Abstract
BACKGROUND AND OBJECTIVES
Gaucher disease (GD) is caused by the deficiency of glucosidase beta acid (GBA). Three clinical forms of GD are available. Some mutations in the GBA gene have a high frequency in specific populations. The aim of this study was to analyze the characteristics of phenotypes and genotypes of GD in Syrian pediatric patients and assess whether a genotype-phenotype relationship could be helpful in treatment decision-making.
DESIGN AND SETTINGS
A cross-sectional clinical genetic study of 19 Syrian children admitted to Children’s Hospital, Damascus University.
PATIENTS AND METHODS
Nineteen Syrian children with GD were enrolled in the study; DNA was extracted from peripheral blood leukocytes. The GBA gene was amplified by polymerase chain reaction, and the 9 most common mutations were studied using a Gaucher Disease Strip Assay (ViennaLab Diagnostics GmbH, Vienna, Austria).
RESULTS
The majority of children had an early age of onset. A total of 17 patients presented severe hematological and skeletal complications. Neurological involvement was encountered in 2 patients. Twelve patients (63, 2%) were homozygous for the L444P mutation, 1 patient (5.3%) was homozygous for the N370S mutation, and 1 patient (5.3%) was heterozygous for the N370S mutation. Five patients (26.3%) had unknown mutations.
CONCLUSION
L444P/L444P was the most common genotype in the studied patients. GD3 with severe visceral presentation in childhood was the dominant phenotype; N370S was found in the heterozygote state in 1 case and in the homozygote state in 1 case. This phenotype and genotype pattern is encountered in the Middle East. There was no genotype-phenotype correlation.
Gaucher disease (GD) is the most common sphingolipidosis.1 Three clinical subtypes of GD are delineated by the absence or presence and progression of neurologic manifestations.2 GD1, which accounts for more than 90%, has a high incidence in Ashkenazi Jews.2,3 The other 2 types are less common (GD2: 1%, GD3: 5%).3 and have no ethnic predilection.2 All types are autosomal recessive traits.
GD phenotype is complex.4 GD1 or the adult non-neuropathic form is characterized by hepatosplenomegaly, anemia, thrombocytopenia, and bone involvement, with a broad range of severity and age of onset. GD2 or the acute neuropathic form is characterized by early onset of symptoms before the age of 6 months with a rapidly progressing brainstem dysfunction, associated with organomegaly, and leading to death before the age of 2. GD3, the juvenile or sub-acute neuropathic form is characterized by severe visceromegaly and progressive encephalopathy (oculomotor apraxia, epilepsy, and ataxia). Signs appear during childhood or adolescence. The fetal form manifests with a decrease or absence of fetal movements or anasarca.5,6 Gaucher-like disease presents with progressive calcification of the aorta and the aortic and/or mitral valves as its main feature.7
GD is due to a deficiency in lysosomal glucosidase beta acid (GBA). It leads to the accumulation of glucosylceramidase deposits in the cells of the reticuloendothelial system (Gaucher cells) of the liver, spleen, bone marrow, and cerebral gray matter.2,8
The GBA gene, a 7-kb gene, is located on 1q21 with 11 exons.9 There is a highly homologous (96% identity) pseudogene (5 kb) located 16-kb downstream to the GBA gene.10 The abnormal alleles include exonic missense and nonsense mutations, splice junction mutations, deletions and insertions of 1 or more nucleotides, and complex alleles resulting from gene conversion or recombination.8
N370S, the most common mutation in Ashkenazim, is also frequent in Caucasian populations. The second most frequent mutation, L444P, was first described in Norbottnian GD3.8
Genotype-phenotype correlations have been noted.2 They are complex, and the molecular analysis has often shown a poor correlation between genotype and phenotype.11 There is a high inter-individual variance in the severity of the clinical manifestations of the same genotype including N370S homozygote.12,13
The L444P mutation is more frequently associated with GD2 and GD3.8 Complex alleles due to genetic rearrangements are more often associated with severe forms and perinatal lethal forms.8
Later-onset effects of Gaucher disease, such as Parkinsonism and peripheral neuropathies, have been described.14 Even heterozygosis for a GBA gene mutation may predispose to Parkinson disease.15,16
The biochemical assay of GBA activity is the most reliable method to diagnose GD. However, it can be supplemented by molecular diagnosis.10 The unfolded protein response assay has been shown to correlate with disease severity.17
Enzyme replacement therapy (ERT) and substrate reduction therapy are 2 available treatments for GD1 and to some extent GD3; they are ineffective for GD2.8,18
The prognosis is good in GD1, while death usually occurs before the age of 2 in GD2. Without a specific treatment, death progresses within a few years in GD3. ERT has no effect on the neurological manifestations.8,19 The magnitude and time course of responses to therapy are also variable.20
This study is the first of the GBA gene mutations in the Syrian population. It aimed to analyze the characteristics of the phenotypes and the genotypes of GD in Syrian pediatric cohort, compare the results with those in the Middle East and the rest of the world, and assess if any genotype-phenotype relationship could be found. The national health system in Syria would refer to this study to introduce GD treatment in its policy in the near future.
