Abstract
Prenatal programming models have rarely been applied to research on children with prenatal substance exposure, despite evidence suggesting that prenatal drug exposure is a form of stress that impacts neurodevelopmental outcomes and risk for psychopathology. Utilizing data from two longitudinal multisite studies comprising children prenatally exposed to substances as well as a nonexposed comparison group (Maternal Lifestyle Study, n = 1,388; Infant Development, Environment, and Lifestyle study, n = 412), we tested whether early phenotypic indicators of hypothesized programming effects, indexed by growth parameters at birth and infant temperament, served as a link between prenatal substance exposure and internalizing and externalizing behavior at age 5. Latent profile analysis indicated that individual differences in reactivity and regulation for infants prenatally exposed to substances was best characterized by four temperament profiles. These profiles were virtually identical across two independent samples, and demonstrated unique associations with adjustment difficulties nearly 5 years later. Results of path analysis using structural equation modeling also showed that increased prenatal substance exposure was linked to poorer growth parameters at birth, profiles of temperamental reactivity in infancy, and internalizing and externalizing behavior at age 5. This pathway was partially replicated across samples. This study was among the first to link known individual-level correlates of prenatal substance exposure into a specific pathway to childhood problem behavior. Implications for the developmental origins of a child’s susceptibility to psychopathology as a result of intrauterine substance exposure are discussed.
The origins of health and behavior are traceable, in part, to development in utero. The prenatal period is characterized by rapid development; organs and physiological systems of a developing fetus are plastic and amendable to environmental exposures during this time of widespread maturation and consolidation. Modest perturbations of the fetal environment may affect how multiple systems, which regulate metabolic, immune, neurobehavioral, and cardiovascular activity, become organized. Seminal epidemiologic research linking fetal undernutrition to metabolic outcomes in adulthood paved the way for investigations into the prenatal determinants of later health and well-being (Barker, Osmond, Winter, Margetts, & Simmonds, 1989; Barker et al., 1993). These efforts have been critical not only for instantiating the notion that prenatal adversity has enduring consequences for adult physical health outcomes but also for revealing links with mental health outcomes (e.g., Davis & Sandman, 2012; Thompson, Syddall, Rodin, Osmond, & Barker, 2001). In other words, features of the early environment may alter fetal and neonatal programming and affect developing systems in ways that have enduring consequences for their structure, function, and behavioral expression. In spite of observed associations between prenatal adversity and mental health outcomes, relatively little is known about the pathways linking prenatal adversity to the development of psychopathology. One context that may be particularly important for investigating mechanisms through which prenatal adversity may influence development is intrauterine substance exposure.
A wealth of research supports the notion that prenatal substance exposure may perturb fetal growth and maturation (Behnke, Smith, Committee on Substance Abuse, & Committee on Fetus and Newborn, 2013; Lester et al., 2002; Smith et al., 2006). Prenatal substance exposure has been tied to a range of developmental differences including poor birth outcomes (e.g., reduced growth parameters, shorter gestational terms, and birth complications), neurobehavioral dysregulation (e.g., hypo- and hyperarousal, and negative reactivity), neurodevelopmental differences (e.g., alterations in brain biochemistry, morphology, and neuronal development), and childhood emotional and behavioral problems (see Behnke et al., 2013; Crocker, Fryer, & Mattson, 2013; Minnes, Lang, & Singer, 2011; Ross, Graham, Money, & Stanwood, 2015, for recent reviews). Lester and Padbury (2009) have proposed that exposure to prenatal substances such as cocaine may impact fetal development in three distinct ways: through neurochemical, vasoconstrictive, and programming mechanisms. Neurochemically, substances may interfere with fetal development, for example, by blocking presynaptic uptake of neurotransmitters such as dopamine, norepinephrine, and serotonin, thereby increasing levels of extracellular neurotransmitter concentrations in ways that contribute to malformation of the developing brain. Furthermore, substances may also exert indirect vasoconstrictive effects, in which physiological derangements secondary to intrauterine substance exposure result in the restriction of blood flow (and constituent nutrients and oxygen) to the fetus (Lester & Padbury, 2009).
Another, and far less studied, potential pathway by which prenatal substance exposure may impact fetal development is through programming effects (Lester & Padbury, 2009). Distinct from the neurochemical and vasoconstrictive mechanisms, which are known to damage developing structures and systems, programming mechanisms differ importantly in that they prompt the differential development of structures and systems in accordance with environmental needs. More specifically, intrauterine substances are thought to act as stressors, or challenges that disrupt fetal-placental homeostasis, thus prompting the fetus to make compensatory adjustments. These homeostatic adjustments in turn result in the reprogramming, or recalibration, of physiological systems that may in turn alter children’s physical and behavioral phenotypes. Some evidence suggests that intrauterine substances may act on the hypothalamic–pituitary–adrenal axis, for example, by altering expression of placental genes such as the norepinephrine transporter (NET) and 11β-HSD-2 (Lester & Padbury, 2009). These alterations, in turn, program the hypothalamic–pituitary–adrenal axis in ways that alter the set point for physiologic, metabolic, and behavioral outcomes. If this is the case, early phenotypic indicators of programming effects, such as growth restriction and temperament, may mediate associations between prenatal substance exposure and subsequent emotional and behavioral problems (Lester & Padbury, 2009). Although technology does not yet afford the possibility to definitively test whether associations between prenatal substance exposure and infant temperament are attributable to vasoconstictive, neurochemical, or programming mechanisms, the theory that programming effects specifically affect the set point of the stress response system, associations between early phenotypic indicators (e.g., growth restriction), and infants’ stress responsivity (e.g., temperament) secondary to prenatal substance exposure suggests that programming effects are likely in play. The current study draws on data from two independent multisite, longitudinal studies of children prenatally exposed to substances to test pathways, informed by programming models, linking prenatal substance exposure with childhood psychopathology.
Effects of Prenatal Substance Exposure on Childhood Emotionality and Behavior
Pregnant women who use substances often do not limit use to a single substance, which makes polysubstance use the rule rather than the exception (Lester et al., 2001; Oei, Abdel-Latif, Clark, Craig, & Lui, 2010). It is therefore likely that polydrug use, rather than use of a specific substance in isolation, drives the problematic behavior trajectories seen in some children with prenatal drug exposure. Moreover, it can be difficult to separate the effect of maternal substance use from confounding factors such as fetal undernutrition, which itself has been associated with health in adulthood (e.g., Roseboom et al., 2000), due to the fact that various substances are known to affect weight and appetite (e.g., methamphetamine). Among the most commonly used substances are tobacco, alcohol, and marijuana, and, to a lesser extent, cocaine and methamphetamine. Substance exposure in utero may operate through a variety of pathophysiological mechanisms, which may differ based on the timing, frequency, and nature of substance exposure (Minnes et al., 2011; Ross et al., 2015).
From early childhood through adolescence, children who were prenatally exposed to substances tend to exhibit more externalizing behavior, compared to their unexposed peers (Ashford, van Lier, Timmermans, Cuijpers, & Koot, 2008; Bada et al., 2007; Bennett, Bendersky, & Lewis, 2007; Billings, Eriksson, Jonsson, Steneroth, & Zetterström, 1994; Eze et al., 2016; Goldschmidt, Day, & Richardson, 2000; La-Gasse et al., 2012; Lester et al., 2009; O’Connor & Paley, 2009; Richardson, Goldschmidt, Leech, & Willford, 2011; Robinson et al., 2008; Tsang, Lucas, Olson, Pinto, & Elliott, 2016). The association between prenatal substance exposure and childhood internalizing behaviors has received relatively less attention, though research has shown drug exposure to be associated with increased internalizing behaviors (Robinson et al., 2008; Tsang et al., 2016). Furthermore, prenatal substance exposure has been shown to affect the development of problem behavior in children as young as 3 and 5 years old, as well as the trajectory of adjustment difficulties across early childhood (Bada et al., 2007; LaGasse et al., 2012; Lester et al., 2009).
The building blocks of childhood psychopathology for children exposed to substances prenatally may be set in place well before a child’s behavior becomes problematic. Individual differences in fetal growth and temperament are observable in the first months of postnatal life, and may serve as early-emerging, phenotypic traits indicating risk for childhood psychopathology. Fetal growth, often indexed by birth weight and other birth parameters, has been linked to behavioral adjustment difficulties in childhood (Alati et al., 2009; Groen-Blokhuis, Middeldorp, van Bijsterveldt, & Boomsma, 2011), though this association may depend, in part, on the sex of the child (Chatterji, Lahiri, & Kim, 2014; Hultman et al., 2007). For instance, recent work from Gupta, Deding, and Lausten (2013) showed that male children had more psychosocial difficulties at age 11 if they were low birth weight; there was no effect among female children. Reduced fetal growth is common among substance-exposed newborns (Bada et al., 2002; Bauer et al., 2005; Behnke, Eyler, Garvan, & Wobie, 2001; El Marroun et al., 2009; Hayatbakhsh et al., 2012; Patra et al., 2011; Salmasi, Grady, Jones, & McDonald, 2010; Shankaran et al., 2004; Smith et al., 2006; Zarén, Lindmark, & Gebre-Medhin, 1996), and may therefore represent early-emerging indicators of programming effects with downstream consequences for children’s socioemotional development. Temperamentally, children who were prenatally exposed to substances also tend to display irregular patterns of reactivity and responsivity during the neonatal period in comparison to nonexposed peers (Bauer et al., 2005; Law et al., 2003; Lester et al., 2002; Oberlander et al., 2010; Smith et al., 2008; Stroud et al., 2009), as well as increased negative emotionality and reactivity in infancy (Lester et al., 2009; O’Connor, 2001; Richardson, Goldschmidt, & Willford, 2008; Schuetze & Eiden, 2007).
Taken together, evidence to date supports intrauterine substance exposure as a predictor of behavioral and emotional outcomes in childhood, the antecedents of which may be identifiable by reduced fetal growth, indexed by birth parameters, and infant neurobehavioral reactivity (i.e., temperament). The effect of substance exposure on the prenatal environment may be due, in part, to programming of fetal growth and physiology.
