Abstract
Aging is associated with an increase in stroke risk. Melatonin, a potent free radical scavenger and broad spectrum antioxidant, has been shown to counteract inflammation and apoptosis in brain injury. However, little is known on the possible protective effects of melatonin in aged individuals affected by brain ischemia. Also, using melatonin before or after an ischemic stroke may result in significantly different molecular outcomes. The objective of the present study was to compare the effects of pre-ischemia vs. post-ischemia melatonin administration in an ischemic lesion in the cortex and hippocampus of senescent Wistar rats. An obstruction of the middle cerebral artery (MCA) to 18-month-old animals was performed. In general, animals treated with melatonin from 24 h prior to surgery until 7 days after the surgical procedure (PrevT) experienced a significant decrease in the levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), glial fibrillary acidic protein (GFAP), Bcl-2-associated death promoter (BAD), and Bcl-2-associated X protein (BAX) in both cortex and hippocampus, while hippocampal levels of sirtuin 1 (SIRT1) and B-cell lymphoma 2 (Bcl-2) increased. Treatment of animals with melatonin only after surgery (AT) resulted in similar effects, but to a lesser extent than in the PrevT group. In any case, melatonin acted as a valuable therapeutic agent protecting aged animals from the harmful effects of cerebral infarction.
Keywords: aging, brain, ischemia, melatonin, middle cerebral artery blockade
1. Introduction
Stroke is one of the leading causes of death in developed countries [1] causing permanent disability in adults worldwide [2]. The sudden occlusion of a blood vessel by a thrombus or embolism is the most common cause of stroke, resulting in the loss of oxygen and glucose supply to the cerebral tissue. Although stroke is a complex pathological process, increasing evidence shows that ischemic injury coursing with inflammation plays a pivotal role [3]. Cerebral ischemia triggers an ischemic cascade causing irreversible neuronal damage in the ischemic core within minutes of the onset [4]. However, a much larger volume of brain tissue surrounding the ischemic core, known as the penumbra, can be recovered if the blood flow is promptly restored.
Ischemic stroke leads to oxidative stress, microvascular injury, blood-brain barrier dysfunction and post-ischemic inflammation causing ultimately the death of glia, neurons and endothelial cells. The extent of permanent damage depends mainly on the duration and the degree of ischemia and the ability of the brain to repair itself and recover from the insult [4].
Excitotoxicity and oxidative stress activate astrocytes and microglia which react by secreting chemokines, cytokines and matrix metalloproteases (MMP). These molecules lead to upregulation of cell adhesion molecules in endothelial cells, allowing blood-derived inflammatory cells to infiltrate the ischemic area. Neutrophils also secrete cytokines which cause further activation of glial cells. These processes result in neuronal cell death, enhancing brain damage. Increased levels of pro-inflammatory cytokines and decreased anti-inflammatory cytokine production are related to greater damage and poorer clinical outcome. In this regard, it has been observed that interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) appear to intensify cerebral injury [5,6,7], whereas transforming growth factor-β (TGF-β) and IL-10 may be neuroprotective [8,9].
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone produced by the pineal gland. It has been shown to have several potential therapeutic benefits, including its potential to counteract age-related alterations [10,11]. Both animal and human studies have shown that its acute toxicity is extremely low. Based on its ability to function as a free radical scavenger, it has been suggested that this indoleamine may act as a neuroprotective agent, protecting the brain from lipid peroxidation and free radical damage induced by reactive oxygen species (ROS) during brain ischemia [12]. This is in agreement with previous results of our group showing that melatonin treatment was able to diminish inflammation and apoptosis after brain stroke [7].
Although significant advance has been made in the understanding of the pathophysiology of cerebral ischemia, therapeutic options for acute ischemic stroke remain very limited [2]. Consequently, successful treatment of acute ischemic stroke remains one of the major challenges in clinical medicine. This is of great significance since the total incidence of stroke is projected to rise substantially over the years as a result of the expanding elderly population. Nonetheless, elderly people have generally been underrepresented in clinical trials, despite age being considered one of the most significant prognostic markers for poor stroke outcome, maybe as a result of the very few experimental studies performed in aged animals, which have created many uncertainties and less optimal medical care for elderly patients. In relation to this, the prescription and use of melatonin as a preventive drug against cerebrovascular accidents in aged individuals is far from being widespread in the clinical context. In addition, using melatonin before or after ischemic stroke may result in significantly different molecular changes, something that needs further clarification. Therefore, the present study aimed to compare the effect of pre-ischemia versus post-ischemia melatonin administration in aged rats subjected to ischemic brain injury.
2. Results
In previous studies, we observed that expression of TNF-α, IL-1β, glial fibrillary acidic protein (GFAP), Bcl-2-associated death promoter (BAD) and Bcl-2-associated X protein (BAX) increased significantly after right middle cerebral artery (MCA) occlusion, whereas sirtuin 1 (SIRT1) and B-cell lymphoma 2 (Bcl-2) significantly decreased [7].
