Abstract
Metals and pesticides are common pollutants and the modulation of biomarkers can indicate sub-lethal influences on the physiology of organisms inhabiting impacted aquatic systems. We examined the effects of mercury and the organophosphate pesticide dimethoate on EROD, MROD, glutathione S-transferase (GST), acetylcholine esterase (AChE), metallothionein (MT) and glutathione (GSH) in the signal crayfish (Pacifastacus leniusculus). Crayfish were injected with mercury chloride or dimethoate (0.3, 0.6, 0.9 μg kg−1) and dissected after 72 h. EROD activity in the hepatopancreas did not change in response to mercury chloride treatment but exhibited a dose dependent decrease at all concentrations of dimethoate tested. MROD (hepatopancreas) exhibited a significant decrease at the 0.9 μg kg−1 treatment for both chemicals. GST (hepatopancreas) demonstrated a significant dose dependent decrease at all concentrations of both mercury chloride and dimethoate. AChE (tail muscle) decreased at the 0.6 and 0.9 μg kg−1 concentrations of dimethoate and 0.9 μg kg−1 mercury chloride. In gill tissue, MT increased in response to 0.3 and 0.6 μg kg−1 of mercury chloride but no effect was observed at the 0.9 μg kg−1 concentration of mercury chloride or any concentrations of dimethoate tested. MT did not change in response to mercury or dimethoate in tail tissue. Furthermore, neither chemical modulated GSH concentrations. Our results indicate that, apart from GSH, these markers are sensitive to the pollutants tested and that animals exposed in the wild are potentially compromised in their ability to detoxify environmental contaminants and carry out normal cellular processes.
Keywords: Crayfish, cytochrome P-450, glutathione, metallothionein, glutathione S-transferase, acetylcholine esterase
1. Introduction
Damage to aquatic ecosystems due to contaminants is a concern and sub-lethal changes in biochemical endpoints in conjunction with contaminant levels can be used to help assess whether exposure causes biological responses in organisms inhabiting impacted habitats (Fernandes et al., 2002; Gunderson et al., 2016). Phase I and II enzyme activities, glutathione (GSH) and metallothionien (MT) concentrations, and acetylcholine esterase (AChE) activities are commonly used biomarkers to assess whether wildlife populations are impacted by contaminants present in the ecosystems in which they live. Species and tissue specific patterns often exist in the expression of these biomarkers thus necessitating careful characterization in a model organism before use as an assessment tool.
In this study, we examined the regulation of the above-mentioned biomarkers by dimethoate and mercury in signal crayfish, Pacifastacus leniusculus. Signal crayfish inhabit streams, rivers and lakes throughout the Pacific Northwest and are considered keystone species due to the fact that their omnivorous diet (i.e. macro-invertebrates, detritus, animal matter and aquatic vegetation) makes them an important link between aquatic energy sources and aquatic and terrestrial predators (Larson and Olden, 2011). Signal crayfish occupy relatively small home ranges (Guan and Wiles, 1997; Holdich, 2002) making them good model organisms for field based ecotoxicology studies since exposure to contaminants can be localized to a specific area or field site. Heavy metals and organophosphate pesticides are common pollutants in regions where signal crayfish reside due to extensive mining, industrial and agricultural activities. Mercury concentrates in aquatic biota and is classified as a toxic heavy metal that causes severe adverse neurological and health effects in wildlife and humans (Mebane and MacCoy, 2013). Mercury enters aquatic systems through atmospheric deposition and to a lesser extent geologic and point source pollution and is present in crayfish and fish tissues throughout the western United States (Abbott et al., 2004; Abbott et al., 2008; Abbott et al., 2002; Abbott et al., 2003; Hothem et al., 2007; Kouba et al., 2010; Mebane and MacCoy, 2013; Mueller and Serdar, 2002). Organophosphate pesticides, such as dimethoate, are commonly used in agriculture and can cause adverse motor, reproductive, and neurotoxic effects to non-target organisms (Joshi and Sharma, 2011).
Cytochrome p-450 activity associated with phase I detoxification is often used as a bioindicator of contaminant exposure, however further work is needed to characterize the regulation of this family of enzymes in invertebrates (James and Boyle, 1998; Snyder, 2000). Cytochrome p-450 monooxygenases are hemoproteins associated primarily with the endoplasmic reticulum (and to a lesser extent other membranes) and are involved in the biotransformation of a broad range of both endogenous substrates (steroids, fatty acids, cholesterol) as well as xenobiotics. Changes in cytochrome p-450 activity can suggest exposure to compounds such as dioxin, dibenzofurans, polychlorinated biphenyls (PCBs) and organic compounds present in pulp mill effluent although inhibition by metals and detergents have been reported and thus need to be factored into the interpretation of field data measuring endpoints such as EROD activity (Newman, 1998; Sen and Semiz, 2007). Cytochrome p-450s have been described in several crayfish species and appear to respond to known inducers (Ashley et al., 1996; Escartin and Porte, 1996b; Jewell et al., 1997; Jewell and Winston, 1989; Lindstrom-Seppa and Hanninen, 1986). Field studies using red swamp crayfish have demonstrated increased EROD activity in hepatopancreas microsomes prepared from crayfish inhabiting areas sprayed with the organophosphate pesticide fenitrothion and significantly increased cytochrome p-450 and higher (but non-significant) EROD activity in crayfish hepatopancreas collected 4 km downstream from a sewage treatment plant (Fernandes et al., 2002; Porte and Escartin, 1998).
Glutathione S-transferase (GST) is a phase II detoxification enzyme modulated by xenobiotics and thus has been suggested for use as a biomarker indicative of environmental contaminant exposure (Aksu et al., 2015; Frasco and Guilhermino, 2002; Gunderson et al., 2004; Gunderson et al., 2016). GST attaches the polar molecule glutathione to foreign chemicals thus facilitating clearance through the urine and bile (Newman, 1998). GST has been purified in crustaceans such as the African River Prawn (Macrobrachium vollenhovenii) (Adewale and Afolayan, 2005) and is modulated by organophosphate pesticides and heavy metals in invertebrate and vertebrate models (Birmelin et al., 1998; Elia et al., 2006; Elia et al., 2003; Elumalai et al., 2007; Sen and Semiz, 2007).
Evidence suggests that exposure to xenobiotics can lead to increased reactive oxygen species (ROS) production and tissue damage thus making non-enzymatic antioxidants such as GSH useful biomarkers for exposure to contaminants (Bagchi et al., 1995; Dickinson and Forman, 2002; Wilhelm et al., 2001). GSH is a tripeptide containing cysteine, glutamate and glycine that can act directly as an endogenous antioxidant that reacts with oxyradicals or is attached to xenobiotics by glutathione S-transferase in phase II detoxification (Newman, 1998). Exposure to oxyradicals leads to increased GSH through GSH synthesis (Dickinson and Forman, 2002). Variation among species as well as intra-species differences in contaminant induced changes in GSH concentrations are described in the literature emphasizing the importance of characterizing its regulation in each model being studied (Ferrari et al., 2007; Kristoff et al., 2008; Matos et al., 2007; Pena-Llopis et al., 2002; Velki and Hackenberger, 2013b). In crayfish, total GSH is detectable in hepatopancreas, gill and muscle tissues and is considered to be one of several biomarkers useful for examining the glutathione redox status in this group (Kovacevic et al., 2008).
