Abstract
We sought a regimen that incorporates optimal novel agents in transplant-ineligible patients that balances efficacy with toxicity. Our study evaluated modified lenalidomide-bortezomib-dexamethasone (RVD lite) in this population. RVD lite was administered over a 35-day cycle. Lenalidomide 15 mg was given orally days 1–21; bortezomib 1.3 mg/m2 weekly subcutaneously (SC) on days 1, 8, 15, and 22; and dexamethasone 20 mg orally day of and after bortezomib for 9 cycles followed by 6 cycles of consolidation with lenalidomide and bortezomib. Primary objective was to evaluate overall response rate (ORR). Secondary objectives included safety, progression free survival (PFS), and overall survival (OS). Fifty-three eligible patients screened between 4/17/13 and 5/20/15; 50 received at least one dose of therapy. Median age at study entry was 73 years (range 65–91). The ORR was 86% and 66% of patients achieved a very good partial response (VGPR) or better. Median PFS was 35.1 months (95% CI, 30.9 - ∞) and median OS was not reached at a median follow-up of 30 months. Peripheral neuropathy was reported in 31 (62%) patients with only 1 patient experiencing grade 3 symptoms. RVD lite is a well-tolerated and highly effective regimen in the transplant-ineligible population with robust PFS and OS.
Keywords: multiple myeloma, transplant-ineligible
Introduction
Multiple myeloma (MM) is a malignant proliferation of plasma cells. It is the second most common hematologic malignancy, accounting for an estimated 30,280 cases and 12,590 deaths in the US in 2017.(Siegel, et al 2017) MM is a disease of older adults with a median age at diagnosis of 66 years.(Kyle, et al 2003) Over the last 10 years, autologous stem-cell transplantation and the availability of effective new drugs such as the immunomodulatory drugs (thalidomide, lenalidomide, and pomalidomide) and the proteasome inhibitors (bortezomib, carfilzomib, and ixazomib) have greatly advanced treatment paradigms and improved the quality and duration of life for patients with this disease. However, the survival benefits are limited in elderly or frail individuals with MM. This may be a reflection of associated comorbidities in the older patient population, differences in disease biology, as well as the lack of participation in clinical trials because of intensity of visit schedule.(Kumar, et al 2014)
Melphalan and prednisone-based regimens were historically the most widely accepted treatment option since the 1960s in the older population ineligible for high-dose therapy.(Facon, et al 2006) More recent data from the FIRST trial established continuous lenalidomide and dexamethasone (Rd) as the new standard of care for transplant-ineligible patients with newly-diagnosed MM (NDMM).(Benboubker, et al 2014) Motivated by in vitro data showing synergistic activity between bortezomib and lenalidomide,(Mitsiades, et al 2002) a major step forward has been combining lenalidomide, bortezomib, and dexamethasone (RVD).(Kumar, et al 2012, Richardson, et al 2010) Among patients treated at the maximum tolerated dose of lenalidomide 25 mg days 1–14, bortezomib 1.3 mg/m2 IV days 1, 4, 8, 11, dexamethasone 20 mg day of and day after bortezomib on a 21-day cycle, the ORR rate was 100% with rates of VGPR or better seen in 74% patients. The Southwest Oncology Group (SWOG) study S0777 validated this triplet regimen, showing superior PFS, OS, and ORR with the triplet combination of RVD versus the doublet combination of Rd alone in patients with NDMM without intention for immediate autologous stem cell transplant (SCT).(Durie, et al 2017)
However, the dosing schedule of RVD may be more challenging for older patients. In patients older than 75 years in this trial (N = 4), dose reductions were required in all patients.7 A recently published phase II study of upfront lower-dose lenalidomide for relapsed, refractory (RR) MM supports the idea of treatment attenuation in older patients to minimize toxicity while maintaining efficacy.(Quach, et al 2017) In patients with RRMM treated with 15 mg of lenalidomide, the ORR was 71%, and the PFS and OS were 8.9 and 30.5 months, respectively. This compared favorably and without statistical difference from a matched cohort of patients from the phase III MM009/MM010 trial with a reduction in rates of neutropenia, infection, and venous thromboembolism.(Dimopoulos, et al 2009)
With a view to exploit the synergy seen in preclinical and clinical studies and to maximize tolerability and convenience in transplant-ineligible patients with NDMM, we propose a reduction in the intensity of the RVD regimen, RVD lite, combining lenalidomide at a dose of 15 mg days 1–21; bortezomib 1.3 mg/m2 days 1, 8, 15, and 22 and dexamethasone 20 mg days of and the days after bortezomib on a 35-day cycle.
