Abstract
Purpose
Anaplastic thyroid cancer (ATC) is a rare tumor with a lethal clinical course despite aggressive multimodal therapy. Intensity-modulated radiotherapy (IMRT) may achieve a good therapeutic outcome in ATC patients, and the role of IMRT should be assessed. We retrospectively reviewed outcomes for ATC treated with three-dimensional conformal radiotherapy (3D-CRT) or IMRT to determine the optimal treatment option and explore the role of radiotherapy (RT).
Materials and Methods
Between December 2000 and December 2015, 41 patients with pathologically proven ATC received RT with a sufficient dose of ≥40 Gy. Among them, 21 patients (51%) underwent surgery before RT. Twenty-eight patients received IMRT, and 13 received 3D-CRT. Overall survival (OS) and progression-free survival (PFS), patterns of failure, and toxicity were examined.
Results
The median follow-up time for survivors was 38.0 months. The median and 1-year OS and PFS rates were 7.2 months and 29%, 4.5 months and 15%, respectively. Surgery significantly improved the prognosis (median OS: 10.7 vs. 3.9 months, p = 0.001; median PFS: 5.9 vs. 2.5 months, p = 0.007). IMRT showed significantly better PFS and OS than 3D-CRT, even in multivariate analysis (OS: hazard ratio [HR] = 0.30, p = 0.005; PFS: HR = 0.33, p = 0.005). Significantly higher radiation dose could be delivered with IMRT than 3D-CRT (EQD210 66 vs. 60 Gy, p = 0.005). Only 2 patients had grade III dermatitis after IMRT. No other severe toxicity ≥grade III occurred.
Conclusion
Patients with ATC showed better prognosis through multimodal treatment. Furthermore, IMRT could achieve favorable survival rates by safely delivering higher dose than 3D-CRT.
Keywords: Anaplastic thyroid carcinoma, Radiotherapy, Intensity-modulated radiotherapy, Surgery, Progression-free survival
Introduction
Anaplastic thyroid carcinoma (ATC) is a rare tumor, that accounts for <5% of all thyroid cancers. However, it is one of the most aggressive human cancers, causing up to 40% of all thyroid cancer deaths [1,2]. Survival is limited to 3–6 months due to very aggressive tumor biology. ATC can invade adjacent organs, such as the larynx, trachea, esophagus, vessels, and muscles, resulting in dyspnea, vocal cord paralysis, dysphagia, and consequently, mortality. Not only uncontrolled local tumor invasion but also distant metastases (DM) can cause cancer deaths [3-7]. Because of its dismal features, all ATC are classified as stage IV according to the American Joint Committee on Cancer Staging (AJCC) staging system (7th edition), regardless of tumor size, nodal status, and either absence or presence of DM [7-11].
Treatment options for ATC are generally recommended as mu l t imodal ther apies i ncluding surger y and chemoradiotherapy, which might offer opportunities to prolong survival or improve the quality of life. However, even with the very best treatment, an extremely low cure rate has been reported in ATC in many recent studies [12,13]. Thus, the optimal multimodal therapy policy is still debated, and a standardized treatment strategy remains unestablished [12-14].
Considering these unsatisfactory results, there is a need to introduce more advanced treatment technique to enhance therapeutic outcomes in ATC. For radiotherapy (RT) modality, the efficacy of conventional three-dimensional conformal radiotherapy (3D-CRT) is potentially limited by undesirable dose reductions due to toxicity related to the anatomical location of the thyroid gland. Intensity-modulated radiotherapy (IMRT), an advanced form of RT, can successfully deliver high doses to the tumor while surrounding normal tissues receive only low doses of radiation. IMRT might achieve a good therapeutic outcome, and contribute to longer survival when IMRT is combined with the aggressive multimodal therapy [15].
In this study, we investigated institutional therapeutic outcomes of patients with ATC who received RT at our institution and assessed survival outcomes to determine the optimal treatment modality. Furthermore, we attempted to demonstrate the difference in clinical outcomes after performing a modern RT technique.
Materials and Methods
1. Patients
We identified 59 patients with pathologically proven ATC who received RT between December 2000 and December 2015 in Yonsei University Health System. A total of 18 patients who received an insufficient radiation dose <40 Gy due to rapid tumor progression, who refused treatment were excluded; therefore, the outcomes of 41 patients were analyzed in this study. The medical records of these patients were retrospectively reviewed. Initial evaluation included physical examination, routine labs, computed tomography (CT) scans of the neck and chest, and positron emission tomography. The AJCC staging system (7th edition) was used for tumor staging. The final pathologic confirmation was done by surgery in 21 patients, open biopsy in 4 patients, and fine-needle aspiration biopsy in 16 patients. The histologic findings were examined by a pathologist to exclude possible poorly differentiated carcinoma. This study was approved by the Institutional Review Board of the Yonsei University Health System (No. 3-2018-0093). The patient records/information were anonymized and de-identified prior to analysis, and informed consent was not obtained from each participant.
2. Treatment
Surgical therapy for ATC included radical resection for patients with resectable tumors and partial/palliative resection for the remaining patients with unresectable tumors. Radical resection was usually done as total thyroidectomy because ATC usually shows aggressive behavior and extensive tumor burden at presentation. Partial thyroidectomy was performed when there was little tumor burden, and when the tumor is focally limited in the unilateral lobe. For patients with clinically positive cervical lymph nodes, level II to VI neck node dissection was also performed. When patients were unable to undergo surgery due to poor general condition, old age, extensive tumor burden (including DM), or refusal of surgery, they underwent tracheostomy and tumor biopsy alone.
