Abstract
An episode of clinically recovered acute kidney injury (r-AKI) has been identified as a risk factor for future hypertension and cardiovascular disease. Our objective was to assess whether r-AKI was associated with future preeclampsia and other adverse pregnancy outcomes and to identify if severity of AKI or time interval between AKI and pregnancy was associated with pregnancy complications. We conducted a retrospective cohort study of women who delivered infants between 1998 to 2016 at Massachusetts General Hospital. AKI was defined using the 2012 Kidney Disease Improving Global Outcomes (KDIGO) laboratory criteria with subsequent clinical recovery (estimate glomerular filtration rate >90 ml/min/1.73m2 prior to conception). AKI was further classified by severity (KDIGO Stages 1-3) and time interval between AKI episode and the start of pregnancy. Women with r-AKI had an increased rate of preeclampsia compared to women without previous r-AKI (22% versus 9%, p<0.001). Infants of women with r-AKI were born earlier (gestational age 38.2±3.0 versus 39.0±2.2 weeks, p<0.001) and were more likely to be small for gestational age (9% versus 5%, p=0.002). Increasing severity of r-AKI was associated with increased risk of preeclampsia for Stage 2 and 3 AKI (adjusted OR 3.5 95% CI 2.1-5.7 and adjusted OR 6.5 95% CI 3.5-12.0, respectively) but not for Stage 1 (adjusted OR 1.7 95% CI 0.9-3.2). A history of AKI prior to pregnancy, despite apparent full recovery, was associated with increased risk of pregnancy complications. Severity and timing of the AKI episode modified the risk.
Keywords: Acute kidney injury, pregnancy, preeclampsia, gestational hypertension, epidemiology
BACKGROUND
The global burden of acute kidney injury (AKI) is increasing.1 AKI severity, duration and clinical context influence outcomes including the development of hypertension, chronic kidney disease (CKD) and dialysis-dependence.2,3 While AKI is most often studied in elderly and critically ill populations, it is also observed in children and young adults.4,5 Young women have been an under-represented group of study in AKI research, yet the consequences of AKI in young women may be more immediate due to the increased demands on renal function in pregnancy.
Preeclampsia is a multi-system disorder of pregnancy characterized by wide-spread endothelial dysfunction resulting in elevated blood pressure and end-organ damage in the second half of pregnancy.6 Increased placental production of soluble fms-like tyrosine kinase 1 (sFlt-1), an antagonist of vascular endothelial growth factor (VEGF), plays a central role in the pathogenesis of preeclampsia.7,8 Many risk factors for preeclampsia are recognized, including nulliparity, obesity, sociodemographic characteristics and preexisting hypertension.9
In a smaller study, we recently demonstrated that a history of AKI, with subsequent complete clinical and laboratory recovery, is associated with higher rates of future preeclampsia.10 The frequency of fetal complications, including fetal growth restriction, was also higher in these women. Our study identified a novel group of women at high risk for complicated pregnancies and adds to an emerging literature suggesting that subclinical kidney disease is associated with poor pregnancy outcomes.11–13 Previous studies have demonstrated that the severity of AKI affects long-term prognosis, with stage 3 AKI conferring increased risk of incident CKD and mortality compared to stage 1 AKI.14,15 As r-AKI was an infrequent exposure in our original cohort (0.4% of women) we could not address differences in AKI severity and time interval. In the present study, we expanded our cohort to include 18 years of data with the aim of confirming our previous findings and to explore if AKI severity and the time interval between the AKI episode and pregnancy influence outcomes.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects and Data Collection
Massachusetts General Hospital (MGH) is a quaternary care hospital that serves patients from Boston and surrounding New England. The obstetrics service provides both community and high-risk care, with more than 3,500 deliveries each year. We performed a retrospective cohort study in the MGH Obstetric Service Birth Database of all deliveries between September 1, 1998 and March 31, 2016. Clinical information such as medical history, prenatal blood pressure measurements, and delivery information were abstracted into the medical record prospectively and transferred into the study database. We previously reported the outcomes of women from 1998-2007, which was combined with data from 2008-2016 to increase our analytic power.10 Detailed past medical history including previous laboratory results, inpatient and outpatient medical documentation, and billing data were obtained for the 10 years prior to pregnancy through the Partners Research Patient Data Registry.16
The cohort included all singleton pregnancies that continued beyond 20 weeks gestation in women who received prenatal care during the study period. In our main analysis, we only included women who had three or more assessments of renal function prior to pregnancy in order to capture women who had the potential for a diagnosis of AKI prior to pregnancy. We excluded women with chronic kidney disease (estimate glomerular filtration rate (eGFR) less than 90 ml/min/1.73m2 using CKD-EPI equation17 before index pregnancy), patients with structural kidney disease (e.g.: polycystic kidney disease, congenital solitary kidney), kidney transplant donors and recipients, and patients with known glomerulonephritis or proteinuria (>2+ on urine dipstick) at the first prenatal visit. All women who presented for prenatal care after 20 weeks gestational age or who were missing baseline blood pressure, urine dipstick or weight (all part of standard care) at the first prenatal visit were excluded.
