Abstract
BACKGROUND
Status epilepticus (SE) is a common, life-threatening condition. Multiple factors are used to predict its outcome and evaluate its risks, and there have been only a few studies in Saudi Arabia.
OBJECTIVES
Investigate predictors of SE outcome.
DESIGN
Retrospective chart review study.
SETTING
Tertiary center, Riyadh.
PATIENTS AND METHODS
We reviewed all pediatric cases (age 14 years or younger) of SE admitted between January 2005 and December 2015, collecting data on age, sex, date of birth, developmental status, pre-existing neurological diseases, SE etiology, Glasgow Outcome Scale (GOS) scores, and electroencephalogram (EEG) findings. The outcome was categorized as poor based on any decrease in baseline GOS score or moderate-to-severe developmental delay in young children; otherwise outcome was considered good.
MAIN OUTCOME MEASURE
Outcome of SE.
RESULTS
One hundred and sixteen patients (54% boys) with ages from 1 month to 10 years were included in the analysis. Fifty-five (47.4%) had a poor outcome. The overall mortality rate related to SE was 2.6%. Four patients had an SE duration of more than 24 hours. Univariate and multivariate analysis revealed that poor outcome was related to symptomatic SE etiology and a history of epilepsy. Age, sex, SE duration, and EEG findings were not predictors of poor outcome.
CONCLUSION
Pediatric status epilepticus is highly associated with neurological morbidity. The main predictor of outcome is underlying symptomatic etiology of SE and to a lesser degree the presence of a history of epilepsy. Duration does not seem to play a major role.
LIMITATIONS
The main limitation is the retrospective chart review nature of the study with possible bias.
Status epilepticus (SE) is a common neurological emergency in children, which has potential morbidity and mortality.1 The International League Against Epilepsy defines SE as “a single epileptic seizure of longer than 30-minute duration or a series of epileptic seizures during which function is not regained between ictal events in a longer than 30-minute period”. 2 The incidence of SE is thought to be underestimated due to the inability to document all cases.3 In the United States, the reported yearly incidence of status epilepticus ranges from 7 to 41 cases per 100 000.4 The incidence has increased fourfold during the last thirty years.5 The epidemiology of the different types and etiologies has been studied throughout the world.1,6,7
Clinically, status epilepticus is classified into two types: convulsive status epilepticus (CSE) and non-convulsive status epilepticus (NCSE). CSE is the most common type encountered in pediatric emergencies,1 but NCSE has been detected more frequently in the last few years due to the increased application of EEG monitoring. 7 NCSE may be an indicator of worse prognosis.8 Few studies have been reported from Saudi Arabia. In one study in Saudi Arabia, Mah et al found that 28% of admitted pediatric seizure episodes were due to SE.9 Furthermore, there are many classifications of SE with regard to etiology. The commonly used classification of etiology is that of Maytal et al: idiopathic, acute symptomatic, remote symptomatic, febrile, and progressive encephalopathy.10,11 Some etiologies are more commonly encountered in children. Previous studies have shown that among young patients, especially below the age of 3 years, the most common etiology is febrile illness. 7,12 Structural and metabolic causes are the next most common etiologies in children.9 Genetic etiologies have presented in only 3–6% of patients.7,13
In several studies, different etiologies have been associated with an increased risk of mortality,14,15 but no association between etiology and mortality rate was shown by Halawa et al.16 Chevrie and DeLorenzo found that the younger the patient, the higher the risk of mortality,3,17,18 while Halawa found no association between age and mortality.18 Finally, the incidence varies by sex (male-to-female, 2:1).12,16 However, none of the these studies found any association between sex and increased risk of mortality. Morbidity, on the other hand, has been predominantly described by the rate of postictal complications, but recent studies have relied more on functional status and quality of life as descriptors of outcome, which are more representative of the postictal disability and, therefore, more accurately represent outcome.19,20
The mortality rate has been reported to be around 20%.3,14,17 However, a systematic review indicated that studies with high quality showed a lower incidence of mortality, between 2.7% to 5.2% and morbidity of less than 15%.21 The aim of this study was to investigate the outcome of pediatric patients with status epilepticus admitted from 2005 until 2015 to King Abdulaziz Medical City in Riyadh, Saudi Arabia.