Methods
Study Design and Patients
In this phase II, single-arm, multicenter study, we prospectively investigated modified RVD (RVD lite) in transplant-ineligible patients with NDMM. Patients were eligible for the study if they had a new diagnosis of MM by the International Myeloma Working Group (IMWG) diagnostic criteria, were 65 years of age or older and/or ineligible for autologous stem cell transplantation in the opinion of the investigator. Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients were excluded if they had received prior systemic therapy for MM, had a platelet level <50,000/mm3, an absolute neutrophil count (ANC) <1000/mm3, a hemoglobin < 8 g/dL, hepatic impairment (total bilirubin ≤ 1.5 × the upper limit of normal (ULN); transaminases ≤ 2 × ULN), renal insufficiency defined as a serum creatinine > 2.5 mg/dL, or peripheral neuropathy ≥ 2 on clinical examination. Females of child-bearing potential had to be on contraception during the study and have negative pregnancy tests before study enrollment. The primary endpoint was ORR. Secondary endpoints included safety, toxicity, PFS, OS, time to response, HRQoL, as well as, the PK profile of IV versus SC administration of bortezomib in combination with lenalidomide and dexamethasone. All patients provided written informed consent. This study was approved by the Institutional Review Board at each of the participating sites. This trial was registered at clinicaltrials.gov (NCT01782963).
Treatment
Patients received lenalidomide 15 mg orally once daily on days 1–21; bortezomib 1.3 mg/m2 SC on days 1, 8, 15, and 22; and dexamethasone 20 mg orally on days 1, 2, 8, 9, 15, 16, 22, and 23 for patients ≤75 years and days 1, 8, 15, 22 for patients older than 75 years of a 35-day cycle. The first 10 patients received bortezomib IV. Induction consisted of 9 cycles of this three-drug combination followed by 6 cycles of consolidation with lenalidomide 15 mg days 1–21 and bortezomib 1.3 mg/m2 days 1 and 15 on a 28-day cycle (Figure 1). Lenalidomide maintenance was not mandated by the protocol but was continued at the discretion of the investigator until disease progression or the development of unacceptable side effects.
Figure 1.
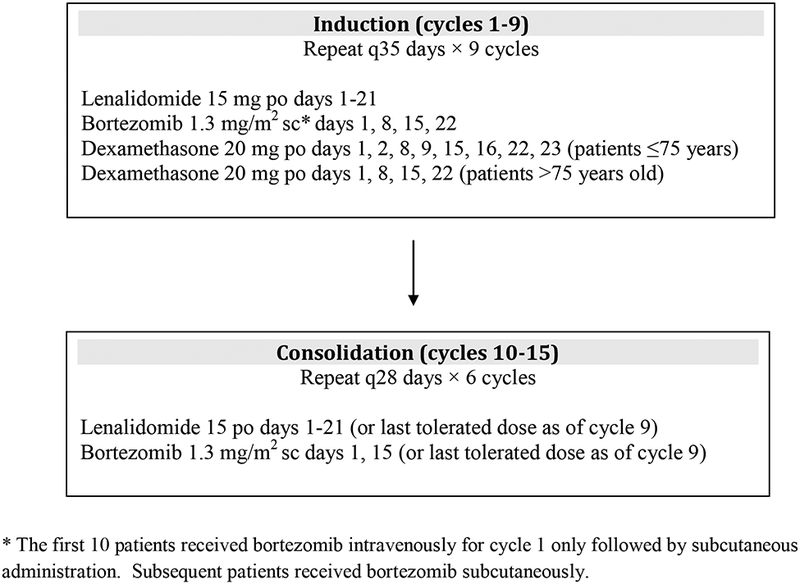
Treatment Schema
Thromboprophylaxis with daily aspirin (81 mg or 325 mg) or low molecular weight heparin was required for all participants. Prophylaxis against herpes zoster with antivirals was also mandated during induction and consolidation cycles. Concomitant bisphosphonate therapy, according to institutional guidelines, was required during induction cycles.
Toxicity assessments were done using the Cancer Therapy Evaluation Program (CTEP) Version 4.0 of the NCI Common Terminology Criteria for Adverse Events (CTCAE). Dose modifications or delays were done based on the toxicity experienced during a cycle of therapy or newly encountered on day 1 of each cycle. Dose modifications for lenalidomide were prespecified for renal insufficiency. Reduction and/or temporary suspension of one agent alone was allowed if toxicity was related primarily to one of the agents. Lenalidomide and bortezomib were held for ≥ Grade 3 neutropenia or thrombocytopenia. Use of granulocyte-colony stimulating factor and platelet transfusions were allowed. For other lenalidomide or bortezomib-related non-hematologic toxicity ≥ Grade 3, the attribution of the toxicity was determined by the investigator and the appropriate therapy was held until resolution of the toxicity to Grade 2 or less before resuming therapy with one level dose reduction.
Patients underwent blood count and serum chemistry monitoring on days 1, 8, 15, and 22 for the first two cycles and on day 1 of each cycle thereafter. Physical examination, serum and urine protein electrophoresis, and serum free light chains were performed on day 1 of each cycle. An independent data and safety monitoring committee reviewed safety and efficacy data throughout the study.