PATIENTS AND METHODS
This study was carried out in Damascus University Children’s Hospital. It comprised a cohort of 19 GD Syrian Children from unrelated families. They were recruited from the General Pediatrics Unit and Pediatric Hematology Unit in a period of 3 years. Diagnosis depended on clinical presentation and laboratory findings compatible with GD.7 These included the presence of organomegaly, neurologic signs, growth failure, blood cytopenia, and high level of acid phosphatase and ferritin. The histologic findings of typical Gaucher cells in bone marrow, liver, or spleen biopsy21 was the principal inclusion criteria. We excluded 4 patients negative for Gaucher cells from the study.
The clinical classification of the patients into GD1or GD2 or GD3 was based on the presence of neurological signs and symptoms and the rate of disease progression.22
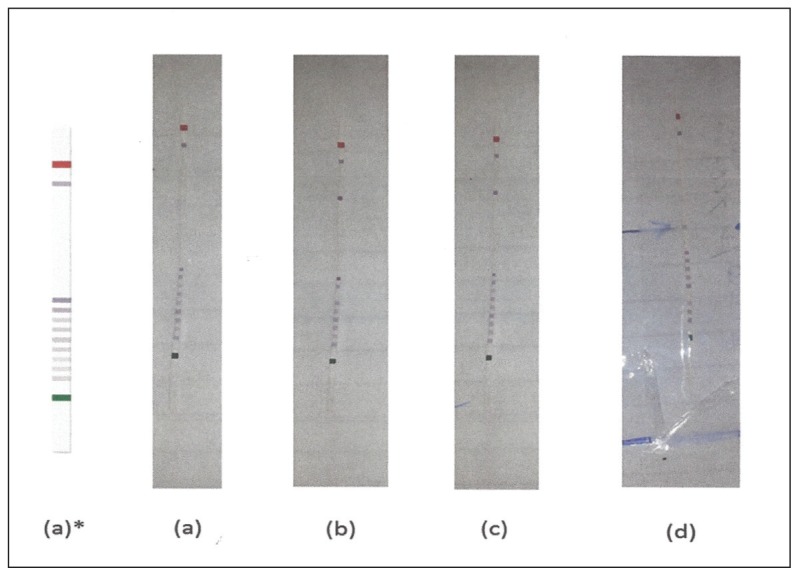
A GBA gene study was performed in the Genetics research laboratory of the Damascus University by using a Kit of Vienna lab Gaucher Disease StripAssay (ViennaLab Diagnostics GmbH, Vienna, Austria). Genomic DNA isolation: DNA was prepared from peripheral blood leukocytes using the salting-out procedure (Miller et al, 1988). The essay includes 3 steps: (1) Polymerase chain reaction amplification using biotinylated primers. (2) Hybridization of amplification products to a test strip containing allele-specific oligonucleotide (ASO) probes immobilized as an array of parallel lines. (3) Bound biotinylated sequences were detected using streptavidin-alkaline phosphatase and color substrates. Affected alleles were detected by allele-specific oligonucleotide hybridization, which allows the identification of homozygote, heterozygote, and normal genotype (Figure 1).
Figure 1.
Gaucher Disease Strip Assay results from Dr. A. Ajlouni’s collection in Damascus. (a) Unknown mutations (b) N370S/N370S (c) N370/Unknown mutation (d) L444P/L444P.
The assay covers 9 common GBA mutations: 84GG [452 +G], IVS2+1 [484 G>A], N370S [1226 A>G], V394L [1297 G>T], D409H [1342 G>C], L444P [1448 T>C], R463C [1504 C>T], R496H [1604 G>A], as well as 2 recombinant alleles derived from crossover between the GBA functional gene and pseudogene (rec NciI, rec TL).
