Abstract
BACKGROUND
There are conflicting results on published randomized controlled trials (RCTs) on the role of vitamin E in the prevention of cancer. We conducted a meta-analysis of RCTs to evaluate the role of vitamin E in the prevention of cancer in adults.
METHODS
We included RCTs in which the outcomes of the intake of vitamin E supplement alone or with other supplements were compared to a control group. The primary outcomes were total mortality, cancer mortality, total incidence of cancer, and incidence of lung, stomach, esophageal, pancreatic, prostate, breast and thyroid cancers. All identified trials were reviewed independently by the two reviewers to determine whether trials should be included or excluded. The quality of all included studies was scored independently by the two reviewers.
RESULTS
Twelve studies, which included 167 025 participants, met the inclusion criteria. There were no statistically significant differences in total mortality (relative risk, 0.99; 95% CI 0.96–1.03), cancer incidence (odds ratio, 0.96; 95% CI 0.92–1.01), and cancer mortality (odds ratio, 1.00; 95% CI, 0.96–1.03) among the different groups of patients included in this meta-analysis. Vitamin E was associated with a significant reduction in the incidence of prostate cancer (relative risk, 0.85; 95% CI, 0.73–0.96, number needed to treat=500), but it did not reduce the incidence of any other types of cancer.
CONCLUSIONS
Vitamin E supplementation was not associated with a reduction in total mortality, cancer incidence, or cancer mortality, but it was associated with a statistically significant reduction in the incidence of prostate cancer. Vitamin E can be used in the prevention of prostate cancer in men who are at high risk of prostate cancer.
Three types of cancer account for at least 50% of new cases in each sex: prostate, lung, and colorectal cancers in males, and breast, lung, and colorectal cancers in females. Almost one-third of cancer deaths in men and almost one-quarter in women are due to lung cancer alone. Cancer is the leading cause of potential years of life lost (PYLL) for men and women in Canada.1 Simple, accessible and safe preventive therapies that will decrease the incidence and mortality of cancer are expected to have a great effect on public health.
Several in vitro studies showed antioxidant vitamins to have a significant protective effect against cancer.2–4 In experimental animals deficiencies of vitamin E were associated with enhanced carcinogenesis, while supplementation of vitamin E inhibited tumor formation.5,6
Alpha-tocopherol is the most common naturally occurring compound of vitamin E. The recommended dietary allowance of vitamin E is 15 mg daily for adult men and women. Each 1 mg of vitamin E equals to 1.5 IU of natural vitamin E and 2.2 IU of synthetic vitamin E. Vegetable oils, nuts, and green leafy vegetables are the main dietary sources of vitamin E.7
METHODS
Inclusion criteria
Data sources were randomized controlled trials (RCTs) in which outcomes related to cancer prevention that were associated with the intake of vitamin E supplements alone or with other supplements were compared to a control group (placebo or control). Participants in studies were adults of either sex (18 years or older). Types of interventions were vitamin E alone or with other supplements versus placebo or no intervention. Supplementation was in capsule or tablet form, to be consumed by mouth.
At least one of the following primary outcomes must have been reported: total mortality, cancer mortality, total incidence of cancer, or incidence of lung, stomach, esophageal, prostate, breast, urinary, hematological or thyroid cancers. Secondary outcomes were the role of high dose (≥300 mg/d) and low dose (<300 mg/d) vitamin E on the primary outcomes.
Search strategy for identification of studies
The following bibliographic databases were searched to identify the relevant primary studies: The Cochrane Controlled Trials Register (CCTR), MEDLINE, and EMBASE. A computerized search of MEDLINE was performed using the OVID platform, to search the MEDLINE database for articles published between January 1966 and June 2005, and the EMBASE database from 1980 to June 2005. The search strategy was conducted using the MeSH terms: “antioxidants” “vitamins”, “vitamin E”, “alpha-tocopherol”, “tocopherol”, “cancer”, “prevention”, and “randomized controlled trials”. These terms were used in various combinations. The Cochrane library was searched for relevant articles using the same search strategy. Relevant articles were retrieved through a manual review of references. No language restrictions were applied.
Study selection
All identified trials were reviewed independently by the two reviewers to assess methodological quality and determine whether trials should be included or excluded. Disagreement was resolved by discussion. All selected studies were published studies. The same two reviewers assessed the methodological quality of each trial according to Jadad score.8 After independent evaluation, the two reviewers discussed the results for each study and any discrepancy was resolved by discussion.
Data abstraction and synthesis
Data were independently extracted by the same reviewers and cross-checked with discrepancies resolved by discussion. The Cochrane Statistics package RevMan, version 4.2 was used for data synthesis. Relative risk (RR) and risk difference (RD) with 95% confidence intervals were reported. If there was a statistically significant RD, the number needed to treat (NNT) and number needed to harm (NNH) were calculated.
Heterogeneity was tested using the Cochran Q statistic with significance at P<0.10. In addition, we tested heterogeneity using the I2 method with a value greater than 50% considered to indicate substantial heterogeneity. 9 Potential sources of heterogeneity of treatment effect were explored using pre-specified subgroup analysis when there were sufficient studies to analyze the dose of vitamin E, the use of other vitamins with vitamin E, and study quality variability. Whenever there was statistically significant between-study heterogeneity the weighted estimate of the typical treatment effect across trials (relative risk) was calculated using the random effects model to ensure robustness of the results.