Assessment
Disease response to treatment was assessed using criteria based on the IMWG uniform response criteria.(Durie, et al 2006, Rajkumar, et al 2011) Responses were assessed on day 1 of each cycle. PFS was defined from the first day of study treatment to disease progression or death from any cause. Toxicity was assessed throughout the study. Plasma concentrations of bortezomib were measured pre-dose and at 5 minutes, 30 minutes, and 5 hours post-dose in 20 patients (10 SC, 10 IV). Age and weight were balanced between the two cohorts. Measurements of QoL were conducted using the The European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire - Core (QLQ-C30), the EORTC Multiple Myeloma (QLQ-MY20) module, and the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT-NTX) side-effects questionnaires. Follow-up assessments were performed every 2 months. These assessments include serum and urine protein electrophoresis, serum and urine immunofixation, immunoglobulins assay, and serum free light chain assay. At these assessments, participants were followed for disease status, survival, and long-term toxicities. Once a patient progressed, disease assessments were no longer required.
Statistical Analysis
The primary endpoint of this study was ORR after 4 cycles of RVD lite. An initial cohort of 10 eligible participants received IV bortezomib. This study used a Simon’s two-stage design to allow for early termination of the study if the combination lacked efficacy. Treatment was considered effective among the 40 eligible participants if 25 or more eligible participants achieve a PR. This design had at least an 87% chance of concluding the treatment was effective when the true response rate was 70% and less than a 10% chance of concluding the treatment was effective when the true response rate was 50% or less. With 40 eligible participants entered on study, the 90% exact binomial confidence interval width for toxicity and response was no wider than 28%.
Statistical analyses for this trial were performed using SAS and R. Patient characteristics were summarized using proportions for discrete data and median for continuous variables with comparisons between arms performed using Fisher’s exact test for discrete data and Mann-Whitney rank sum for continuous data. Time-to-event outcomes were estimated using Kaplan-Meier methods and compared between groups using stratified log rank tests. Logistic regression models and Cox proportional hazard regression models were implemented to evaluate the impact of baseline information on response and time to event outcomes.
QLQ-C30 and QLQ-MY20 domains were scored in accordance with their published guidelines. Results were transformed into scales ranging from 0 to 100 for QLQ-C30 and QLQ-MY20. For the functional scales (Global Health Status and Physical Functioning), higher scores indicate better HRQoL, whereas for the symptom scales (Fatigue, Pain, Disease Symptoms, and Side Effects of Treatment), lower scores indicate a better health state.
Results
Patients
From April 17, 2013 to May 20, 2015, 53 eligible patients were screened at 5 sites; 50 patients received at least one dose of therapy. Median age at study entry was 73 years (range 65–91) with 27 (54%) women and 23 (46%) men (Table 1). ECOG performance status of patients enrolled was 0 in 25 (50%), 1 in 18 (36%), and 2 in 7 (14 %) patients. The International Staging System (ISS) stage was I in 19 (38 %), II in 17 (34%), and III in 14 (28%) pts. High-risk cytogenetics defined as the presence of deletion 17, t(4;14), t(14;16), and/or t(16;20) by fluorescence in-situ hybridization were identified in 6 (12%) patients.
Table 1.
Patient Characteristics
| Characteristic | No. (%) of Patients | |
|---|---|---|
| N=50 | % | |
| Median Age at Diagnosis | 73 | (65–91) |
| Sex | ||
| Female | 27 | 54 |
| Male | 23 | 46 |
| Race | ||
| White | 42 | 84 |
| Black or African-American | 2 | 4 |
| Asian | 3 | 6 |
| Other | 3 | 6 |
| ISS Stage at Diagnosis | ||
| I | 19 | 38 |
| II | 17 | 34 |
| III | 14 | 28 |
| Durie-Salmon Stage | ||
| I | 16 | 32 |
| II | 16 | 32 |
| III | 18 | 36 |
| ECOG Performance Status | ||
| 0 | 25 | 50 |
| 1 | 18 | 36 |
| 2 | 7 | 14 |
| High-Risk Cytogenetics1 | ||
| Yes | 6 | 12 |
| No | 42 | 84 |
| Unknown | 2 | 4 |
| Serum Heavy/Light Chain | ||
| IgG | 34 | 68 |
| IgA | 9 | 18 |
| Light-chain only | 5 | 10 |
| Unknown | 2 | 4 |
| Median Body Mass Index | 28 | (18–45) |
Includes del 17, t(4;14), t(14;16), t(14;20)
Efficacy
Overall Response Rate.
The primary endpoint was ORR after four cycles of RVD lite (Table 2). Fifty patients received at least one cycle of therapy. Three patients withdrew from the study after less than 1 cycle. Of these patients, one withdrew for worsened adrenal insufficiency, one for rash attributed to lenalidomide, and one for neurosurgical intervention who was removed at the investigator’s discretion. One additional patient completed 4 cycles of treatment but did not return for C5D1 visit so response was based on response after cycle 3. The ORR was 86% for the 50 patients, 66% of whom achieve a VGPR or better. Of the 46 patients evaluable for response after 4 cycles of therapy, the ORR was 94% and 72% achieved a VGPR or better.