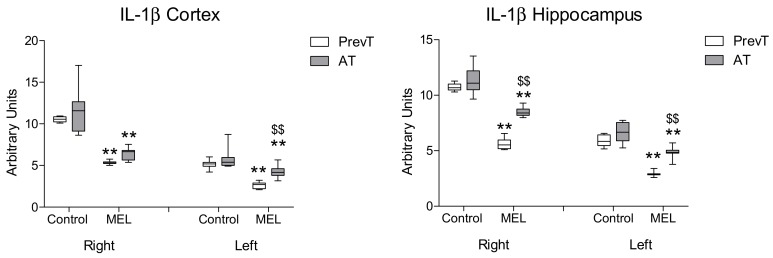
In the present study, both pre- and post-ischemia melatonin administrations were able to decrease IL-1β levels in both hemispheres in a significant manner (p < 0.01). In the hippocampus, pre-ischemia melatonin administration showed a significantly higher reduction in IL-1β levels, as compared to the post-ischemia treatment (p < 0.01). In cortex, no significant differences were found between the melatonin-treated groups in the ipsilateral (right) hemisphere (Figure 1). However, in the contralateral (left) side, PrevT (pre-ischemia treatment) animals showed significantly (p < 0.01) lower levels of this cytokine.
Figure 1.
Right and left cortical and hippocampal mRNA expression of IL-1β in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. ** p < 0.01 with respect to the values obtained in the control group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
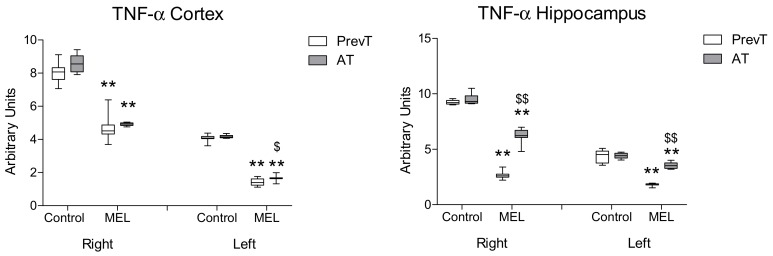
In the case of hippocampal TNF-α expression, pre-ischemia melatonin administration showed a significantly higher reduction (p < 0.01) as compared to the AT group. However, post-ischemia melatonin administration was also able to decrease significantly TNF-α levels in both right and left hippocampus, as compared to their respective controls (Figure 2). A similar effect was observed in the cortex, although here the levels of this pro-inflammatory marker showed a significant reduction (p < 0.05) in the left cortex of PrevT animals in comparison with the AT group.
Figure 2.
Right and left cortical and hippocampal mRNA expression of TNF-α in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. ** p < 0.01 with respect to the values obtained in the control group; $ p < 0.05 with respect to the values obtained in the PrevT group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
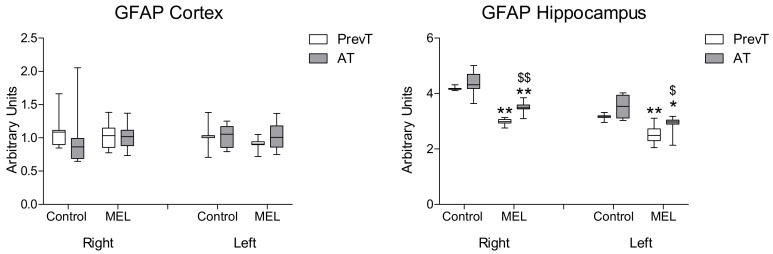
Regarding GFAP (Figure 3), no significant differences were found in cortex with either pre- or post-ischemia treatments. However, post-ischemia melatonin administration was able to decrease significantly GFAP levels in both right (p < 0.01) and left hippocampus (p < 0.05). The PrevT group experienced a significantly higher reduction in GFAP levels, as compared to the AT treated group. This difference was more significant in the ipsilateral (right) side of the lesion (p < 0.01).
Figure 3.