The protocol was reviewed and approved by the Faculty of Medicine Ethics Committee. A written Informed consent was taken from subjects primary caregivers prior to inclusion in the study.
Statistical study
The statistical analysis of data was done using SPSS, version 14.0 (SPSS Inc., Chicago, IL USA). Qualitative data were presented as frequency and proportions, whereas quantitative data were presented as mean (standard deviation). The Spearman rho correlation coefficient test was used to measure the strength of association between the detected genotypes and disease phenotypes. Results were considered significant at the 0.05 level (2-tallied).
RESULTS
This study includes 19 unrelated Syrian patients comprising 12 females and 7 males. The effect of parents’ consanguinity was observed in 16 cases, out of which, 13 parents were first cousins. The family history of GD was positive in 2 cases, and the occurrence of unexplained deaths in infants was observed in 2 of these families (Table 1). The mean age of diagnosis was 19.3 (12.4) months.
Table 1.
Detailed clinical and genotype finding in 19 children with Gaucher disease.
| Genotype | Gaucher typical cell (+) | Consanguinity | Familial history | Symptoms and sings (main presentations) | Age at study (Y m/12) | Age at diagnosis (Y m/12) | Sex | Patient’s number |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| L444P/L444P | BM, L, S | +b | +a1 | Severe viscerald | 4 | 10/12 | M | 1 |
| L444P/L444P | BM, L | +c | − | Severe visceral | 1 6/12 | 1 6/12 | F | 2 |
| L444P/L444P | BM | +c | − | Severe visceral | 2 6/12 | 2 6/12 | F | 3 |
| L444P/L444P | BM | − | + | Severe visceral | 1 6/12 | 1 6/12 | F | 4 |
| L444P/L444P | BM | +b | − | Severe visceral | 2 | 2 | F | 5 |
| L444P/L444P | BM | +c | +a2 | Severe visceral | 1 2/12 | 1 2/12 | M | 6 |
| N370S/Unknown mutation | BM, L | − | +a3 | Early severe visceral | 2 6/12 | 2 6/12 | F | 7 |
| L444P/L444P | BM, S | +b | − | Severe viscerald Bone pain |
3 | 1 4/12 | F | 8 |
| L444P/L444P | BM, L, S | +b | − | Severe viscerald | 1 6/12 | 1 6/12 | F | 9 |
| L444P/L444P | BM, L | +b | − | Severe visceral | 2 | 2 | F | 10 |
| Unknown mutations | BM | +b | − | Severe visceral | 1 | 1 | F | 11 |
| Unknown mutations | BM | − | +a3 | Psychomotor regression Mild visceral |
2 | 2 | F | 12 |
| Unknown mutations | BM | +b | − | Severe visceral | 7/12 | 7/12 | M | 13 |
| N370S/N370S | BM | +b | − | Severe viscerald Bone pain |
9 | 5 | F | 14 |
| Unknown mutations | BM | +b | − | Severe visceral Interstitial pneumonia |
6/12 | 6/12 | F | 15 |
| L444P/L444P | BM | +b | − | Severe visceral | 6/12 | 6/12 | M | 16 |
| L444P/L444P | BM, L | +b | − | Severe visceral | 1 2/12 | 1 2/12 | M | 17 |
| L444P/L444P | BM | +b | − | Early visceral | 8/12 | 8/12 | M | 18 |
| Unknown mutations | BM | +b | − | Early psychomotor regression Spastic palsy Death |
2 | 2 | M | 19 |
M, male; F, female; BM, bone marrow; L, liver, S, spleen.
Three cousins with Gaucher disease;
One cousin with Gaucher disease diagnosed at 6 months of age;
Unexplained early deaths in the fraternity;
Consanguinity degree III;
Consanguinity degree IV or more;
Splenectomy.
A total of 15 patients were presented with the chief complaint of large abdominal mass. All the 19 patients had hepatosplenomegaly; it was considered to be extremely large in 8 cases, large in 5 cases, moderate in 4 cases, and mild in 2 cases. Complications such as underweight and/or failure to thrive were found in 8 cases. One patient presented with bone complications after undergoing splenectomy with an osteonecrosis lesion. Four patients needed splenectomy at different ages (1.5, 3, 4, and 9 years), and 1 patient presented with interstitial pneumonia.