For chemotherapeutic regimens, doxorubicin, cisplatin, and paclitaxel-based concurrent chemoradiotherapy (CCRT) was usually administered before and after surgery in our institution according to the clinicians’ preferences and patients’ condition. If patients could not tolerate CCRT, physicians sometimes decided to perform RT only. Sequential chemotherapy with doxorubicin, cisplatin, and paclitaxel after CCRT was also considered if it was necessary.
For RT, all patients were in the supine position using a thermoplastic mask, and a contrast-enhanced 3 mm-thickness slice cut CT scan was obtained. The CT scan was imported to the Pinnacle3 planning system version 9.4 (Philips Medical Systems, Cleveland, OH, USA) and MIM software version 6.5.8 (MIM Software Inc., Cleveland, OH, USA), and the target volume was delineated on each of the axial CT images by an experienced radiation oncologist. In the 2000s, patients were mainly treated with the 3D-CRT technique, but as IMRT began to be covered by Korean national health insurance, IMRT gradually became the predominant modality.
For the RT field, gross tumor volume (GTV) was defined as the primary tumor and metastatic lymph nodes in patients without surgery whereas in patients with surgery, it was defined as postoperative tumor bed. The clinical target volume (CTV) included the thyroid area and regional lymph node areas for elective treatment (e.g., bilateral neck level II–VI, upper mediastinum). The planning target volume (PTV) was defined as CTV plus a 0.3-cm margin. For 3D-CRT, the median total radiation dose to the GTV for definitive treatment was 60.0 Gy (range, 51.3 to 67.0 Gy), delivered in single daily fractions of median 2.0 Gy (range, 1.8 to 2.5 Gy), 5 days a week, for a median total of 30 fractions (range, 24 to 36 fractions). For postoperative cases, median dose to the tumor bed was 55.8 Gy (range, 53.1 to 63.7 Gy), delivered in single daily fractions of 1.8 Gy for a median total of 32 fractions (range, 30 to 36 fractions). The dose administered to regional lymph nodes areas was median 40.7 Gy (range, 30 to 67.0 Gy), with a median fractional dose of 1.8 Gy (range, 1.8 to 2.5 Gy), for a median 22 fractions (range, 15 to 30 fractions). For IMRT, the median radiation dose to the GTV for definitive treatment was 67.1 Gy (range, 43.5 to 74.8 Gy), delivered in single daily fractions of median 2.5 Gy (range, 2.0 to 3.0 Gy), 5 days a week, for a median total of 26 fractions (range, 20 to 33 fractions). For postoperative cases, median dose to the tumor bed was 64.2 Gy (range, 57.3 to 67.7 Gy) delivered in single daily fractions of median 2.2 Gy (range, 2.0 to 2.7 Gy), for a median total of 30 fractions (range, 22 to 32 fractions). The dose administered to regional lymph nodes areas was median 48.9 Gy (range, 35.4 to 60.4 Gy) with a median fractional dose of 1.8 Gy (range,1.6 to 2.2 Gy), for a median 30 fractions (range, 20 to 33 fractions). The simultaneous integrated boost-IMRT (SIB-IMRT) technique was used for IMRT. Therefore, some patients with IMRT received a higher dose in the GTV area using the SIB technique than did patients with 3D-CRT, while simultaneously delivering a lower dose to the normal tissues.
3. Treatment outcome and toxicity
Clinicians routinely examined patients at least once a week during the RT, and 1 month following the completion of RT for toxicity assessment, which included a complete blood count (CBC) test. If clinically indicated, other blood tests or imaging studies such as CT scans were obtained. Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE v.4.0). Acute toxicity was defined as an event occurring during RT or within 90 days from the start of RT. Late toxicity was defined as an event occurring after 90 days from the start of RT. Acute or late toxicity ≥grade 3 was considered severe.
For follow-up, patients were evaluated at 3-month intervals for the first 2 years, and every 6 months thereafter. The first treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors Group (RECIST) at the first visit after completing RT. Routine follow-up included neck and chest CT scans and CBC. All patients were followed until death or time of analysis. When recurrence or metastasis was suspected, imaging studies including CT scan were performed for further assessment. Locoregional failure (LRF) was defined as any recurrence inside the neck area (thyroid tumor bed as ‘local failure’ and lymph nodes as ‘regional failure’) with or without DM. All other recurrences outside the neck area were defined as DM.
4. Statistical analysis
Progression-free survival (PFS) was defined as the time from the date of first treatment to any recurrence or last follow-up. Overall survival (OS) was defined as the time from the date of first treatment to the time of death from any cause or last follow-up. Survival rates were calculated using the Kaplan-Meier method, and prognostic impacts of clinical factors were analyzed with the log-rank test. Cox regression was used for analysis of independent prognostic factors. After univariate analysis of survival, we chose significant factors and generated multivariate cox regression model. Comparisons of basic characteristics between groups were done using the chi-square test. Student t-test analysis was also performed to assess the differences between the continuous variables of two different groups. SPSS version 23 (IBM, Armonk, NY, USA) was used for statistical analyses. The p-values less than 0.05 were considered statistically significant.