Right and left cortical and hippocampal mRNA expression of GFAP in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. * p < 0.05 with respect to the values obtained in the control group; ** p < 0.01 with respect to the values obtained in the control group; $ p < 0.05 with respect to the values obtained in the PrevT group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
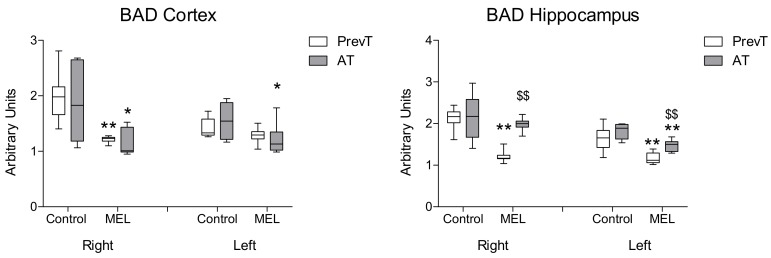
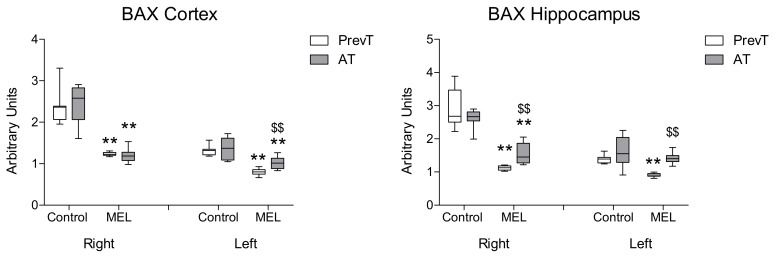
Melatonin treatment also reduced the expression of pro-apoptotic markers BAD (Figure 4) and BAX (Figure 5). In right and left hippocampus, a significantly higher reduction (p < 0.01) in these expressions was found in the PrevT group, as compared to the post-ischemia treatment. Regarding cortex, there were no significant differences between the melatonin-treated groups in BAD cortical levels; in the case of BAX, the pre-ischemia treatment achieved a significantly higher decrease in the contralateral (left) hemisphere (p < 0.01).
Figure 4.
Right and left cortical and hippocampal mRNA expression of BAD in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. * p < 0.05 with respect to the values obtained in the control group; ** p < 0.01 with respect to the values obtained in the control group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
Figure 5.
Right and left cortical and hippocampal mRNA expression of BAX in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. ** p < 0.01 with respect to the values obtained in the control group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
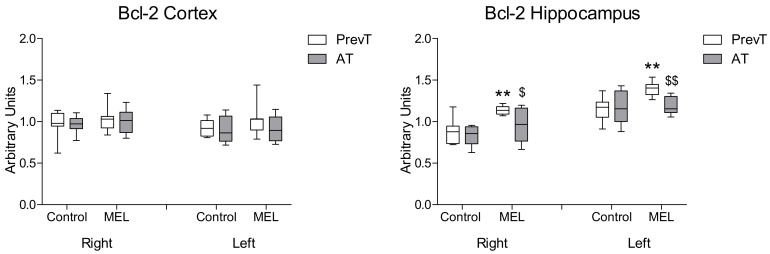
With respect to the anti-apoptotic marker Bcl-2 (Figure 6), no significant differences were found in cortex after melatonin treatment. In hippocampus, the administration of this indoleamine resulted in PrevT values being significantly higher in right (p < 0.05) and left (p < 0.01) hemispheres than those observed in the AT group.
Figure 6.
Right and left cortical and hippocampal mRNA expression of Bcl-2 in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. ** p < 0.01 with respect to the values obtained in the control group; $ p < 0.05 with respect to the values obtained in the PrevT group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
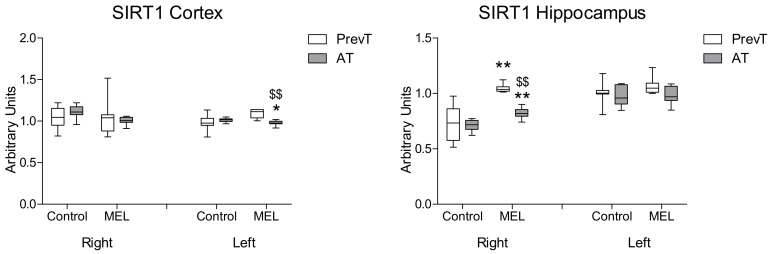
Regarding SIRT1 expression (Figure 7), both pre- and post-ischemia melatonin treatments were able to increase significantly the levels of SIRT1 (p < 0.01) in the right hippocampus, with a greater effect obtained in the PrevT animals (p < 0.01). In the left hippocampus and in both right and left cortex, both pre- and post-ischemia melatonin treatments did not increase SIRT1 levels.
Figure 7.
Right and left cortical and hippocampal mRNA expression of SIRT1 in 18-month-old rats in control (non-treated) conditions, after treatment with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (PrevT), and treated only after surgery during 7 days (AT). Each value represents the median, interquartile and full range of five determinations performed in duplicate. * p < 0.05 with respect to the values obtained in the control group; ** p < 0.01 with respect to the values obtained in the control group; $$ p < 0.01 with respect to the values obtained in the PrevT group.