Ascertainment of Exposures and Outcomes
We defined Acute Kidney Injury using clinical laboratory data obtained prior to pregnancy. AKI cases were identified using the Kidney Disease Improving Global Outcomes (KDIGO) laboratory definition of AKI as “an increase in serum creatinine by 0.3 mg/dl or more within 48 hours or an increase in serum creatinine to 1.5 times baseline, which is known or presumed to have occurred within the prior 7 days”.18 For AKI diagnosed in the outpatient setting, an increase in serum creatinine to 1.5 times baseline was used to establish the diagnosis.19 Stage of AKI was similarly defined using KDIGO criteria (Stage 1: serum creatinine elevation of more than 0.3mg/dl within 48 hours or 1.5-1.9x baseline; Stage 2: serum creatinine elevation of 2.0-3.0x baseline, Stage 3: serum creatinine elevation 3.0x or more from baseline or need for renal replacement therapy). Only the first pregnancy following AKI was considered for analysis. Medical records for all women who met laboratory criteria for AKI in the cohort were reviewed in detail to confirm AKI diagnosis. Prior to chart review we identified all women who had a normal eGFR (> 90 ml/min/1.73m2) at the closest time point prior to the start of the index pregnancy. Women who had a GFR < 90 ml/min/1.73m2 were considered to have chronic kidney disease. Cause of AKI was determined by independent chart review (including clinical documentation, laboratory and radiology review) by two nephrologists who were blinded to outcomes. Time interval from AKI to pregnancy was calculated from peak serum creatinine during the AKI episode to estimated date of last menstrual period (delivery date minus gestational age at delivery). Pre-existing hypertension was defined as a blood pressure prior to 20 weeks gestation greater than or equal to 140/90 mmHg, use of antihypertensive medications or documentation of hypertension in the OB medical record at initial pre-natal visit. Pre-existing diabetes was defined based on documentation in the OB medical record at initial pre-natal visit or use of insulin or oral hypoglycemic prior to pregnancy.
Preeclampsia was defined based on blood pressure and spot urine protein measurements made at prenatal visits. In women who were normotensive at their first prenatal visit (blood pressure less than 140/90 mmHg), and lacked a diagnosis of chronic hypertension, gestational hypertension was defined as blood pressure greater than or equal to 140/90 mmHg after 20 weeks gestation.20 In women who were hypertensive at their first prenatal visit (blood pressure greater than or equal to 140/90 mmHg) gestational exacerbation of hypertension was defined by the presence of a rise in systolic blood pressure greater than 30 mmHg or a rise in diastolic blood pressure greater than 15 mmHg after 20 weeks gestation. Preeclampsia was defined as the presence of gestational hypertension and 2+ or greater proteinuria after 20 weeks gestation or gestational hypertension and 1+ proteinuria after 20 weeks gestation with confirmation of the diagnosis in the electronic delivery record. Preterm preeclampsia and early preterm preeclampsia were defined as preeclampsia requiring delivery before 37 weeks gestation and 34 weeks gestation, respectively. Small for gestational age (SGA) was defined as birthweight in the 5th percentile or lower for completed week of gestational age based on national standards.21 Perinatal death was defined as fetal death at greater than 20 weeks gestation or infant death that occurred at fewer than 7 days of age. The composite fetal outcome was defined as preterm delivery (<37 weeks), neonatal intensive care unit (NICU) admission, SGA or perinatal death.
Statistical Analyses
Baseline characteristics and primary outcomes in women with and without r-AKI were compared using Student’s t-test for continuous variables and Fisher’s exact tests for categorical variables. Univariate and multivariate logistic regression was used to compare the odds of preeclampsia, preterm delivery, delivery by cesarean section, SGA infants, perinatal death, NICU admission and the composite fetal outcome. Multivariate logistic regression models included variables associated with adverse pregnancy outcomes based on prior literature and included maternal age, body mass index (BMI), first trimester diastolic blood pressure, pre-pregnancy diabetes status, race and parity. The association between maternal baseline characteristics and preeclampsia are summarized in S1. The effect estimates in this cohort were similar to previous literature.22 In a secondary analysis, we included all women who met our initial inclusion criteria regardless of assessment of kidney function prior to pregnancy (Figure 1). Additionally, we conducted a subgroup analysis that included only nulliparous women given the possibility that a prior undocumented pregnancy with preeclampsia could have altered our results.
Figure 1. Cohort Design.