Description of studies and methodological quality
Twelve trials, which included 167 025 individuals, met the inclusion criteria (Table 1). More than 76 000 (45%) of the participants were females. These trials were performed in many countries (Finland, Italy, Canada, China, UK, US, Denmark, Germany, Ireland, Netherlands, Norway, Spain, Sweden, Switzerland, and Mexico). Vitamin E dose varied between 50 mg/d to 800 mg/d. Study duration ranged between 510 days to 10 years. Two studies were open-label trials, but outcome assessment was blinded in these studies.18,20 All studies were analyzed using the intention to treat principle.
Table 1.
Characteristics of 12 trials included in the meta-analysis.
| Study | Duration | Participants | Interventions | Jadad Score |
|---|---|---|---|---|
| Women’s Health Study, 200510 | 10.1 years | 39 876 healthy US women | Vitamin E or placebo and aspirin or placebo, using 2×2 factorial design | 5 |
| Blot et al, 199311 | 5.25 years | 29 594 healthy adults | Four combinations of vitamins including vitamin E, using 2×4 fractional design | 5 |
| ATBC, 1994;12 Heinonen et al, 1998;15 Albanes et al, 2000;13 Virtamo et al, 200014 | 6.1 years | 29 133 male smokers | Vitamin E (50 mg/d), or beta-carotene (20 mg/day), both or placebo | 5 |
| MRC/BHF Heart Protection Study, 200216 | 5 years | 20 536 UK adults with coronary artery disease, other occlusive arterial disease, or diabetes | Vitamin E 600 mg/d, vitamin C 250 mg, and B-carotene 20 mg daily, or matching placebo | 5 |
| Herberg et al, 200417 | 7.5 years | 13 017 French adult men and women | Vitamin E 30 mg, B-carotene 6000 μg, vitamin C 120 mg, selenium 100 μg, zinc 20 mg or placebo | 5 |
| GISSI-Prevenzione, 199918 | 3.5 years | 11 324 Patients with recent (<3 months) MI | Vitamin E (300 mg daily, n=2830), n-3 PUFA (1g daily, n=2,836), both (n=2,830), or none (control n=2,828) | 3 |
| HOPE, 200019 | 4.5 years | 9541 patients at high risk for cardiovascular disease. | Vitamin E 400 IU or matching placebo | 5 |
| PPP, 200120 | 3.6 years | 4495 patients with one or more risk factors for cardiovascular disease | Vitamin E (300 mg/d), and no vitamin E groups, and aspirin (100 mg/day) and no aspirin groups | 3 |
| AREDS, 200121 | 6.3 years | 4754 healthy adults | Vitamin E 400IU, vitamin C 500mg, and beta carotene, and placebo | 5 |
| Li et al, 199322 | 6 years | 3318 patients with cytological evidence of esophageal dysplasia | Daily supplementation with 14 vitamins including vitamin E (60 IU/d) and 12 minerals, or matching placebo | 5 |
| Davey et al, 199823 | 510 days | 2002 patients with angiographically proven coronary atherosclerosis | Vitamin E (800 IU/d for first 546 patients, 400 IU/d for reminder), or matching placebo | 5 |
| Boaz et al, 200024 | 519 days | 196 hemodialysis patients with pre-existing cardiovascular disease | Vitamin E 800 IU/d, or matching placebo | 5 |
RESULTS
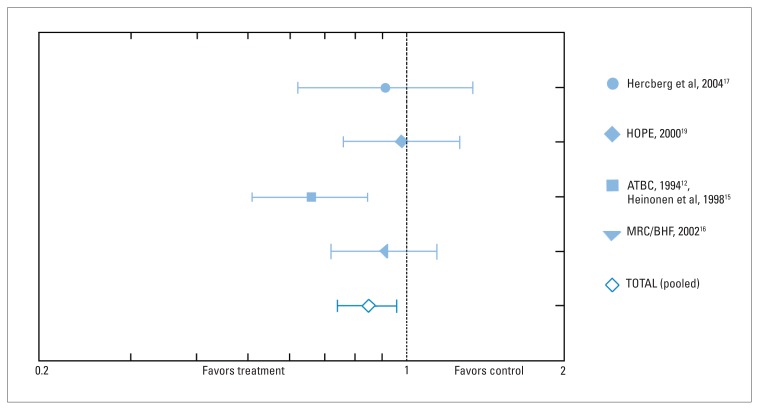
For vitamin E alone or with other supplements versus control (all 12 studies), vitamin E was associated with a significant reduction in the incidence of prostate cancer, but did not reduce the incidence of any other types of cancer (Table 2). Four studies (including 71 759 individuals) reported on the role of vitamin E in the prevention of prostate cancer (Figure 1, Table 3).15–19 One study showed a statistically significant reduction in the incidence of prostate cancer in individuals receiving vitamin E supplements (RR 0.66; 95% CI 0.51, 0.84).15 When all studies were combined there was a significant reduction in the incidence of prostate cancer (RR 0.85; 95% CI 0.74, 0.96, RD=-0.002, NNT=500, test for heterogeneity: P=0.13, I2=47%)
Table 2.
Summary of results of meta-analysis.
| Outcomes | No. of studies | No. of participants | Relative risk (fixed) (95% CI) |
|---|---|---|---|
| Vitamin E (all studies) vs. control | |||
| Total mortality | 12 | 161 349 | 0.99 (0.96, 1.03) |
| Cancer mortality | 6 | 124 495 | 1.02 (0.95, 1.09) |
| Cancer incidence | 8 | 151 372 | 0.98 (0.94, 1.02) |
| