, correlated significantly with both PRNFLT (P < 0.001 and P = 0.002, respectively) and macular volume (P = 0.001). This indicates that the neurodegenerative process runs in parallel in the brain and the retina in PD. Comparing the UPDRS subscales with the OCT findings showed that UPDRS-I (mentation, behavior, and mood) and subscale-IV (complications of treatment) did not correlate well with RNFL thinning or macular volume loss, thereby implying that these two parameters did not parallel the extent of neurodegeneration. The baseline score (when not on drugs) or “off scores” for motor examination (subscale III) obtained in 28 out of the 34 study subjects showed that correlation with RNFL thickness was not significant (P = 0.142) while that with macular volume was P = 0.002. Since the baseline motor score or off-score can be taken as a reliable indicator of disease severity and thereby the duration of disease, the relation with macular thinning can be accepted, especially considering the fact that other parameters of disease severity in the UPDRS (subscales V and VI) also had significant relation to retinal thinning. However, the discrepancy between the level of significance of RNFL thinning and macular volume loss is difficult to explain. The small study sample could have probably led to this bias.
This study had a few limitations. The number of patients included, though larger than previously published studies, still leaves room for larger multicenter studies. Furthermore, we could not include patients in very early or presymptomatic stages of disease owing to the inherent design of our study. It would also have been of more interest if we could compare the RNFL thinning pattern in PD patients with that of secondary parkinsonism and so-called Parkinson-plus cases. The study design being cross-sectional also leaves room for improvement. A longitudinal study using repeated OCT on the same patients will provide better information on the course of the disease. We also could not use Electroretinogram (ERG) due to its nonavailability and hence could not parallely assess the functional impairment in visual processing along with OCT changes in cases and controls.
Nevertheless, we have included a significant number of patients, provided important data for Indian patients and found out significant associations which can be used for further research and clinical work.
CONCLUSION
The present study showed a significant reduction of PRNFL thickness and macular volume in PD patients compared to age-matched healthy controls. The thinning was significant in all sectors being more on the temporal half compared to the nasal half. There is sparing of the central macula. Severity of RNFL thinning and macular volume paralleled the clinical severity and duration of Parkinsonism in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Parkinson J. An essay on the shaking palsy 1817. J Neuropsychiatry Clin Neurosci. 2002;14:223–36. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Kempster PA, Hurwitz B, Lees AJ. A new look at James Parkinson's essay on the shaking palsy. Neurology. 2007;69:482–5. doi: 10.1212/01.wnl.0000266639.50620.d1. [DOI] [PubMed] [Google Scholar]
- 3.Björklund A, Dunnett SB. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Hornykiewicz O. The discovery of dopamine deficiency in the Parkinsonian brain. J Neural Transm Suppl. 2006;70:9–15. doi: 10.1007/978-3-211-45295-0_3. [DOI] [PubMed] [Google Scholar]
- 5.Birkmayer W, Hornykiewicz O. The L-3, 4-dihydroxyphenylalanine (DOPA) effect in Parkinson-akinesia. Wien Klin Wochenschr. 1961;73:787–8. [PubMed] [Google Scholar]
- 6.Birkmayer W, Hornykiewicz O. The effect of l-3,4-dihydroxyphenylalanine (=DOPA) on akinesia in Parkinsonism. Parkinsonism Relat Disord. 1998;4:59–60. doi: 10.1016/s1353-8020(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 7.Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism – Chronic treatment with L-dopa. N Engl J Med. 1969;280:337–45. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro ML, Cunha LP, Costa-Cunha LV, Maia OO, Jr, Oyamada MK. Relationship between optical coherence tomography, pattern electroretinogram and automated perimetry in eyes with temporal hemianopia from chiasmal compression. Invest Ophthalmol Vis Sci. 2009;50:3535–41. doi: 10.1167/iovs.08-3093. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro ML, Fernandes DB, Apóstolos-Pereira SL, Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:3959–66. doi: 10.1167/iovs.11-9324. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro ML, Afonso CL. Macular thickness measurements with frequency domain-OCT for quantification of axonal loss in chronic papilledema from pseudotumor cerebri syndrome. Eye (Lond) 2014;28:390–8. doi: 10.1038/eye.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahn S, Elton RL. UPDRS Development Committee. The unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan; 1987. pp. 153–63. [Google Scholar]
- 12.Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009;127:737–41. doi: 10.1001/archophthalmol.2009.106. [DOI] [PubMed] [Google Scholar]
- 13.Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004;44:2793–7. doi: 10.1016/j.visres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Yavas GF, Yilmaz O, Küsbeci T, Oztürk F. The effect of levodopa and dopamine agonists on optic nerve head in Parkinson disease. Eur J Ophthalmol. 2007;17:812–6. doi: 10.1177/112067210701700520. [DOI] [PubMed] [Google Scholar]
- 15.Altintaş O, Işeri P, Ozkan B, Caǧlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson's disease. Doc Ophthalmol. 2008;116:137–46. doi: 10.1007/s10633-007-9091-8. [DOI] [PubMed] [Google Scholar]
- 16.Kirbas S, Turkyilmaz K, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in Parkinson disease. J Neuroophthalmol. 2013;33:62–5. doi: 10.1097/WNO.0b013e3182701745. [DOI] [PubMed] [Google Scholar]
- 17.Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol. 2011;21:24–9. doi: 10.5301/ejo.2010.1318. [DOI] [PubMed] [Google Scholar]
- 18.Bodis-Wollner I. Diagnosing and treating early: Is there a precardinal stage in Parkinson's disease? Parkinson Rep. 2008;19:9–10. [Google Scholar]