Pathways From Prenatal Exposure to Problem Behavior
Barker and colleagues were among the first to find that variations in fetal growth, as indexed by birth weight, have important implications for the health of the individual across the life span (Barker & Osmond, 1986; Barker et al., 1989, 1993). They found that prenatal exposure to severe undernutrition was related to elevated LDL cholesterol and triglyceride levels among adult females (Lumey, Stein, Kahn, & Romijn, 2009), and increased rates of coronary heart disease (Roseboom et al., 2000). These studies provided initial theoretical and empirical support for the “fetal origins hypothesis” (also known as the Barker hypothesis). It was found that disease resulted only when the prenatal environment did not match the postnatal environment. In the case of undernutrition, when a fetus was exposed to fewer calories in utero and was later reared in a calorie-rich postnatal environment, the fetus was more likely to develop metabolic disease (e.g., Hales et al., 1991). For example, maternal undernutrition as a result of the Dutch Hunger Winter was related to lower birth weight and an increased incidence of coronary heart disease in offspring with low birth weights. In this case, a metabolic trade-off in the form of a slower metabolism may have occurred that altered fetal growth, with implications for immediate postnatal survival. However, when the newborn was exposed to a calorie-rich environment as is typical in Western countries, the mismatch between a slower metabolic system combined with exposure to calorie-rich foods made it more likely that infants with low birth weight developed metabolic disease (Hales et al., 1991). One function of programming efforts may therefore be to adjust fitness-promoting physiological parameters, while constraining growth elsewhere, in an attempt to yield a fetal phenotype adapted to the postnatal environment (Gluckman, Hanson, Cooper, & Thornburg, 2008).
Thus, a critical component of programming theories is the degree to which the prenatal environment matches the postnatal one; the best outcomes should occur when the pre- and postnatal environments are matching (Sandman, Davis, Buss, & Glynn, 2012). The fetal origins hypothesis was therefore revised and renamed the developmental origins of health and disease hypothesis (Barker & Thornburg, 2013; Padmanabhan, Cardoso, & Puttabyatappa, 2016; Wadhwa, Buss, Entringer, & Swanson, 2009). According to this evolutionary-developmental theory, fetal development is regulated by mechanisms that differentially allocate metabolic resources to increase evolutionary fitness (e.g., survival and reproductive success) in accordance with environmental demands. This occurs through the adjustment of key physiological systems in response to environmental cues (e.g., hormones) from the mother regarding the quality of the postnatal environment. It has been posited that insults trigger a biochemical response from the developing fetus, which leads to adjustments in the fetal developmental strategy (Gluckman et al., 2008).
It is still unclear whether prenatal substance exposure, which has traditionally been assumed to result in deficits for the developing child, functions as a programming factor in the same manner as other factors, such as undernutrition. Prenatal substance exposure does appear to be associated with postnatal outcomes through similar intrauterine pathophysiological mechanisms, and is expressed across levels of analysis (e.g., epigenome, stress physiology, and behavior), in an attempt to produce “adapted” phenotypic traits (Lester & Padbury, 2009; Padmanabhan et al., 2016). How these traits are expressed postnatally will have important implications for a child’s health and behavior. However, postnatal pathways via individual-level phenotypic profiles (i.e., temperament) remain to be specified.
Temperament: An Early-Emerging Indicator of Risk for Psychopathology
Children’s temperamental characteristics, or dispositional proclivities for reactivity and regulation (Rothbart & Bates, 2006), may provide behavioral markers of children’s susceptibility for later adjustment problems. How a child’s temperament is expressed is thought to be the result of a dynamic interplay between biology and context that unfolds over time and across development, and reflects a multifaceted neurobehavioral profile characterized by proclivities for activity, affectivity, attention, and regulation (Shiner et al., 2012).
Although much of extant research has focused on clarifying the influence of postnatal contextual factors on temperament expression over time, a growing body of research suggests that prenatal factors may also play a formative role (Bergman, Sarkar, O’Connor, Modi, & Glover, 2007; Davis et al., 2004; Huizink, Robles De Medina, Mulder, Visser, & Buitelaar, 2002). Prenatal substance exposure may heighten risk for negative reactivity, which in turn may heighten risk for later internalizing and externalizing problems (Lester et al., 2009; Locke et al., 2016; O’Connor, 2001). For instance, one study by Lester et al. (2009) found a path that linked prenatal substance exposure (i.e., cocaine, tobacco, alcohol, and marijuana) with behavioral reactivity at 1 month, negative reactivity at 4 months, and behavioral problems at 3 and 7 years. Similarly, O’Connor (2001) found a path that linked prenatal alcohol exposure with infant negative affect at 12 months and depressive symptoms at age 6. Thus, among substance-exposed children, negative reactivity in infancy may represent an important indicator of risk for childhood problem behavior.
A major criticism of temperament research thus far is that extant temperament characterizations have been flawed (see Scott et al., 2016, for a discussion). Specifically, although researchers generally agree about the domains that comprise childhood temperament (Shiner et al., 2012), disagreement still exists about how to best represent this complex neurobehavioral profile. Extant temperament research has tended to employ one of two strategies. The typographical approach (e.g., Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984; Thomas & Chess, 1977), which categorizes children’s temperament into types or groups based on practically emerging behavioral patterns (e.g., “difficult temperament” or “behaviorally inhibited”), has strengths in its ability to provide holistic, practical characterizations of children that are intuitive and interpretable. However, this approach focuses more on conceptual, rather than statistical, methods for extracting temperament groups, and has not always been effective in capturing the full range of variability in temperament types (e.g., Thomas, Chess, & Birch, 1970). In contrast, the dimensional approach (e.g., Goldsmith, 1996; Rothbart, 1981), which describes children’s proclivities on a number of dimensions (e.g., fear, soothability, and approach), has strengths in that dimensions have typically been derived from data-driven (i.e., factor analytic) statistical techniques, and thus more fully capture the range of temperament characteristics. However, because so many dimensions have emerged, research considering temperament dimensions has often focused only on a few dimensions (e.g., fear or anger) or their broader factors (e.g., negative reactivity and effortful control), and thus has been limited in its ability to fully characterize children in practically meaningful ways.
More recent temperament research has begun to employ data-driven, typological approaches for characterizing temperament profiles, such as by latent class analysis (e.g., Beekman et al., 2015; Gartstein et al., 2017; Scott et al., 2016). These strategies have typically yielded between two and five types of temperament profiles (e.g., Aksan et al., 1999; Beekman et al., 2015; Caspi & Silva, 1995; Stifter, Putnam, & Jahromi, 2008), some of which have been found to mirror the theoretically based temperament profiles described previously (e.g., inhibited temperament; Stifter et al., 2008). Because temperament research has only recently begun to incorporate these innovative statistical techniques for characterizing temperament profiles, much remains to be clarified about the predominant profiles that emerge across samples, their relations to the prenatal environment, and subsequent implications for children’s adjustment.
The Current Study
Physical and psychological outcomes associated with prenatal substance exposure are well documented. We sought to build upon the evidence base by examining the possible developmental origins of psychopathology among children prenatally exposed to substances. We focused on whether early phenotypic indicators of hypothesized programming effects (i.e., birth outcomes and temperament) served as a link between prenatal substance exposure and problem behavior in early childhood. Furthermore, we assessed how consistent these results were across two independent samples of children who were prenatally exposed to substances, an important strength of our research given the replicability crisis within the social sciences (Open Science Collaboration, 2015). Data were utilized from the Maternal Lifestyle Study (MLS), the largest prospective study on prenatal cocaine exposure (Lester et al., 2002, 2009), and the Infant Development, Environment, and Lifestyle (IDEAL) study, the largest prospective study of prenatal methamphetamine exposure (Smith et al., 2006, 2015). The current study thus represents one of the largest tests of a specific pathway to childhood psychopathology conducted for this high-risk population. Taken together, we examined behavioral outcomes in a total of 1,801 children with prenatal substance exposure.
The present study had three objectives. First, we explored whether children could be characterized by phenotypic profiles of neurobehavioral activity (i.e., temperament) using latent class analysis. Second, we sought to examine a developmental path to later problem behavior using structural equation modeling. We hypothesized that increased substance exposure in utero would be associated with birth outcomes suggestive of fetal growth restriction; that birth outcomes would be related to neurobehavioral profiles conferring elevated temperamental reactivity in infancy; that profiles conferring elevated reactivity would confer risk for later adjustment difficulties; and finally, that birth outcomes and temperament mediated the association between prenatal substance exposure and internalizing and externalizing behavior at age 5. Third, we tested whether our findings were consistent across two independent samples of substance-exposed children.
Method
Study 1: MLS
Participants
The primary objective of the first study was to examine the effects of fetal cocaine exposure on developmental outcomes. Data for the MLS was collected in four US cities (Detroit, MI; Memphis, TN; Miami, FL; and Providence, RI) from May 1993 to May 1995. The institutional review board at each site approved the study’s protocol and consent forms. A certificate of confidentiality was obtained from the National Institute on Drug Abuse to ensure confidentiality regarding a mother’s use of illicit substances.
A detailed description of MLS recruitment is presented elsewhere (Lester et al., 2001, 2002). In brief, mothers were screened for eligibility within 24 hours of giving birth. A total of 19,079 mother-infant dyads were identified for inclusion in the study. Of these mother-infant dyads, 16,988 were eligible and 11,811 gave consent to participate in the study. A total of 658 neonates were classified as cocaine exposed (exposure status described below). A non-cocaine-exposed comparison group was identified, and included 730 neonates. Nonexposed newborns were matched with their exposed counterparts on race, sex, and gestational age within each study site.
Measures
Prenatal substance exposure
Infant prenatal substance exposure to cocaine, opiates, tobacco, alcohol, and marijuana was determined based on maternal report of substance use during pregnancy (tobacco, alcohol, and marijuana) and gas chromatography–mass spectrometry of a presumptive positive meconium screen for substance metabolites (amphetamine, cocaine, opiates, cannabinoids, and phencyclidine). Mothers reported on substance use during pregnancy and sociodemographic information during a structured interview, which was conducted in the hospital following consent. Meconium samples were collected while in the hospital and shipped to a central laboratory for processing (ElSohly Laboratories, Inc.). Maternal use of each of the substances was dichotomized (1 = yes, 0 = no) based on the results of either maternal report (tobacco, alcohol, and marijuana) or meconium toxicology screen (cocaine and opiates).