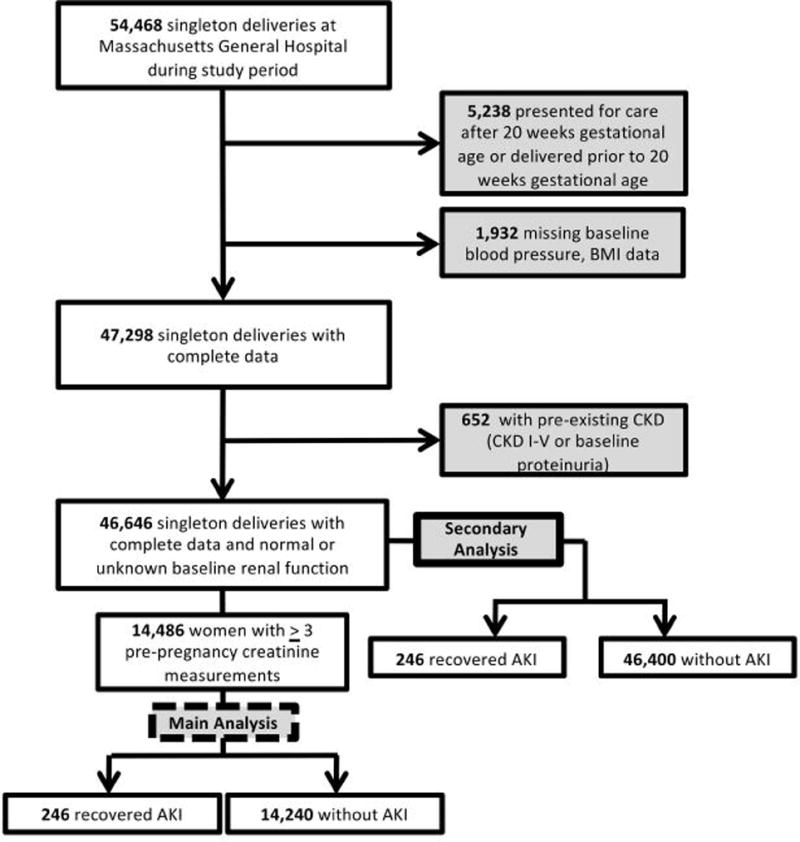
Flow of patients into cohort.
To assess the relationship between AKI severity and adverse pregnancy outcomes, we modeled AKI stage by KDIGO classification as a 4-category exposure variable (no AKI, Stage 1-3 AKI). Main outcomes were compared between groups using analysis of variance for continuous variables and Fisher’s exact tests for categorical variables. Multivariate logistic regression models included variables associated with adverse pregnancy outcomes listed previously.
To assess the relationship between time interval from AKI to pregnancy and risk for adverse pregnancy outcomes, we compared outcomes stratified by time interval less than or equal to 18 months from AKI episode to pregnancy and greater than 18 months from AKI to pregnancy. Eighteen months was chosen as this was the upper limit of the lowest quartile of time interval between AKI and pregnancy. Primary outcomes were compared between time groups using analysis of variance (ANOVA) for continuous variables and Fisher’s exact tests for categorical variables. In an analysis looking only at women with r-AKI, time interval was treated as a continuous variable in a multivariate logistic regression model including variables associated with adverse pregnancy outcomes listed previously. Statistical analyses were conducted using STATA 14 (Stata Corporation, College Station, TX).
RESULTS
Participant Characteristics
From the initial population of 54,468 singleton deliveries at Massachusetts General Hospital during the study period, 14,486 women met the inclusion criteria for entry into the study cohort (Figure 1). Two-hundred and forty-six women met the criteria for r-AKI (105 women from 1998-2007 and 141 women from 2008-2016). Characteristics of the two cohorts (1998-2007 and 2008-2016) were similar with respect to both baseline demographics and outcomes.
Baseline characteristics of women with and without r-AKI are summarized in Table 1. Women with r-AKI were of similar age and BMI at first prenatal visit. Women with r-AKI were more likely to have pre-existing diabetes (9% versus 5%, p=0.006). Etiology of AKI episodes are described in S2. AKI developed as a complication of a prior pregnancy in 7% of women in the cohort.
Table 1.
Characteristics in patients with r-AKI versus no AKI.
| Characteristic | r-AKI N=246 |
No AKI N=14,240 |
|||||
|---|---|---|---|---|---|---|---|
| Stage 1 N=98 |
Stage 2 N=99 |
Stage 3 N=49 |
|||||
| Demographics | |||||||
| Age at first prenatal visit – years | 31.0±6.3 | 31.1±6.2 | p=0.901 | ||||
| 31.3±5.4 | 31.0±6.8 | 28.7±6.4 | |||||
| Non-white race | 36% (89) | 42% (6,033) | p=0.051 | ||||
| 33% (32) | 37% (37) | 40% (20) | |||||
| Married | 59% (144) | 66% (9,427) | p=0.012 | ||||
| 64% (63) | 57% (56) | 51% (25) | |||||
| Initial Prenatal Visit Characteristics | |||||||
| Gestational age at visit – weeks | 10.7±2.6 | 11.2±4.1 | p=0.062 | ||||
| 10.3±2.6 | 11.3±2.8 | 10.7±2.5 | |||||
| BMI– kg/m2 | 26.8±6.1 | 26.7±5.7 | p=0.835 | ||||
| 26.7±6.0 | 26.5±5.2 | 27.3±5.6 | |||||
| Nulliparous | 38% (94) | 43% (6,176) | p=0.105 | ||||
| 32% (31) | 36% (36) | 55% (27) | |||||
| Pre-existing Hypertension | 3% (9) | 6% (826) | p=0.153 | ||||
| 1% (1) | 4% (4) | 8% (4) | |||||
| Pre-existing Diabetes | 9% (21) | 5% (673) | p=0.006 | ||||
| 3% (3) | 9% (9) | 18% (9) | |||||
| Systolic blood pressure – mmHg | 111±12 | 110±12 | p=0.392 | ||||
| 109±11 | 110±12 | 113±14 | |||||
| Diastolic blood pressure – mmHg | 68±9 | 68±8 | p=0.767 | ||||
| 67±9 | 68±9 | 72±9 | |||||
| Pre-conception Creatinine – mg/dl | 0·65±0·14 | 0.66±0.11 | p=0.231 | ||||
| 0.67±0.12 | 0.64±0.12 | 0.56±0.15 | |||||
| Peak Creatinine – mg/dl | 1·7±0·8 | n/a | n/a | ||||
| 1.2±0.34 | 1.5±0.46 | 2.6±1.3 | |||||
| AKI to LMP– months | 32 [18-60] | n/a | n/a | ||||
| 32 [18-56] | 39 [18-68] | 27 [12-42] | |||||
Data are presented as % (n), the mean ± SD or median [IQR]. P-value reflect test of association between all r-AKI and no AKI. LMP = last menstrual period. Peak Creatinine reflects highest creatinine level reported during AKI episode.