3. Discussion
Stroke is a cerebrovascular accident that may cause long-term disability and death. Stroke risk factors include aging, diabetes, elevated lipids, smoking and heavy drinking, high blood pressure, coronary artery disease and heart diseases. Ischemic stroke is the most common form, accounting for around 85% of strokes. The effects of ischemic stroke are relatively rapid because the brain does not store glucose and is unable to perform anaerobic metabolism [13]. Within an hour of the ischemic insult, a core of infarction area occurs, surrounded by an oligemic zone called the ischemic penumbra where autoregulation is ineffective. The critical time period during which the brain tissue is at risk is referred to as the “window of opportunity” since, as previously mentioned, the neurological damage caused by ischemia can be partially or fully reversed by reperfusing the ischemic, yet viable, brain tissue [13,14,15,16,17,18].
When ischemia occurs, the mitochondrial membrane potential and the normal proton gradient are disrupted, resulting in excessive ROS generation via electron transfer for ATP synthesis [19]. This process defeats mitochondrial oxidant-scavenging capacity establishing a vicious positive feedback loop in which increased ROS induce more ROS production. ROS also activate inflammatory responses and damage mitochondrial DNA leading to cell swelling and death.
Inflammatory response to brain injury is initiated by the rapid production of several inflammatory mediators. It has been suggested that pro-inflammatory cytokines including TNF-α and IL-1 play a pivotal role in the pathology of ischemia/reperfusion-induced brain injury. Pro-inflammatory cytokines may contribute to brain damage both directly or indirectly [20].
Melatonin is a ubiquitous hormone that is secreted by the pineal gland primarily during darkness. A number of studies suggest that melatonin and its metabolites are highly effective physiological antioxidants and free radical scavengers [21,22]. Many biochemical and histopathological findings have revealed that melatonin exerts neuroprotective effects following experimental and clinical oxidative injury [23,24,25]. Furthermore, lower circulating levels of melatonin exaggerate the oxidative damage to tissues that are subjected to increased oxidative stress [26]. Melatonin exerts anti-inflammatory effects by inhibiting the synthesis of inflammatory cytokines such as IL-1, IL-6 and TNF-α [10,12].
We had previously demonstrated that melatonin, administered in a preventive fashion, was able to protect against brain ischemia/reperfusion-induced injury [7]. Here, this preventive effect was compared to that generated by the post-ischemia administration of melatonin after the induction of a brain ischemic lesion in the cortex and hippocampus of senescent rats. Immediately after surgery, all animals showed limb-use asymmetry and lateralization signs. Although all groups recovered after surgery, we observed that melatonin-treated animals did it faster than non-treated rats. In addition, some of the non-treated animals died in the first 72 h after surgery while animals receiving melatonin survived. However, no significant differences were observed in the mortality rate among groups. It was seen that both types of treatments were able to decrease the inflammatory response, i.e., TNF-α and IL-1β levels, in the ipsilateral and contralateral ischemic areas of both tissues, but more clearly in the PrevT group. Although the effect was more pronounced with that treatment, the findings of the present study also revealed a protective effect of post-ischemia melatonin administration against the aforementioned pro-inflammatory markers, mainly in the penumbra area. These results may be related to melatonin direct ROS-scavenging effects or its ability to regulate lymphocyte migration.
Regarding GFAP, a biomarker synthesized by astrocytes in response to physical or metabolic injuries, including those under pro-inflammatory or oxidative stress conditions, no significant differences were found in the cortex in melatonin-treated rats. Interestingly, in hippocampus a significant decrease was observed in both PrevT and AT groups. This effect was more evident when melatonin administration started 1 day before surgery (PrevT animals). Considering that during ischemia the formation of a colateral circulation that partially irrigates the ischemic lesion occurs, the ability of the penumbral neurons to be reperfused again appears to be evident and melatonin, therefore, may exert its most beneficial effects on them, limiting the extent of the lesion.
Both oxidative stress and inflammatory response can induce cell death. The two processes by which damaged neurons are known to die are necrosis and apoptosis. Apoptotic mechanisms begin within 1 h after ischemic injury, whereas necrosis begins by 6 h after arterial occlusion. This is important because, hypothetically, neuronal death can be prevented by modifying the process responsible for apoptosis. MCA blockade has been shown to cause a significant increase in the expression of pro-apoptotic markers in aged rats [7]. In the present study, this effect was inhibited by the administration of melatonin. Although the effect of pre-ischemia melatonin treatment was generally higher in both hippocampus and cortex, it is interesting to note that similar results were also obtained with post-ischemia treatment. This suggests that melatonin, by reducing the expression of apoptotic markers, could decrease neuronal damage. This would be in agreement with previous studies of other authors describing that the administration of melatonin after head injury prevented both detectable tissue damage and the cognitive deficits that it produced [27,28].