Metallothioneins (MTs) are cysteine rich, non-enzymatic metal binding proteins that are inducible by heavy metals, thus making them potential markers for assessment of metal exposure in aquatic biota inhabiting impacted environments (Khati et al., 2012; Roesijadi, 1992, 1994) Numerous physiological roles have been ascribed to MTs. They are believed to play a homeostatic role for essential metals such as zinc and copper by acting as a reservoir as well as contributing to metal tolerance through the detoxification of both essential and non-essential trace metals (Amiard et al., 2006). Furthermore, they provide protection from cellular damage by either acting as antioxidants and scavenging free radicals or binding heavy metals, and thus sequester the metals within the cell (Amiard et al., 2006). MTs have been measured and characterized in numerous invertebrate taxa that include annelid worms, crustaceans and molluscs and differences and inconsistencies exist both in basal levels of expression as well as inducibility by metals such as cadmium, mercury, zinc and copper. Studies report the presence of cadmium binding proteins as well as cadmium induced MTs (or cadmium binding proteins) in crayfish hepatopancreas, both through injection (Astacus astacus) and water exposure (Procambus clarkii) (Delramo et al., 1989a; 1989b; Martinez et al., 1993; Pedersen et al., 1996).
AChE is used as a biomarker in field-based studies to screen populations for exposure to neurotoxic compounds and inhibition of cholinesterases by organophosphate compounds (OP) and metals is well documented in vertebrate and invertebrate organisms (Escartin and Porte, 1996a; Fornstrom et al., 1997; Richetti et al., 2011; Velki and Hackenberger, 2012, 2013a, b). For example, the organophosphate compounds terbufos and fenitrothion both decrease AChE activity in red swamp crayfish (Procambarus clarkii), with terbufos also increasing mortality and aberrant behavior (loss of equilibrium and motor control) (Escartin and Porte, 1996a; Fornstrom et al., 1997). AChE hydrolyzes the neurotransmitter acetylcholine in the synapse and inhibition of this enzyme can lead to altered neuro-signaling. Care needs to be taken when using AChE as a marker for OP exposure as other chemicals that include surfactants, detergents and metals are known to inhibit AChE (Diamantino et al., 2000; Guilhermino et al., 2000; Guilhermino et al., 1996). It is thus important to characterize the response of this enzyme to different classes of chemicals, as well as identify tissue specific and temporal variation in the patterns of expression in the model organism(s) utilized in ecotoxicology studies (Kirby et al., 2000; Matozzo et al., 2005; Sturm et al., 1999).
In this study, we examined whether low doses of mercury chloride or dimethoate injected directly into the hemolymph have dose dependent effects on phase I and II enzyme activities, GSH and MT concentrations and AChE activity in signal crayfish tissues to determine whether these endpoints can serve as useful sub-lethal biomarkers indicative of contaminant exposure in this species. As discussed above, these endpoints are used in ecotoxicology studies and it is important to characterize responses to environmentally relevant compounds in the model organism being studied.
2. Methods
2.1 Animal collection and exposure
Animals were collected by kick netting from the Boise River under Idaho Department of Fish and Game permits F-12-04-13 and F-12-04-14. The animals collected for the dimethoate treatment were collected in June 2013 whereas animals used for the mercury chloride exposure were collected in March 2014. Animals were acclimated in dechlorinated tap water for at least two weeks at room temperature with timed lights to simulate summer day length in southwestern Idaho (June ~6:30 am −10:30 pm). After acclimation, animals were transferred to 7 L aerated fishbowls filled with de-ionized water supplemented with 200 mg L−1 Instant Ocean™. Animals were injected at the base of the third leg with dimethoate or mercury chloride (mercury chloride or dimethoate (0.3, 0.6, and 0.9 μg kg−1) using a saline vehicle (400 mOsm). Water was changed each day (~24 h) during the treatment cycle. At the end of the 72 h treatment, animals were euthanized (chilled on ice and decapitated) and the tissues were harvested, flash frozen, and stored at −80°C until analysis.
2.2 EROD, MROD and glutathione S-transferase (GST) activity
2.2.1 Tissue preparation
Ethoxyresorufin O-deethylase (EROD), methoxyresorufin O-deethylase (MROD) and GST activities were measured in hepatopancreas microsomes (EROD and MROD) and cytosol (GST) using previously published protocols with modifications to the procedures (Gunderson et al., 2004; Gunderson et al., 2016). Approximately 0.33g of hepatopancreas was thawed on ice and homogenized in 1 mL of cold homogenization buffer (100 mM Tris, pH 7.4, 0.1 M KCl, 1 mM EDTA, 1mM PMSF, 5 μM aprotinin, 10 μM pepstatin) 10–20 zirconium oxide beads using a Bullet Blender (Next Advance, Inc., BBUC3137) (Speed 7 for 4 min). The blended tissue was first centrifuged for 15 minutes at 9,491g (4°C) (Eppendorf 5418). The supernatant was then poured into a separate tube and centrifuged for an additional 20 min at 14,3265g (4°C). Finally, the supernatant was collected and centrifuged for 1 hour at 100,350g (4 °C) for 60 min (L8-80M Beckman Ultracentrifuge). The cytosolic fraction was aliquoted and stored at −80 °C to be used in the GST assay. The pellet was re-suspended in ice cold microsome buffer (100 mM potassium phosphate, pH 7.25; 1 mM EDTA, 1 mM DTT, 20% v/v glycerol) and stored at −80°C for use in the EROD and MROD assays. Total protein concentration was measured using the Bradford method (Bradford, 1976).
2.2.2 EROD and MROD assays
A 96-well plate fluorometric assay was used to measure EROD and MROD activities as previously described (Gunderson et al., 2004) with slight modifications. Briefly, 50 μl of microsome sample (diluted to 5 μg/μl of total protein in microsome buffer) was added to 0.2 ml incubation buffer (50 mM Tris (pH 7.8), 1M NaCl, 3 μM resorufin ethyl or methyl ether) in triplicate (black Costar 96 well polystyrene assay plate, with clear flat bottom). The reaction was started by adding 5 μl of start buffer (50 mM Tris (pH 7.8), 1M NaCl, 5 mM NADPH) and allowed to equilibrate at room temperature for 10 minutes. Fluorescence was then measured (530 nm excitation wavelength and 590 nm emission wavelength) initially and every 10 minutes for 40 minutes with a 10 second mix before each read (Biotek Synergy HT fluorometer with Gen 5 software). Preliminary work demonstrated the reaction was linear for at least 40 minutes after a 10-minute incubation (data not shown). Vmax values were calculated, and relative fluorescence units were converted to pM resorufin using a 0–1 μM resorufin standard curve (0.15 ml incubation buffer, 50 μl of corresponding resorufin dilution generated by serial dilution). Final values were reported in pM Resorufin/min*μg protein.
2.2.3 Glutathione S-transferase (GST) activity
GST activity (EC 2.5.1.18) was measured using a 96-well plate colorimetric assay read kinetically on a BioRad Benchmark Plus © spectrophotometer microplate reader at 340 nm using a previously published protocol (Gunderson et al., 2004). Samples were run in triplicate on 96-well plates with 2.5 μg of total cytosolic protein loaded per reaction. Activity was calculated as previously described (Gunderson et al., 2004) and was determined to be linear for at least 40 minutes at room temperature after a 10-minute incubation.