Maternal and Fetal Outcomes
Pregnancy outcomes in women with and without r-AKI are summarized in Table 2. Women with r-AKI had increased rates of preeclampsia and preterm preeclampsia (22% versus 9%, p<0.001 and 9% versus 2%, p<0.001, respectively). Rates of delivery by cesarean section were similar between the groups (38% versus 31%, p=0.190). After adjustment for maternal age, BMI, race, parity, history of diabetes and diastolic blood pressure at first prenatal visit, r-AKI remained significantly associated with adverse outcomes. Namely, r-AKI was associated with a 3-fold increased odds of preeclampsia and pre-term preeclampsia compared to women without AKI (adjusted OR 2.9 95% CI 1.9-4.4 for preeclampsia and adjusted OR 3.6 95% CI 1.8-7.1 for preterm preeclampsia). While rates of early preterm preeclampsia (<34 weeks) were higher in women with r-AKI (4% versus 1%, p<0.001), this association did not persist after multivariate adjustment in a logistic regression model (adjusted OR 1.2 95% CI 0.2-8.7).
Table 2.
Primary maternal-fetal outcomes in patients with r-AKI vs no AKI.
| Outcome | r-AKI (N=246) | No AKI N=14,240 |
Unadjusted Odds | Adjusted Odds | |||
|---|---|---|---|---|---|---|---|
| Stage 1 N=98 |
Stage 2 N=99 |
Stage 3 N=49 |
|||||
| Maternal Outcomes | |||||||
| Cesarean section | 38% (94) | 31% (4,430) | 1.4 [1.1-1.8] | 1·3 [0.9-1.9] | p=0.190 | ||
| 36% (35) | 40% (40) | 39% (19) | |||||
| Preeclampsia | 22% (55) | 9% (1,274) | 3.0 [2.2-4.0] | 2·9 [1.9-4.4] | p<0.001 | ||
| 12% (12) | 23% (23) | 40% (20) | |||||
| Preterm Preeclampsia (<37 weeks) | 9% (22) | 2% (330) | 4.1 [2.6-6.5] | 3·6 [1.8-7.1] | p<0.001 | ||
| 4% (4) | 9% (9) | 18% (20) | |||||
| Early Preterm Preeclampsia (<34 weeks) | 4% (9) | 1% (95) | 5.7 [2.8-11.3] | 1·2 [0.2-8.7] | p=0.878 | ||
| 1% (1) | 4% (4) | 8% (4) | |||||
| Gestational Hypertension | 22% (51) | 12% (1,621) | 2.0 [1.5-2.7] | 2.3 [1.7-3.3] | p<0.001 | ||
| 12% (12) | 25% (24) | 33% (15) | |||||
| Fetal Outcomes | |||||||
| Gestation age at delivery – weeks | 38.2±3.0 | 39.0±22 | n/a | n/a | p<0.001 | ||
| 38.7±2.4 | 38.1±3.3 | 37.1±3.5 | |||||
| Baby weight – grams | 3,010±690 | 3,350±620 | n/a | n/a | p<0.001 | ||
| 3,240±600 | 3,040±730 | 2,940±730 | |||||
| Birth weight <10th percentile | 20% (50) | 9% (1,299) | 2.5 [1.8-3.5] | 2.7 [1.7-4.3] | p<0.001 | ||
| 16% (16) | 24% (24) | 20% (10) | |||||
| Birth weight <5h percentile | 9% (23) | 5% (718) | 1.5 [0.9-2.4] | 2.2 [1.4-3.4] | p=0.001 | ||
| 7% (7) | 10% (10) | 12% (6) | |||||
| Birth weight <3rd percentile | 3% (8) | 3% (397) | 1.2 [0.6-2.4] | 1.3 [0.6-2.6] | p=0.495 | ||
| *** | *** | *** | |||||
| Perinatal Death | **** | 1% (108) | 2.2 [0.8-5.9] | 1.9 [0.7-5.5] | p=0.209 | ||
| Neonatal ICU Admission | 19% (46) | 10% (1,364) | 2.2 [1.6-3.0] | 2.0 [1.4-2.8] | p<0.001 | ||
| 13% (13) | 19% (19) | 30% (14) | |||||
| Composite Fetal Outcome | 39% (96) | 18% (2,621) | 2.3 [1.8-3.1] | 1.9 [1.4-2.6] | p<0.001 | ||
| 25% (25) | 32% (32) | 39% (19) | |||||
Data are presented as % (n), the mean ± SD and OR (95% CI). Gestational hypertension outcome included only for women without pre-existing hypertension. Outcomes are adjusted for age, race, BMI, diastolic blood pressure at first prenatal visit, history of diabetes and parity. P-values reflect the adjusted analysis for dichotomous outcome variables and t-tests between for continuous outcome variables entire r-AKI cohort and no AKI cohort. Numbers of perinatal deaths and birthweight < 3rd percentile in r-AKI group suppressed to preserve confidentiality due to low numbers.