Results
1. Clinical and pathological characteristics
The patient and tumor characteristics are summarized in Table 1. There were 41 patients, including 24 females and 17 males with a ratio of 1.4:1.0. The age ranged from 43 to 86 years old, with a median of 67.2 years. Five patients had stage IVA disease (tumor limited to the thyroid), 20 patients had IVB disease (thyroid capsule penetrated), and 16 patients had IVC disease (exhibiting distant metastasis). For nodal status, 7 patients had N0 disease, 6 patients had N1a disease, and 28 patients had N1b disease. Among 16 patients with stage IVC, the most common distant metastases sites were lung only (n = 10), followed by bone only (n = 4), and multiple sites simultaneously (n = 2).
Table 1.
Patient and treatment characteristics
| Variable | Value |
|---|---|
| Age (yr) | 67.2 (43–86) |
| <65 | 13 (31.7) |
| ≥65 | 28 (68.3) |
| Sex | |
| Male | 17 (41.5) |
| Female | 24 (58.5) |
| ECOG PS | |
| 0–1 | 28 (68.3) |
| 2–4 | 13 (31.7) |
| T stage | |
| T4a | 6 (14.6) |
| T4b | 35 (85.4) |
| N stage | |
| N0 | 7 (17.1) |
| N1a | 6 (14.6) |
| N1b | 28 (68.3) |
| M stage | |
| M0 | 25 (61) |
| M1 | 16 (39) |
| Stage | |
| IVA | 5 (12.2) |
| IVB | 20 (48.8) |
| IVC | 16 (39) |
| Treatment modality | |
| Surgery + RT + CTx | 16 (39) |
| Surgery + RT | 5 (12.2) |
| RT + CTx | 15 (36.6) |
| RT alone | 5 (12.2) |
| Surgery | |
| Total thyroidectomy | 18 (43.9) |
| Partial thyroidectomy | 3 (7.3) |
| No surgery | 20 (48.8) |
| Chemotherapy | |
| Concurrent | 28 (68.3) |
| Sequential | 3 (7.3) |
| No chemotherapy | 10 (24.4) |
| RT modality | |
| IMRT | 28 (68.3) |
| 3D-CRT | 13 (31.7) |
| Total dose (EQD210, Gy) | 63.7 (43.5–74.8) |
| <62.0 | 13 (31.7) |
| ≥62.0 | 28 (68.3) |
Values are presented as median (range) or number (%).
ECOG PS, Eastern Cooperative Oncology Group performance score; RT, radiation therapy; CTx, chemotherapy; IMRT, intensity-modulated radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy.
Twenty-one out of 41 patients underwent surgery initially. Total thyroidectomy was performed in 18 patients, and partial thyroidectomy in 3 patients. Four patients underwent tracheostomy alone, without thyroidectomy. Subsequently, 31 out of 41 patients received chemotherapy. Among them, 12 patients received doxorubicin-based CCRT, 9 patients received cisplatin-based CCRT, 6 patients received paclitaxel-based CCRT, and 1 patient received doxorubicin-cisplatin combination CCRT. Three received sequential chemotherapy after RT (1 with doxorubicin, 1 with cisplatin, and 1 with paclitaxel).
All patients received RT (n = 41). For modality, 28 patients received IMRT and 13 received 3D-CRT. The median total EQD210 dose was 63.7 Gy, and the dose varied from 43.5 to 74.8 Gy. The median dose per fraction was 2.2 Gy (range, 1.8 to 3.0 Gy), and the median number of fractionations was 30 (range, 20 to 36 fractions). The median dose for IMRT was significantly (p = 0.005) higher than that of 3D-CRT (66.0 Gy and 60.0 Gy, respectively).
1. Survival and prognostic factors
The median follow-up time was 38.0 months (range, 5.1 to 60.2 months) for surviving patients and 7.2 months (range, 1.2 to 60.2 months) for the entire cohort. Six out of 41 patients were alive at the last follow-up date. Among 35 deaths, the most common cause of death was an aggravation of lung metastases in 19 patients. Only 2 patients died of local tumor progression, and 1 of them underwent salvage surgery for this. The other causes of death included brain metastasis, infection including sepsis, and poor general condition.