Only 2 patients displayed neurological involvement, resulting in early childhood death in 1 case. Neurological abnormalities were spasticity, squint, and swallowing difficulties in 1 patient and developmental delay in 2 patients (Table 2).
Table 2.
Symptoms and signs in 19 Gaucher disease patients.
| Symptoms and signs | Number of patients | Percentage (%) |
|---|---|---|
|
| ||
| Hepatosplenomegaly | 19 | 100 |
| Large abdomen | 15 | 79 |
| Failure to thrive | 8 | 42 |
| Underweight | 8 | 42 |
| Splenectomy | 4 | 21 |
| Recurrent fever | 3 | 15.7 |
| Respiratory distress | 2 | 10.5 |
| Bone pain | 2 | 10.5 |
| Interstitial pneumonia | 1 | 5.2 |
| Osteonecrosis | 1 | 5.2 |
| Psychomotor regression | 2 | 10.5 |
| Spastic palsy | 1 | 5.2 |
| Pyramidal sings | 1 | 5.2 |
| Severe malnutrition | 1 | 5.2 |
| Chronic diarrhea | 1 | 5.2 |
| Osteonecrosis lesion | 1 | 5.2 |
| Adenopathy | 1 | 5.2 |
| Death | 1 | 5.2 |
Hypochromic microcytic anemia was found in all patients, with the hemoglobin concentration of 8.4 (1.79) g/dL, platelet count of 115 157.8 (86 113.7) cells/mm2, and ferritin concentration of 166 (84.1) ng/mL (Table 3). The level of acid phosphatase was found to be elevated in 18 patients. Nineteen patients were found to be Gaucher cell positive (Table 1).
Table 3.
Laboratory finding in 19 children with Gaucher disease.
| Descriptive statistics | Minimum | Maximum | Mean | STDV |
|---|---|---|---|---|
|
| ||||
| Age (mo) | 6 | 60 | 19.3 | 12.4 |
| Ferretin (ng/mL ) | 65 | 380 | 166 | 84.1 |
| PLT (Cells/mm3 ) | 8000 | 289 000 | 115 157.8 | 86 113.7 |
| WBC (Cells/mm3) | 1900 | 12 700 | 6040.5 | 3219.7 |
| HB (g/L) | 4.3 | 11.4 | 8.4 | 1.7 |
PLT, Platelet count; WBC, white blood count; HB: hemoglobin; STDEV, standard deviation.
Clinically 17/19 patients (89.5%) presented with an early severe visceral form. They did not display any neurologic symptoms; phenotype classification of these cases remained uncertain (GD1 or GD3). One patient (5.3%) presented with acute neuropathic form (GD2), and 1 patient (5.3%) presented with chronic neuropathic form (GD3).
L444P was found in 24/38 studied alleles (63.1 %) for a GBA gene mutation assay, N370S was found in 3/38 studied alleles (7.9%), and 11/38 studied alleles (28.9%) remained unknown (Figure 1).
One patient (5.3%) was heterozygous for N370S mutation, and 12/19 patients (63.2%) were homozygous for L444P mutation. They presented no neurologic manifestations at the time of the study. One patient (5.3%) was homozygous for N370S; he was presented with a severe non-neuropathic form. The genotype assay was negative in 5 patients (26.3%): 3 had a severe visceral non-neuropathic presentation, 1 had a chronic neuropathic form, and 1/5 patient had an acute neuropathic form (Tables 1 and 4).
Table 4.
Genotypes and phenotypes in 19 Syrian children with Gaucher disease.
| Genotype | Number of patients | Phenotypea | ||
|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | ||
|
| ||||
| L444P/L444P | 12 | − | − | 12 |
| N370S/Unknown mutations | 1 | − | − | 1 |
| N370S/N370S | 1 | 1 | − | − |
| Unknown mutations | 5 | − | 1 | 4 |
| 19 | 1 | 1 | 17 | |
Correlation value of phenotype and genotype; r=−0.358, P=.133.