Offspring of mothers with r-AKI were born earlier (gestational ages 38.2±3.0 weeks versus 39.0±2.2 weeks, p<0.001). Mean offspring weights in women with and without r-AKI were 3,010±690 and 3,350±620 grams, respectively (p<0.001). Women with r-AKI were more likely to have SGA infants compared with women without AKI (9% vs 5%, p=0.002) and have neonates admitted to the neonatal intensive care unit (19% vs 10%, p<0.001). The association between r-AKI and SGA infants persisted after excluding women who developed preeclampsia. There was no significant difference in the rates of perinatal deaths between groups (adjusted OR 1.9, 95% CI 0.7-5.5). Recovered AKI was associated with increased odds of SGA infants, requirement for NICU admission and the composite neonatal adverse outcome (adjusted OR 2.2, 95% CI 1.4-3.4, adjusted OR 2.0, 95% CI 1.4-2.8 and adjusted OR 1.9, 95% CI 1.4-2.6, respectively).
Outcomes by r-AKI Stage
Baseline characteristics of women by KDIGO AKI stage are summarized in Table 1. Women with stage 3 r-AKI were on average younger, had a higher BMI and were more likely to have diabetes prior to pregnancy compared to women with no AKI or a history of less severe AKI. Table 2 summarizes the main maternal and fetal outcomes by r-AKI stage. The rate of preeclampsia increased with increasing stage of r-AKI (12% in stage 1, 23% in stage 2 and 40% in stage 3, p<0.001). Gestational age at delivery and mean neonatal birthweights decreased with increasing AKI stage (38.7±2.4 weeks and 3,240±600 grams for stage 1 r-AKI, 38.1 + 3.3 weeks and 3,040±730 grams for stage 2 r-AKI and 37.1±3.5 weeks and 2,940±730 grams for stage 3 r-AKI, p<0.001 respectively). Rates of perinatal death did not significantly differ across r-AKI stage (p=0.161). After multivariate adjustment, odds of preeclampsia were highest in women with stage 3 r-AKI (adjusted OR 6.5, 95% CI 3.5-12.0) and intermediate in women with stage 2 r-AKI (adjusted OR 3.5, 95% CI 1.2-5.7). There was no statistically significant difference in odds of preeclampsia, preterm delivery of need for neonatal ICU admission in women with a history of stage 1 AKI as compared to women with no AKI (Figure 2).
Figure 2. Association between KDIGO AKI Stage and Main Maternal-Fetal Outcomes.
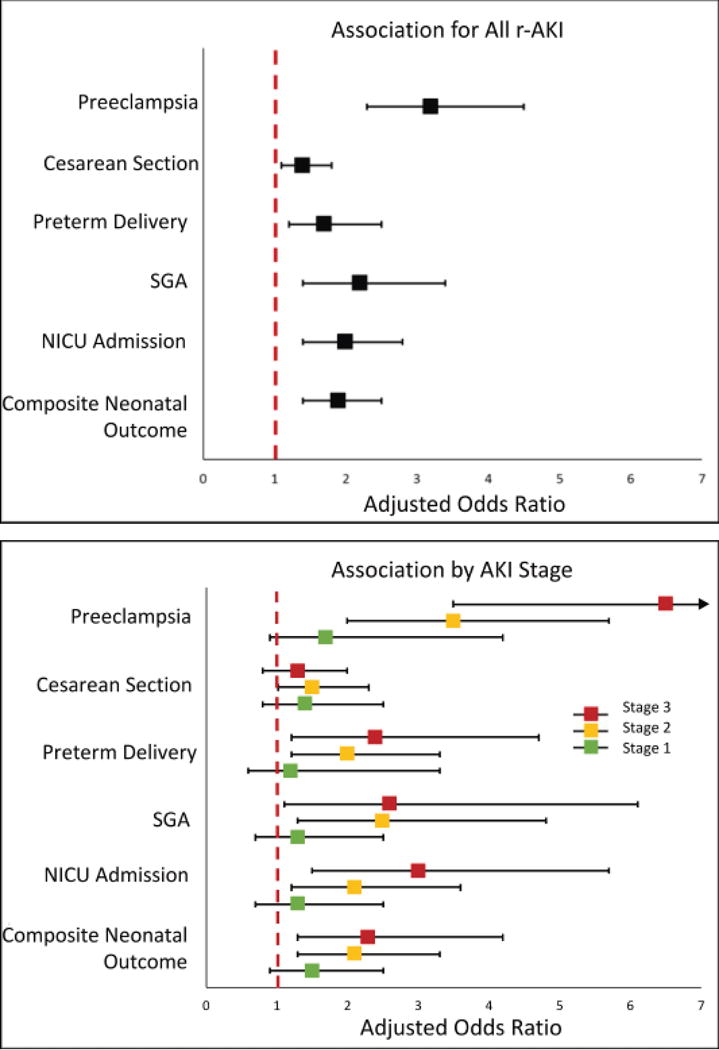
Association of adverse outcomes with r-AKI from logistic regression by severity of AKI episode (KDIGO Stage 1-3). Please refer to online supplement for point estimates and 95% CIs.