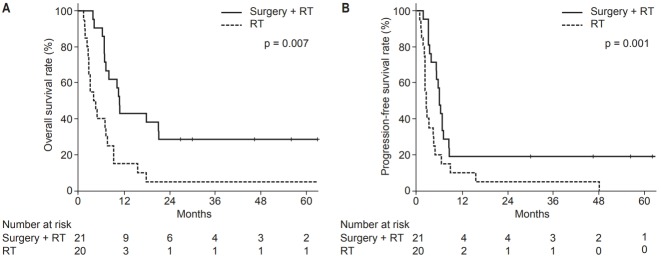
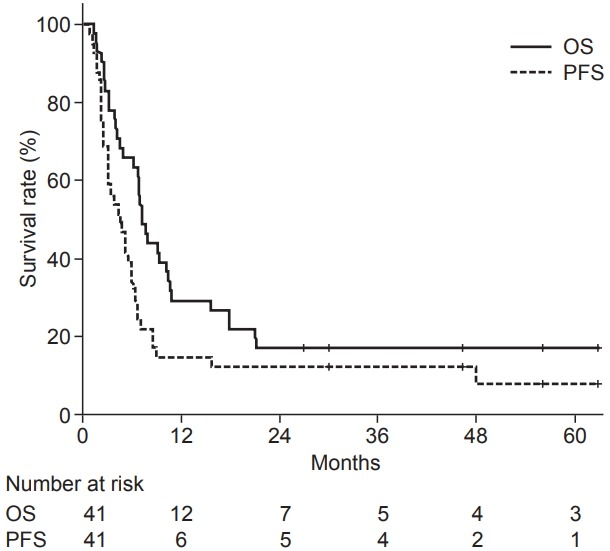
The median OS was 7.2 months, and the 1-year OS was 29% (Fig. 1). In univariate analysis, AJCC stage, and whether the patient had undergone surgery were also significant factors. Stage IVA patients had a better OS (median 69.0 months, 1-year 80%) than stage IVB (median 8.0 months, 1-year 25%) or stage IVC patients (median 3.9 months, 1-year 19%) (p = 0.007). Patients who underwent surgery had a better OS (median 10.7 months, 1-year 43%) than patients without surgery (median 3.9 months, 1-year 15%) (p = 0.001) (Fig. 2A). In multivariate analysis of OS, stage IVA (HR 0.04, p = 0.004 to others), surgery (HR 0.27, p = 0.001), and IMRT (HR 0.30, p = 0.005) were found to be independently significant factors.
Fig. 1.
Kaplan-Meier survival analysis of all 41 patients with anaplastic thyroid carcinoma. OS, overall survival; PFS, progression-free survival.
Fig. 2.
Kaplan-Meier survival analysis on overall survival (A) and progression-free survival (B) in patients with surgery or not. RT, radiation therapy.
The median PFS was 4.5 months, and the 1-year PFS was 15% (Fig. 1). In univariate analysis, AJCC stage, RT modality, and whether the patient had undergone surgery were significant prognostic factors. Stage IVA patients had a better PFS (median 48.0 months, 1-year 60%) than stage IVB (median 5.1 months, 1-year 10%) or stage IVC patients (median 2.3 months, 1-year 6%) (p = 0.010). Patients who were treated with IMRT had a better PFS (median 5.1 vs. 2.6 months, 1-year 18% vs. 8%) (p = 0.049). Patients who underwent surgery had a better PFS (median 5.9 months, 1-year 19%) than those without surgery (median 2.5 months, 1-year 10%) (p = 0.007) (Fig. 2B). In multivariate analysis, stage IVA (hazard ratio [HR] = 0.14, p = 0.005 to others), surgery (HR = 0.47, p = 0.026), and IMRT (HR = 0.33, p = 0.005) were still found to be significant factors. The survival analyses of potential variables are summarized in Table 2.
Table 2.
Univariate and multivariate analysis of survival rates
| Variable | OS |
PFS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≥65 yr) | 1.44 | 0.69–3.01 | 0.328 | 1.38 | 0.68–2.80 | 0.370 | ||||||
| Sex (female) | 0.96 | 0.49–1.89 | 0.896 | 0.78 | 0.40–1.52 | 0.466 | ||||||
| ECOG PS (≥2) | 1.42 | 0.70–2.87 | 0.327 | 1.67 | 0.85–3.30 | 0.132 | ||||||
| Stage (IVA vs. others) | 0.10 | 0.01–0.76 | 0.026* | 0.04 | 0.00–0.36 | 0.004* | 0.26 | 0.08–0.88 | 0.021* | 0.14 | 0.04-0.55 | 0.005* |
| Surgery | 0.32 | 0.16–0.64 | 0.001* | 0.27 | 0.13–0.58 | 0.001* | 0.42 | 0.22–0.81 | 0.007* | 0.47 | 0.24-0.91 | 0.026* |
| IMRT | 0.61 | 0.29–1.25 | 0.173 | 0.30 | 0.13–0.69 | 0.005* | 0.51 | 0.26–1.01 | 0.049* | 0.33 | 0.15-0.72 | 0.005* |
| Chemotherapy | 0.62 | 0.28–1.37 | 0.235 | 0.91 | 0.41–2.02 | 0.809 | ||||||
| EQD210 dose (≥62 Gy) | 0.87 | 0.42–1.83 | 0.719 | 1.08 | 0.52–2.24 | 0.836 | ||||||
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; IMRT, intensity-modulated radiotherapy.
p < 0.05.
2. Patterns of failure
During the median follow-up duration of 7.4 months, recurrences developed in 32 patients (78%). In 32 patients with failures, DM was the most dominant failure pattern (n = 25/32, 78%) followed by LRF (n = 20/32, 63%). DM was observed most commonly in the lungs (n = 22), followed by the non-regional lymph nodes (n = 4), bone (n = 3), liver (n = 1), brain (n = 1), and heart (n = 1). Local failure occurred in 13 patients (41%), and regional failure occurred in 7 patients (22%). The LRF rate was reduced significantly after surgery with RT than after RT only (1 year 80% vs. 44%, p = 0.019). However, the DM rate did not differ significantly with treatment modality (with surgery vs. without surgery: 28% vs. 24%). There was no difference between IMRT and 3D-CRT in the LRF rate (p = 0.280) or DM rate (p = 0.960).