In the case of SIRT1, a protein marker of cell viability, a significant decrease after surgery had been observed in both right and left hippocampus after the MCA occlusion [7]. Like melatonin production, SIRT1 has an association with aging. However, few studies have investigated the relationship between melatonin and SIRT1 with age. Gutierrez-Cuesta et al. investigated the effects of melatonin on pro-survival processes observing that melatonin was able to enhance SIRT1 protein levels, which suggests that melatonin suppressed oxidative stress in SAMP8 mice allowing SIRT1 levels to increase [29]. According to this, in this study we observed that pre-ischemia melatonin administration induced a higher increase in SIRT1 levels, as compared to the post-ischemia treatment in right hippocampus. Nonetheless, and remarkably, in the left hippocampus, no significant differences were found between the pre- and the post-ischemia melatonin treatments. Only a few of the molecular mechanisms involved in sirtuin regulation by melatonin have been addressed in recent studies [30], suggesting that the melatonin membrane receptor pathway may be involved in the upregulation of SIRT1 activity [31]. Moreover, recent studies have shown that SIRT1 activation protects against early brain injury after experimental subarachnoidal hemorrhage in rats [32]. In particular, SIRT1 activation by resveratrol has been reported to reduce brain edema and neuronal apoptosis [33]. Melatonin-induced upregulation of SIRT1 has also been associated with an increase in the anti-apoptotic factor Bcl-2 and a reduction in the pro-apoptotic factor BAX [34], which may be related to the protective effects exerted by melatonin in the animal model used in this study. In fact, numerous findings report a rise in SIRT1 activity in a diversity of cells and animal models after melatonin treatment, with the exception of some tumor cells, where the effect is inhibitory [30].
The clinical outcomes resulting from cerebral ischemia are wide-ranging and include from short-term effects such as death, hypoxic-ischemic encephalopathy and seizures, to long-term ones including cerebral palsy and sensory, cognitive, developmental, neuroendocrine and behavioral deficits which are permanent and currently incurable. Little is known, however, on the possible protective effects of melatonin in elderly individuals affected by cerebral ischemia. For this reason, a possible strength of this study appears to be the use of aged animals in the ischemic brain injury performed experimentally after MCA occlusion. Nonetheless, a limitation is that neurological or behavioral deficits were not evaluated. However, a number of studies have reported that melatonin treatment shows a suitable profile to prevent and/or ameliorate these deficits, including spatial learning and memory performance, severe loss of pyramidal neurons, preservation of cytoarchitectural characteristics in prefrontal cortex and hippocampal CA1 pyramidal neurons, and a better display of place learning and working memory [35]. Further investigation including the use of immunofluorescence, immunohistochemical, and Western-blot techniques, together with the evaluation of neuronal post-treatment cell death/survival, neuroinflammatory astrocyte and microglial changes, as well as performing neurological and behavioral tests, are needed to elucidate the exact role of melatonin in the aging animal model used in the present study.
4. Materials and Methods
4.1. Animals
Eighteen-month-old male Wistar rats obtained from Harlan Ibérica (Barcelona, Spain) were housed under standard conditions with a 12/12 h light/dark photoperiod. Access to standard rodent chow (A04; Panlab, Barcelona, Spain) and water was permitted ad libitum. Animals were subjected to a blockade of the right MCA as a model of ischemic brain injury and divided into 3 groups: Control (non-treated), treated with a daily dose of melatonin (10 mg/kg b.w.) from 24 h before until 7 days after surgery (pre-ischemia treatment, PrevT), and treated only after surgery during 7 days (post-ischemia treatment, AT). The study was approved by the Ethical Committee of the Complutense University of Madrid (27 January 2011, Madrid, Spain) in accordance with the European directive on the protection of animals used for scientific purposes (2010/63/EU).
4.2. Surgical Procedure
The surgery was carried out following a protocol previously described [36]. Briefly, after premedication (fentanyl and medetomidine, 0.3/0.3 mg/kg b.w.), animals were intubated and maintained under isoflurane anesthesia. Cervical ventral area was shaved and animals were placed in supine position with the forelimbs open. The surgical field was prepared and sterilized. A midline incision from the laryngeal area up to 2 mm cranially to the xiphoid process was made and lengthened 1.5 cm to the right. The right external carotid artery was exposed, carefully separated from the soft tissues of the area, and ligated with a silk 3/0 suture placed caudally to the dissected portion. A microvascular clip was then placed cranially to the ligature. The carotid was opened in the middle point between the ligature and the microvascular clip with a 30 g needle. A 5/0 nylon filament was inserted up to the microvascular clip, which was removed to allow filament insertion into its final position. After assessing that the portion of filament introduced into the carotid artery was correctly positioned, a ligature was placed cranially to the site where the filament had been inserted. Absence of vascular bleeding was then checked and suture in layers (continuous subcutaneous–vicryl 3/0– and skin with interrupted stitches–vicryl or silk 3/0 3/0–) was performed. Isoflurane supply was interrupted, anesthesia was reversed with atipamezole (0.3 mg/kg b.w.) and a broad-spectrum antibiotic (enrofloxacin injectable solution) and an opioid analgesic (buprenorphine) were supplied. Analgesia was repeated at 12 and 24 h after surgery. Throughout the surgical procedure and in the post-surgical recovery, the animals were placed on a heating blanket.