Birth outcomes
Trained research staff who were blind to exposure status conducted physical examinations and obtained newborn growth measurements. Gestational age was determined based on the estimated date of delivery or, in cases where prenatal care was absent or inadequate, based on a postnatal examination conducted by the participants’ physician.
Temperament
Mothers reported on their infants’ temperament during the 4-month time point using the Infant Behavior Questionnaire (IBQ; Rothbart, 1981), an established caregiver-report measure of emotionality and behavior for 3- to 12-month-old infants. The IBQ assesses six domains of infant temperament: activity level, distress to limitations, distress to novelty, duration of orienting, smiling/laughter, and soothability (see Table 2 for descriptive information). Maternal reports of infant temperament were available for 1,085 infants.
Table 2.
Descriptive Information
| MLS (n = 1388)
|
IDEAL (n = 412)
|
Z-score | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Mother Characteristics | |||||||
| Age (years) | 28.35 | 5.82 | 18–48 | 25.17 | 5.61 | 18–41 | −9.99*** |
| Education (% high school or more) | 60.6% | 58.0% | ns | ||||
| Socioeconomic Status (% low Hollingshead) | 23.3% | 22.7% | ns | ||||
| Relationship Status (% Partnered)a | 19.2% | 55.1% | 13.45*** | ||||
| Race | |||||||
| % White | 15.9% | 38.8% | 8.98*** | ||||
| % Black | 76.6% | – | |||||
| % Hispanic | 6.3% | 22.3% | 7.43*** | ||||
| % Asian or Pacific Islander | – | 31.1% | – | ||||
| % Other | 1.2% | 7.8% | 4.84*** | ||||
| Prenatal Substance Use (% used) | |||||||
| Methamphetamine | – | 49.5% | – | ||||
| Cocaine | 43.7% | – | – | ||||
| Opiates | 8.4% | – | – | ||||
| Tobacco | 53.9% | 52.9% | ns | ||||
| Alcohol | 59.4% | 25.7% | −13.35*** | ||||
| Marijuana | 23.3% | 18.4% | −2.20* | ||||
| Infant Characteristics | |||||||
| Sex (% male) | 52.4% | 53.4% | ns | ||||
| Birth weight (grams) | 2629.75 | 818.24 | 519–4880 | 3247.50 | 598.27 | 1482–5273 | 16.80*** |
| Birth length (cm) | 46.74 | 5.01 | 28–57 | 50.40 | 3.33 | 33–60 | 17.21*** |
| Head Circumference | 32.12 | 3.03 | 20.5–41 | 33.89 | 1.82 | 28–39 | 14.53*** |
| Gestational Age | 36.26 | 4.02 | 21–42 | 38.65 | 2.06 | 28–43 | 16.17*** |
| Temperament Typesb | |||||||
| Mod. Low Reactive, Mod Dysregulated | 35.6% | 40.8% | 1.72† | ||||
| General Reactive, Well Regulated | 9.8% | 27.9% | 6.93*** | ||||
| Negative Reactive, Dysregulated | 13.4% | 10.5% | ns | ||||
| High Positive Affect, Well Regulated | 41.2% | 20.7% | −7.68*** | ||||
| Externalizing Problems T score (5yrs) | 55.74 | 10.98 | 33–77 | 54.16 | 10.89 | 28–82 | −2.03* |
| Internalizing Problems T score (5yrs) | 49.90 | 9.45 | 30–84 | 54.16 | 10.02 | 29–79 | 6.32*** |
Note: Means, standard deviations (SD), and mean differences were computed using maximum likelihood with robust standard errors. MLS, Maternal Lifestyle Study. IDEAL, Infant Development, Environment, and Lifestyle study.
Percent married (MLS) or partnered (IDEAL).
Temperament types at 4 months (MLS) or 12 months (IDEAL).
p < .01.
p < .001.
p < .10.
Problem behavior
Internalizing and externalizing behaviors were assessed at age 5 using the Child Behavior Checklist (Achenbach & Rescorla, 2000). Standardized T scores for internalizing and externalizing behavior are reported in Table 2. Information about child behavioral problems was available for 822 children.
Study 2: IDEAL study
Participants
The primary objective of the second study was to examine the effects of fetal methamphetamine exposure on developmental outcomes. Data for the IDEAL study was collected in four US cities (Los Angeles, CA; Honolulu, HI; De Moines, IA; and Tulsa, OK) from September 2002 to August 2003. Clinical sites were selected in geographical areas known to have increased use of methamphetamine at the time of study initiation. The institutional review board at each site approved the study’s protocol and consent forms. A certificate of confidentiality was obtained from the National Institute on Drug Abuse to ensure confidentiality regarding a mother’s use of illicit substances.
A detailed description of IDEAL recruitment and exclusion criteria is presented elsewhere (Smith et al., 2006, 2015). In brief, mothers were screened for eligibility within 48 hours of giving birth. A total of 34,833 mother–infant dyads were identified for inclusion in the study, of whom 26,999 were screened for eligibility. Of these mother–infant dyads, 3,705 were eligible and gave consent to participate in the study. A total of 204 neonates were classified as methamphetamine exposed (exposure status described below). A non-methamphetamine-exposed comparison group was identified, and included 208 neonates. Nonexposed newborns were matched with their exposed counterparts on maternal race, infant birth weight, insurance type, and maternal educational status within each study site. Demographic data for the sample is provided in Table 2.
Measures
Prenatal substance exposure
Infant prenatal exposure to methamphetamine, tobacco, marijuana, and alcohol was assessed through maternal report (tobacco, marijuana, and alcohol) and gas chromatography–mass spectrometry of a presumptive positive meconium screen for substance metabolites (amphetamines, cannabinoids, cocaine, cotinine, and opiates). Mothers reported on substance use history and sociodemographic information during a structured interview conducted in the hospital after delivery following consent. Meconium samples were collected while in the hospital and shipped to a central laboratory for processing (US Drug Testing Laboratory). Maternal use of each of the substances was dichotomized (1 = yes, 0 = no) based on the results of either maternal report (tobacco, alcohol, and marijuana) or meconium toxicology screen (methamphetamine).
Birth outcomes
Newborn growth measurements (i.e., head circumference, length, and weight) were recorded during the structured maternal interview following enrollment (Smith et al., 2006). Similar to MLS, gestational age was determined based on the estimated date of delivery or, in cases where prenatal care was absent or inadequate, based on a postnatal examination conducted by the participants’ physician. When there were discrepancies in gestational dating, the estimate obtained by the physicians’ postnatal assessment was used. Descriptive birth outcome data is presented in Table 2.
Temperament
Mothers’ reported on their infants’ temperament during the 12-month time point using the IBQ (Rothbart, 1981), an established caregiver-report measure of emotionality and behavior for 3- to 12-month-old infants. Maternal reports of infant temperament were available for 333 infants.
Problem behavior
Internalizing and externalizing behaviors were assessed at age 5 using the Child Behavior Checklist (Achenbach & Rescorla, 2000). Mothers reported whether statements about their children’s behavior and emotions over the past 6 months were not true, somewhat true/sometime true, or very/often true. Standardized T scores for internalizing and externalizing behavior are reported (see Table 2 for descriptive statistics). Information about child behavioral problems was available for 304 children.
Analytic approach
Temperament profiles
Statistical analyses were completed using maximum likelihood with robust standard errors in Mplus 7.31 (Muthen & Muthen, 1998-2012). Variables were inspected for normality and the presence of outliers. Outliers on maternal subjective report variables were winsorized at 1.5 × interquartile range (IQR). Categorical variables were dummy coded to reduce nonessential multicollinearity (Cohen, Cohen, West, & Aiken, 2003). Thereafter, a series of latent class analyses were run on standardized IBQ dimension scores. Model fit was evaluated by comparing entropy (Celeux & Soromenho, 1996), the Bayesian information criterion (BIC; Schwarz, 1978), and the Vuong–Lo–Ruben likelihood ratio test (VLRT; Lo, Mendell, & Rubin, 2001). The best fitting model was determined a priori to be the model with the best fit overall as determined by higher entropy values, lower BIC values, statistically significant VLRT values, and an interpretable solution. Posterior probabilities of class membership from the best fitting solution were recorded for use in the subsequent path analysis model.
Path models
Analyses for the primary study aim was completed with a path analysis model using structural equation modeling (SEM). To test the general fit of the proposed conceptual model, a χ2 test of fit, comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR) were examined. Good fit was defined pre-hoc as χ2 test probability > .05, CFI > 0.95, RMSEA < 0.06, and SRMR < 0.08 (Hu & Bentler, 1999). Latent variables were specified to reflect prenatal substance exposure and infant birth outcomes, and were run separately prior to path analyses to ensure adequate model fit. Prenatal methamphetamine exposure was specified as the indicator variable for the latent prenatal substance exposure variable; loadings for tobacco, alcohol, and marijuana exposure were freely estimated. Infant birth weight, which was square-root transformed to facilitate model convergence, was specified as the indicator variable for the latent birth outcomes variable; loadings for birth length, head circumference, and gestational age were freely estimated. Factor scores representing infant birth outcomes and prenatal substance exposure were computed using factor loadings from the respective latent models and were correlated with demographic and key study variables to identify covariates for inclusion in the full SEM model. Pre-hoc we determined that any variables that were significantly correlated with any pair of endogenous and exogenous variables would be adjusted for in study analyses. In addition, the possible presence of sex differences was tested by comparing model fit (corrected test) for two multigroup models grouped by children’s female or male sex. In the first model, all specified paths were constrained to be equal. In the second model, all specified paths were freely estimated. Finally, when indicated, the statistical significance of indirect (mediation) effects was tested using the MODEL INDIRECT command.
Results
Study 1: MLS
Temperament profiles
Descriptive statistics for maternal ratings of infant temperament are presented in Table 1. Twenty-one data points were adjusted for 20 infants with dimension scores above or below the 1.5 × IQR cutoff. Model fit indices and entropy are presented in Table 3. Entropy was highest for the three- and four-class solutions, BIC was lowest for the five-class solution, and VLRT was statistically significant for the two-, three-, and four-class solutions. Because the four-class solution demonstrated the best fit and entropy overall across solutions, it was determined to be the best fitting solution.