PATIENTS AND METHODS
We reviewed the records of all SE cases admitted between January 2005 and December 2015 at the Pediatric Department of King Abdulaziz Medical City (KAMC) in Riyadh, Saudi Arabia. The cases were identified by diagnostic codes. All pediatric patients with presentation of seizure that fulfilled the definition of SE as a single epileptic seizure lasting longer than 30 minutes or a series of epileptic seizures during which function was not regained between ictal events in a period longer than 30 minutes were enrolled in the study. Cases with electrographic features of SE but with no loss of consciousness, a syndrome of continuous spike and wave discharges in slow wave sleep, and epilepsia partialis continua with no loss of consciousness were excluded. Cases with refractory and super-refractory SE were enrolled in the study. Refractory SE represents seizure episodes lasting for 1–24 hours occurring in 23–43% of the patients with SE.22 Super-refractory SE is defined as SE that continues for 24 hours or more. The incidence of super-refractory SE is 12–22% of all episodes of SE and there is a higher morbidity and mortality. 22,23
There was no written protocol for the treatment of pediatric SE, but there was a consensus among treating physicians on using IV benzodiazepines for 1–2 doses as a first-line treatment followed by phenytoin and phenobarbital as the second line. As third-line treatment, there were differences in preference for using midazolam IV drip, IV thiopental drip or other antiepileptic drugs.
We performed a systematic chart review of all the cases that were identified through medical records via the coding system for the following data: demographics including sex and date of birth, developmental status, pre-existing neurological diseases, etiology type, duration of SE, Glascow Outcome Scale (GOS) scores, neurological assessment, lab, neuroimaging, and electroencephalogram findings; and outcome at follow-up.
In children 6 years or older, the outcome was categorized as good or bad depending on GOS score with “good” being a score of 5, and “poor” being a score of 4 or less (Table 1). While in younger children, assessment of development was used as indicator of neurological outcome. Development was assessed using the Denver Developmental Screening Test and clinical evaluation, and was classified as either normal or delayed. The Denver test was mainly used because it is available at bedside in the clinics, and it was done on all patients while more advanced psychometric tests were used only on a few patients. Developmental delay, if identified, was categorized according to severity. Developmental delay was considered mild if the child possessed ≥66% developmental functioning for their chronological age, whereas those with development ≤33% for chronological age were considered to have severe delay. Cases with development of 33%-66% were considered to have moderate delay. Outcomes were considered to be unfavorable or bad for patients who were found to have moderate or severe delay in development. Conversely, those with no developmental delay and those classified as mild developmental delay were considered to have a favorable or good outcome.
Table 1.
Glasgow Outcome Scale.
| Grade | Glasgow Outcome Scale |
|---|---|
|
| |
| 1 | Dead |
| 2 | Vegetative |
| 3 | Severely disabled |
| 4 | Moderately disabled |
| 5 | Good |
Data is presented as descriptive statistics, including absolute and relative frequencies, means and standard deviations. Univariate analysis and multivariate analysis were applied. Ninety-five percent confidence intervals were calculated, and all statistical tests were considered significant at P<.05. All analyses were conducted using SPSS version 22 (IBM Corp., Armonk, NY).