3. Subgroup analyses according to treatment modality
Subgroup analyses were performed for patients with surgery (‘Adjuvant RT’, n = 21) or not (‘Primary RT’, n = 20). The patient and treatment characteristics between the two groups are compared in Table 3. There were significantly more stage IVC patients in the primary RT group (65.0% vs. 14.3%, p = 0.002).
Table 3.
Patients’ characteristics according to whether they underwent surgery
| Variable | Primary RT (n = 20) | Adjuvant RT (n = 21) | p-value |
|---|---|---|---|
| Age (yr) | |||
| <65 | 6 (30.0) | 7 (33.3) | 0.819 |
| ≥65 | 14 (70.0) | 14 (66.7) | |
| Sex | |||
| Male | 10 (50.0) | 7 (33.3) | 0.279 |
| Female | 10 (50.0) | 14 (66.7) | |
| ECOG PS | |||
| 0–1 | 13 (65.0) | 15 (71.4) | 0.658 |
| 2–4 | 7 (35.0) | 6 (28.6) | |
| Stage | |||
| IVA | 1 (5.0) | 4 (19.0) | 0.002 |
| IVB | 6 (30.0) | 14 (66.7) | |
| IVC | 13 (65.0) | 3 (14.3) | |
| Chemotherapy | |||
| Yes | 15 (25.0) | 16 (76.2) | 0.999 |
| No | 5 (75.0) | 5 (23.8) | |
| RT modality | |||
| IMRT | 11 (55.0) | 17 (81.0) | 0.074 |
| 3D-CRT | 9 (45.0) | 4 (19.0) | |
| EQD210 dose (Gy) | |||
| <62.0 | 6 (30.0) | 7 (33.3) | 0.999 |
| ≥62.0 | 14 (70.0) | 14 (66.7) |
Values are presented as number (%).
RT, radiation therapy; ECOG PS, Eastern Cooperative Oncology Group performance score; IMRT, intensity-modulated radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy.
In the adjuvant RT group, there was no significant prognostic factor for OS and PFS, although there was a tendency of better survival when treated with IMRT (IMRT vs. 3D-CRT: 1-year OS 47% vs. 25%, p = 0.949; 1-year PFS 24% vs. 0%, p = 0.323).
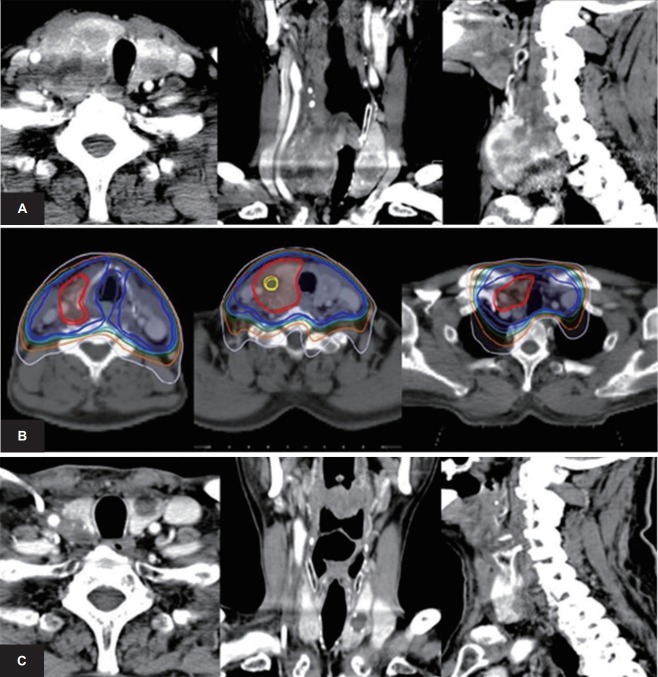
In contrast, there were different results in the primary RT group (Table 4). In the univariate analyses of PFS, high-dose RT with EQD210 dose ≥62 Gy showed a significant better survival than EQD210 dose <62 Gy (HR = 0.32, p = 0.031). Performing chemotherapy also showed a better survival than no chemotherapy (HR = 0.14, p = 0.002). There were similar results in the analyses of OS. RT with EQD210 dose ≥62 Gy also showed a significantly better OS than EQD210 <62 Gy (HR = 0.16, p = 0.003). Furthermore, performing chemotherapy showed a significant better 1-year OS than not performing chemotherapy (HR = 0.07, p = 0.001). According to RECIST criteria, complete response, partial response, and stable disease were noted in 41%, 31%, and 28%, respectively. In Fig. 3, we present a case of 67-year-old male patient in primary RT group with EQD210 dose ≥62 Gy, chemotherapy, and IMRT. This patient lived for 17.8 months.
Table 4.
Univariate analysis of survival in the primary RT group
| Variable | OS |
PFS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≥65 yr) | 1.00 | 0.37–2.76 | 0.993 | 1.02 | 0.38–2.75 | 0.965 |
| Sex (female) | 0.77 | 0.31–1.91 | 0.567 | 0.78 | 0.31–1.96 | 0.603 |
| ECOG PS (≥2) | 1.13 | 0.43–3.00 | 0.807 | 1.07 | 0.40–2.86 | 0.899 |
| Stage (IVA vs. others) | 0.03 | 0.00–11.7 | 0.253 | 0.03 | 0.00–11.8 | 0.253 |
| IMRT | 0.58 | 0.22–1.49 | 0.254 | 0.68 | 0.27–1.72 | 0.413 |
| Chemotherapy | 0.07 | 0.01–0.35 | 0.001* | 0.14 | 0.04–0.49 | 0.002* |
| EQD210 dose (≥62 Gy) | 0.16 | 0.05–0.53 | 0.003* | 0.32 | 0.11–0.90 | 0.031* |
RT, radiation therapy; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; IMRT, intensity-modulated radiotherapy; OS, overall survival; PFS, progression-free survival.
p < 0.05.