Table 2.
Response to RVD lite Therapy
| Best Overall Response | N=50 | % |
|---|---|---|
| Stringent Complete Response | 6 | 12 |
| Complete Response | 16 | 32 |
| Very Good Partial Response | 11 | 22 |
| Partial Response | 10 | 20 |
| Minimal Response | 1 | 2 |
| Stable Disease | 3 | 6 |
| Not Evaluable1 | 3 | 6 |
| ORR | 43 | 86 |
| VGPR or better | 33 | 66 |
Received less than 4 cycles of therapy
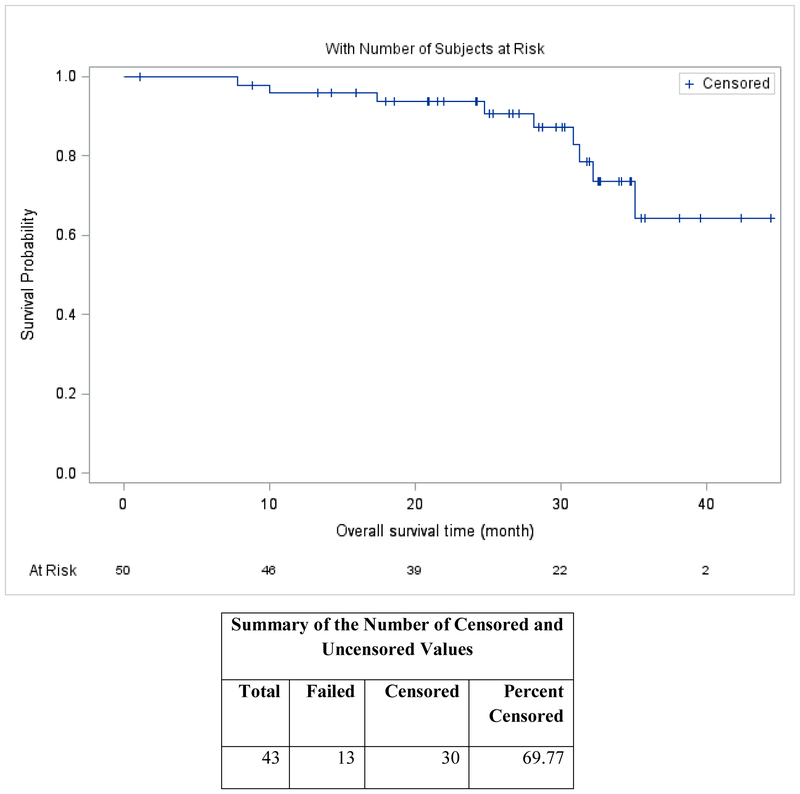
The median follow-up time was 30 months (95% confidence interval [CI], 25–33) based on inverse Kaplan-Meier estimation. Median PFS was 35.1 months (95% CI, 30.9 - ∞) (Figure 2A), and median OS (Figure 2B) was not reached. The median time to response was 1.1 months. Sixty-six percent of patients received lenalidomide maintenance.
Figure 2A.
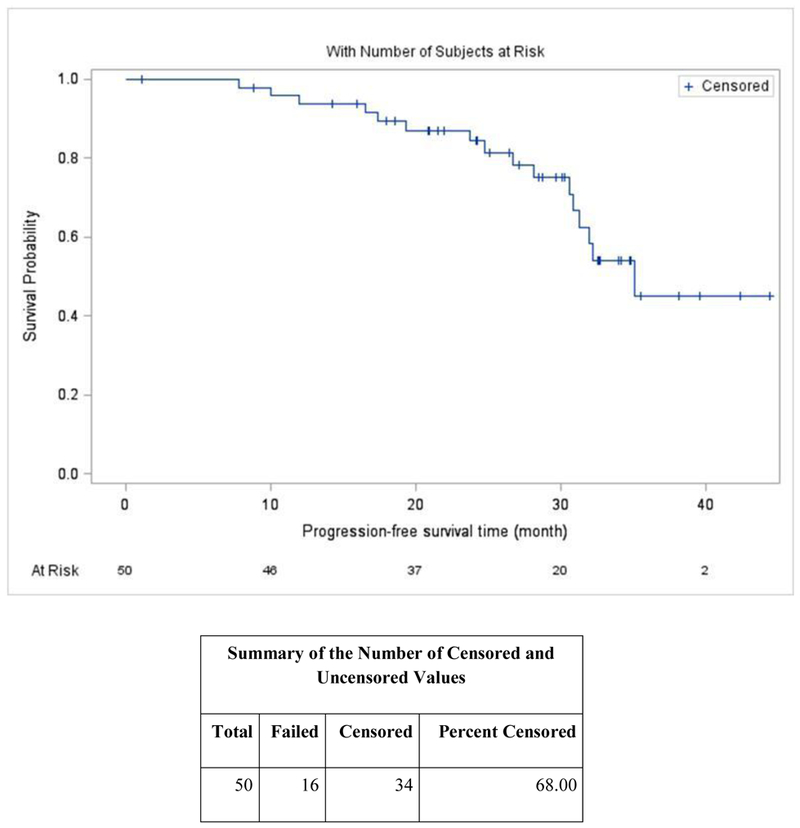
Progression Free Survival
Figure 2B.