DISCUSSION
This study highlights the pattern of possible GBA gene mutations present in a Syrian pediatric cohort. It is known that 9 common mutations promise the genetic diagnosis in only 50% to 70% of non-Jewish people.23,24 Although the L444P mutation is most common worldwide,25 some studies of the rest of world demonstrate the highest prevalence of N370S compared with L444P like in Brazil,26 Mexico,27 Colombia,28 Argentina,29 Romanian,30 and France.31 L444P was encountered in more than the half of the muted allele in Egypt11 and Saudi Arabia.32 Furthermore, homozygous for L444P mutations was present in more than half of the patients in a pediatric Egyptian study.33 Other known common mutation such as RecNcil that was encountered in high frequency in some population like Egypt,11 Tunisia,34 and Argentina29 was not encountered in our study.
The sequencing of GBA gene is needed for all negative results. It is important to define if other rare mutations or some new mutations could be found,24 which are expected in Non-Ashkenazi people.35
Although many studies reported early severe visceral GD with the absence of neurological signs in L444P/L444P genotype.30,34 it is accepted that the onset of neurological findings is delayed in GD3. The 12 patients homozygous for L444P who succumbed to early severe visceral GD could be first classified as having GD1.36,37
Furthermore, the prevalence of neuropathic GD appears to be higher in those who are not of European origin including the Middle East, India, China, Japan, and Korea.22,38 However, subtle neurological abnormalities can make the diagnosis of neurologic GD difficult.39 Therefore, among the 17 patients with early isolated visceral presentation, only the patient with N370S/N370S genotype could be considered as GD1; the other 16 patients (12 patients with L444P/L444P and 1 patient with N370S/Unknown mutation Genotypes) may need to be reclassified as GD3.40
The Spearman rho factor to assess genotype-phenotype correlation showed no significance (r: –0.358, 2-tallied: 0.133, P value >.05). Anyway, the ability to predict patient outcome on the basis of DNA studies is often limited.1
These Syrian genotype-phenotype pattern results could not be considered for the therapeutic judgments.24 Otherwise, ERT may have some benefits in GD3.8 This treatment is well indicated when patients have a progressive visceral form.41
In conclusion, GD3 is the most encountered phenotype, L444P is the most common allele, and L444P/L444P genotype is the most common genotype in studied Syrian children with GD. This phenotypegenotype pattern is encountered in the Middle East. No genotype-phenotype correlation was found; further advanced studies of the GBA gene are needed.
In addition, the treatment decision in the early visceral presentation of GD3 will depend on the clinical course of the disease, and genotype should not interfere in the decision of treatment.
REFERENCES
- 1.Sidransky E, Ginns EI. Phenotypic and genotypic heterogeneity in gaucher diseases: implication for genetic counseling. J Genet Couns. 1994;1(3):13–22. doi: 10.1007/BF01414603. [DOI] [PubMed] [Google Scholar]
- 2.Mc Govern MM, Desnick RJ. Lipidoses (Lysosomal Storage Disorders) In: Kliegman RM, Stanton B, St Geme J, Schor NF, editors. Nelson textbook of pediatric. 19th ed. Philadelphia (PA): Elsevier Saunders; 2011. pp. 487–8. [Google Scholar]
- 3.Charrow J, Andersson C, Kaplan P, Kolodnry E, et al. The Gaucher Registry. Demographic and Disease Characteristic of 1698 Patient With Gaucher Disease. Arch Intern Med. 2000;160(18):2835–43. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- 4.Mistry P. Phenotype variations in Gaucher disease. Rev Med Interne. 2006;27(1):S3–10. doi: 10.1016/s0248-8663(06)80002-0. [DOI] [PubMed] [Google Scholar]
- 5.Andersson H, Kaplan P, Kacena K, Yee J. Eight-Year Clinical Outcomes of Long-Term Enzyme Replacement Therapy for 884 Children With Gaucher Disease Type 1. Pediatrics. 2008;122(6):1182–90. doi: 10.1542/peds.2007-2144. [DOI] [PubMed] [Google Scholar]