Fig. 3.
Results after intensity-modulated radiation therapy: a patient in the primary RT group. A male patient received a diagnosis of anaplastic thyroid cancer at the age of 67 years. Fine-needle aspiration biopsy revealed an anaplastic carcinoma of right thyroid gland. (A) There was a 5.5cm sized lobulated bulky mass at right thyroid gland with direct invasion of trachea at the time of diagnosis. (B) Concurrent chemoradiotherapy with paclitaxel was administered. The isodose lines represent 106% of prescribed dose (EQD210 dose of 70 Gy, yellow), 100% (66 Gy, red), 100% of the prescribed dose at lymph node area (48 Gy, blue). (C) Six months after radiation therapy, the primary right thyroid mass showed significant response. This patient managed to survive for 17.8 months. RT, radiation therapy.
The patient and treatment characteristics of the 3D-CRT (n = 13) and IMRT (n=28) group are compared in Table 5. More patients received EQD210 dose ≥62 Gy in the IMRT group than the 3D-CRT group (IMRT 82.1% vs. 3D-CRT 38.5%, p = 0.005). The median EQD210 dose for the 3D-CRT group was 60.0Gy, whereas the EQD210 dose for the IMRT group was 66.0 Gy.
Table 5.
Patients’ characteristics according to radiation modality
| Variable | 3D-CRT (n = 13) | IMRT (n = 28) | p-value |
|---|---|---|---|
| Age (yr) | |||
| <65 | 3 (23.1) | 10 (35.7) | 0.418 |
| ≥65 | 10 (76.9) | 18 (64.3) | |
| Sex | |||
| Male | 6 (46.2) | 11 (39.3) | 0.678 |
| Female | 7 (53.8) | 17 (60.7) | |
| ECOG PS | |||
| 0–1 | 8 (61.5) | 20 (71.4) | 0.527 |
| 2–4 | 5 (38.5) | 8 (28.6) | |
| Stage | |||
| IVA | 2 (15.4) | 3 (10.7) | 0.663 |
| IVB | 5 (38.5) | 15 (53.6) | |
| IVC | 6 (46.1) | 10 (35.7) | |
| Chemotherapy | |||
| Yes | 8 (61.5) | 23 (82.1) | 0.153 |
| No | 5 (38.5) | 5 (17.9) | |
| RT aim | |||
| Primary RT | 9 (69.2) | 11 (39.3) | 0.074 |
| Adjuvant RT | 4 (30.8) | 17 (60.7) | |
| EQD210 dose (Gy) | |||
| <62.0 | 8 (61.5) | 5 (17.9) | 0.005 |
| ≥62.0 | 5 (38.5) | 23 (82.1) |
Values are presented as number (%).
3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; ECOG PS, Eastern Cooperative Oncology Group performance score; RT, radiation therapy.
4. Toxicity
Acute toxicities included anorexia, nausea, vomiting, fatigue, esophagitis, skin reaction, and xerostomia. Thirty-two patients (78%) experienced acute toxicities, and esophagitis/pharyngitis was the most common side effect (27/41, 66%). All esophagitis/pharyngitis were grade I or II toxicities only (n = 7 and n = 20, respectively), and none required tube feeding support. Skin reaction developed in 12 patients (29%). Among them, grade I occurred in 2 patients, grade II in 8 patients, and grade III in 2 patients. One patient with grade III dermatitis was admitted and received daily dressing. After 3 weeks of rest, this patient was able to restart RT. In the other patient with grade III dermatitis, 2 weeks of rest from RT was required, and RT could be successfully continued and finished with the skin-sparing adaptive plan after 25 fractions. Except for these cases of grade III dermatitis, no other acute toxicity ≥grade III occurred in all patients. There were no late adverse effects of therapy until the last follow-up date. Furthermore, there was no difference in toxicity according to RT modality.
Discussion and Conclusion
This study reviewed the institutional treatment outcome of ATC after RT. A better survival was noted in the patients who received aggressive multimodal therapy including surgical resection and chemotherapy. The IMRT group showed a better prognosis than the 3D-CRT group by safely delivering a higher radiation dose.
ATC is a malignant thyroid tumor that has a dismal clinical course and very low survival rate compared to other thyroid malignancies. To date, there have been no published phase III trials of ATC yet. The rarity of this disease entity combined with its aggressive nature makes randomized clinical trials difficult. Most of the available evidence is from retrospective studies, which suggest that the combination of surgery, RT, and chemotherapy may yield favorable results. According to the recent National Comprehensive Cancer Network (NCCN) Guidelines, the recommended treatment of ATC is surgical resection and adjuvant therapy including RT and chemotherapy [16].