Table 1.
Infant Temperament Descriptives
| Dimension | MLS 4-month (n = 1085)
|
IDEAL 12-month (n = 333)
|
Z-score | ||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | ||
| Activity Level | 3.24 | 0.64 | 1.60–4.90 | 3.34 | 0.65 | 1.88–5.00 | 0.00 |
| Smiling and Laughter | 3.52 | 0.72 | 1.61–5.00 | 3.92 | 0.54 | 2.42–5.00 | 0.13 |
| Fear | 2.37 | 0.65 | 1.00–4.25 | 2.51 | 0.62 | 1.00–4.00 | 0.00 |
| Distress to Limitations | 2.53 | 0.57 | 1.00–4.06 | 2.69 | 0.56 | 1.25–4.10 | −0.07 |
| Soothability | 3.47 | 0.70 | 2.00–5.00 | 3.46 | 0.75 | 1.50–5.00 | 0.04 |
| Orienting | 3.01 | 0.59 | 1.25–4.64 | 2.72 | 0.54 | 1.33–4.19 | −0.01 |
Note: Means and standard deviations (SD) for variables with missing data were computed using maximum likelihood with robust standard errors. None of the mean differences were statistically different at the p < .05 level. MLS, Maternal Lifestyle Study. IDEAL, Infant Development, Environment, and Lifestyle study.
Table 3.
Model Fit for Latent Class Analyses of Infant Temperament
| Classes | Entropy | BIC | VLRT
|
||
|---|---|---|---|---|---|
| value | p | ||||
| MLS | 1 | – | 17505.27 | – | – |
| 2 | 0.63 | 17033.62 | 520.58 | <.001 | |
| 3 | 0.66 | 16890.92 | 191.62 | 0.01 | |
| 4 | 0.65 | 16847.03 | 92.82 | 0.01 | |
| 5 | 0.62 | 16830.41 | 65.54 | 0.13 | |
| IDEAL | 1 | – | 5573.07 | – | – |
| 2 | 0.63 | 5465.71 | 148.02 | 0.003 | |
| 3 | 0.63 | 5435.44 | 70.93 | 0.42 | |
| 4 | 0.67 | 5424.04 | 52.06 | 0.06 | |
| 5 | 0.68 | 5442.01 | 22.70 | 0.58 | |
Note: MLS, Maternal Lifestyle Study. IDEAL, Infant Development, Environment, and Lifestyle study. BIC, Bayesian information criterion. VLRT, Vuong–Lo–Ruben likelihood ratio test.
Temperament profiles corresponding to each of the temperament types are presented in Figure 1. Given the lack of naming conventions among researchers utilizing this approach, we labeled temperament profiles based on manifest reactivity and regulation, respectively. The temperament type describing the majority of infants was the high positive affect, well-regulated type (41.7%, n = 452), and comprised infants with mean levels of activity, high smiling and laughter, low negative affect, and above mean levels of regulation. The second most predominant temperament type (36.1%; n = 392) was labeled the moderately low reactive, moderately dysregulated type, and comprised infants with below mean levels of positive reactivity, negative reactivity, and regulation. Next, the temperament type labeled negative reactive, dysregulated (13.2%, n = 143), comprised infants with mean levels of activity, high smiling and laughter, low negative affect, and above mean levels of regulation. Finally, the least common temperament type (9.0%, n = 98) was labeled the reactive, well-regulated type (9.0%), and comprised infants with above mean levels of positive reactivity, negative reactivity, and regulation.
Figure 1.
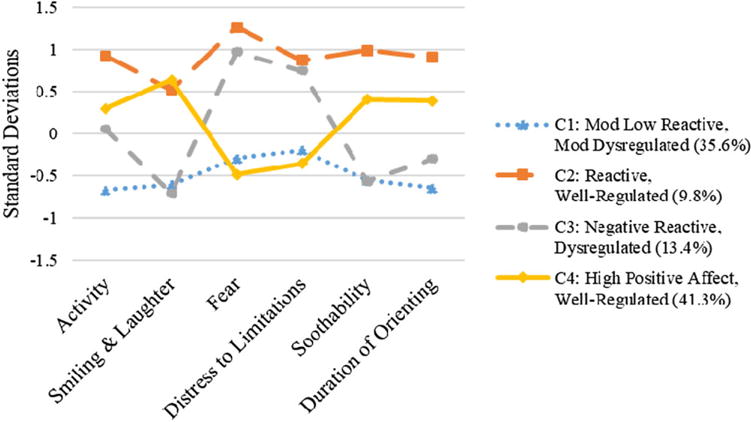
Maternal Lifestyle Study infant temperament profiles at 4 months. Mean standardized scores on each of the temperament dimensions are presented.
Path model
Descriptive statistics and correlations are presented in Tables 2 and 3, respectively. Covariates included site location, maternal age, maternal education, maternal socioeconomic status, marital status, Black and Hispanic race, and infant sex, each of which was found to be significantly associated with pairs of independent and dependent variables. Because a test comparing the fit of multiple-group models investigating the possible presence of sex differences indicated the presence of statistically significant sex differences, corrected , p = .02, MODINDICES from the fully constrained model were used to identify the specific paths that differed significantly (p < .05) between the model for girls and boys, which indicated that paths linking C1 temperament type with externalizing behaviors, C3 temperament type with internalizing behaviors, and Michigan site with internalizing behaviors were significantly different. Therefore, each of these paths was allowed to be freely estimated; all others were constrained to be equal.
Results from the SEM model are presented in Figure 2. Fit statistics indicated that the model fit the data well: χ2 (339) = 814.47, p < .001; RMSEA = 0.05; CFI = 0.95; SRMR = 0.04. The manifest variables corresponding to the latent prenatal substance exposure variable loaded significantly well at the p < .001 level both for girls (cocaine, β = 0.75, SE = 0.03; opiates, β = 0.12, SE = 0.03; tobacco, β = 0.74, SE = 0.03; alcohol, β = 0.37, SE = 0.03; marijuana, β = 0.43, SE = 0.03) and for boys (cocaine, β = 0.72, SE = 0.03; opiates, β = 0.12, SE = 0.03; tobacco, β = 0.71, SE = 0.03; alcohol, β = 0.35, SE = 0.03; marijuana, β = 0.42, SE = 0.03). Likewise, the manifest variables corresponding to the latent birth outcomes variables loaded significantly well at the p < .001 level both for girls (birthweight, β = 0.98, SE = 0.00; birth length, β = 0.93, SE = 0.01; head circumference, β = 0.92, SE = 0.02; gestational age, β = 0.88, SE = 0.01) and for boys (birthweight, β = 0.98, SE = 0.00; birth length, β = 0.93, SE = 0.01; head circumference, β = 0.92, SE = 0.01; gestational age, β = 0.88, SE = 0.01).
Figure 2.
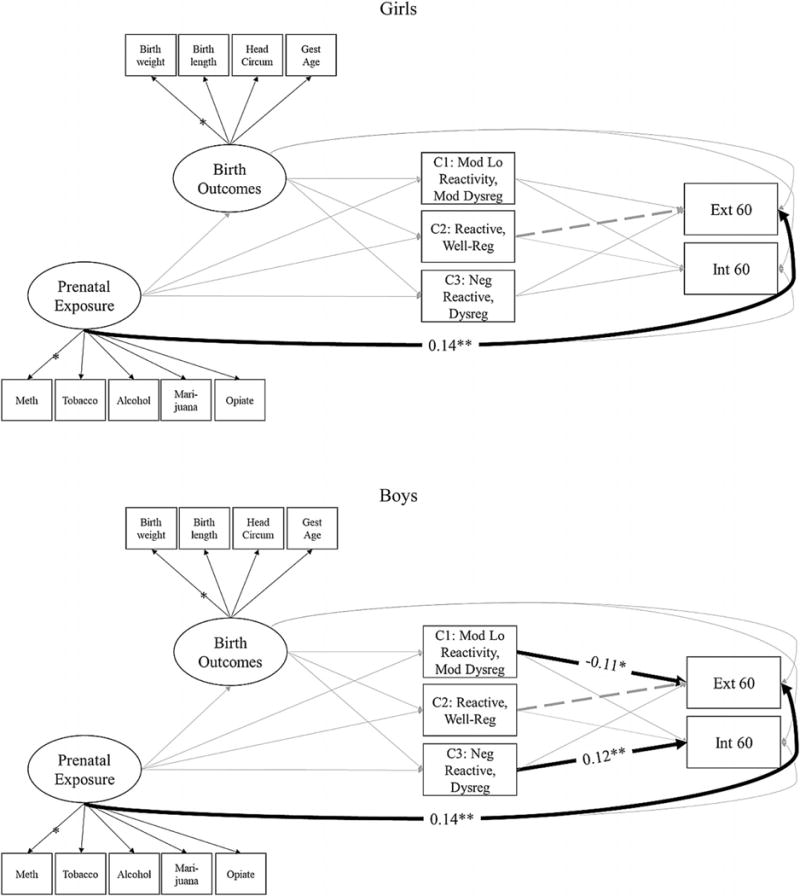
Pathways linking prenatal substance exposure with 5-year-olds’ internalizing and externalizing symptoms (Maternal Lifestyle Study) for girls (top) and boys (bottom). Standardized coefficients are only presented for statistically significant paths to aid interpretability. Posterior probabilities of membership in temperament types 1–3 were allowed to covary. Externalizing and internalizing problems were also allowed to covary. Covariates were regressed on each of the key study variables.
In the model for girls, prenatal substance exposure was significantly associated with externalizing symptoms. No other significant associations emerged. In the model for boys, prenatal substance exposure was again significantly associated with externalizing symptoms. Higher probability of classification as moderately low reactive, moderately dysregulated temperament type was associated with fewer externalizing symptoms. Higher probability of classification as negative reactive, dysregulated temperament type was associated with more internalizing symptoms. No other significant associations emerged.
Study 2: IDEAL study
Temperament profiles
Descriptive statistics for maternal ratings of infant temperament are presented in Table 1. Ten data points were adjusted for nine infants with dimension scores above or below the 1.5 × IQR cutoff.
Model fit indices and entropy are presented in Table 3. Entropy was highest for the four- and five-class solutions, BIC was lowest for the four-class solution, and VLRT was statistically significant for the two-class solution, and marginally significant for the four-class solution. Because the four-class solution demonstrated the best fit and entropy overall across solutions, it was determined to be the best fitting solution.
Temperament profiles corresponding to each of the temperament types are presented in Figure 3. The same four temperament types emerged in the IDEAL data as did in the MLS data (see online-only Supplementary Figure S.1, in which both sets of profiles from MLS and IDEAL are included in the same figure). The temperament type describing majority of infants (40.8%, n = 136) was labeled the moderately low reactive, moderately dysregulated type, followed by the reactive, well-regulated type (27.9%, n = 93), the high positive affect, well-regulated type (20.7%, n = 69), and the negative reactive, dysregulated type (10.5%, n = 35). Although mean levels of each of the temperament dimensions corresponding to the four temperament types were more similar than different across studies, the reactive, well-regulated infants in the MLS in particular exhibited markedly higher mean levels of distress to limitations and fear.
Figure 3.
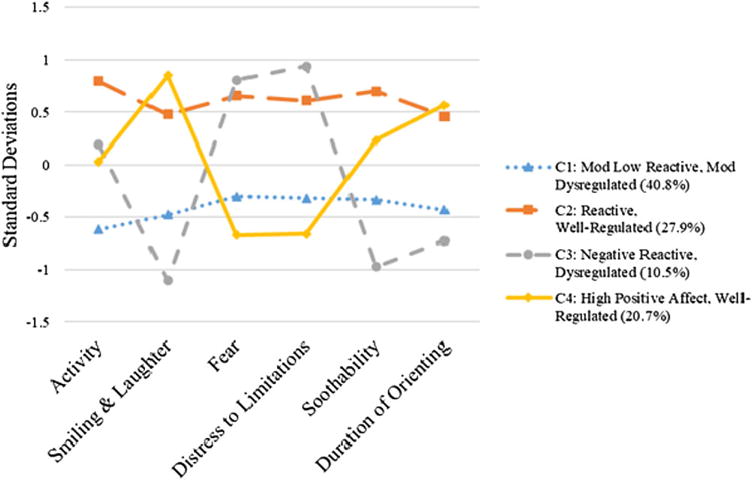
Infant Development, Environment, and Lifestyle study infant temperament profiles at 12 months. Mean standardized scores on each of the temperament dimensions are presented.
Path model
Data points were adjusted for five infants with externalizing T scores above the 1.5 × IQR cutoff; no other outliers were identified. Correlations among demographic variables and key study variables are presented in Table 4. Maternal age, socioeconomic status, infant sex, and participation at the Iowa site were significantly associated with pairs of independent and dependent variables. Therefore, maternal age, maternal socioeconomic status, and Iowa site were included as covariates for the path model. A test comparing the fit of multiple-group models investigating the possible presence of sex differences indicated the absence of statistically significant sex differences, corrected , p = .11. Therefore, sex was included as a covariate instead of as a moderator.
Table 4.
Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother Characteristics | |||||||||||||||||
| 1. Age | – | .16 | −.02 | .18 | −.06 | −.04 | – | .00 | .30 | .01 | −.05 | .12 | −.03 | −.03 | −.17 | −.04 | .00 |
| 2. Educationa | .22 | – | −.34 | .24 | −.09 | −.07 | – | .03 | −.20 | .02 | .03 | .07 | −.11 | −.07 | .02 | −.08 | −.04 |
| 3. SESb | .00 | −.31 | – | −.18 | .12 | .02 | – | −.05 | .11 | −.02 | −.06 | −.10 | .12 | .01 | .00 | .10 | .08 |
| 4. Relationship Statusc | .02 | .05 | −.18 | – | −.32 | .06 | – | .05 | −.21 | .02 | .06 | .10 | −.04 | −.04 | −.01 | −.15 | −.10 |
| 5. Blackd | – | – | – | – | −.47 | – | −.20 | .02 | −.08 | −.17 | −.11 | .11 | .07 | −.00 | .04 | −.05 | |
| 6. Hispanicd | −.03 | −.21 | .08 | −.07 | – | – | – | −.03 | −.04 | .07 | .11 | −.03 | .02 | .00 | −.01 | −.07 | .05 |
| 7. Asian or Pacific Islanderd | −.05 | −.08 | .16 | .08 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 8. Otherd | −.06 | .10 | −.03 | −.07 | – | – | – | – | .00 | .00 | −.02 | .02 | −.05 | .01 | .00 | .01 | .02 |
| 9. Prenatal substance use | .06 | −.05 | .24 | −.25 | – | −.06 | .01 | .05 | – | −.05 | .04 | .02 | .00 | −.02 | .15 | .09 | |
| Infant Characteristics | |||||||||||||||||
| 10. Sexe | −.04 | −.04 | −.05 | −.04 | – | .01 | −.06 | −.02 | −.03 | – | .12 | .01 | −.06 | −.03 | .01 | −.06 | .08 |
| 11. Birth outcomes | −.12 | −.04 | −.11 | .04 | – | −.04 | −.04 | .03 | −.15 | .11 | – | −.03 | −.04 | −.03 | .54 | −.07 | −.04 |
| 12. Temp C1f | .01 | .07 | −.01 | .01 | – | .08 | −.13 | .00 | .02 | −.02 | .06 | – | – | – | – | −.07 | −.04 |
| 13. Temp C2g | .01 | −.09 | .10 | −.01 | – | .02 | .15 | .01 | .00 | .02 | −.12 | – | – | – | – | .09 | .04 |
| 14. Temp C3h | .03 | −.09 | .07 | −.01 | – | .05 | −.05 | .02 | .03 | .04 | .06 | – | – | – | – | .00 | .04 |
| 15. Temp C4i | −.12 | −.09 | −.16 | .02 | – | −.16 | .03 | −.03 | −.05 | −.03 | .04 | – | – | – | – | .03 | −.07 |
| 16. Externalizing T-score | −.07 | −.18 | .14 | −.01 | – | .01 | .00 | −.03 | .15 | .17 | .00 | −.06 | .11 | .15 | −.17 | – | .67 |
| 17. Internalizing T-score | −.13 | −.16 | .11 | −.04 | – | .02 | .06 | .05 | .13 | .08 | .02 | −.07 | .11 | .13 | −.15 | .69 | – |
Note: Correlations for the Maternal Lifestyle Study (MLS) are presented above the diagonal, and correlations for the Infant Development, Environment, and Lifestyle study (IDEAL) are presented below the diagonal.
Correlations were computed using maximum likelihood with robust standard errors. Bolded values indicate alpha significance at p < .05.
1 = less than high school, 2 = high school, 3 = greater than high school (MLS), and 1 = high school or greater (IDEAL).
Socioeconomic status 1 = Hollingshead 5.
1 = is married (MLS) or has romantic partner (IDEAL).
White racewas used as a reference group.
1 = male sex.
Moderately low reactive, moderately dysregulated.
General reactive, well regulated.
Negative reactive, dysregulated.
High positive affect, well regulated.
Results from the full SEM model are presented in Figure 4. Because inclusion of the posterior probabilities corresponding to each of the four temperament types would have caused perfect collinearity, the model included only three of the four types, and opted to exclude the high positive affect, well-regulated type to serve as a reference group. Fit statistics indicated that the model fit the data well: χ2 (73) = 131.48, p < .001; RMSEA = 0.04; CFI = 0.96; SRMR = 0.03.
Figure 4.
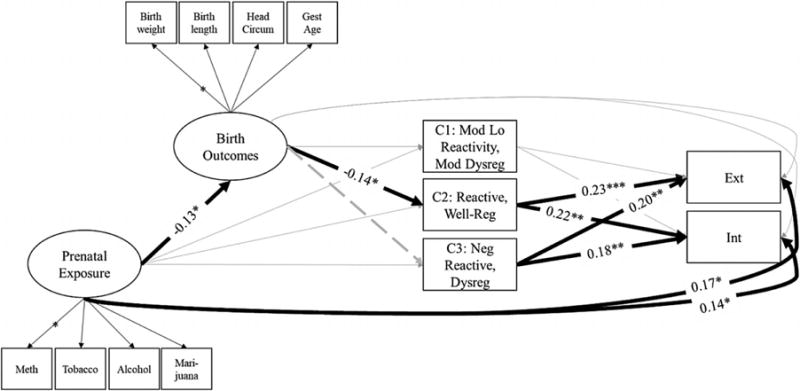
Pathways linking prenatal substance exposure with 5-year-olds’ internalizing and externalizing symptoms (Infant Development, Environment, and Lifestyle study). Standardized coefficients are only presented for statistically significant paths to aid interpretability. Posterior probabilities of membership in temperament types 1–3 were allowed to covary. Externalizing and internalizing problems were also allowed to covary. Covariates were regressed on each of the key study variables.
Each of the manifest variables corresponding to the latent prenatal substance exposure (methamphetamine, β = 0.87, SE = 0.05; tobacco, β = 0.62, SE = 0.05; alcohol, β = 0.33, SE = 0.05; marijuana, β = 0.43, SE = 0.05) and birth outcomes variables (birthweight, β = 0.96, SE = 0.05; birth length, β = 0.78, SE = 0.04; head circumference, β = 0.78, SE = 0.02; gestational age, β = 0.67, SE = 0.04) loaded significantly well at the p < .001 level. More prenatal substance exposure was significantly associated with poorer infant birth outcomes, and more externalizing and internalizing symptoms at 5 years. Infants’ poorer birth outcomes was significantly associated with greater probability of classification in the reactive, well-regulated type (Class 2). Poorer birth outcomes were also associated with lower probability of classification in the negative reactive, dysregulated (Class 3) temperament types at 12 months, although this effect was trending toward significance (β = 0.09, SE = 0.05, p = .06). Greater probability of classification in either the general reactive, well-regulated or the negative reactive, dysregulated type was significantly associated with more externalizing and internalizing symptoms at age 5. No other significant associations emerged.