Outcomes by Time Interval
Baseline characteristics of women with AKI stratified by time interval between AKI and pregnancy are summarized in S3. Women who conceived within 18 months of AKI were more likely to be of non-white race and had higher rates of pre-existing hypertension. Rates of preeclampsia, preterm delivery, delivery by cesarean section and need for NICU admission were higher in women who conceived within 18 months of the AKI episode (Figure 3). After multivariate adjustment, women who conceived within 18 months of AKI were at 7-fold increased risk of preeclampsia and 4-fold increased risk of having an infant admitted to the NICU. Women who conceived more than 18 months from the AKI episode had increased odds of preeclampsia compared to women without r-AKI, however the magnitude of the risk was attenuated. A similar association was also observed for preterm delivery. The risk of an SGA infant did not differ between time intervals. Amongst women with r-AKI, risk of preeclampsia decreased by 16% for each 6-month interval time increase between AKI and day of last menstrual period (adjusted OR 0.84, 95% CI 0.77-0.92).
Figure 3. Association between Time Interval and Main Maternal-Fetal Outcomes.
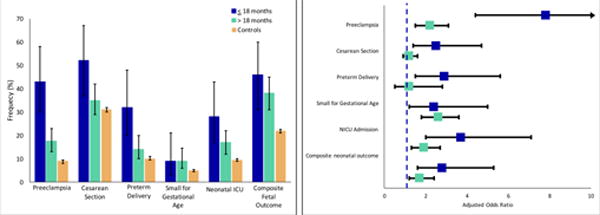
Left panel: Frequency (%) of adverse outcomes between r-AKI (stratified by time interval of less than 18 months or great then 18 months from AKI event to pregnancy). Error bars represents 95% CI. Right panel: Association of adverse outcomes with r-AKI stratified by time interval from logistic regression. Please refer to online supplement for point estimates and 95% CIs.
Secondary Analyses
We performed a secondary analysis of women who met our initial inclusion criteria except for three or more pre-pregnancy serum creatinine measurements. The frequency of pre-existing diabetes and hypertension was lower in the expanded cohort compared with the restricted population used in the main analysis (S4). After multivariate adjustment, we observed a similar association between r-AKI and adverse outcomes when using the expanded cohort (S5).
In an analysis of only nulliparous women, women with r-AKI remained at 2-fold increased risk for both preeclampsia and SGA infant. In a subgroup analysis by AKI severity in the nulliparous cohort (N=94 r-AKI), only women with a history of stage 3 AKI were at increased risk for adverse outcomes (S6).
DISCUSSION
Despite global improvements in maternal mortality in the last decade, rates of maternal death in the U.S. are increasing. Hypertensive disorders of pregnancy, including preeclampsia, are a leading cause of maternal morbidity and mortality.23–25 The effects of preeclampsia are not limited to the duration of pregnancy: women with preeclampsia are at increased risk for future cardiovascular disease and their offspring, if born premature or at low birth weight, are at increased risk for chronic disease in adulthood.26 The results of our study provide further evidence on the association between r-AKI and adverse pregnancy outcomes including preeclampsia and premature delivery, even when creatinine has completely recovered prior to gestation, supplementing prior work. New to this study, we demonstrated that severe episodes of AKI and shorter interval between AKI episode and pregnancy were associated with higher risks of preeclampsia.