RESULTS
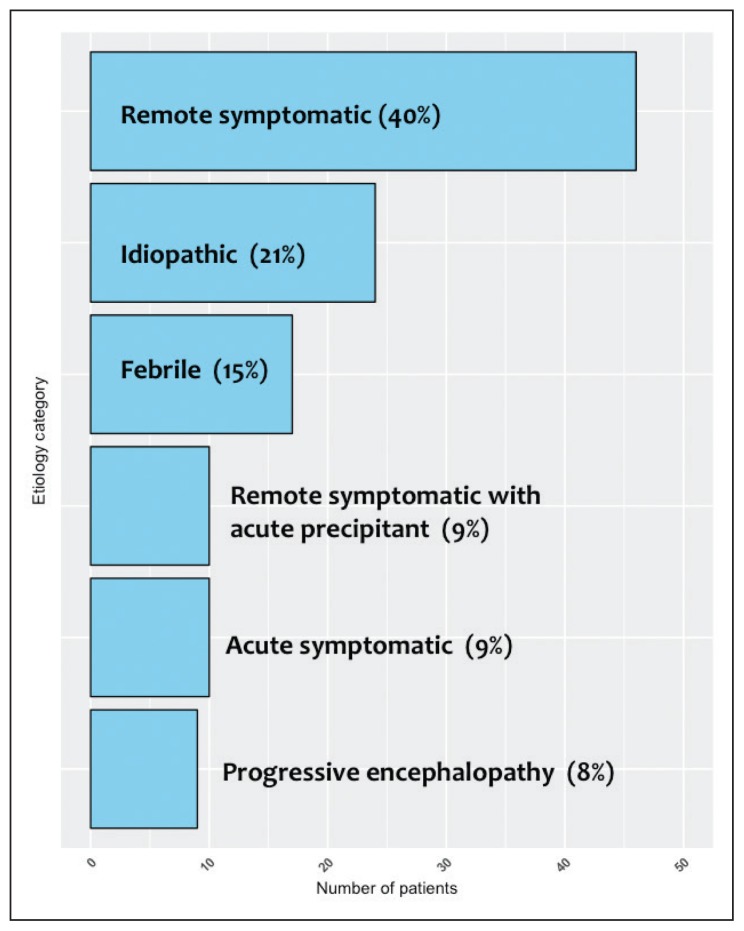
Of 135 patients admitted with SE, 19 cases were excluded for various causes, leaving 116 cases with SE available for analysis. Boys accounted for 63 (54%) of 116 cases (Table 2). Age at presentation ranged from 1 month to 10 years (mean: 5.3 [3.9] years) with median age of 4.5 years. SE with a duration of 30 to 60 minutes was seen in 87 (75%) cases while refractory SE with a duration of 1 to 24 hours was seen in 25 (21.6%) cases. Super-refractory SE with a duration more than 24 hours was seen in four (3.4%) cases. Etiologic categories are shown in Figure 1. The identifiable causes were as follows: 13 (11.2%) cases of hypoxic ischemic encephalopathy, 12 (10.3%) monogenetic/syndromic, 9 (7.7%) brain malformation, 8 (6.9%) acute CNS inflammation, 8 (6.9%) hydrocephalus-related complications, 4 (3.4%) traumatic brain injury, and 2 (1.7%) periventricular leukomalacia. Unidentifiable, presumably a genetic etiology, was encountered in 19 cases (16.3%). Fifty-five patients had poor outcome and 61 patients had favorable outcomes (Table 2). Three patients (2.6%) died as a direct result of SE. There was a statistically significant difference in duration of SE, etiology, epilepsy prior to SE, and significant neuroimaging abnormalities. However, the stepwise logistic regression confirmed two independent predictors: symptomatic etiology (OR=84.6; 95% CI=10.81–662.19) and history of epilepsy (OR=3; 95% CI=1.02–8.86) (Table 3).
Table 2.
Demographic and clinical variables, including predictors of outcome of status epilepticus.
| Factor | Good outcome (n=61) | Poor outcome (n=55) | P value |
|---|---|---|---|
|
| |||
| Male (n=63) | 36 (59%) | 27 (49%) | .28 |
| Female (n=53) | 25 (47%) | 28 (53%) | NS |
| Age,* years | 5.1 (3.9) | 5.5 (4.0) | .56 |
| Duration | |||
| SE | 51 (84%) | 36 (65%) | <.04 |
| Refractory SE | 10 (16.4%) | 15 (27%) | <.25 |
| Super-refractory SE | 0 | 4 (7) | <.04 |
| Etiology | |||
| Idiopathic | 23 (37.7%) | 1 (2%) | <.0001 |
| Febrile | 17 (27.8%) | 0 | <.0001 |
| Acute symptomatic | 4 (6.5%) | 6 (11%) | .73 |
| Remote symptomatic | 17 (27.8%) | 39 (71%) | <.0001 |
| Epilepsy before SE | 29 (48%) | 45 (82%) | .001 |
Age upon episode. Unless indicated otherwise, data are mean (standard deviation) or number (%).