Overall Survival
Safety.
Table 3 lists the most commonly reported adverse events (AEs). Fatigue was the most commonly reported toxicity occurring in 37 (74%) patients, and was mostly grade 1 or 2 in 29 (58%). Peripheral neuropathy of any grade was reported in 31 (62%) patients. Neuropathy appeared after a median of 6 cycles. Seventeen (34%) patients experience grade 1 and 9 (18%) grade 2 sensory peripheral neuropathy symptoms. Only one patient experienced grade 3 sensory neuropathy symptoms. Three patients (6%) reported grade 1 peripheral motor neuropathy and 3 (6%) grade 2 motor neuropathy. Upper respiratory infections occurred in 6 (12%) of patients. Grade 3 or greater toxicities occurring in 10% or more of patients included hypophosphatemia in 17 (34%), fatigue 8 (16%), neutropenia 7 (14%), and rash in 5 (10%) patients. Dose modifications occurred in 39 (78%) patients: dose reductions were made in 19 (38%) patients for bortezomib, 27 (54%) for lenalidomide, and 32 (64%) for dexamethasone. Two patients (4%) discontinued treatment due to toxicity. One patient developed a retinal artery thrombosis that was attributed to lenalidomide and the other patient developed grade 3 peripheral neuropathy attributed to bortezomib.
Table 3.
Treatment-related Adverse Events with Overall Incidence Greater Than 15%
| Any grade | Grade 3 or higher | |||
|---|---|---|---|---|
| Adverse event | Total | % | Total | % |
| Fatigue | 37 | 74 | 8 | 16 |
| Peripheral Neuropathy | 30 | 60 | 1 | 2 |
| Hypophosphatemia | 23 | 46 | 17 | 34 |
| Neutropenia | 22 | 44 | 7 | 14 |
| Diarrhea | 19 | 38 | 0 | 0 |
| Peripheral Edema | 18 | 36 | 1 | 2 |
| Insomnia | 17 | 34 | 1 | 2 |
| Rash | 17 | 34 | 5 | 10 |
| Anemia | 14 | 28 | 1 | 2 |
| Thrombocytopenia | 12 | 24 | 1 | 2 |
| Constipation | 12 | 24 | 0 | 0 |
| Dysgeusia | 12 | 24 | 0 | 0 |
| Hyperglycemia | 12 | 24 | 2 | 4 |
| Nausea | 9 | 18 | 0 | 0 |
| Depression | 8 | 16 | 0 | 0 |
| Psychiatric Disorder, other | 8 | 16 | 2 | 4 |
| Generalized Muscle Weakness | 8 | 16 | 2 | 4 |
Treatment Exposure.
Patients received a median of 15 (range 0–15) complete cycles of RVD lite. Thirty-two (64%) of patients completed all 15 cycles of therapy. Thirty-seven (74%) of patients completed induction. Of the 18 patients that discontinued therapy prior to completion, 6 (12%) had progressive disease, 4 (8%) withdrew consent, 3 (6%) discontinued at the discretion of the investigator, 2 (4%) discontinued due to toxicity, 1 (2%) experienced a toxicity unrelated to study drug and was removed from study, 1 (2%) wished to travel for the winter, and 1 (2%) switched to non-protocol therapy.
Pharmacokinetics.
Plasma concentrations of bortezomib were significantly higher for the IV route, 98.8 v 13.0 mg/mL (p<0.0002) at 5 minutes, respectively but higher for the SC route at 30 minutes, 20.5 v 8.73 ng/mL (p<0.0082). There was no significant difference in plasma concentrations of bortezomib at 5 hours. In the SC route, high body mass index (BMI) patients tended to have low concentration at both the 5 and 30 minute measures but not at 5 hours. There was no correlation with BMI using the IV route (Supp. Table 1 and Figure 1). There was no correlation between age and concentration level.
Patient-Reported Outcomes.
Patient-reported QoL, functional, and symptom assessment scores comparing baseline and end of treatment were reported in 30 study patients (Figure 3, Supp. Table 2). At the end of treatment, there were statistically significant improvements in scores of physical functioning (p=0.013), future perspective (p=0.023) and disease symptoms (p=0.001) when compared to baseline. Patients reported fewer symptoms across all symptom domains with the exception of diarrhea. EORTC global QoL scores showed improvement over the course of therapy though it did not reach statistical significance. There were no statistically significant changes in QoL using the FACT-GOG instrument over the course of treatment.
Figure 3.