Studies assessing long-term complications of AKI in humans have largely omitted young populations.27–30 AKI is most often studied in elderly and critically ill populations with high co-morbid rates of hypertension, diabetes and vascular disease.28,30,31 AKI, however, is also observed in children.32 The concept that reversible AKI has long-term consequences has been demonstrated with respect to incident CKD, ESRD and death. In a study of clinically recovered hospitalized patients with AKI from a large health system, individuals with r-AKI were at nearly 6-fold increased risk for incident stage 3 CKD.30 AKI has also been associated with increased mortality.33,34
Given that AKI stage was associated with higher risk for gestational hypertensive disorders, one hypothesis is that women with clinically “recovered” AKI, especially those with severe AKI, have residual subclinical kidney disease and lower nephron mass prior to entering pregnancy. Isolated measurements of serum creatinine perform poorly in estimating GFR in individuals with normal kidney function. Nephron number can be reduced by 50% before serum creatinine rises above the normal range. Low nephron number and surrogates for low renal mass have been linked with adverse long-term health consequences including hypertension and CKD.26 Low nephron number also appears to be a risk factor for pregnancy complications: both living kidney donors and women with a solitary congenital kidney have been identified as groups at high risk for gestational hypertension and preeclampsia.11,35 This association is especially relevant in the transplant donor population, who are generally healthy and receive careful medical assessment prior to donation. Pregnancy is associated with profound changes in renal plasma flow that results in a 50% rise in GFR by mid-gestation. Impairments in gestational hyperfiltration have been identified as a risk factor for preeclampsia, preterm birth and low birthweight.36 Of note, women with stage 3 AKI had a lower pre-pregnancy serum creatinine than women with milder stages of AKI in our cohort. One hypothesis is that women who recover from more severe AKI were in a state of hyperfiltration prior to entering pregnancy due to reduced nephron number, which may further lead to impaired renal adaptation during pregnancy. Because creatinine measurements are not part of routine care prenatal care, we were not able to assess GFR changes during pregnancy in this cohort. In addition, the kidney plays a key role in gestational plasma volume expansion which is recognized to be inadequate in early pregnancy in women with preeclampsia. Subclinical reduced renal function may also contribute to impaired hemodynamic adaptation associated with reduced placental perfusion. One potential approach to identify at-risk women would be to assess renal functional reserve prior to conception in women with a history of severe AKI.
The mechanisms of recovery after AKI are not fully understood. While renal tubular epithelium can regenerate after an ischemic insult, endothelial cells regeneration may not be complete.37–39 In animal models, significant vascular dropout has been observed following ischemic AKI despite clinical recovery.40 AKI is emerging as a systemic disease with generalized inflammation and endothelial injury.41 Studies in experimental animals suggest that many of the systemic consequences of AKI persist in the long-term.42 It is possible that women with r-AKI represent a unique group of patients who have underlying endothelial dysfunction and therefore more susceptible preeclampsia. These patients may be particularly susceptible to develop endothelial injury even at lower levels of placental soluble factors made during pregnancy. We identified that shorter time interval between the episode of AKI and pregnancy was associated with increased risk of preeclampsia. Longer time interval may allow for resolution of endothelial dysfunction. Delaying pregnancy after an AKI episode may be one strategy to reduce the risk of preeclampsia in this population, however our findings need to be confirmed in other cohorts before such a recommendation can be made. Additionally, this finding should prompt increased efforts to prevent AKI in young women.
The strengths of our study include our use of a large pregnancy database with detailed clinical information on all participants. This allowed us to control for important confounders such as early-pregnancy blood pressure, weight and previous chronic medical conditions such as diabetes and hypertension. AKI cases were identified based on biochemical definitions. Additionally, all cases were confirmed by independent chart review by two nephrologists.
Our study does have limitations. As this is a retrospective observational study, there is potential for bias and residual confounding in our analysis despite efforts to reduce this in our analytic design. The diagnosis of AKI and preeclampsia may not be captured completely. Our definition of preeclampsia deviates from the most recent changes in the definition by ACOG.20 As a majority of our data was collected prior to the current version of these guidelines, this represented a reproducible definition of preeclampsia that was not subject to bias of selection of lab testing and diagnostic coding by providers. We could only identify AKI events that happened in our health network. To diagnose AKI, laboratory samples must be obtained; women with fewer interactions with the health care system or who are viewed as healthier by their medical providers may not have renal function checked when presenting with similar types of illnesses. To address this limitation, only women with previous creatinine measurements were included in the main analysis.
Supplementary Material
PERSPECTIVES.
An episode of AKI, followed by clinical recovery prior to conception, is a risk factor for pregnancy complications including preeclampsia. Severity of the AKI episode and duration between AKI episode and pregnancy influence the risk for adverse outcomes. This study adds to the growing literature that reversible AKI is not harmless. Our study has important implications for pregnancy counseling. Practitioners should actively assess for episodes of AKI and counsel women on increased risk. Our findings also highlight the importance of AKI prevention, especially in young women.
NOVELTY AND SIGNIFICANCE.
1) What Is New
This study confirms are previous findings that an episode of kidney injury followed by complete clinical recovery is associated with increased risk of complications in pregnancy including preeclampsia.
We also found that the severity of kidney injury and the timing of the kidney injury modifies the risk of pregnancy complications. Women with the most severe episodes of kidney injury (Stage 3 AKI) and who conceived within 1 year of the kidney injury episode were at the highest risk for preeclampsia and infant growth restriction.
2) What Is Relevant?
Preeclampsia is a leading cause of maternal morbidity and mortality across the globe. The findings from our study suggest that some women may develop hypertensive disorders in pregnancy as a consequence of prior kidney injury. Additionally, the results of this study will help providers better counsel women with previous kidney injury who are planning pregnancy.
3) Summary
A history of AKI prior to pregnancy, despite clinical recovery, is associated with increased risk of gestational hypertensive disorders including preeclampsia.