Statistically significant P values are bolded.
Figure 1.
Etiologic categories for the 116 patients with status epilepticus.
Table 3.
Stepwise logistic regression.
| Question | P value | OR | 95% C.I. for OR | |
|---|---|---|---|---|
|
| ||||
| Symptomatic etiology? | No (42) | .001 | Reference | 13.3–796.9 |
| Yes (74) | 102.9 | |||
| Prior history of epilepsy? | No (41) | .001 | Reference | 2.1–11.6 |
| Yes (75) | 5.0 | |||
Symptomatic etiology −2 log likelihood: 98.346 Nagelkerke R Square: .554; Prior history of epilepsy −2 log likelihood: 145.205 Nagelkerke R Square: .165
DISCUSSION
This study provided an up-to-date perspective into SE with a sample of 116 patients. The distribution of sexes was relatively equal. Most incidents lasted for 30–60 min, while a duration of more than 24 hours was the least common. In terms of etiology of SE, most cases were remote symptomatic, while the least common etiology was that of progressive encephalopathy. Of all cases studied, the perceived cause with the highest frequency was unidentifiable, presumably a genetic factor, with the next highest cause being hypoxic ischemic encephalopathy, and the least two frequently encountered causes being traumatic brain injury and periventricular leukomalacia. There was no significant effect of treatment modality on the short-term outcome of status epilepticus in our cohort (as per personal communication with Majed Al-Jeresy an author of a different un-published study on the same cohort on the short-term outcome of pediatric SE).
The last study done in Saudi Arabia to investigate pediatric SE was in 1999 by Mah. Mah’s study showed similar demographic distribution per sex and history of epilepsy compared to our study. However, the distribution of etiology differed. We found that a remote symptomatic etiology accounted for 40% of cases, as opposed to only 5% in Mah’s study.9 Previous studies have found the acute symptomatic and febrile etiologies to be the most prevalent causes of SE, while in our study, they only accounted for 8.6% and 14.7%, respectively. 12,22 The low percentage of febrile SE in our study could be the result of being a referral center where the number of febrile SE is usually low and as such may have influenced the final result. The idiopathic etiology was identified as the main etiology of SE in a similar retrospective study;24 it accounted for 21% in our study.
Outcomes relating to SE are poor in the published literature based on overall mortality and postictal neurological sequelae. Both Raspall-Chaure and Claassen found symptomatic etiology to be the most significant predictor of mortality in SE.10,14 Furthermore, both studies established the acute symptomatic etiology as being associated with the highest mortality. Our study provides additional descriptors, with a “poor” outcome being defined by GOS score and developmental outcomes. This afforded us the advantage of identifying outcomes beyond the scope of post-SE mortality only. We found that symptomatic etiology was significantly associated with poor outcomes: low GOS score and moderate-to-severe developmental delay. These findings indicate that SE of symptomatic etiology both carries an increased risk of mortality and is associated with a lower GOS score and moderate-to-severe developmental delay. It remains essential, however, to observe outcomes in patients with SE as a combination of mortality, structural neurological consequences, and developmental deficits.
It is worth noting that 75% of patients had a SE duration between 30 and 60 minutes, which is likely due to improved management, but regardless of duration, we did not find an association between duration and poor outcome. One may intuitively anticipate that duration would have a deteriorating effect, but its role on SE outcome is controversial, as some studies have found it to be an independent risk factor,25–27 while others have not.24,28,29 We cannot provide a conclusive explanation on this lack of association, but it is likely due to the fact that those with super refractory SE already had significant neurological disability, which meant that the SE episode had no effect on their baseline.