4.3. Administration of Melatonin
Melatonin (Actafarma, Madrid, Spain) was dissolved in absolute ethanol, diluted with water to a final concentration of 0.1%, and added to the drinking water at a dose allowing the supply of 10 mg/kg b.w./day in 25–30 mL. The dose of 10 mg/kg b.w. melatonin has been extensively used in scientific literature and it corresponds approximately to a dose of 1.62 mg/kg b.w. in humans, i.e., around 100 mg/day for a 60 kg person [37]. Previous studies observed that the daily administration to adults of doses up to 300 mg of melatonin produced no significant adverse reactions [38]. Moreover, follow-up studies of more than 10 years have reported no side effects [39]. Eighteen-month-old animals (treated groups) started the supply of this melatonin solution 24 h before (PrevT) or immediately after (AT) surgical procedure and during 7 days of post-treatment period until sacrifice. Drinking water with 0.1% ethanol only was available for the non-treated group. At the end of the treatment period, animals were sacrificed by decapitation and cortex and hippocampus of both brain hemispheres were carefully dissected. Tissues were immediately frozen in liquid nitrogen and kept at −80 °C until analyzed.
4.4. RNA Isolation and RT-PCR
mRNA expression of IL-1β, TNF-α, BAD, BAX, GFAP, Bcl-2 and SIRT1 was measured by means of reverse transcription-polymerase chain reaction (RT-PCR). RNA was isolated from cortex and hippocampus samples using the TRI Reagent Kit (Molecular Research Center, Inc., Cincinnati, OH, USA), following the manufacturer’s protocol. The purity of the RNA was estimated with 1% agarose gel electrophoresis, and the RNA concentration and purity were determined with spectrophotometry (260/280 nm). Reverse transcription of 2 µg of RNA for cDNA synthesis was performed using the Reverse Transcription System (Promega, Madison, WI, USA), and a pd(N) 6 random hexamer. RT-PCR was performed using an Applied Biosystems 7500 Fast apparatus with the SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) and 300 nM concentrations of specific primers (Table 1). RT-PCR amplifications were performed as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, 60 °C for 30 s and 95 °C for 15 s. Normalization of cDNA loading in the PCR was performed using the amplification of 18S ribosomal RNA. Relative changes in gene expression were calculated using the 2−ΔΔCt method.
Table 1.
Primers used in RT-PCR experiments. 18S was used as a housekeeping gene to compare the samples.
| Marker | Primers | Sequence (5′–3′) |
|---|---|---|
| 18S | Forward | GGTGCATGGCCGTTCTTA |
| Reverse | TCGTTCGTTATCGGAATTAACC | |
| IL-1β | Forward | TGTGATGAAAGACGGCACAC |
| Reverse | CTTCTTCTTTGGGTATTGTTTGG | |
| TNF-α | Forward | ATGAGAAGTTCCCAAATGGC |
| Reverse | CTCCACTTGGTGGTTTGCTA | |
| BAD | Forward | GCCCTAGGCTTGAGGAAGTC |
| Reverse | CAAACTCTGGGATCTGGAACA | |
| BAX | Forward | GTGAGCGGCTGCTTGTCT |
| Reverse | GGTCCCGAAGTAGGAGAGGA | |
| GFAP | Forward | ACAGACTTTCTCCAACCTCCAG |
| Reverse | CCTTCTGACACGGATTTGGT | |
| Bcl-2 | Forward | CAGGTATGCACCCAGAGTGA |
| Reverse | GTCTCTGAAGACGCTGCTCA | |
| SIRT1 | Forward | TCGTGGAGACATTTTTAATCAGG |
| Reverse | GCTTCATGATGGCAAGTGG |
4.5. Statistical Analysis
Data are expressed as median, interquartile and full range of the number of determinations carried out in duplicate. The results were analyzed by ANOVA followed by Fisher’s test. Statistical analyses were carried out using the SPSS 24.0 computer program (SPSS, Chicago, IL, USA). The level of statistical significance was established at p < 0.05.
5. Conclusions
Melatonin treatment may mediate neuroprotection against ischemic brain injury by limiting the consequential inflammatory and apoptotic responses in both brain hemispheres. Although the effects of melatonin were found to be more evident when treating animals in a preventive way, post-ischemia melatonin administration also showed a significant reduction in inflammation and apoptosis, as compared to the non-treated groups. Thus, melatonin appears as a valuable therapeutic agent that may protect the elderly from the damaging effects of stroke, either before or after injury occurrence, with potential actions in preventing the extent of the lesion.
Acknowledgments
The authors would like to express their thanks to Daniel Campón and Rocío Campón for their skillful technical assistance, and to medical student Blanca Bermudo for her continuous interest and co-operation in our work.
Author Contributions
Conceptualization, P.G., C.R.-B. and J.A.F.T.; Formal analysis, E.V.; Funding acquisition, E.V. and J.A.F.T.; Investigation, L.R., S.D.P., C.G., P.G., C.R.-B., M.C.-S. and B.H.; Methodology, L.R., S.D.P., C.G., P.G., C.R.-B., M.C.-S. and B.H.; Project administration, E.V. and J.A.F.T.; Supervision, E.V. and J.A.F.T.; Validation, E.V. and J.A.F.T.; Writing—original draft, L.R. and S.D.P.; Writing—review & editing, L.R., S.D.P., E.V. and J.A.F.T.
Funding
Fundación Mutua Madrileña: Ayudas a la Investigación 2011; Fundación Rodríguez Pascual: Ayudas a la Investigación Biomédica 2011; Red Cooperativa de Envejecimiento y Fragilidad 2013–2016—Instituto de Salud Carlos III: RETICEF-RD12/0043/0032; Universidad Complutense de Madrid-Banco de Santander: Programa de Financiación para Grupos de Investigación UCM 2013.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lo E.H., Dalkara T., Moskowitz M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 2.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 3.Muir K.W., Tyrrell P., Sattar N., Warburton E. Inflammation and ischaemic stroke. Curr. Opin. Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 5.Ferrarese C., Mascarucci P., Zoia C., Cavarretta R., Frigo M., Begni B., Sarinella F., Frattola L., De Simoni M.G. Increased cytokine release from peripheral blood cells after acute stroke. J. Cereb. Blood Flow Metab. 1999;19:1004–1009. doi: 10.1097/00004647-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Caso J.R., Moro M.A., Lorenzo P., Lizasoain I., Leza J.C. Involvement of IL-1beta in acute stress induced worsening of cerebral ischemia in rats. Eur. Neuropsychopharmacol. 2007;17:600–607. doi: 10.1016/j.euroneuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Paredes S.D., Rancan L., Kireev R., González A., Louzao P., González P., Rodríguez-Bobada C., García C., Vara E., Tresguerres J.A. Melatonin Counteracts at a Transcriptional Level the Inflammatory and Apoptotic Response Secondary to Ischemic Brain Injury Induced by Middle Cerebral Artery Blockade in Aging Rats. Biores. Open Access. 2015;4:407–416. doi: 10.1089/biores.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y., Yang G.Y., Ahlemeyer B., Pang L., Che X.M., Culmsee C., Klumpp S., Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J. Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spera P.A., Ellison J.A., Feuerstein G.Z., Barone F.C. IL-10 reduces rat brain injury following focal stroke. Neurosci. Lett. 1998;251:189–192. doi: 10.1016/S0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 10.Puig Á., Rancan L., Paredes S.D., Carrasco A., Escames G., Vara E., Tresguerres J.A. Melatonin decreases the expression of inflammation and apoptosis markers in the lung of a senescence-accelerated mice model. Exp. Gerontol. 2016;75:1–7. doi: 10.1016/j.exger.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Paredes S.D., Forman K.A., García C., Vara E., Escames G., Tresguerres J.A. Protective actions of melatonin and growth hormone on the aged cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014;18:79–88. doi: 10.1515/hmbci-2014-0016. [DOI] [PubMed] [Google Scholar]
- 12.Reiter R.J., Tan D.X., Fuentes-Broto L., Paredes S.D., Sanchez-Barcelo E., Mediavilla M.D. Melatonin salvages neural tissue from ischemia/reperfusion injury. Open Neurendocrinol. J. 2010;3:112–120. [Google Scholar]
- 13.Jones T.H., Morawetz R.B., Crowell R.M., Marcoux F.W., FitzGibbon S.J., DeGirolami U., Ojemann R.G. Thresholds of focal ischemia in awake monkeys. J. Neurosurg. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- 14.Astrup J., Seisjo B.K., Symon L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.STR.12.6.723. [DOI] [PubMed] [Google Scholar]
- 15.Wise R.J., Bernardi S., Frackowiak R.S., Legg N.J., Jones T. Serial observations on the pathophysiology of acute stroke: The transition from ischaemia to infarction as reflected in regional oxygen extraction. Brain. 1983;106:197–222. doi: 10.1093/brain/106.1.197. [DOI] [PubMed] [Google Scholar]
- 16.Heros R. Stroke: Early pathophysiology and treatment. Summary of the Fifth Annual Decade of the Brain Symposium. Stroke. 1994;25:1877–1881. doi: 10.1161/01.STR.25.9.1877. [DOI] [PubMed] [Google Scholar]