Single-modality therapy has been shown to have limited effect on locoregional disease recurrence and survival [17]. Therefore, multimodal therapy has progressively become the treatment of choice for ATC, with surgery being the cornerstone of patient management. Moreover, due to the threat of early metastatic spread, therapeutic options in ATC must combine local and systemic treatments. In a retrospective study in Germany [18], 9 patients with ATC underwent multimodal treatment consisting of surgery followed by postoperative RT with or without chemotherapy. An improved survival at 24 months was found in univariate analysis for CCRT (p = 0.018) than RT alone and symptoms were controlled at the end of RT (p = 0.030). Similarly, in our study, adjuvant RT after surgery was administered to 21 patients, and better prognosis was noted among them. Moreover, 3 patients who underwent aggressive multimodal therapy despite of the stage IVC disease showed favorable OS of 56, 21, and 6 months, respectively.
The total radiation dose is known to be related to the LRF of ATC, which leads to dyspnea and tracheostomy. In a retrospective study on 75 patients with ATC, Swaak-Kragten et al. [19] reported that the median OS of patients who had received a total dose of >40 Gy versus ≤40 Gy, was 5.4 and 1.7 months, respectively (p < 0.001). Another study indicated that the median survival of 24 patients who received ≥40 Gy was longer than that of 34 patients who received <40 Gy (9 vs. 3 months) [20]. In a meta-analysis of 3,552 patients in the National Cancer Database by Glaser et al. [21], a radiation dose greater than 59.4 Gy showed significantly better survival. Although it is difficult to directly compare outcomes among studies due to the differences in surgery extent and RT aim, a higher radiation dose seems to contribute to a better survival outcome. Similarly, although our results showed no difference in outcome in the entire cohort, significantly higher survival rates were seen with EQD210 ≥62 Gy in the primary RT group.
IMRT provides a relatively more conformal high dose to the gross tumor and high-risk areas with better homogeneity, compared to the 3D-CRT technique. At the same time, normal organs at risk receive less radiation using the IMRT technique, which achieves superior therapeutic ratio. Lim et al. [8] retrospectively reviewed the treatment outcome of 13 consecutive patients who treated in our institution between 2006 and 2010. Among them, 5, 2, and 6 patients had stage IVA, IVB, and IVC disease, respectively. They received 3D-CRT and chemotherapy plus surgery, and an unfavorable outcome was reported (median PFS and OS: 2.8 and 3.8 months, respectively). Foote et al. [22] reported 10 patients with regionally confined disease who were treated with aggressive therapy combining surgery, IMRT, and chemotherapy. Five patients (50%) were alive and disease-free, and OS at 1 and 2 years was 70% and 60%, respectively. He et al. [15] examined the treatment results of 13 patients treated with IMRT. At a median follow-up duration of 12 months, the median survival was 9 months. The locoregional disease was in complete remission in 2 patients, partial remission in 1 patient, stabilized in 7 patients and progressive disease in 3 patients. Distant metastases were found in 7 patients.
When we compare our results with those of Lim et al. [8], 3D-CRT with or without aggressive multimodal therapy showed similar PFS and OS (3.1 and 3.9 months, respectively) with a different patient cohort, whereas IMRT showed better PFS and OS (5.9 and 8.0 months, respectively). These outcomes are favorable results similar to other previous IMRT-related reports. Furthermore, more patients received an EQD210 dose ≥62 Gy in the IMRT group than in the 3D-CRT group (IMRT 82.1% vs. 3D-CRT 38.5%, p = 0.005), while significant higher survival rates were seen with EQD210 >62 Gy in the primary RT group (HR 0.16, p = 0.003 for OS; HR = 0.32, p = 0.031 for PFS) in univariate analysis. This improved prognosis is probably from the conformity and dose difference between IMRT and 3D-CRT (IMRT 66.0 Gy vs. 3D-CRT 60.0 Gy, p = 0.005), even though it was not possible to calculate the exact conformity index between the two groups in this study.
Chemotherapy is one of the effective treatment strategies proposed for ATC. Haigh et al. [23] confirmed that adjuvant CCRT after surgery can improve the survival rate. Busnardo et al. [24], also suggested that neoadjuvant CCRT improves the tumor resection rate and prognosis. However, McIver et al. [25] found that comprehensive treatment with surgery and CCRT did not significantly prolong survival time. Our study also could not reveal that chemotherapy prolonged PFS and OS in the entire cohort. The positive effect of chemotherapy was suggested only in the subgroup without surgery. Randomized, prospective trials with larger sample sizes are required to confirm whether modern chemotherapy has a survival benefit for ATC.
There are several limitations of this study. First, the sample size was small, because of the rarity of ATC and low referral rates for RT before the role of aggressive multimodal therapy including RT was established. However, compared to other retrospective studies on ATC, our sample size was relatively large. The second limitation is the relatively short follow-up duration of a median 7.4 months for the entire cohort, and 46.0 months for living patients. However, compared to other previous studies, this follow-up duration is not short for discussing the outcome of ATC, because ATC is a disease with a very aggressive course. Third, since our cohort included only patients who received RT, there was a possibility of a selection bias. Patients who only underwent surgery without RT were not included; therefore, patients who did not need to receive RT (e.g., those with small tumors limited to the thyroid) were excluded from this study. Furthermore, we included patients with advanced-stage tumors, who might not have been able to afford surgery. This might result in a shorter survival rate compared to previous studies dealing only with postoperative RT cases. Further studies based on more diverse cases and sufficient follow-up periods would help confirm the results of our study. The last limitation is that there might be a selection bias. Surgery was a significant factor of OS and PFS in univariate and multivariate analysis, but it is not clear if surgery directly improves survival. Since resectability depends on extent of involved structures, potential morbidity, and mortality associated with resection, whether a patient can undergo surgery or not might have an influence on survival.