The test of the indirect effect linking prenatal substance exposure, birth outcomes, and the reactive, well-regulated temperament type indicated that it was not statistically significant at the p < .05 level, 95% confidence level (CI) [−0.003, 0.034]. Because this indirect effect was not statistically significant, the test of the indirect effect linking prenatal substance exposure, birth outcomes, the reactive, well-regulated type, and behavioral symptoms was not indicated. The test of the indirect effect linking birth outcomes, the reactive, well-regulated type, and behavioral symptoms likewise indicated that neither was statistically significant at the p < .05 level: externalizing, 95% CI [−0.046, 0.254]; internalizing 95% CI [−0.132, 0.010]. A trend emerged in which the indirect effect of birth outcomes on the reactive, well-regulated temperament type on internalizing symptoms was marginally significant, 90% CI [−0.121, 0.001].
Discussion
The goal of the present study was to examine the programming effects of prenatal substance exposure on psychopathology using two independent samples. We focused on a specific pathway that linked prenatal substance exposure with growth parameters at birth, temperament in infancy, and behavioral problems in early childhood. Development during the prenatal period may induce persistent changes in brain and behavioral functioning that increases risk for psychopathology (Padmanabhan et al., 2016; Wadhwa et al., 2009). Research to date, however, has yet to define the perinatal pathways to childhood health and psychopathology. Our findings add to the burgeoning evidence base by highlighting how infant phenotypic traits manifest, in part, as a consequence of prenatal substance exposure, and how individual differences in these traits are implicated in the development of problem behavior at age 5. By leveraging two multisite, longitudinal studies of children with prenatal substance exposure, our findings lend credence to the association between infant temperament profiles and risk for problem behavior, while highlighting areas for future research on the developmental origins of psychopathology among children prenatally exposed to substances.
Prior to examining our development pathways of interest, we tested whether infants could be grouped based on phenotypic profiles of neurobehavioral activity, that is, temperament. This is the first study, to our knowledge, to reproduce the same temperament profiles across independent samples of children who exposed to varying levels of intrauterine substance exposure. Our results support the use of latent class analysis for capturing individual differences in temperament among young children (Beekman et al., 2015; Scott et al., 2016). This approach moves beyond the effects of a single temperament trait, or trait-by-trait interactions, in the prediction of later behavioral adjustment, and instead utilized a data-driven approach that captured a child’s functioning across numerous dimensions. Although, remarkably, the same four profiles emerged in these independent data sets, there were meaningful differences in the proportion of profile membership among the samples we examined. For instance, a greater percentage of IDEAL infants were categorized as reactive, well-regulated and moderately low reactive, moderately dysregulated, compared to the MLS study infants. In contrast, more MLS infants belonged to the high positive affect, well-regulated temperament type (41.3%) compared to the IDEAL study infants (20.4%). It is unclear as to why these discrepancies existed across samples of substance-exposed children, particularly in regard to the high proportion of infants from the IDEAL sample categorized in the high positive affect, well-regulated type. These differences may be due, in part, to the type, timing, and nature of substance exposure in utero, which we were unable to assess in the present study, as well as differences in the timing of temperament assessment. The action or coaction of certain substances (e.g., alcohol) at various points during pregnancy may have exerted a differential influence on children in each sample, resulting in disparities in temperament profile membership. This hypothesis is speculative, however, and will require a more detailed record of prenatal substance use to parse out in future research.
Remarkably, the nature of the identified profiles largely paralleled those in the two other studies by Beekman et al. (2015) and Gartstein et al. (2017) that have employed a data-driven typological approach to examining profiles of infant temperament. Drawing from a sample of over 500 9-month-old infants who were adopted, Beekman et al. (2015) likewise identified four temperament profiles, each of which corresponded directly with the four profiles emerging in the current study. Gartstein et al. (2017) drew data from nine different data sets with infants spanning 3-12 months, and divided the sample into groups of younger (i.e., 3 to 8 months; n = 731) and older infants (i.e., 9 to 12 months, n = 625). In the younger age group, Gartstein et al. (2017) identified three profiles, each of which again corresponded directly with three of our four profiles. In the older age group, they identified five profiles, three of which corresponded with ours. Specifically, the high positive affect, well-regulated and moderately low reactive, moderately dysregulated groups emerged across each of our five samples (“positive reactive” and “typical low expressive” in Beekman et al., 2015; “high positive/regulated,” “high approach/soothable,” and “fear-less/low positive/low orienting,” “low pleasure/low approach/difficult to calm” in Gartstein et al., 2017). The negative reactive, dysregulated and generally reactive, regulated groups emerged across four of our five samples (all but the older group and younger group in Gartstein et al., respectively). Important, however, mean levels of each of the temperament dimensions corresponding to each of the profiles differed modestly across samples, including between the two samples from the current study. Taken together, the consistent emergence of these four profiles of infant temperament across our five samples suggests that they may reflect average prototypes of temperament in infancy, and that slight variations in mean levels may emerge across samples as a function of unique sample characteristics.
The composition of temperament is known to be multifaceted and composed of multiple interacting traits; how these traits are organized and expressed may be a more robust marker of later behavioral adjustment than any trait independently (Stifter & Dollar, 2016). Our study supports this notion, and is among the first to use a data-driven approach to examine how the constellation of traits relate to behavioral adjustment (Stifter et al., 2008). Given that temperament is often defined by a child’s proclivities to reactivity and regulation across dimensions (Rothbart & Bates, 2006; Shiner et al., 2012), the use of latent profile analysis to classify a child’s temperament is an important direction for temperament research. We identified two infant temperament profiles (the reactive, well-regulated and negative reactive, dysregulated types) that were associated with increased behavior problems nearly 5 years later.
Both of these profiles were characterized by high levels of fear and anger reactivity, traits that interact with regulatory capabilities to predict problem behavior in childhood (see Stifter & Dollar, 2016, for review). Children in the reactive, well-regulated type, however, also exhibited high levels of positive affect and regulation. It is unclear why this profile characterized by high levels of regulation was associated with risk for childhood psychopathology. It may be that these children are especially sensitive to threat, both real and perceived, in the environment and become aroused quickly. Then, as evidenced by their high level of orienting, these infants may have a difficult time disengaging from the arousing stimulus, which may prolong reactivity and/or thwart recovery. This aberrant pattern may lead to prolonged periods of unresolved reactivity and risk for problem behavior as a function of early environmental exposures. Yet, these infants may be highly sensitive to any stimulation in the environment, as evidenced by their elevated scores on all temperament domains. Infants in the negative reactive, dysregulated profile had similarly high levels of negative affect, but tended to exhibit lower activity levels along with less positive affect and regulatory abilities. This profile may correspond to the behaviorally inhibited profile described by Kagan and colleagues (Garcia-Coll, Kagan, & Reznick, 1984; Kagan, 2012). Over time, these patterns of reactivity and regulation may manifest on either the internalizing or the externalizing spectrum based on the quality of early rearing conditions, and whether fear or anger is the dominant trait expressed (Braungart-Rieker, Hill-Soderlund, & Karrass, 2010; Slagt, Dumas, Deković, & van Aken, 2016). More research is needed to determine the intricate ways in which a child’s unique constellation of temperament traits function together to influence socioemotional development.
The etiology of problem behavior is complex and attributable to a dynamic interplay between biology and context that unfolds over early development. Part of this phenotypic development may begin in utero, as evidenced by results from the present study as well as other studies that have linked prenatal stressors directly to childhood psychopathology (e.g., Davis & Sandman, 2012; Fisher et al., 2011). Nevertheless, due to the long latency between exposure and the onset of behavior problems, it seems likely that a variety of individual- and environmental-level processes operate as intermediary steps linking prenatal conditions to childhood health and pathology (Cicchetti, 2008; Gottlieb, 2007), supporting the notion of multifinality (Cicchetti & Rogosch, 2002). Our findings suggest that an infant’s phenotypic traits, characterized by growth parameters at birth and temperamental reactivity in infancy, may serve as early indicators of risk along this developmental trajectory. This study was among the first to link known individual-level correlates of prenatal substance exposure (e.g., low birth weight and temperament) into a specific pathway to childhood problem behavior (Lester et al., 2009).
Although the indirect path from prenatal conditions to birth outcomes, infant temperament, and childhood problem behavior was not reproduced across samples, our results provided initial support for a path to childhood psychopathology. Both studies did lend support to a temperament–psychopathology link, though this association was limited to only boys in the MLS sample. The observed sex differences may be attributable, in part, to the timing of the temperament assessment, which differed between the samples and may signal that temperamental risk for prenatally substance-exposed boys emerges earlier than for girls. This point is speculative and requires further examination. Regardless, multiple pathways to childhood psychopathology may exist for this population of children, and may operate via individual (e.g., temperament) and environmental (e.g., poverty) mechanisms (Abar et al., 2013; Eze et al., 2016; Fisher et al., 2011; Lester et al., 2009). Disentangling the postnatal pathways associated with various outcomes for substance-exposed children has proven challenging due to the myriad of co-occurring factors that are more common among women who used substances during pregnancy, which include psychiatric comorbidities, housing instability, and legal system involvement, among other stressors (Oei et al., 2010). Future research should therefore consider examining how individual-level factors, including those identified in this study, transact with environment risk across early development in the prediction of problem behavior for children with prenatal substance exposure (Cicchetti, 2008; Gottlieb, 2007).
Support is growing for the prenatal period as one point of origin of risk for childhood problem behavior; the function and mechanisms associated with intrauterine adversity, however, still require clarification. One possibility is that intrauterine experiences serve as a guide that directs fetal development toward a strategy that “matches” conditions in the anticipated postnatal environment (Gluckman et al., 2008; Sandman et al., 2012). Many woman who use substances during pregnancy continue using substances postnatally (e.g., Conradt et al., 2016), suggesting that fetal neurobehavioral adjustments in response to this prenatal stressor may be an attempt to prepare the fetus for a postnatal environment characterized by sustained stress exposure. Compared to other established programming factors (e.g., undernutrition), the programming influence of prenatal substance exposure may be unique, in that it “may be developmentally disruptive with no long-term adaptive value, although the fetus may make homeostatic adjustments that confer immediate survival advantage” (Lester & Padbury, 2009, p. 25). These adjustments include reduced body composition at birth and temperamental reactivity as observed in the present study, as well as newborn neurobehavioral functioning (Bauer et al., 2005; Law et al., 2003; Lester et al., 2002; Oberlander et al., 2010; Smith et al., 2008; Stroud et al., 2009). Reduced fetal growth requires less metabolic resources, which means the infant would have an increased chance of survival in a potentially stressful (e.g., nutrient poor) postnatal environment. Behaviorally, what may be considered an aberrant pattern of reactivity and regulation may instead function to arouse attention from the caregiver, which may also increase the likelihood of survival. Testing the mechanisms through which prenatal substance exposure affects fetal growth and infant behavioral reactivity is a promising avenue for investigation (Lester & Padbury, 2009), and may provide insight into whether these phenotypic traits are actually adaptive for prenatally substance-exposed children.