2.3 Glutathione (GSH) concentrations
Extracts of hepatopancreas tissue were prepared by homogenizing 0.11–0.12 grams of tissue in 5 volumes of 10 mM EDTA buffer (pH 8.04) using a Bullet Blender (Next Advance, Inc., BBUC3137) (10–20 zirconium oxide beads, Speed 7 for 4 min @ 4°C). The blended tissue was then centrifuged at 9,491g (Eppendorf 5418) for 15 minutes (4°C). The supernatant was transferred to a separate tube and centrifuged again at 16,873g for 15 minutes (4°C). The supernatant was then added to tubes containing 20% sulfosalycylic acid (SSA) (32.5 μL of 20% SSA for every 100 μL of supernatant) and centrifuged at 8,609g for 10 minutes (4°C).
GSH concentrations were measured using a 96 well colorimetric assay using the reaction between GSH and DTNB to quantify GSH concentrations in hepatopancreas tissue preparations. For each sample, 30 μL of 0.5M NaHPO4 buffer, 95 μL of the extract (see above), and 125 μL of 10 mM DTNB (5,5′-Dithiobis(2-nitrobenzoic acid)) was added and the plate was read at 415 nm (BioRad iMark© microplate reader) after 5 minutes of incubation. A GSH standard curve was used to quantify GSH concentrations in the tissue extracts. GSH dilutions were prepared in 0.5M NaHPO4 buffer (2.5, 5.0, 10, 20, 40 and 80 nmol). A standard curve was run on each plate by adding 125 μL of each GSH dilution and 125 μL of 10 mM DTNB each well. All samples and standards were run in quadruplicate.
2.4 Metallothionein (MT) concentrations
MT from gill tissue samples was purified and isolated as described in (Gunderson et al., 2016) with slight modifications for signal crayfish. Approximately 0.3 grams of gill tissue was homogenized, and supernatants centrifuged to purify MT. Colorimetric absorbance assay measured at 412 nm was done using DTNB to quantify concentration of MT. Signal crayfish MT was assumed to have 18 cysteine residues (Faria et al., 2010).
2.5 Acetylcholine esterase (AChE) activity
AChE activity was measured in tail muscle using previously described protocols (Ellman et al., 1961; Xuereb et al., 2009) with minor modifications. Tail muscle (0.02 grams) was weighed and blended using a Bullet Blender (Next Advance, Inc., BBUC3137) in 1.0 mL of 0.1 M potassium phosphate buffer, pH 8.0 at −20°C (10–20 zirconium oxide beads (Speed 7 for 4 min)). Following modifications from (Xuereb et al., 2009), samples were spun in a centrifuge (Eppendorf 5418) at 9,000g for 15 minutes (4°C). Supernatant was collected and stored at −80°C overnight, while pellet material was discarded. Total protein assays were run in quadruplicate on AChE supernatant as described (Bradford, 1976). The AChE assay was determined in quadruplicate for each sample using a microplate according to the original protocol (Ellman et al., 1961). Briefly, 0.26 mL of buffer (0.1 M potassium phosphate buffer (pH 8.0)), 20 μL of the reagent (coloring agent 0.01 M dithiobisnitrobenzoic acid (DTNB): 0.1 M potassium phosphate buffer, pH 7.0 + 0.0792 g DTNB/20 mL 0.1M Potassium Phosphate + 0.030 g sodium bicarbonate/20 mL 0.1 M Potassium Phosphate) and 40 μL supernatant were each added to a 96-well plate. AChE activity was measured upon addition of 10 μL of the substrate (0.075 M acetythiocholine iodide (ACTI)) at 415 nm using a BioRad iMark© microplate reader. Substrate solution was kept for approximately 14 days, before a new solution was made. An assay blank included 300 μL buffer, 20 μL reagent and 10 μL substrate. After validation, absorbance readings were taken at 0 minutes and again at 25 minutes, while the hydrolysis of ACTI and the formation of the yellow products (5-mercapto-2-nitrobenzoate and 2-nitrobenzoate-mercapto-thiocholine) took place. After 25 minutes, the difference between the final absorbance (at 25 minutes) and the initial absorbance (at 0 minutes) was determined. The change in absorbance was then divided by the molar extinction coefficient (ε) of DTNB (1.36 × 10−4) to determine the concentration (in M) of DTNB hydrolyzed. The concentration was then divided by 25 minutes as well as the weight of tissue initially prepared to determine the rate per gram tissue (Ellman et al., 1961).
2.6 Statistical Analysis
The EROD, MROD, GST, GSH, MT, and AChE datasets were determined to be parametric or non-parametric using a Shapiro-Wilk normality test and a Brown-Forsythe equal variance test. A One-Way ANOVA with Holm-Sidak method for multiple comparisons was used to compare treatment groups if the data was parametric. A Kruskal-Wallis One Way ANOVA on Ranks with Dunn’s method was used to analyze non-parametic datasets. Either a t-test (parametric dataset) or Mann-Whitney Rank Sum Test (non-parametric dataset) was used to compare saline controls for each study. All statistical analyses were carried out using the software program SigmaPlot 13.0.
3. Results
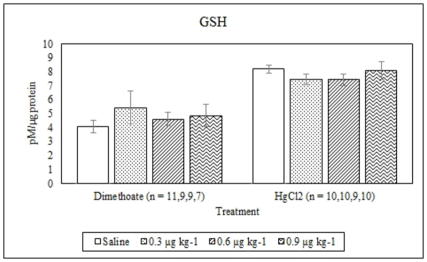
Dimethoate treatment decreased EROD activity at all concentrations tested compared to the saline control (p ≤ 0.005) whereas mercury chloride had no significant effect (p = 0.64) (Figure 1). Animals treated with 0.9 μg kg−1 dimethoate 0.9 μg kg−1 and mercury chloride demonstrated significant decreases in MROD activity compared to saline controls (p ≤ 0.04) although no significant effects were observed at the lower concentrations (p > 0.05) (Figure 2). Both dimethoate and mercury chloride treatments significantly decreased GST activity at all concentrations tested (p < 0.001) (Figure 3). Dimethoate and mercury chloride had no effect on GSH concentrations at the doses tested in this study (p ≥ 0.47) (Figure 4). In gill tissue, MT demonstrated no response to dimethoate at the concentrations tested (p = 0.08) (Figure 5). Mercury chloride significantly increased MT concentrations in gill tissue at the two lower doses (0.3 and 0.6 μg kg−1) (p ≤ 0.02) although no effect was observed at the highest exposure concentration (p = 0.32) compared to the saline controls (Figure 5). Neither dimethoate nor mercury chloride modulated MT in tail tissue when compared to the saline controls (p > 0.05) (Figure 6). Dimethoate treatment decreased AChE activity at the two highest doses (p ≤ 0.04) with no effect being observed at the 0.3 μg kg−1 treatment concentration (p = 0.41) (Figure 7). Mercury chloride decreased AChE activity at 0.9 μg kg−1 (p = 0.001) with no effect being observed at the lower concentrations (p ≥ 0.53).
Figure 1.