In conclusion, our study reviewed the institutional outcomes of ATC treated with RT, using either IMRT or 3D-CRT. We observed increased OS and PFS with surgery or IMRT. Aggressive multimodal therapy including surgery, modern chemotherapy, and IMRT might further improve the prognosis in ATCs, which is known to have a dismal prognosis.
Acknowledgments
This study was supported by the National Research Foundation of Korea grant funded by the Korean government (No. NRF2017M2A2A4A03083634).
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84:4043–9. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 2.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–64. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Zivaljevic V, Tausanovic K, Paunovic I, et al. Age as a prognostic factor in anaplastic thyroid cancer. Int J Endocrinol. 2014;2014:240513. doi: 10.1155/2014/240513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon J, Kim BH, Jung HW, Besic N, Sugitani I, Wu HG. The prognostic impacts of postoperative radiotherapy in the patients with resected anaplastic thyroid carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2016;59:34–45. doi: 10.1016/j.ejca.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22:486–97. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sykorova V, Dvorakova S, Vcelak J, et al. Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Res. 2015;35:2029–36. [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer staging manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 8.Lim SM, Shin SJ, Chung WY, et al. Treatment outcome of patients with anaplastic thyroid cancer: a single center experience. Yonsei Med J. 2012;53:352–7. doi: 10.3349/ymj.2012.53.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wein RO, Weber RS. Anaplastic thyroid carcinoma: palliation or treatment? Curr Opin Otolaryngol Head Neck Surg. 2011;19:113–8. doi: 10.1097/MOO.0b013e328343af3d. [DOI] [PubMed] [Google Scholar]
- 10.Akaishi J, Sugino K, Kitagawa W, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid. 2011;21:1183–9. doi: 10.1089/thy.2010.0332. [DOI] [PubMed] [Google Scholar]
- 11.Derbel O, Limem S, Segura-Ferlay C, et al. Results of combined treatment of anaplastic thyroid carcinoma (ATC) BMC Cancer. 2011;11:469. doi: 10.1186/1471-2407-11-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito K, Hanamura T, Murayama K, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. 2012;34:230–7. doi: 10.1002/hed.21721. [DOI] [PubMed] [Google Scholar]
- 13.Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY, Lang BH. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann Surg Oncol. 2008;15:2500–5. doi: 10.1245/s10434-008-0005-0. [DOI] [PubMed] [Google Scholar]
- 14.McLarnon A. Thyroid cancer: pazopanib alone is not effective against anaplastic thyroid cancer. Nat Rev Endocrinol. 2012;8:565. doi: 10.1038/nrendo.2012.136. [DOI] [PubMed] [Google Scholar]
- 15.He X, Li D, Hu C, Wang Z, Ying H, Wu Y. Outcome after intensity modulated radiotherapy for anaplastic thyroid carcinoma. BMC Cancer. 2014;14:235. doi: 10.1186/1471-2407-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network . Fort Washington, PA: National Comprehensive Cancer Network; c2018. NCCN Guidelines - thyroid carcinoma [Internet] [cited 2018 Jun 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ thyroid.pdf. [Google Scholar]
- 17.Veness MJ, Porter GS, Morgan GJ. Anaplastic thyroid carcinoma: dismal outcome despite current treatment approach. ANZ J Surg. 2004;74:559–62. doi: 10.1111/j.1445-2197.2004.03062.x. [DOI] [PubMed] [Google Scholar]
- 18.Kasmann L, Bolm L, Janssen S, Rades D. Prognostic factors for survival in patients treated with multimodal therapy for anaplastic thyroid cancer. Anticancer Res. 2016;36:4697–700. doi: 10.21873/anticanres.11023. [DOI] [PubMed] [Google Scholar]
- 19.Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. Multimodality treatment for anaplastic thyroid carcinoma: treatment outcome in 75 patients. Radiother Oncol. 2009;92:100–4. doi: 10.1016/j.radonc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Goutsouliak V, Hay JH. Anaplastic thyroid cancer in British Columbia 1985-1999: a population-based study. Clin Oncol (R Coll Radiol) 2005;17:75–8. doi: 10.1016/j.clon.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, Beriwal S. Anaplastic thyroid cancer: prognostic factors, patterns of care, and overall survival. Head Neck. 2016;38 Suppl 1:E2083–90. doi: 10.1002/hed.24384. [DOI] [PubMed] [Google Scholar]
- 22.Foote RL, Molina JR, Kasperbauer JL, et al. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid. 2011;21:25–30. doi: 10.1089/thy.2010.0220. [DOI] [PubMed] [Google Scholar]
- 23.Haigh PI, Ituarte PH, Wu HS, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91:2335–42. [PubMed] [Google Scholar]
- 24.Busnardo B, Daniele O, Pelizzo MR, et al. A multimodality therapeutic approach in anaplastic thyroid carcinoma: study on 39 patients. J Endocrinol Invest. 2000;23:755–61. doi: 10.1007/BF03345066. [DOI] [PubMed] [Google Scholar]
- 25.McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 200;130:1028–34. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]