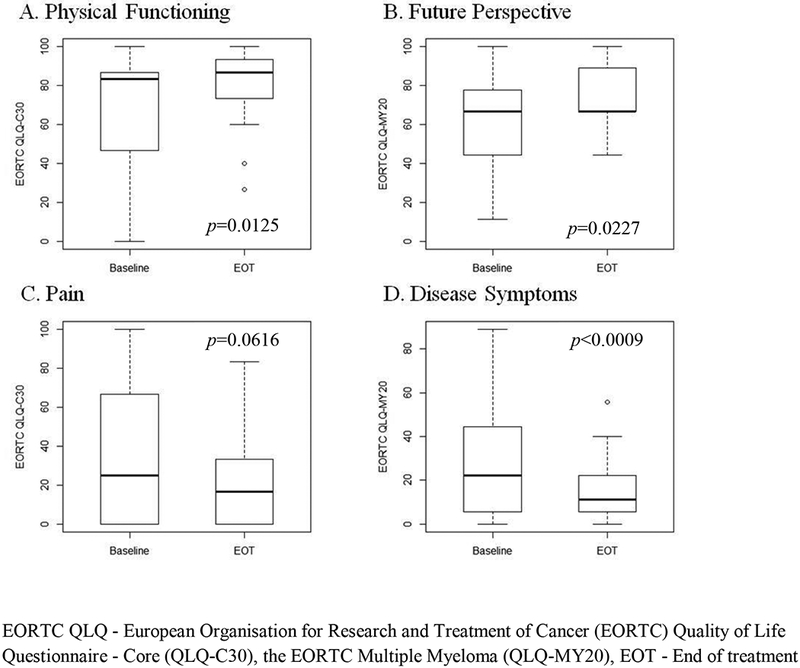
Patient-Reported Quality of Life and Symptom Outcomes
Discussion
One of the goals of treatment in newly-diagnosed patients is to achieve the deepest response possible, as outcomes correlate with depth of response. In this phase II study in transplant-ineligible NDMM, we showed that patients could be treated with dose-adjusted RVD (RVD lite) with comparable efficacy and better tolerability than standard induction RVD. This is all the more significant given the older patient population in our study. The ORR after 4 cycles of RVD lite was 86% in patients with 66% achieving a VGPR or better. Putting our data in the context of other studies of the RVD combination, in the EVOLUTION study, patients receiving RVD had an ORR of 85% and a VGPR or better in 51% after 4 cycles of RVD at standard doses.(Kumar, et al 2012) Ninety-four percent of these patients were considered transplant-eligible at the time of study entry. In the phase II portion of the single-arm study of RVD in NDMM the ORR was 100% with 74% achieving a VGPR or better after 4 cycles of therapy.(Richardson, et al 2010) Furthermore, in the SWOG S0777 study, the ORR in the cohort receiving RVD for 8 cycles was 82% with 43.5% achieving a VGPR or better.(Durie, et al 2017) The median PFS in the SWOG study was superior at 43 months, however, this was a younger study population with a median age of 63 with only 43% of patients over the age of 65. Similarly, in two recent studies of ixazomib in combination with lenalidomide and dexamethasone in NDMM where transplant was either deferred or the patients were ineligible, our results showed superior ORR and PFS than the all oral combinations that would conceptually be an attractive regimen for the older population. Specifically, when ixazomib was administered once weekly in combination with Rd, the ORR was 80% and the median PFS 25.3 months in those not proceeding to SCT.(Kumar S 2017) With twice weekly ixazomib in combination with RD, the ORR was 92%, however, the median PFS 24.9 months for patients not proceeding to SCT.(Richardson P 2017)
Acknowledging the limitation of drawing conclusions from the comparisons of trials with different investigation combinations, schedules, and patient populations, our results do compare favorably with previous trials focusing specifically on transplant-ineligible patients. The median PFS in our study was 35.1 months, which is the longest PFS reported in a trial of transplant in-eligible patients. By comparison, the FIRST Trial showed a PFS for continuous Rd of 25.5 months. The median age for our trial was 73, which is the same as the FIRST trial. RVD lite also compares favorably with bortezomib-based combinations of bortezomib-thalidomide-dexamethasone (VTD), bortezomib-melphalan-prednisone (VMP), and bortezomib-dexamethasone examined in the transplant-ineligible population in the UPFRONT trial conducted in the community setting.(Niesvizky, et al 2015) In this trial, the longest median PFS was 17.3 months for VMP and the best ORR was 80% for VTD. In the CLARION trial, 955 patients with NDMM were randomized to melphalan, prednisone, and either carfilzomib or bortezomib on a twice-weekly schedule.(FaconT 2017) The median age of participants was 72 years. The overall response rates were 84.3% vs. 78.8% (odds ratio [OR] = 1.41; 95% CI 1.01–1.97), respectively, and the PFS 22.3 months (95% CI 20.9–26.7) in the carfilzomib arm, compared with 22.1 months (95% CI 20.8–24.4) in the bortezomib arm, for a non-significant hazard ratio of 0.906 (95% CI 0.75–1.1; p=0.159). Treatment discontinuation because of an AE occurred in 16.7 percent of carfilzomib-treated patients and 14.7 percent of bortezomib-treated patients.