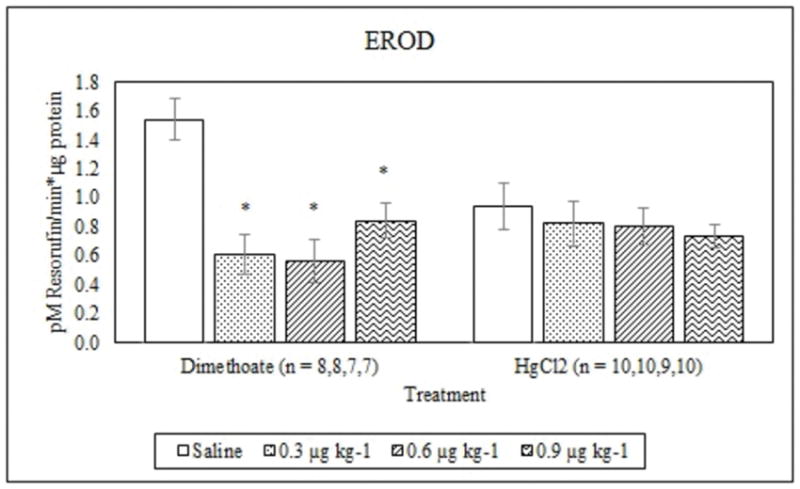
Average EROD activity (mean +/− S.E.) in signal crayfish hepatopancreas 72 h after dimethoate and mercury chloride treatments. * Denotes statistical significance (p≤ 0.05) compared to the saline control.
Figure 2.
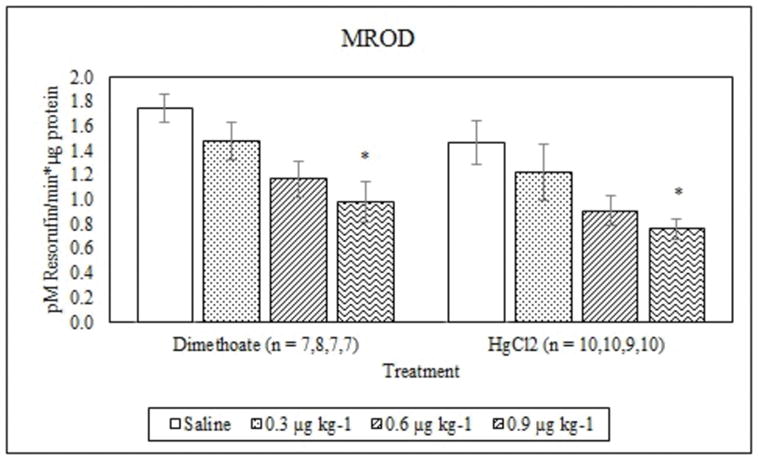
Average MROD activity (mean +/− S.E.) in signal crayfish hepatopancreas 72 h after dimethoate and mercury chloride treatments. * Denotes statistical significance (p≤ 0.05) compared to the saline control.
Figure 3.
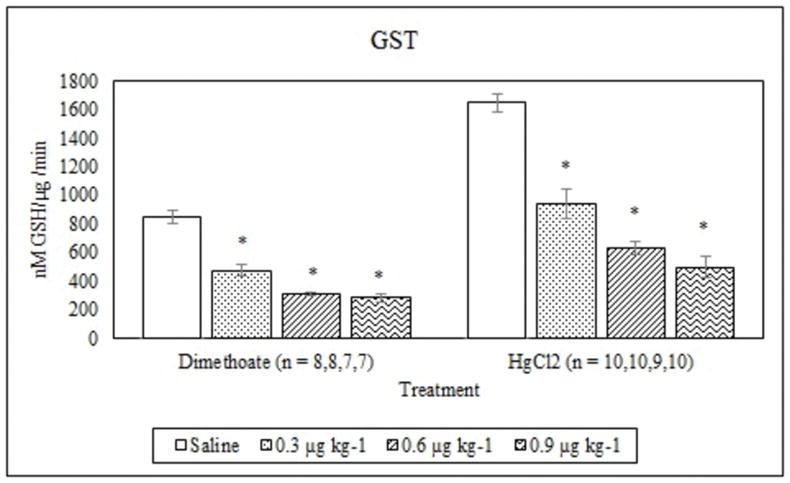
Average GST activity (mean +/− S.E.) in signal crayfish hepatopancreas 72 h after dimethoate and mercury chloride treatments. * Denotes statistical significance (p≤ 0.05) compared to the saline control.
Figure 4.
Average GSH concentrations (mean +/− S.E.) in signal crayfish hepatopancreas 72 h after dimethoate and mercury chloride treatments.
Figure 5.
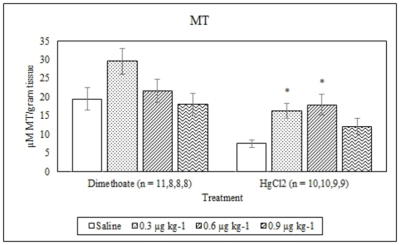
Average MT concentrations (mean +/− S.E.) in signal crayfish gill tissue 72 h after dimethoate and mercury chloride treatments. * Denotes statistical significance (p≤ 0.05) compared to the saline control.
Figure 6.
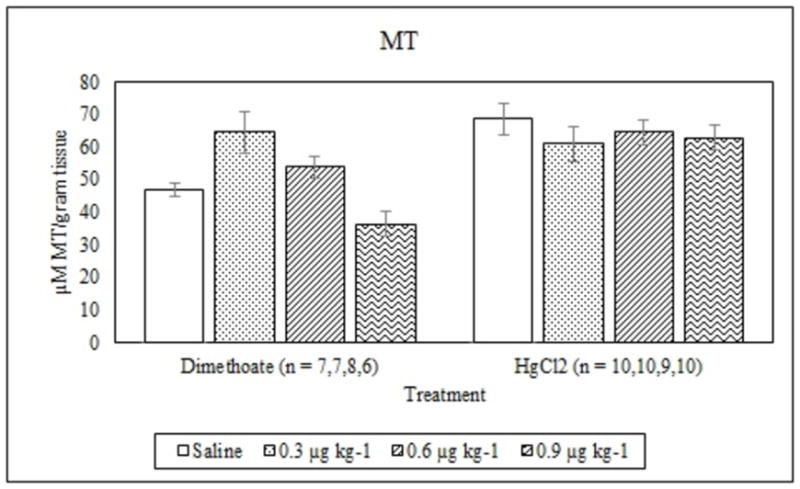
Average MT concentrations (mean +/− S.E.) in signal crayfish tail tissue 72 h after dimethoate and mercury chloride treatments.
Figure 7.
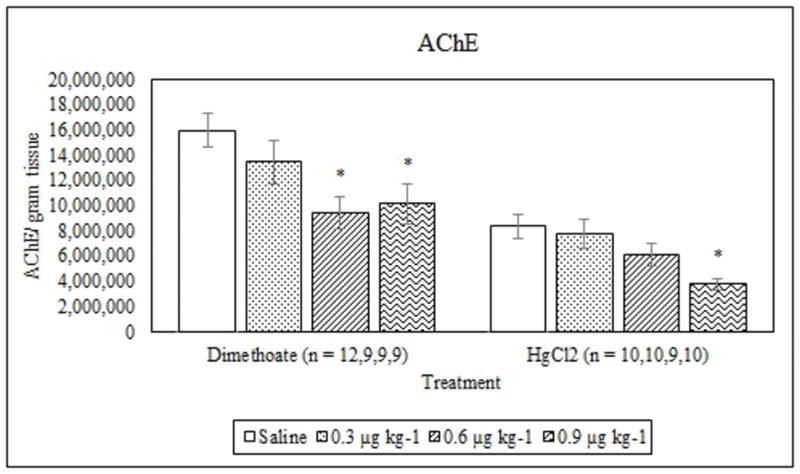
Average AChE activity (mean +/− S.E.) in signal crayfish tail tissue 72 h after dimethoate and mercury chloride treatments. * Denotes statistical significance (p ≤ 0.05) compared to the saline control.