Moghaddasi found that patients with a history of epilepsy had higher mortality rates than those without.30 Furthermore, Kang found that a history of epilepsy was associated with neurological sequelae to a further extent than a history of acute symptomatic SE. Our study identified a history of epilepsy to be an independent predictor of outcome in SE. Moreover, because our study describes outcomes in relation to GOS and developmental stage, it is possible to hypothesize that underlying structural neurological insults occur with higher frequency in patients with SE with history of epilepsy and could also account for the developmental aberrations postSE. Additionally, it remains to be answered whether underlying structural defects that may be seen in patients with epilepsy predispose to an increased risk of accumulating further structural insults and also predispose patients with epilepsy to delayed development postSE. It is also worth mentioning that in the these studies, a history of epilepsy was considered as a distinct entity from acute symptomatic etiology; thus, patients with acute symptomatic etiology who went on to develop epilepsy could not be identified. Through our analysis, however, we were able to isolate a history of epilepsy as an independent predictor of outcome in SE with regard to GOS and developmental outcomes.
One strength of this study is that it was conducted in a well-developed tertiary center. Another strength is that outcomes were measured objectively, independent of mortality, using the GOS and functional development as an assessment tool. This allowed us to take into account postictal functional status and residual impairment. However, this method of assessment is not sensitive enough to detect milder brain dysfunction as a result of SE. Further studies with more sensitive methods like the Bayley Scales of Infant Development and the Wechsler Intelligence Scale for Children (WISC-V) in a more homogenous group of patients with idiopathic etiology would be more sensitive to detect more subtle brain dysfunction. The main limitation is the retrospective nature of the study, which might introduce bias. Other limitation of the study was the size of the sample, which reflected the experience of one center with a relatively younger age than reported in other studies and as such would not be adequately representative of the intended population. Therefore, our findings cannot be generalized. Larger multicenter prospective studies are warranted. The final limitation involves the long-term follow-up. Studies with a more detailed and longer follow-up period are needed. Pediatric SE is highly associated with neurological morbidity. The main predictor of outcome is an underlying symptomatic etiology and to a lesser degree a history of epilepsy. Duration does not seem to affect outcome but larger, prospective studies are needed.
REFERENCES
- 1.Santos MI, Nzwalo H, Monteiro JP, Fonseca MJ. Convulsive status epilepticus in the pediatric emergency department: five year retrospective analysis. Acta Med Port. 2012;25(4):203–6. [PubMed] [Google Scholar]
- 2.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34(4):592–6. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 3.DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995 Jul;12(4):316–25. [PubMed] [Google Scholar]
- 4.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006 Jul 15;368(9531):222–9. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 5.Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care. 2014 Jun;20(3):476–83. doi: 10.1007/s12028-013-9935-x. [DOI] [PubMed] [Google Scholar]
- 6.Freilich ER, Schreiber JM, Zelleke T, Gaillard WD. Pediatric status epilepticus: identification and evaluation. Curr Opin Pediatr. 2014 Dec;26(6):655–61. doi: 10.1097/MOP.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 7.Asadi-Pooya AA, Poordast A. Etiologies and outcomes of status epilepticus in children. Epilepsy Behav. 2005 Nov;7(3):502–5. doi: 10.1016/j.yebeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Abend NS, Arndt DH, Carpenter JL, Chapman KE, Cornett KM, Gallentine WB, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013 Jul 23;81(4):383–91. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mah JK, Mah MW. Pediatric status epilepticus: a perspective from Saudi Arabia. Pediatr Neurol. 1999 May;20(5):364–9. doi: 10.1016/s0887-8994(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 10.Raspall-Chaure M, Chin RF, Neville BG, Scott RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol. 2006 Sep;5(9):769–79. doi: 10.1016/S1474-4422(06)70546-4. [DOI] [PubMed] [Google Scholar]