- 17.Garcia J.H., Yoshida Y., Chen H., Li Y., Zhang Z.G., Lian J., Chen S., Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am. J. Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- 18.Hossmann K.A. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 19.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doll D.N., Rellick S.L., Barr T.L., Ren X., Simpkins J.W. Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J. Neurochem. 2015;132:443–451. doi: 10.1111/jnc.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu A.W., Maldonado M., Sanchez-Hidalgo M., Tan D.X., Reiter R.J. Protective effects of melatonin in experimental free radical-related ocular diseases. J. Pineal Res. 2006;40:101–109. doi: 10.1111/j.1600-079X.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 22.Tan D.X., Manchester L.C., Terron M.P., Flores L.J., Reiter R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 23.Montilla P.L., Túnez I.F., Muñoz de Agueda C., Gascón F.L., Soria J.V. Protective role of melatonin and retinol palmitate in oxidative stress and hyperlipidemic nephropathy induced by adriamycin in rats. J. Pineal Res. 1998;25:86–93. doi: 10.1111/j.1600-079X.1998.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 24.Okatani Y., Wakatsuki A., Kaneda C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000;28:89–96. doi: 10.1034/j.1600-079X.2001.280204.x. [DOI] [PubMed] [Google Scholar]
- 25.Reiter R.J., Tan D.X., Terron M.P., Flores L.J., Czarnocki Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007;54:1–9. [PubMed] [Google Scholar]
- 26.Reiter R.J., Tan D.X., Cabrera J., D’Arpa D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv. Exp. Med. Biol. 1999;467:379–387. doi: 10.1007/978-1-4615-4709-9_48. [DOI] [PubMed] [Google Scholar]
- 27.Mésenge C., Margaill I., Verrecchia C., Allix M., Boulu R.G., Plotkine M. Protective effect of melatonin in a model of traumatic brain injury in mice. J. Pineal Res. 1998;25:41–46. doi: 10.1111/j.1600-079X.1998.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Wakatsuki A., Okatani Y., Shinohara K., Ikenoue N., Fukaya T. Melatonin protects against ischemia/reperfusion-induced oxidative damage to mitochondria in fetal rat brain. J. Pineal Res. 2001;31:167–172. doi: 10.1034/j.1600-079x.2001.310211.x. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez-Cuesta J., Tajes M., Jiménez A., Coto-Montes A., Camins A., Pallàs M. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model. J. Pineal Res. 2008;45:497–505. doi: 10.1111/j.1600-079X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 30.Mayo J.C., Sainz R.M., González Meléndez P., Cepas V., Tan D.X., Reiter R.J. Melatonin and sirtuins: A ‘not-so unexpected’ relationship. J. Pineal Res. 2017;62:e12391. doi: 10.1111/jpi.12391. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L., Liu H., Yue L., Zhang J., Li X., Wang B., Lin Y., Qu Y. Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-KB signaling pathway following subarachnoid hemorrhage in mice. Mol. Neurobiol. 2017;54:1612–1621. doi: 10.1007/s12035-016-9776-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X.S., Wu Q., Wu L.Y., Ye Z.N., Jiang T.W., Li W., Zhuang Z., Zhou M.L., Zhang X., Hang C.H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016;7:e2416. doi: 10.1038/cddis.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian C., Jin J., Chen J., Li J., Yu X., Mo H., Chen G. SIRT1 activation by resveratrol reduces brain edema and neuronal apoptosis in an experimental rat subarachnoid hemorrhage model. Mol. Med. Rep. 2017;16:9627–9635. doi: 10.3892/mmr.2017.7773. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y., Jiang S., Dong Y., Fan C., Zhao L., Yang X., Li J., Di S., Yue L., Liang G., et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015;58:61–70. doi: 10.1111/jpi.12193. [DOI] [PubMed] [Google Scholar]
- 35.Ramos E., Patiño P., Reiter R.J., Gil-Martín E., Marco-Contelles J., Parada E., de Los Rios C., Romero A., Egea J. Ischemic brain injury: New insights on the protective role of melatonin. Free Radic. Biol. Med. 2017;104:32–53. doi: 10.1016/j.freeradbiomed.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Cipolla M.J., Lessov N., Hammer E.S., Curry A.B. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: Effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.STR.32.7.1658. [DOI] [PubMed] [Google Scholar]
- 37.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 38.Weishaupt J.H., Bartels C., Pölking E., Dietrich J., Rohde G., Poeggeler B., Mertens N., Sperling S., Bohn M., Hüther G., et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006;41:313–323. doi: 10.1111/j.1600-079X.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 39.Buscemi N., Vandermeer B., Pandya R., Hooton N., Tjosvold L., Hartling L., Baker G., Vohra S., Klassen T. Melatonin for treatment of sleep disorders. Evid. Rep. Technol. Assess. 2004;108:1–7. doi: 10.1037/e439412005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]