4. Discussion
We examined whether the environmental pollutants mercury and dimethoate modulate biomarkers commonly utilized in field-based ecotoxicology studies in the signal crayfish (Pacifastacus leniusculus) with the goal of determining whether they could serve as useful indicators of exposure to these pollutants in impacted populations. We found that dimethoate decreases EROD, MROD, GST and AChE activities and mercury chloride exposure leads to decreased MROD, GST and AChE activities and increased MT concentrations in gill tissue at lower concentrations of exposure. Patterns of modulation differ in some cases from reports in other taxa and, although we did not design the study to examine seasonal variation in these markers, there is some indication that these markers vary throughout the year based on natural cycles or seasonal changes in contaminant exposure.
Studies have reported variable responses of phase I and II enzymes to organophosphate pesticides with increases, decreases, and no changes being observed depending on the chemical, species, research model and experimental design. We report that phase I (represented by EROD and MROD assays) and II (GST) enzymes in signal crayfish decrease in response to dimethoate exposure. Studies in the red swamp crayfish reported an increase in EROD in response to the organophosphate pesticide fenitrothion (whole animal exposure) both under controlled and field conditions (Escartin and Porte, 1996b; Fernandes et al., 2002). Interestingly, a later study using hepatopancreas primary cell culture found a decrease in EROD activity in response to fenitrothion (Birmelin et al., 1998). EROD activity was inhibited by the organophosphate methidathion in the gudgeon (Gobio gobio) but dimethoate exposure did not inhibit EROD activity in guppies (Poecilia reticulate), although the authors suggest a slight dose dependent decrease was present (Flammarion et al., 1998; Frasco and Guilhermino, 2002). The authors of these studies propose that the mechanism of EROD inhibition by organophosphate pesticides is likely based on the mechanism of action described for parathion which involves the covalent bonding of the sulfur molecule, released during the activation of the organophosphate compound which forms an oxon analogue, to the cytochrome P-450 enzyme, leading to inhibition of EROD activity (Flammarion et al., 1998; Frasco and Guilhermino, 2002). The decrease observed in GST activity in signal crayfish in this study is opposite to the response to fenitrothion observed in red swamp crayfish and consistent with decreased GST in response to dimethoate in guppies (Poecilia reticulate) (Birmelin et al., 1998; Frasco and Guilhermino, 2002). In the case of GST inhibition by dimethoate in guppies, the authors suggest that inhibition could involve the negative regulation of GST gene expression or be related to the role of GST in scavenging toxins (Frasco and Guilhermino, 2002). The variability in the response of these enzymes to organophosphate compounds is important to note when interpreting field-based ecotoxicology studies using phase I and II enzymes as biomarkers and emphasizes the value of careful characterization as well as using suites of bioindicators when examining biological responses in impacted populations.
Mercury exposure resulted in decreases in MROD and GST activities and no change in EROD activity which differs from responses reported in the literature for some taxa, again illustrating variations in responses. Mercury is toxic to enzymes and postulated mechanisms of inhibition include the binding of sulfhydryl groups by divalent ions or the generation of reactive oxygen species (ROS) that then lead to oxidative damage (Bozcaarmutlu and Arinc, 2004; Haniu et al., 1989; Viarengo et al., 1997). Studies in fish or HEPG2 cells report the inhibition of either the induction of EROD (by β-napthoflavone or benzo[a]pyrene) or un-induced EROD activity by mercury (Bozcaarmutlu and Arinc, 2004; Guilherme et al., 2008; Oliveira et al., 2004; Vakharia et al., 2001; Viarengo et al., 1997; Vieira et al., 2009). MROD activity appears to be more sensitive to mercury exposure than EROD in signal crayfish since it decreased in animals in this study and interestingly responds to mercury in a manner consistent with EROD in vertebrates (Bozcaarmutlu and Arinc, 2004; Guilherme et al., 2008; Vieira et al., 2009). The lack of a response of EROD to mercury chloride is consistent with a study conducted on bivalves (Chlamys farreri) that reported a decrease in EROD activity with exposure to lead but not mercury or copper (Zhang et al., 2010). The EROD saline controls for the mercury chloride study, which were collected in March, were significantly lower (p < 0.05) than the controls for the dimethoate study that were collected in June. We did not design the study to examine seasonal variation in these endpoints (discussed below), but it should be noted that the lack of EROD inhibition by mercury could be due to the lower overall activity in animals collected during a different season, a difference that was not observed in MROD. Finally, as noted above, GST decreased in response to mercury in signal crayfish which is opposite to responses reported in other taxa where mercury increased GST activity in catfish (Ictalurus melas) and green shore crabs (Carcinus maenas) (Elia et al., 2003; Elumalai et al., 2007).
GSH expression, as well as other endogenous antioxidants, may affect the response of cytochrome P-450 and additional enzymes to heavy metals as well as to known inducers. GSH protects against the metal induced inhibition of EROD, presumably by direct binding of the sulfhydryl groups to the metals or through its role as an antioxidant, which reacts with ROS generated by metal exposure (Bozcaarmutlu and Arinc, 2004; Guilherme et al., 2008; Oliveira et al., 2004; Viarengo et al., 1997). This could provide an explanation for the lack of a response of EROD to mercury in crayfish in this study given that GSH was detectable in all hepatopancreas samples, although it did not change in response to dimethoate or mercury at the concentrations tested in this study. The lack of change in GSH is consistent with studies that demonstrate no change in GSH in response to contaminants in Nile Tilapia, gastropods and worms exposed to contaminants (Kristoff et al., 2008; Matos et al., 2007; Velki and Hackenberger, 2013b). The GSH results indicate that although ROS production is not induced to an extent capable of modulating GSH concentrations at the doses of dimethoate and mercury tested in this study, it is likely that GSH (and MT) is providing a protective effect. Therefore, MROD as well as GST and AChE (discussed below) may be more sensitive to mercury exposure than EROD.
Tissue type, class of chemical and dose need to all be carefully considered when interpreting field data using MT as a biomarker in signal crayfish. Dimethoate treatment did not modulate MT concentrations in the gill or muscle tissue of experimental animals indicating that it is not a sensitive biomarker in signal crayfish for exposure to this compound at the concentrations tested in this study. Mercury chloride treatment increased MT concentrations in the gill tissue at the low and middle doses but not the highest concentration (0.9 μg kg−1) and did not alter MT in muscle tissue at any of the doses tested in this study. Gill tissue is more sensitive to mercury exposure which could be in part due to the lower overall expression of MT in gill relative to tail muscle. We do not have an explanation for why animals did not respond with an increase in MT at the highest dose of mercury in gill tissue, although it is interesting to note as nonmonotonic dose response curves have been described in the context of endocrine disrupting compounds (Vandenberg et al., 2012). MROD, GST and AChE all decreased significantly at the highest mercury chloride concentration in the same study animals indicating that they did in fact receive a higher dose. Higher concentrations should be tested in future studies to determine whether this pattern persists.