- 6.Nyhan WL, Barshop BA, Al-Aqeel AI. Gaucher disease. In: Nyhan WL, Barshop BA, Al-Aqeel AI, editors. ATLAS OF INHERITED METABOLIC DISEASES. third ed. London (UK): Hodder Arnold; 2012. pp. 698–707. [Google Scholar]
- 7.Elstein D, Abrahamov A, Dweck A, Hadas-Halpern I, Zimran A. Gaucher disease: pediatric concerns. Paediatr Drugs. 2002;4(7):417–26. doi: 10.2165/00128072-200204070-00001. [DOI] [PubMed] [Google Scholar]
- 8.Vanier MT, Caillaud C. Disorders of Sphingolipid Metabolism and Neuronal Ceroid-Lipofuscinoses. In: Saudubray JM, Berghe G, Walter JH, editors. Inborn Metabolic Diseases Diagnosis and Treatment. Fifth ed. Berlin Heidelberg: Springer-Verlag; 2012. pp. 557–9. [Google Scholar]
- 9.Malini E, Grossi S, Deganuto M, Rosano C, Parini R, Dominisini S, et al. Functional analysis of 11 novel GBA alleles. Eur J Hum Genet. 2014;22(4):511–6. doi: 10.1038/ejhg.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goker-Alpan O, Hruska KS, Orvisky E, Kishnani PS, Stubblefield BK, Schiffmann R, et al. Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet. 2005;42(6):e37. doi: 10.1136/jmg.2004.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Morsy Z, Khashaba MT, El-Sayed Soliman O, Yahia S, Abd El-Hady D. Glucosidase acid beta gene mutations in Egyptian children with Gaucher disease and relation to disease phenotypes Mansoura, Egypt. World J Pediatr. 2011;7(4):326–30. doi: 10.1007/s12519-011-0309-1. [DOI] [PubMed] [Google Scholar]
- 12.Sidransky E. Gaucher disease: complexity in a simple disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Amato D, Stachiw T, Clarke JT, Rivard GE. Gaucher disease: variability in phenotype among siblings. J Inherit Metab Dis. 2004;27:659–69. doi: 10.1023/b:boli.0000042983.60840.f3. [DOI] [PubMed] [Google Scholar]
- 14.Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15(2):181–8. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. N Engl J Med. 2009;361:1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(7):1783–94. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maor G1, Rencus-Lazar S, Filocamo M, Steller H, Segal D, Horowitz M. Unfolded protein response in Gaucher disease: from human to Drosophila. Orphanet J Rare Dis. 2013;8:140. doi: 10.1186/1750-1172-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vellodi A, Tylki-Szymanska A, Davies EH, et al. Management of neuropathic Gaucher disease: revised recommendations. J Inherit Metab Dis. 2009;32:660–4. doi: 10.1007/s10545-009-1164-2. [DOI] [PubMed] [Google Scholar]
- 19.Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with nonneuronopathic Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113(2):112–9. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 20.Barranger JA, O’Rourke E. Lessons learned from the development of enzyme therapy for Gaucher disease. J Inherit Metab Dis. 2001;24:89–96. doi: 10.1023/a:1012440428282. [DOI] [PubMed] [Google Scholar]
- 21.Giraldo P, Pocovi M, Perez-Calvo JI, Rubio-Felix D, Giralt M. Report of the Spanish Gaucher’s disease registry: clinical and genetic characteristics. Haematologica. 2000;85(8):792–9. [PubMed] [Google Scholar]
- 22.Pastores GM, Hughes DA. Gaucher Disease. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 2000. Jul 27, [Updated 2013 Sep 19] http://www.ncbi.nlm.nih.gov/books/NBK1269/ [PubMed] [Google Scholar]
- 23.Beutler E, Grabowski GA. Gaucher Disease. In: Scriver CR, Brandet AL, Sly SW, Valle D, editors. The metabolic and molecular bases of inherited diseases. Eighth edition. New York: McGraw-Hill; 2001. pp. 3635–57. [Google Scholar]
- 24.Koprivica V, Stone DL, Park JK. Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet. 2000;66(6):1777–86. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz M, Tzuri G, Eyal N, Berebi A, Kolodny EH, Brady RO, et al. Prevalence of Nine Mutations among Jewish and Non-Jewish Gaucher Disease Patients. Am J Hum Genet. 1993;53(4):921–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Sobreira E, Pires RF, Cizmarik M, Grabowski GA. Phenotypic and genotypic heterogeneity in Gaucher disease type 1: A comparison between Brazil and the rest-of-the-world. Mol Genet Metab. 2007;90(1):81–6. doi: 10.1016/j.ymgme.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Carbajal-Rodriguez L, Voirol-García A, Mora-Magaña I, Rodríguez-Herrera R, Zarco-Román J. Gaucher disease in Mexico. Epidemiologic overview. Acta Pediatr Mex. 2011;32(5):277–80. [Google Scholar]