Acknowledgments
SOURCES OF FUNDING:
J.S.T. is supported by funding through the American Kidney Fund Clinical Scientist in Nephrology Fellowship. W.A.H. A. received support from the International Society of Nephrology fellowship program. C.E.P. is supported by career development awards from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K23DK113218-01) and the Robert Wood Johnson Foundation’s Harold Amos Medical Faculty Development Program (74256).
Footnotes
DISCLOSURES:
None
References
- 1.Li PK, Burdmann EA, Mehta RL, World Kidney Day Steering C Acute kidney injury: global health alert. Kidney Int. 2013;83:372–6. doi: 10.1038/ki.2012.427. [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis. 2007;50:712–20. doi: 10.1053/j.ajkd.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS. Elevated BP after AKI. J Am Soc Nephrol. 2016;27:914–23. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGregor TL, Jones DP, Wang L, et al. Acute Kidney Injury Incidence in Noncritically Ill Hospitalized Children, Adolescents, and Young Adults: A Retrospective Observational Study. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131–8. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. New England Journal of Medicine. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Maynard SE, Min J-Y, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A Systematic Review and Meta-Analysis of Outcomes of Pregnancy in CKD and CKD Outcomes in Pregnancy. Clin J Am Soc Nephrol. 2015;10:1964–78. doi: 10.2215/CJN.09250914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangren JS, Powe CE, Ankers E, et al. Pregnancy Outcomes after Clinical Recovery from AKI. J Am Soc Nephrol. 2017;28:1566–74. doi: 10.1681/ASN.2016070806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg AX, Nevis IF, McArthur E, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124–33. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccoli GB, Cabiddu G, Attini R, et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. Journal of the American Society of Nephrology: JASN. 2015;26:2011–22. doi: 10.1681/ASN.2014050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendrick J, Holmen J, You Z, Smits G, Chonchol M. Association of Unilateral Renal Agenesis With Adverse Outcomes in Pregnancy: A Matched Cohort Study. Am J Kidney Dis. 2017;70:506–11. doi: 10.1053/j.ajkd.2017.02.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Delgado JC, Esteve F, Torrado H, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Critical care (London, England) 2013;17:R293. doi: 10.1186/cc13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney international. 2011;79:1361–9. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annual Symposium proceedings AMIA Symposium. 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AWG KDIGO. KDIGO clinical practice guideline for acute kidney injury. Kidney International. 2012;(Supplement 17):1–138. [Google Scholar]
- 19.Leither MD, Murphy DP, Bicknese L, et al. The impact of outpatient acute kidney injury on mortality and chronic kidney disease: a retrospective cohort study. Nephrol Dial Transplant. 2018 doi: 10.1093/ndt/gfy036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 21.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ (Clinical research ed) 2016:353. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens I, Basit S, Lykke JA, et al. Association Between Hypertensive Disorders of Pregnancy and Later Risk of Cardiomyopathy. JAMA. 2016;315:1026–33. doi: 10.1001/jama.2016.1869. [DOI] [PubMed] [Google Scholar]
- 24.Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep. 2013;15:114–21. doi: 10.1007/s11906-013-0329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–9. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 26.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 27.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–56. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney International. 2011;79:1361–9. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clinical Journal of the American Society of Nephrology. 2011;6:2567–72. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M. Association of Complete Recovery From Acute Kidney Injury With Incident CKD Stage 3 and All-Cause Mortality. American Journal of Kidney Diseases. 2012;60:402–8. doi: 10.1053/j.ajkd.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chertow GM. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. Journal of the American Society of Nephrology. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 32.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2016 doi: 10.1056/NEJMoa1611391. 0:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney international. 2010;78:478–85. doi: 10.1038/ki.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta RH, Honeycutt E, Patel UD, et al. Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. The American journal of cardiology. 2010;106:1728–34. doi: 10.1016/j.amjcard.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Kendrick J, Holmen J, You Z, Smits G, Chonchol M. Association of Unilateral Renal Agenesis With Adverse Outcomes in Pregnancy: A Matched Cohort Study. Am J Kidney Dis. 2017 doi: 10.1053/j.ajkd.2017.02.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S, Lee SM, Park JS, et al. Midterm eGFR and Adverse Pregnancy Outcomes: The Clinical Significance of Gestational Hyperfiltration. Clin J Am Soc Nephrol. 2017;12:1048–56. doi: 10.2215/CJN.12101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little MH, Kairath P. Does Renal Repair Recapitulate Kidney Development? J Am Soc Nephrol. 2017;28:34–46. doi: 10.1681/ASN.2016070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu KD, Brakeman PR. Renal repair and recovery. Critical care medicine. 2008;36:S187–92. doi: 10.1097/CCM.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- 39.Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:628–34. doi: 10.1097/MNH.0b013e3283588f24. [DOI] [PubMed] [Google Scholar]
- 40.Basile DP, Friedrich JL, Spahic J, et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. American journal of physiology Renal physiology. 2011;300:F721–33. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Druml W. Systemic consequences of acute kidney injury. Current opinion in critical care. 2014;20:613–9. doi: 10.1097/MCC.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 42.Shiao CC, Wu PC, Huang TM, et al. Long-term remote organ consequences following acute kidney injury. Critical care (London, England) 2015;19:438. doi: 10.1186/s13054-015-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