RVD lite demonstrated excellent tolerability. Toxicities were generally manageable and were consistent with the types of toxicities reported historically with this combination. The only grade 3 toxicity noted in > 10% of patients was hypophosphatemia which was manageable and did not result in any dose modifications. Peripheral neuropathy of any kind was reported in 62% of patients with only one patient (2%) experiencing grade 3 symptoms. This compares favorably with twice weekly RVD with IV bortezomib reporting neuropathy in 80% of patients(Richardson, et al 2010) and the EVOLUTION study reporting grade 3 or greater neuropathy in 17% of patients.(Kumar, et al 2012) The reduction in rates and severity of peripheral neuropathy is likely the combination of the SC route of administration and the once weekly schedule. Though many patients did receive dose-modifications, this was done pro-actively to allow patients to continue therapy which is reflected in the very low discontinuation rate. The treatment discontinuation rate due to adverse events from drug toxicity was low at 4%.
In a subset of patients, this study compared pharmacokinetics of SC versus IV administration and supported previously reported findings that the SC route of administration was well-tolerated with excellent response rates and minimal high-grade peripheral neuropathy.(Moreau, et al 2011) Though the absolute rates of peripheral neuropathy in our trial exceeded that of both the SC and IV routes of administration in the trial by Moreau et al. (5% SC and 15% IV), only one patient (2%) had grade 3 neuropathy.
RVD lite was associated with stable to improved HRQoL in patient-reported assessments. Few studies in MM have reported prospective HRQoL assessments to-date. In the FIRST trial comparing Rd with MPT, HRQoL was a secondary endpoint. Overall, ccontinuous Rd was associated with a clinically meaningful improvement in HRQoL compared with MPT. In our study, statistically significant changes were also seen in physical functioning, future perspective, and disease symptoms. Global health status improved though did not meet statistical significance. It is recognized that when analyzing HRQoL data, a statistically significant change does not necessarily imply a clinically significant change. An assessment of minimal important difference (MID) can augment the interpretations of changes in HRQoL by translating statistical differences into clinically significant differences.(King 2011) Pain associated with MM is a common and significant problem for this population. Though improvements in pain assessments did not meet statistical significance, the MID associated with pain did and suggests a clinically significant improvement in this domain for this patient population as well.(Delforge, et al 2015, Kvam, et al 2010a, Kvam, et al 2010b) Though paired samples were only available for 30 out of the 50 enrolled patients, the overall finding support stable to improved HRQoL over the course of RVD lite therapy.
There are several limitations to this study. Specifically, it is a single-arm study that lacks a comparator arm. Further, the study did not mandate maintenance therapy, although the majority of patients (66%) did receive lenalidomide maintenance. This lack of standardization of maintenance therapy was largely due to the fact that the study was opened prior to the increasing use of lenalidomide as maintenance or continuous therapy.
RVD lite is a highly effective and well-tolerated regimen for previously untreated, transplant-ineligible MM patients and may represent the new standard of care for treating this patient population. We demonstrate that we can bring the benefits of more effective combination strategies observed in younger fitter transplant-eligible patients to older, transplant-ineligible patients with modifications in dose and schedule, without compromising efficacy. Ongoing studies are underway looking at other novel combinations in the transplant-ineligible population including trials of daratumumab, lenalidomide, and dexamethasone (NCT02252172) and ixazomib, lenalidomide, and dexamethasone (NCT01850524). An area of future exploration will be the addition of other novel therapies such as monoclonal antibodies to this regimen with the goal of creating a curative platform for the disease.
Supplementary Material
Acknowledgements:
Research support was provided by Takeda Pharmaceuticals.
Footnotes
Conflict-of-interest disclosure: E.O. Takeda consultancy. J.L Celgene consultancy. I.G. Takeda consultancy. N.M Celgene and Takeda consultancy. K.A. Celgene and Takeda consultancy. P.R. Celgene and Takeda consultancy and research funding. N.R. Celgene and Takeda consultancy and honoraria.