- 28.Pomponio RJ, Cabrera-Salazar MA, Echeverri OY, Miller G, Barrera LA. Gaucher disease in Colombia: Mutation identification and comparison to other hispanic populations. Mol Genet Metab. 2005;86(4):466–72. doi: 10.1016/j.ymgme.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Cormand B, Harboe TL, Gort L, Campoy C, Blanco M, Chamoles N, et al. Mutation Analysis of Gaucher Disease Patients From Argentina: High Prevalence of the RecNciI Mutation. Am J Med Genet. 1998;80(4):343–51. doi: 10.1002/(sici)1096-8628(19981204)80:4<343::aid-ajmg8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Drugan C, Procopciuc L, Jebeleanu G, Grigorescu-Sido P, Dussau J, Poenaru L, Caillaud C. Gaucher disease in Romanian patients: incidence of the most common mutations and phenotypic manifestations. Eur J Hum Genet. 2002;10(9):511–5. doi: 10.1038/sj.ejhg.5200845. [DOI] [PubMed] [Google Scholar]
- 31.Stirnemann J, Vigan M, Hamroun D, Heraoui D, Rossi-Semerano L, Berger MG, et al. The French Gaucher’s disease registry: clinical characteristics, complications and treatment of 562 patients. Orphanet J Rare Dis. 2012;7(1):77. doi: 10.1186/1750-1172-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaya N, Al-ZAHRANI F, Al-Odaib A. Identification of Gaucher disease mutations found in Saudi Arabia. Blood Cells Mol Dis. 2008;41(2):200–1. doi: 10.1016/j.bcmd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Khalifa A, Tantawy A, Shawky R, Monir E, Elsayed S, Fateen E, et al. Outcome of enzyme replacement therapy in children with Gaucher disease: The Egyptian experience. Egypt J Med Hum Genet. 2011;12(1):9–14. [Google Scholar]
- 34.Ben Turkia H, Riahi I, Azzouz H, Ladab S, Cherif W, Ben Chehida A, et al. Phenotype and mutational spectrum in Tunisian pediatric Gaucher disease. Tunis Med. 2010;88(3):158–62. [PubMed] [Google Scholar]
- 35.Choy FY, Zhang W, Shi HP, Zay A, Campbell T, Tang N, Ferreira P. Gaucher disease among Chinese patients: Review on genotype/phenotype correlation from 29 patients and identification of novel and rare alleles. Blood Cells Mol Dis. 2007;38(3):287–93. doi: 10.1016/j.bcmd.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Filocamo M, Mazzotti R, Stroppiano M, Seri M, Giona F, Parenti G, et al. Analysis of the glucocerebrosidase gene and mutation profile in 144 Italian gaucher patients. Hum Mutat. 2002;20(3):234–5. doi: 10.1002/humu.9058. [DOI] [PubMed] [Google Scholar]
- 37.Erdos M, Hodanova K, Taskó S, Palicz A, Stolnaja L, Dvorakova L, et al. Genetic and clinical features of patients with Gaucher disease in Hungary. Blood Cells Mol Dis. 2007;39(1):119–23. doi: 10.1016/j.bcmd.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Tylki-Szymañska A, Keddache M, Grabowski GA. Characterization of neuronopathic Gaucher disease among ethnic Poles. Genet Med. 2006;8(1):8–15. doi: 10.1097/01.gim.0000196443.42899.25. [DOI] [PubMed] [Google Scholar]
- 39.Schiffmann R. Neuronopathic phenotypes of Gaucher disease. In: Grabowski Gregory A., editor. Advances in Gaucher Disease: Basic and Clinical Perspectives. 1st ed. London: Future Medicine; 2013. pp. 12–24. http://www.future-sciencegroup.com/_img/pics/Advances_in_Gaucher_Disease_Basic_and_Clinical_Perspectives.pdf. [Google Scholar]
- 40.Giraldo P, Capablo JL, Pocovi M. Neuronopathic Forms in Subjects with Mutations in GBA Gene. In: Plaseska-Karanfilska D, editor. Human Genetic Diseases. Croatia: In Tech; 2011. pp. 91–108. http://www.intechopen.com/books/human-genetic-diseases/neuronopathic-forms-in-subjects-with-mutations-in-gba-gene. [Google Scholar]
- 41.Weinthal JA. Prognosis of Patients with Gaucher Disease. Clin Adv Hematol Oncol. 2012;10(6):10–11. [PubMed] [Google Scholar]