Traditionally, phenotypic traits that result from intrauterine substance exposure have been considered deficits. From this perspective, intrauterine substance exposure is thought to result in the degradation of various neurobehavioral systems, which affects subsequent functional development of the child. Children who possess traits that manifest as a result of these insults would then be expected to exhibit problematic behavior, particularly if they are exposed to subsequent stressors (e.g., diathesis-stress; Monroe & Simons, 1991). Alternatively, developmental programming models would suggest that programming efforts alter biobehavioral development in an attempt to adapt the fetus to postnatal conditions, which has important implications for phenotypic trait expression postnatally. As a potent stressor in the intrauterine environment, fetuses with prenatal substance exposure may be programmed for stressful postnatal conditions. In accordance with evolutionary–developmental perspectives (Del Giudice & Ellis, 2016), the profiles of fetal growth, temperamental reactivity, and problem behavior that emerged among children with prenatal substance exposure in this study may therefore constitute adapted phenotypic traits well suited for the harsh and/or unpredictable ecological conditions frequently experienced by these children (Abar et al., 2013; Fisher et al., 2011; Oei et al., 2010). However, given the mental, physical, and financial toll of childhood behavior problems in modern society (National Research Council and Institute of Medicine, 2009), the desirability of this programmed neurobehavioral profile for substance-exposed children outside of an evolutionary–developmental perspective are unclear.
Deficit models would also argue that certain temperament types would reflect a vulnerability to environmental conditions. Children who are temperamentally reactive and/or high in negativity are more likely to display internalizing and externalizing behaviors across development (Eisenberg et al., 2009; Gartstein, Putnam, & Rothbart, 2012; Kochanska & Kim, 2013; Oldehinkel, Hartman, de Winter, Venstra, & Ormel, 2004). While this perspective continues to gain empirical support, it may only represent half of the story (see Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011, for discussion). Recent theoretical work has posited that certain children may possess traits that make them more sensitive to both negative and positive environmental influences (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Ellis et al., 2011). Although we were unable to directly examine differential susceptibility in the present study, the combination of traits that comprise the reactive, well-regulated and the negative reactive, dysregulated profiles (e.g., high negativity) may make these children disproportionately susceptible to the quality of their rearing environment. If so, it may be that the path to problem behavior for these prenatally substance-exposed children may operate through their susceptibility to the stressful conditions often experienced during the first years of life (Abar et al., 2013; Fisher et al., 2011; Oei et al., 2010). In contrast, children with either the moderately low reactive, moderately dysregulated or the high positive affect, well-regulated profiles may constitute hardier counterparts who will function adequately regardless of the quality of their early conditions. Whether these profiles do function as neurobehavioral susceptibility factors remains an open question. The differing functional implications for temperament profiles identified in this study underscore the importance of examining individual differences when assessing children who were prenatally exposed to substances, which we plan to do in follow-up studies.
Limitations
Our results should be interpreted within the context of limitations of the present study. Temperament and childhood problem behavior were based on maternal report, which may present reporting bias even though they were sampled 5 years apart. However, it may be that mothers who engaged in substance use during pregnancy may be more likely to report elevated trait expression in their infants. Future research in this area should consider supplementing maternal reports of behavior with objective measures such as behavioral coding. Furthermore, the entropy values for the best fitting models in the MLS and IDEAL study were relatively low, at .65 and .67, respectively, suggesting that class membership was not always clearly defined. Nonetheless, these values of entropy are similar to those reported in Beekman et al. (2015), with entropy values at 9 months ranging from .68 to .74, and at 18 months ranging from .54 to .65. It is conceivable that lower entropy values in studies concerning temperament may reflect the considerable variability of infants’ temperament expression, especially in diverse samples. In other words, although temperament types appear to capture frequently co-occurring constellations of temperament at the group level, infants continue to evidence variability within those temperament types at the individual level. This hypothesis is consistent with the observation discussed previously that mean levels of each of the temperament dimensions within prototypic temperament types show some variability across samples.
Perhaps related, although the present study utilized independent samples that were racially and socioeconomic diverse relative to the general US population, and thereby included individuals who may be underrepresented in research, it is unclear if the number of temperament profiles we identified will generalize to populations of children who were not exposed to substances in utero. In addition, this study did not directly test whether temperament operated as a susceptibility factor, or whether substance exposure functions strictly as a prenatal insult or as a programming factor. These are important implications of this line of research, and should be examined in future research with this population of children. Finally, although we conceptualize prenatal substance exposure as an intrauterine stressor (Lester & Padbury, 2009), differences still remain between the type, timing, frequency, and combination of substances used during pregnancy and their effects on the offspring. For example, as mentioned above, the majority of the MLS sample reported alcohol use during pregnancy, compared to approximately one-quarter of the IDEAL sample mothers. The known teratogenic effect of alcohol exposure and increased use in the MLS sample may have exerted a differential effect on these children, which may have affected our ability to detect the anticipated developmental pathway in this sample. Future research should consider how various combinations of substance use influence childhood outcomes.
Conclusions
The etiology of childhood psychopathology for children with prenatal substance exposure is complex, and likely operates through multiple developmental pathways originating prenatally. Research over the past two decades has converged on programming efforts in utero as an important determinant of health and behavior. The present study contributed to the extant literature by outlining a developmental pathway to problem behavior via birth outcomes and infant temperament. These early-emerging phenotypic traits may be programmed by intrauterine exposure to substances, with implications for mental and physical health across the life span. Identifying pathways from prenatal substance exposure to psychopathology may ultimately clarify targets for intervention to reduce the negative sequelae for substance-exposed children (e.g., temperament in infancy), while aiding parents and pediatricians in supporting socioemotional development. Any future intervention efforts will undoubtedly need to be augmented by social policy aimed at reducing the impact of early adversity exposure on this high-risk group of children.
Acknowledgments
This work was supported by the following NIH grants: National Institute of Child Health and Human Development (NICHD) Neonatal Research Network and an interinstitute agreement with the National Institute on Drug Abuse (NIDA) through cooperative agreements: U10-DA-024119-01 and U10-HD-27904 (to B.M.L.); NICHD contract N01-HD-2-3159 (to B.M.L) and 1RO1DA014918 (to L.L.); and a Career Development Award from the NIDA 7K08DA038959-02 (to E.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the NIDA, or the NIH.
Footnotes
Supplementary Material
To view the supplementary material for this article, please visit https://doi.org/10.1017/S0954579418000391.
References
- Abar B, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, Lester BM. Examining the relationships between prenatal methamphetamine exposure, early adversity, and child neurobehavioral disinhibition. Psychology of Addictive Behaviors. 2013;27:662–673. doi: 10.1037/a0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Aksan N, Goldsmith HH, Smider NA, Essex MJ, Clark R, Hyde JS, Vandell DL. Derivation and prediction of temperamental types among preschoolers. Developmental Psychology. 1999;35:958–971. doi: 10.1037//0012-1649.35.4.958. [DOI] [PubMed] [Google Scholar]
- Alati R, Najman JM, O’Callaghan M, Bor W, Williams GM, Clavarino A. Fetal growth and behaviour problems in early adolescence: Findings from the Mater University study of pregnancy. International Journal of Epidemiology. 2009;38:1390–1400. doi: 10.1093/ije/dyp252. [DOI] [PubMed] [Google Scholar]
- Ashford J, van Lier PA, Timmermans M, Cuijpers P, Koot HM. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:779–787. doi: 10.1097/CHI.0b013e318172eefb. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, Wright LL, Maza PL. Gestational cocaine exposure and intrauterine growth: Maternal Lifestyle Study. Obstetrics and Gynecology. 2002;100:916–924. doi: 10.1016/S0029-7844(02)02199-3. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;327:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Winter PD, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;334:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clinical Obstetrics and Gynecology. 2013;56:511–519. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, Verter J. Acute neonatal effects of cocaine exposure during pregnancy. Archives of Pediatric and Adolescent Medicine. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- Beekman C, Neiderhiser JM, Buss KA, Loken E, Moore GA, Leve LD, Reiss D. The development of early profiles of temperament: Characterization, continuity, and etiology. Child Development. 2015;86:1794–1811. doi: 10.1111/cdev.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke M, Eyler FD, Garvan CW, Wobie K. The search for congenital malformations in newborns with fetal cocaine exposure. Pediatrics. 2001;107:1–6. doi: 10.1542/peds.107.5.e74. [DOI] [PubMed] [Google Scholar]
- Behnke M, Smith VC, Committee on Substance Abuse & Committee on Fetus and Newborn Prenatal substance abuse: Short- and longterm effects on the exposed fetus. Pediatrics. 2013;131:e1009–e1024. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. Journal of Developmental and Behavioral Pediatrics. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Billings L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse & Neglect. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, Karrass J. Fear and anger reactivity trajectories from 4 to 16 months: The roles of temperament, regulation, and maternal sensitivity. Developmental Psychology. 2010;46:791–804. doi: 10.1037/a0019673. [DOI] [PubMed] [Google Scholar]
- Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: Longitudinal evidence from a birth cohort. Child Development. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13:195–212. doi: 10.1007/bf01246098. [DOI] [Google Scholar]