Inhibition of AChE by mercury and dimethoate could lead to increased mortality or altered physiological function in this species. Dimethoate and mercury chloride both decreased AChE activity in tail muscle. This is consistent with studies reporting inhibition of this enzyme by organophosphate pesticides in fish (Frasco and Guilhermino, 2002) and metals such as chromium, molybdenum and cadmium in Daphnia magna (Diamantino et al., 2000). A depression of 20% or more in AChE activity is considered to be indicative of organophosphate exposure and a 50% or greater decrease in AChE can be life threatening (Day and Scott, 1990). In this study, treatment with 0.3, 0.6 and 0.9 μg kg−1dimethoate led to decreases of approximately 16%, 41% and 37% respectively, with the higher two doses being significant (p < 0.05). Likewise, treatment with 0.3, 0.6 and 0.9 μg kg−1 mercury chloride resulted in a decrease in AChE of 7%, 27% and 55% respectively, with the highest dose being significantly different from the saline controls (p < 0.05). The fact that mercury chloride decreased AChE activity at the highest dose illustrates that inhibition of AChE is not a response unique to OPs in this species and thus must be factored into field studies using AChE as an endpoint using signal crayfish as an ecotoxicology model.
Seasonal variation in the endpoints described in this study needs to be assessed in future studies (Kerambrun et al., 2016; Osse et al., 2018). Although, we did not design this study to examine seasonal variation we did observe a difference in the values measured for the saline controls used in the dimethoate and mercury chloride studies. Animals for the dimethoate study were collected in June whereas animals used in the mercury chloride study were collected in March. The saline controls were significantly different (p < 0.05) between the studies for EROD, GST, GSH, MT in both gill and muscle, and AChE. Seasonal variation has been reported in both GSH concentrations and GST activity in the red swamp crayfish and certainly warrants investigation in the signal crayfish when using these markers in field studies (Elia et al., 2006).
The variability in responses to contaminants across taxa, classes of chemicals and potentially season in the markers examined in this study should be considered when conducting field -based studies. We report modulations by dimethoate and mercury of common endpoints used as biomarkers. Patterns of modulation differ, in some cases, from reports in other taxa which can complicate the interpretation of results obtained from field studies. Based on our results, we can conclude that EROD, MROD, GST and AChE are sensitive to dimethoate exposure and that MROD, GST, MT and AChE are modulated by mercury chloride in this species. GSH concentrations did not vary among treatments. Dimethoate and mercury both decreased phase I and II enzymes as well as AChE, potentially impacting physiological function and the animals’ ability to biotransform other contaminants. Seasonal variation may exist in the expression of these markers although future experiments specifically designed to test this question should be conducted.
Highlights.
EROD, MROD, GST and AChE are sensitive to dimethoate exposure in signal crayfish
MROD, GST, MT and AChE are modulated by mercury chloride
Decreased phase I and II enzymes could impact overall biotransformation capability
AChE inhibition could cause increased mortality or altered physiological function
Acknowledgments
The project described was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #s P20RR016454 and P20GM103408 (to MPG) as well as funds from the M.J. Murdock Charitable Trust (#2006188LJVZL11/16/06) and Kathryn Albertson Foundation (to MPG). We thank Collin Clovis, Alicia Latta and Patrick Erstad for their help with animal collection and Quinlan McLaughlin and Jennifer Liou for their help with editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott M, Einerson J, Schuster P, Susong D, Taylor HE. Trace elements and common ions in southeastern Idaho snow: Regional air pollutant tracers for source area emissions. Fuel Processing Technology. 2004;85:657–671. [Google Scholar]
- 2.Abbott ML, Lin CJ, Martian P, Einerson JJ. Atmospheric mercury near salmon falls creek reservoir in southern Idaho. Applied Geochemistry. 2008;23:438–453. [Google Scholar]
- 3.Abbott ML, Susong DD, Krabbenhoft DP, Rood AS. Mercury deposition in snow near an industrial emission source in the western US and comparison to ISC3 model predictions. Water Air and Soil Pollution. 2002;139:95–114. [Google Scholar]
- 4.Abbott ML, Susong DD, Olson M, Krabbenhoft DP. Mercury in soil near a long-term air emission source in southeastern Idaho. Environmental Geology. 2003;43:352–356. [Google Scholar]
- 5.Adewale IO, Afolayan A. Purification and catalytic properties of glutathione transferase from the hepatopancreas of crayfish Macrobrachium vollenhovenii (Herklots) J Biochem Mol Toxicol. 2005;18:332–344. doi: 10.1002/jbt.20044. [DOI] [PubMed] [Google Scholar]
- 6.Aksu O, Yildirim NC, Yildirim N, Danabas D, Danabas S. Biochemical response of crayfish Astacus leptodactylus exposed to textile wastewater treated by indigenous white rot fungus Coriolus versicolor. Environmental Science and Pollution Research. 2015;22:2987–2993. doi: 10.1007/s11356-014-3550-z. [DOI] [PubMed] [Google Scholar]
- 7.Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat Toxicol. 2006;76:160–202. doi: 10.1016/j.aquatox.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Ashley CM, Simpson MG, Holdich DM, Bell DR. 2,3,7,8-Tetrachloro-dibenzo-p-dioxin is a potent toxin and induces cytochrome P450 in the crayfish, Pacifastacus leniusculus. Aquat Toxicol. 1996;35:157–169. [Google Scholar]
- 9.Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 10.Birmelin C, Escartin E, Goldfarb PS, Livingstone DR, Porte C. Enzyme effects and metabolism of fenitrothion in primary cell culture of the red swamp crayfish Procambarus clarkii. Marine Environmental Research. 1998;46:375–378. [Google Scholar]
- 11.Bozcaarmutlu A, Arinc E. Inhibitory effects of divalent metal ions on liver microsomal 7-ethoxyresorufin O-deethylase (EROD) activity of leaping mullet. Marine Environmental Research. 2004;58:521–524. doi: 10.1016/j.marenvres.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Day KE, Scott IM. Use of acetylcholineesterase activity to detect sublethal toxicity in stream inverebrates exposed to low concentrations of organophosphate insecticides. Aquat Toxicol. 1990;18:101–114. [Google Scholar]
- 14.Delramo J, Pastor A, Torreblanca A, Medina J, Diazmayans J. Cadmium binding protein induced in exposed freshwater crayfish Procambarus clarkii Biol. Trace Elem Res. 1989a;21:75–80. doi: 10.1007/BF02917238. [DOI] [PubMed] [Google Scholar]
- 15.Delramo J, Pastor A, Torreblanca A, Medina J, Diazmayans J. Cadmium binding proteins in midgut gland of freshwater crayfish Procambus clarkii Bull. Environ Contam Toxicol. 1989b;42:241–246. doi: 10.1007/BF01699406. [DOI] [PubMed] [Google Scholar]