- 11.Maytal J, Shinnar S, Moshé SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989 Mar;83(3):323–31. [PubMed] [Google Scholar]
- 12.Saz EU, Karapinar B, Ozcetin M, Polat M, Tosun A, Serdaroglu G, et al. Convulsive status epilepticus in children: etiology, treatment protocol and outcome. Seizure. 2011 Mar;20(2):115–8. doi: 10.1016/j.seizure.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Tabarki B, Yacoub M, Selmi H, Oubich F, Barsaoui S, Essoussi AS. Infantile status epilepticus in Tunisia. Clinical, etiological and prognostic aspects. Seizure. 2001 Jul;10(5):365–9. doi: 10.1053/seiz.2000.0495. [DOI] [PubMed] [Google Scholar]
- 14.Claassen J, Lokin JK, Fitzsimmons BF, Mendelsohn FA, Mayer SA. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002 Jan 8;58(1):139–42. doi: 10.1212/wnl.58.1.139. [DOI] [PubMed] [Google Scholar]
- 15.DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996 Apr;46(4):1029–35. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 16.Halawa EF, Draz I, Ahmed D, Shaheen HA. Predictors of Outcome of Convulsive Status Epilepticus Among an Egyptian Pediatric Tertiary Hospital. J Child Neurol. 2015 Nov;30(13):1736–42. doi: 10.1177/0883073815579706. [DOI] [PubMed] [Google Scholar]
- 17.Garzon E, Fernandes RM, Sakamoto AC. Analysis of clinical characteristics and risk factors for mortality in human status epilepticus. Seizure. 2003 Sep;12(6):337–45. doi: 10.1016/s1059-1311(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 18.Chevrie JJ, Aicardi J. Convulsive disorders in the first year of life: persistence of epileptic seizures. Epilepsia. 1978 Feb;19(1):67–74. doi: 10.1111/j.1528-1157.1978.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 19.Kang BS, Kim DW, Kim KK, Moon HJ, Kim YS, Kim HK, et al. Prediction of mortality and functional outcome from status epilepticus and independent external validation of STESS and EMSE scores. Crit Care. 2016 Jan 27;20:25. doi: 10.1186/s13054-016-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitinger M, Kalss G, Rohracher A, Pilz G, Novak H, Höfler J, et al. Predicting outcome of status epilepticus. Epilepsy Behav. 2015 Aug;49:126–30. doi: 10.1016/j.yebeh.2015.04.066. [DOI] [PubMed] [Google Scholar]
- 21.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology. 2002 Feb 26;58(4):537–41. doi: 10.1212/wnl.58.4.537. [DOI] [PubMed] [Google Scholar]
- 22.Boggs JG. Mortality Associated with Status Epilepticus. Epilepsy Curr. 2004 Jan;4(1):25–27. doi: 10.1111/j.1535-7597.2004.04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubey D, Kalita J, Misra UK. Status epilepticus: Refractory and super-refractory. Neurol India. 2017;65(Supplement):S12–S17. doi: 10.4103/neuroindia.NI_958_16. [DOI] [PubMed] [Google Scholar]
- 24.Kang DC, Lee YM, Lee J, Kim HD, Coe C. Prognostic factors of status epilepticus in children. Yonsei Med J. 2005 Feb 28;46(1):27–33. doi: 10.3349/ymj.2005.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson KJ, Koivikko MJ. Status epilepticus in children: aetiology, treatment, and outcome. Dev Med Child Neurol. 1997 Oct;39(10):652–8. doi: 10.1111/j.1469-8749.1997.tb07358.x. [DOI] [PubMed] [Google Scholar]
- 26.Dunn DW. Status Epilepticus in Children: Etiology, Clinical Features, and Outcome. J Child Neurol. 1988 Jul;3(3):167–73. doi: 10.1177/088307388800300303. [DOI] [PubMed] [Google Scholar]
- 27.Maegaki Y, Kurozawa Y, Hanaki K, Ohno K. Risk factors for fatality and neurological sequelae after status epilepticus in children. Neuropediatrics. 2005 Jun;36(3):186–92. doi: 10.1055/s-2005-865611. [DOI] [PubMed] [Google Scholar]
- 28.Barnard C, Wirrell E. Does Status Epilepticus in Children Cause Developmental Deterioration and Exacerbation of Epilepsy? J Child Neurol. 1999 Dec 1;14(12):787–94. doi: 10.1177/088307389901401204. [DOI] [PubMed] [Google Scholar]
- 29.Maytal J, Shinnar S, Moshé SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989 Mar;83(3):323–31. [PubMed] [Google Scholar]
- 30.Moghaddasi M, Joodat R, Ataei E. Evaluation of Short-term Mortality of Status Epilepticus and Its Risk Factors. J Epilepsy Res. 2015 Jun 30;5(1):13–6. doi: 10.14581/jer.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]