- Chatterji P, Lahiri K, Kim D. Fetal growth and neurobehavioral outcomes in childhood. Economics & Human Biology. 2014;15:187–200. doi: 10.1016/j.ehb.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. A multiple-levels-of-analysis perspective on research in development and psychopathology. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. Hoboken, NJ: Wiley; 2008. pp. 27–57. [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70:6. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Conradt E, Beauchaine TP, Abar B, Lagasse L, Shankaran S, Bada HS, Lester BM. Early caregiving stress exposure moderates the relation between respiratory sinus arrhythmia reactivity at 1 month and biobehavioral outcomes at age 3. Psychophysiology. 2016;53:83–96. doi: 10.1111/psyp.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker NA, Fryer SL, Mattson SN. Exposure to teratogens as a risk factor for psychopathology. In: Beauchaine TB, Hinshaw SH, editors. Child and adolescent psychopathology. Hoboken, NJ: Wiley; 2013. pp. 285–316. [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. [Google Scholar]
- Del Giudice M, Ellis BJ. Evolutionary foundations of developmental psychopathology. In: Cicchetti D, editor. Developmental psychopathology: Developmental neuroscience. 3rd. Vol. 2. Hoboken, NJ: Wiley; 2016. pp. 1–58. [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, Losoya SH. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Steegers EAP, Jaddoe VWV, Hofman A, Verhulst FC, Huizink AC. Intrauterine cannabis exposure affects fetal growth trajectories: The Generation R Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- Eze N, Smith LM, LaGasse LL, Derauf C, Newman E, Arria A, Lester BM. School-aged outcomes following prenatal methamphetamine exposure: 7.5-year follow-up from the Infant Development, Environment, and Lifestyle study. Journal of Pediatrics. 2016;170:34–38. doi: 10.1016/jpeds.2015.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Lester BM, Degarmo DS, LaGasse LL, Lin H, Bauer CR, Higgins R. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Development and Psychopathology. 2011;23:777–788. doi: 10.1017/S0954579411000290.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Development. 1984;55:1005–1019. [Google Scholar]
- Gartstein MA, Prokasky A, Bell MA, Calkins S, Bridgett DJ, Braungart-Rieker J, Seamon E. Latent profile and cluster analysis of infant temperament: Comparisons across person-centered approaches. Developmental Psychology. 2017;53:1811–1825. doi: 10.1037/dev0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Putnam SP, Rothbart MK. Etiology of preschool behavior problems: Contributions of temperament attributes in early childhood. Infant Mental Health Journal. 2012;33:197–211. doi: 10.1002/imhj.21312. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicology and Teratology. 2000;22:325–336. doi: 10.1016/S0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67:218–235. doi: 10.2307/1131697. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Probabilistic epigenesis. Developmental Science. 2007;10:1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Groen-Blokhuis MM, Middeldorp CM, van Bijsterveldt CEM, Boomsma DI. Evidence for a causal association of low birth weight and attention problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:1247–1254. doi: 10.1016/j.jaac.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Gupta ND, Deding M, Lausten M. The effect of low birth weight on height, weight and behavioral outcomes in the medium-run. Economics & Human Biology. 2013;7:1–6. doi: 10.1016/j.ehb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. British Medical Journal. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, Najman JM. Birth outcomes associated with cannabis use before and during pregnancy. Pediatric Research. 2012;71:215–219. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Huizink AC, Robles De Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Torrång A, Tuvblad C, Cnattingius S, Larsson JO, Lichtenstein P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: A prospective Swedish twin study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- Kagan J. The biography of behavioral inhibition. In: Zentner M, Shiner RL, editors. Handbook of temperament. New York: Guilford Press; 2012. pp. 69–82. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. doi: 10.2307/1129793. [DOI] [Google Scholar]
- Kochanska G, Kim S. Difficult temperament moderates links between maternal responsiveness and children’s compliance and behavioral problems in low-income families. Journal of Child Psychology and Psychiatry. 2013;54:323–332. doi: 10.1111/jcpp.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C, Lester BM. Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics. 2012;129:681–688. doi: 10.1542/peds.2011-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KL, Stroud LR, Lagasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- Lester BM, Bagner DM, Liu J, Lagasse LL, Seifer R, Bauer CR, Das A. Infant neurobehavioral dysregulation: Behavior problems in children with prenatal substance exposure. Pediatrics. 2009;124:1355–1362. doi: 10.1542/peds.2008-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, Maza PL. The Maternal Lifestyle Study: Drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Maza PL. The Maternal Lifestyle Study: Effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Locke RL, LaGasse LL, Seifer R, Lester BM, Shankaran S, Bada HS, Bauer CR. Effects of prenatal substance exposure on infant temperament vary by context. Development and Psychopathology. 2016;28:309–326. doi: 10.1017/S0954579415000504. [DOI] [PubMed] [Google Scholar]
- Lumey L, Stein AD, Kahn HS, Romijn J. Lipid profiles in middle-aged men and women after famine exposure during gestation: The Dutch Hunger Winter Families Study. American Journal of Clinical Nutrition. 2009;89:1737–1743. doi: 10.3945/ajcn.2008.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Lang A, Singer L. Prenatal tobacco, marijuana, stimulant, and opiate exposure: Outcomes and practice implications. Addiction Science & Clinical Practice. 2011;6:57–70. [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–25. doi: 10.1037//0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus user’s guide. 7th. Los Angeles: Author; 1998–2012. [Google Scholar]
- National Research Council and Institute of Medicine. Preventing mental, emotional, and behavioral disorders among young people: Progress and possibilities. Washington, DC: National Academic Press; 2009. [PubMed] [Google Scholar]
- Oberlander TF, Jacobson SW, Weinberg J, Grunau RE, Molteno CD, Jacobson JL. Prenatal alcohol exposure alters biobehavioral reactivity to pain in newborns. Alcoholism: Clinical and Experimental Research. 2010;34:681–692. doi: 10.1111/j.1530-0277.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ. Prenatal alcohol exposure and infant negative affect as precursors of depressive features in children. Infant Mental Health Journal. 2001;22:291–299. [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Developmental Disabilities Research Review. 2009;15:225–234. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- Oei J, Abdel-Latif ME, Clark R, Craig F, Lui K. Short-term outcomes of mothers and infants exposed to antenatal amphetamines. Archives of Disease in Childhood—Fetal and Neonatal Edition. 2010;95:F36–F41. doi: 10.1136/adc.2008.157305. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, de Winter AF, Veenstra R, Ormel J. Temperament profiles associated with internalizing and externalizing problems in preadolescence. Development and Psychopathology. 2004;16:421–40. doi: 10.1017/s0954579404044591. [DOI] [PubMed] [Google Scholar]
- Open Science Collaboration. Estimating the reproducibility of psychological science. Science. 2015;349 doi: 10.1126/science.aac4716. [DOI] [Google Scholar]
- Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental programming, a pathway to disease. Endocrinology. 2016;157:1328–1340. doi: 10.1210/en.2016-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J, Bakker R, Irving H, Jaddoe VWV, Malini S, Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)—A systematic review and meta-analyses. BJOG. 2011;118:1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: Effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicology and Teratology. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicology and Teratology. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Oddy WH, Li J, Kendall GE, de Klerk NH, Silburn SR, Mattes E. Pre- and postnatal influences on preschool mental health: A large-scale cohort study. Journal of Child Psychology and Psychiatry. 2008;49:1118–1128. doi: 10.1111/j.1469-7610.2008.01955.x. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JHP, Osmond C, Barker DJP, Ravelli ACJ, Schroeder-Tanka JM, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology. 2015;40:61–87. doi: 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. doi: 10.2307/1129176. [DOI] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology: Social, emotional, and personality development. 6th. Vol. 3. Hoboken, NJ: Wiley; 2006. pp. 99–106. [Google Scholar]
- Salmasi G, Grady R, Jones J, Mcdonald SD. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstetricia et Gynecologica Scandinavica. 2010;89:423–41. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between prenatal exposure to cigarettes and infant and maternal negative affect. Infant Behavior Development. 2007;30:387–398. doi: 10.1016/j.infbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BG, Lemery-Chalfant K, Clifford S, Tein JY, Stoll R, Goldsmith HH. A twin factor mixture modeling approach to childhood temperament: Differential heritability. Child Development. 2016;87:1940–1955. doi: 10.1111/cdev.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Das A, Bauer CR, Bada HS, Lester B, Wright LL, Smeriglio V. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004;114:e226–e234. doi: 10.1542/peds.101.2.229. [DOI] [PubMed] [Google Scholar]
- Shiner R, Buss K, McClowry S, Putnam S, Saudino K, Zentner M. What is temperament now? Assessing progress in temperament research on the twenty-fifth anniversary of Goldsmith et al. (1987) Child Development Perspectives. 2012;6:436–444. doi: 10.1111/j.1750-8606.2012.00254.x. [DOI] [Google Scholar]
- Slagt M, Dubas JS, Dekovic M, van Aken MA. Differences in sensitivity to parenting depending on child temperament: A meta-analysis. Psychological Bulletin. 2016;142:1068–1110. doi: 10.1037/bul0000061. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Lester BM. The Infant Development, Environment, and Lifestyle study: Effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicology and Teratology. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter C, Dollar J. Temperament and developmental psychopathology. In: Damon W, Lerner R, Eisenberg N, editors. Developmental psychopathology: Risk, resilience, and intervention. Vol. 4. Hoboken, NJ: Wiley; 2016. pp. 1–62. [Google Scholar]
- Stifter C, Putnam S, Jahromi L. Exuberant and inhibited toddlers: Stability of temperament and risk for problem behavior. Development and Psychopathology. 2008;20:401–421. doi: 10.1017/S0954579408000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, Lester BM. Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. Journal of Pediatrics. 2009;154:10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Chess S. Temperament and development. New York: Brunner/Mazel; 1977. [Google Scholar]
- Thomas A, Chess S, Birch HG. The origins of personality. Scientific American. 1970;233:102–109. doi: 10.1038/scientificamerican0870-102. [DOI] [PubMed] [Google Scholar]
- Thompson C, Syddall H, Rodin IAN, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. British Journal of Psychiatry. 2001;179:450–55. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- Tsang TW, Lucas BR, Olson HC, Pinto RZ, Elliott EJ. Prenatal alcohol exposure, FASD, and child behavior: A meta-analysis. Pediatrics. 2016;137:1–20. doi: 10.1542/peds.2015-2542. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Seminars in Reproductive Medicine. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarén B, Lindmark G, Gebre-Medhin M. Maternal smoking and body composition of the newborn. Acta Paediatrica. 1996;85:213–219. doi: 10.1111/j.1651-2227.1996.tb13995.x. [DOI] [PubMed] [Google Scholar]