- 16.Diamantino TC, Guilhermino L, Almeida E, Soares A. Toxicity of sodium molybdate and sodium dichromate to Daphnia magna Straus evaluated in acute, chronic, and acetylcholinesterase inhibition tests. Ecotoxicol Environ Saf. 2000;45:253–259. doi: 10.1006/eesa.1999.1889. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 18.Elia AC, Dorr AJM, Mastrangelo C, Prearo M, Abete MC. Glutathione and antioxidant enzymes in the hepatopancreas of crayfish Procambarus clarkii (Girard, 1852) of Lake Trasimeno (Italy) Bull Fr Pech Piscic. 2006:1351–1361. [Google Scholar]
- 19.Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf. 2003;55:162–167. doi: 10.1016/s0147-6513(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 21.Elumalai M, Antunes C, Guilhermino L. Enzymatic biomarkers in the crab Carcinus maenas from the Minho River estuary (NW Portugal) exposed to zinc and mercury. Chemosphere. 2007;66:1249–1255. doi: 10.1016/j.chemosphere.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Escartin E, Porte C. Acetylcholinesterase inhibition in the crayfish Procambarus clarkii exposed to fenitrothion. Ecotoxicol Environ Saf. 1996a;34:160–164. doi: 10.1006/eesa.1996.0058. [DOI] [PubMed] [Google Scholar]
- 23.Escartin E, Porte C. Bioaccumulation, metabolism, and biochemical effects of the organophosphorus pesticide fenitrothion in Procambarus clarkii. Environ Toxicol Chem. 1996b;15:915–920. [Google Scholar]
- 24.Faria M, Huertas D, Soto DX, Grimalt JO, Catalan J, Carmen Riva M, Barata C. Contaminant accumulation and multi-biomarker responses in field collected zebra mussels (Dreissena polymorpha) and crayfish (Procambarus clarkii), to evaluate toxicological effects of industrial hazardous dumps in the Ebro river (NE Spain) Chemosphere. 2010;78:232–240. doi: 10.1016/j.chemosphere.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes D, Potrykus J, Morsiani C, Raldua D, Lavado R, Porte C. The combined use of chemical and biochemical markers to assess water quality in two low-stream rivers (NE Spain) Environ Res. 2002;90:169–178. doi: 10.1006/enrs.2002.4390. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari A, Venturino A, de D’Angelo AMP. Effects of carbaryl and azinphos methyl on juvenile rainbow trout (Oncorhynchus mykiss) detoxifying enzymes. Pest Biochem Physiol. 2007;88:134–142. doi: 10.1016/j.cbpc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Flammarion P, Migeon B, Urios SB, Morfin P, Garric J. Effect of methidathion on the cytochrome P-450 1A in the cyprinid fish gudgeon (Gobio gobio) Aquat Toxicol. 1998;42:93–102. [Google Scholar]
- 28.Fornstrom CB, Landrum PF, Weisskopf CP, LaPoint TW. Effects of terbufos on juvenile red swamp crayfish (Procambarus clarkii): Differential routes of exposure. Environ Toxicol Chem. 1997;16:2514–2520. [Google Scholar]
- 29.Frasco MF, Guilhermino L. Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol Biochem. 2002;26:149–156. [Google Scholar]
- 30.Guan R-Z, Wiles P. The home range of the signal crayfish in a British lowland river. Freshwater Forum. 1997:45–54. [Google Scholar]
- 31.Guilherme S, Valega M, Pereira ME, Santos MA, Pacheco M. Antioxidant and biotransformation responses in Liza aurata under environmental mercury exposure: Relationship with mercury accumulation and implications for public health. Mar Pollut Bull. 2008;56:845–859. doi: 10.1016/j.marpolbul.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Guilhermino L, Lacerda MN, Nogueira AJA, Soares A. In vitro and in vivo inhibition of Daphnia magna acetylcholinesterase by surfactant agents: possible implications for contamination biomonitoring. Sci Total Environ. 2000;247:137–141. doi: 10.1016/s0048-9697(99)00485-4. [DOI] [PubMed] [Google Scholar]
- 33.Guilhermino L, Lopes MC, Carvalho AP, Soares A. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere. 1996;32:727–738. doi: 10.1016/0045-6535(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 34.Gunderson MP, Oberdorster E, Guillette LJ. Phase I and II liver enzyme activities in juvenile alligators (Alligator mississippiensis) collected from three sites in the Kissimmee-Everglades drainage, Florida (USA) Comp Biochem Physiol C-Toxicol Pharmacol. 2004;139:39–46. doi: 10.1016/j.cca.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Gunderson MP, Pickett MA, Martin JT, Hulse EJ, Smith SS, Smith LA, Campbell RM, Lowers RH, Boggs AS, Guillette LJ., Jr Variations in hepatic biomarkers in American alligators (Alligator mississippiensis) from three sites in Florida, USA. Chemosphere. 2016;155:180–187. doi: 10.1016/j.chemosphere.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haniu M, McManus ME, Birkett DJ, Lee TD, Shively JE. Structural and functional analysis of NADPH cytochrome P-450 reductase from human liver - complete sequence of human enzyme and NADPH binding sites. Biochemistry. 1989;28:8639–8645. doi: 10.1021/bi00447a054. [DOI] [PubMed] [Google Scholar]
- 37.Holdich DM. Biology of Freshwater Crayfish. Blackwell Science Ltd; 2002. p. 702. [Google Scholar]
- 38.Hothem RL, Bergen DR, Bauer ML, Crayon JJ, Meckstroth AM. Mercury and trace elements in crayfish from Northern California. Bull Environ Contam Toxicol. 2007;79:628–632. doi: 10.1007/s00128-007-9304-6. [DOI] [PubMed] [Google Scholar]
- 39.James MO, Boyle SM. Cytochromes P450 in crustacea. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol. 1998;121:157–172. doi: 10.1016/s0742-8413(98)10036-1. [DOI] [PubMed] [Google Scholar]
- 40.Jewell CSE, Mayeaux MH, Winston GW. Benzo a pyrene metabolism by the hepatopancreas and green gland of the red swamp crayfish, Procambarus clarkii, in vitro. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol. 1997;118:369–374. doi: 10.1016/s0742-8413(97)00158-8. [DOI] [PubMed] [Google Scholar]
- 41.Jewell CSE, Winston GW. OXYRADICAL PRODUCTION BY HEPATOPANCREAS MICROSOMES FROM THE RED SWAMP CRAYFISH, PROCAMBARUS-CLARKII. Aquat Toxicol. 1989;14:27–46. [Google Scholar]
- 42.Joshi SC, Sharma P. Male reproductive toxicity of organophosphorous compounds: a review. Toxicol Environ Chem. 2011;93:1486–1507. [Google Scholar]
- 43.Kerambrun E, Rioult D, Delahaut L, Evariste L, Pain-Devin S, Auffret M, Geffard A, David E. Variations in gene expression levels in four European zebra mussel, Dreissena polymorpha, populations in relation to metal bioaccumulation: A field study. Ecotoxicol Environ Saf. 2016;134:53–63. doi: 10.1016/j.ecoenv.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Khati W, Ouali K, Mouneyrac C, Banaoui A. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use in biomonitoring. In: Salame C, Aillerie M, Khoury G, editors. Terragreen 2012: Clean Energy Solutions for Sustainable Environment. 2012. pp. 784–794. [Google Scholar]
- 45.Kirby MF, Morris S, Hurst M, Kirby SJ, Neall P, Tylor T, Fagg A. The use of cholinesterase activity in flounder (Platichthys flesus) muscle tissue as a biomarker of neurotoxic contamination in UK estuaries. Mar Pollut Bull. 2000;40:780–791. [Google Scholar]
- 46.Kouba A, Buric M, Kozak P. Bioaccumulation and Effects of Heavy Metals in Crayfish: A Review. Water Air and Soil Pollution. 2010;211:5–16. [Google Scholar]