References
- Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, Pinto A, Weisel K, Ludwig H, Bahlis N, Banos A, Tiab M, Delforge M, Cavenagh J, Geraldes C, Lee JJ, Chen C, Oriol A, de la Rubia J, Qiu L, White DJ, Binder D, Anderson K, Fermand JP, Moreau P, Attal M, Knight R, Chen G, Van Oostendorp J, Jacques C, Ervin-Haynes A, Avet-Loiseau H, Hulin C, Facon T & Team FT (2014) Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. New England Journal of Medicine, 371, 906–917. [DOI] [PubMed] [Google Scholar]
- Delforge M, Minuk L, Eisenmann JC, Arnulf B, Canepa L, Fragasso A, Leyvraz S, Langer C, Ezaydi Y, Vogl DT, Giraldo-Castellano P, Yoon SS, Zarnitsky C, Escoffre-Barbe M, Lemieux B, Song K, Bahlis NJ, Guo S, Monzini MS, Ervin-Haynes A, Houck V & Facon T (2015) Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica, 100, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, Petrucci MT, Yu Z, Olesnyckyj M, Zeldis JB, Knight RD & Weber DM (2009) Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia, 23, 2147–2152. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV & International Myeloma Working G (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, Thakuri M, Reu F, Reynolds CM, Sexton R, Orlowski RZ, Barlogie B & Dispenzieri A (2017) Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet, 389, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facon T, Mary JY, Pegourie B, Attal M, Renaud M, Sadoun A, Voillat L, Dorvaux V, Hulin C, Lepeu G, Harousseau JL, Eschard JP, Ferrant A, Blanc M, Maloisel F, Orfeuvre H, Rossi JF, Azais I, Monconduit M, Collet P, Anglaret B, Yakoub-Agha I, Wetterwald M, Eghbali H, Vekemans MC, Maisonneuve H, Troncy J, Grosbois B, Doyen C, Thyss A, Jaubert J, Casassus P, Thielemans B, Bataille R & Intergroupe Francophone du Myelome, g. (2006) Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood, 107, 1292–1298. [DOI] [PubMed] [Google Scholar]
- FaconT LJ, Moreau P, et al. (2017) Phase 3 study (CLARION) of carfilzomib, melphalan, prednisone (KMP) v bortezomib, melphalan, prednisone (VMP) in newly diagnosed multiple myeloma (NDMM) 16th International Myeloma Workshop; New Delhi, India, Abstract #3730. [Google Scholar]
- King MT (2011) A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res, 11, 171–184. [DOI] [PubMed] [Google Scholar]
- Kumar SBJ, Niesvizky R, et al. (2017) Deep and Durable Responses with Weekly Ixazomib, Lenalidomide, and Dexamethasone in Patients with Newly Diagnosed Multiple Myeloma: Long-term Follow-up of Patients Who Did Not Undergo SCT. EHA Learning Center, Jun 24, 2017.
- Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, Stewart AK, Turturro F, Rifkin R, Wolf J, Estevam J, Mulligan G, Shi H, Webb IJ & Rajkumar SV (2012) Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood, 119, 4375–4382. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, Lust J, McCurdy A, Russell SJ, Zeldenrust SR, Kyle RA & Rajkumar SV (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia, 28, 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvam AK, Fayers P & Wisloff F (2010a) What changes in health-related quality of life matter to multiple myeloma patients? A prospective study. Eur J Haematol, 84, 345–353. [DOI] [PubMed] [Google Scholar]
- Kvam AK, Wisloff F & Fayers PM (2010b) Minimal important differences and response shift in health-related quality of life; a longitudinal study in patients with multiple myeloma. Health Qual Life Outcomes, 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM & Greipp PR (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proceedings, 78, 21–33. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP & Anderson KC (2002) Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood, 99, 4525–4530. [DOI] [PubMed] [Google Scholar]
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W & Harousseau JL (2011) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol, 12, 431–440. [DOI] [PubMed] [Google Scholar]
- Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, Essell J, Gaffar Y, Warr T, Neuwirth R, Zhu Y, Elliott J, Esseltine DL, Niculescu L & Reeves J (2015) Community-Based Phase IIIB Trial of Three UPFRONT Bortezomib-Based Myeloma Regimens. J Clin Oncol, 33, 3921–3929. [DOI] [PubMed] [Google Scholar]
- Quach H, Fernyhough L, Henderson R, Corbett G, Baker B, Browett P, Blacklock H, Forsyth C, Underhill C, Cannell P, Trotman J, Neylon A, Harrison S, Link E, Swern A, Cowan L, Dimopoulos MA & Miles Prince H (2017) Upfront lower dose lenalidomide is less toxic and does not compromise efficacy for vulnerable patients with relapsed refractory multiple myeloma: final analysis of the phase II RevLite study. Br J Haematol. [DOI] [PubMed]
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J & International Myeloma Workshop Consensus, P. (2011) Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood, 117, 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, H. C, Rosenbaum C, et al. (2017) Twice-Weekly Ixazomib plus Lenalidomide-Dexamethasone in Patients with Newly Diagnosed Multiple Myeloma: Long-Term Follow-Up for Patients Who Did not Undergo Stem Cell Transplantation. In: EHA Learning Center, Vol. Jun 25, 2017.
- Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, Avigan DE, Xie W, Ghobrial IM, Schlossman RL, Mazumder A, Munshi NC, Vesole DH, Joyce R, Kaufman JL, Doss D, Warren DL, Lunde LE, Kaster S, Delaney C, Hideshima T, Mitsiades CS, Knight R, Esseltine DL & Anderson KC (2010) Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood, 116, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD & Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin, 67, 7–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.