- 47.Kovacevic TB, Borkovic SS, Pavlovic SZ, Despotovic SG, Saicic ZS. Glutathione as a suitable biomarker in hepatopancreas, gills and muscle of three freshwater crayfish species. Archives of Biological Sciences. 2008;60:59–66. [Google Scholar]
- 48.Kristoff G, Guerrero NRV, Cochon AC. Effects of azinphos-methyl exposure on enzymatic and non-enzymatic antioxidant defenses in Biomphalaria glabrata and Lumbriculus variegatus. Chemosphere. 2008;72:1333–1339. doi: 10.1016/j.chemosphere.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Larson ER, Olden JD. The State of Crayfish in the Pacific Northwest. Fisheries. 2011;36:60–73. [Google Scholar]
- 50.Lindstrom-Seppa P, Hanninen O. Induction of cytochrome P-450 mediated mono-oxygenase reactions and conjugation activities in freshwater crayfish (Astacus astacus). Arch. Toxicol. Suppl. 1986;9:374–377. doi: 10.1007/978-3-642-71248-7_73. [DOI] [PubMed] [Google Scholar]
- 51.Martinez M, Torreblanca A, Delramo J, Pastor A, Diazmayans J. Cadmium induced metallothionein in hepatopancreas of Procambarus clarkii: Quantification by a silver saturation method. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol. 1993;105:263–267. [Google Scholar]
- 52.Matos P, Fontainhas-Fernandes A, Peixoto F, Carrola J, Rocha E. Biochemical and histological hepatic changes of Nile tilapia Oreochromis niloticus exposed to carbaryl. Pest Biochem Physiol. 2007;89:73–80. [Google Scholar]
- 53.Matozzo V, Tomei A, Marin MG. Acetylcholinesterase as a biomarker of exposure to neurotoxic compounds in the clam Tapes philippinarum from the Lagoon of Venice. Mar Pollut Bull. 2005;50:1686–1693. doi: 10.1016/j.marpolbul.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Mebane CA, MacCoy DE. Monitoring plan for mercury in fish tissue and water from the Boise River, Snake River, and Brownlee Reservoir, Idaho and Oregon. USGS Open-File Report 2013 [Google Scholar]
- 55.Mueller KW, Serdar DM. Total mercury concentrations among fish and crayfish inhabiting different trophic levels in Lake Whatcom, Washington. Journal of Freshwater Ecology. 2002;17:621–633. [Google Scholar]
- 56.Newman M. Fundementals of Ecotoxicology. Lewis Publishers; Boca Raton: 1998. [Google Scholar]
- 57.Oliveira M, Santos MA, Pacheco M. Glutathione protects heavy metal-induced inhibition of hepatic microsomal ethoxyresorufin O-deethylase activity in Dicentrarchus labrax L. Ecotoxicol Environ Saf. 2004;58:379–385. doi: 10.1016/j.ecoenv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Osse M, Hamel J-F, Mercier A. Markers of oil exposure in cold-water benthic environments: Insights and challenges from a study with echinoderms. Ecotoxicol Environ Saf. 2018;156:56–66. doi: 10.1016/j.ecoenv.2018.02.076. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen SN, Pedersen KL, Hojrup P, Depledge MH, Knudsen J. Primary structures of decapod crustacean metallothioneins with special emphasis on freshwater and semi-terrestrial species. Biochem J. 1996;319:999–1003. doi: 10.1042/bj3190999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pena-Llopis S, Ferrando MD, Pena JB. Impaired glutathione redox status is associated with decreased survival in two organophosphate-poisoned marine bivalves. Chemosphere. 2002;47:485–497. doi: 10.1016/s0045-6535(01)00323-x. [DOI] [PubMed] [Google Scholar]
- 61.Porte C, Escartin E. Cytochrome P450 system in the hepatopancreas of the red swamp crayfish Procambarus clarkii: a field study. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol. 1998;121:333–338. doi: 10.1016/s0742-8413(98)10054-3. [DOI] [PubMed] [Google Scholar]
- 62.Richetti SK, Rosemberg DB, Ventura-Lima J, Monserrat JM, Bogo MR, Bonan CD. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology. 2011;32:116–122. doi: 10.1016/j.neuro.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Roesijadi G. Metallothioneins in metal regulation and toxicity in aquatic animals Aquat. Toxicol. 1992;22:81–114. [Google Scholar]
- 64.Roesijadi G. Metallothionein induction as a measure of response to metal exposure in aquatic animals Environ. Health Perspect. 1994;102:91–95. doi: 10.1289/ehp.94102s1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sen A, Semiz A. Effects of metals and detergents on biotransformation and detoxification enzymes of leaping mullet (Liza saliens) Ecotoxicol Environ Saf. 2007;68:405–411. doi: 10.1016/j.ecoenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Snyder MJ. Cytochrome P450 enzymes in aquatic invertebrates: recent advances and future directions. Aquat Toxicol. 2000;48:529–547. doi: 10.1016/s0166-445x(00)00085-0. [DOI] [PubMed] [Google Scholar]
- 67.Sturm A, de Assis HCD, Hansen PD. Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination. Marine Environmental Research. 1999;47:389–398. [Google Scholar]
- 68.Vakharia DD, Liu N, Pause R, Fasco M, Bessette E, Zhang QY, Kaminsky LS. Polycyclic aromatic hydrocarbon/metal mixtures: Effect on PAH induction of CYP1A1 in human HEPG2 cells. Drug Metab Dispos. 2001;29:999–1006. [PubMed] [Google Scholar]
- 69.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Velki M, Hackenberger BK. Species-specific differences in biomarker responses in two ecologically different earthworms exposed to the insecticide dimethoate. Comp Biochem Physiol C-Toxicol Pharmacol. 2012;156:104–112. doi: 10.1016/j.cbpc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Velki M, Hackenberger BK. Different sensitivities of biomarker responses in two epigeic earthworm species after exposure to pyrethroid and organophosphate insecticides. Arch Environ Contam Toxicol. 2013a;65:498–509. doi: 10.1007/s00244-013-9930-4. [DOI] [PubMed] [Google Scholar]
- 72.Velki M, Hackenberger BK. Inhibition and recovery of molecular biomarkers of earthworm Eisenia andrei after exposure to organophosphate dimethoate. Soil Biol Biochem. 2013b;57:100–108. [Google Scholar]
- 73.Viarengo A, Bettella E, Fabbri R, Burlando B, Lafaurie M. Heavy metal inhibition of EROD activity in liver microsomes from the bass Dicentrarchus labrax exposed to organic xenobiotics: Role of GSH in the reduction of heavy metal effects. Marine Environmental Research. 1997;44:1–11. [Google Scholar]
- 74.Vieira LR, Gravato C, Soares A, Morgado F, Guilhermino L. Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: Linking biomarkers to behaviour. Chemosphere. 2009;76:1416–1427. doi: 10.1016/j.chemosphere.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Wilhelm D, Torres MA, Tribess TB, Pedrosa RC, Soares CHL. Influence of season and pollution on the antioxidant defenses of the cichlid fish acara (Geophagus brasiliensis) Braz J Med Biol Res. 2001;34:719–726. doi: 10.1590/s0100-879x2001000600004. [DOI] [PubMed] [Google Scholar]
- 76.Xuereb B, Lefevre E, Garric J, Geffard O. Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda): Linking AChE inhibition and behavioural alteration. Aquat Toxicol. 2009;94:114–122. doi: 10.1016/j.aquatox.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Song JM, Yuan HM, Xu YY, He ZP, Duan LQ. Biomarker responses in the bivalve (Chlamys farreri) to exposure of the environmentally relevant concentrations of lead, mercury, copper. Environ Toxicol Pharmacol. 2010;30:19–25. doi: 10.1016/j.etap.2010.03.008. [DOI] [PubMed] [Google Scholar]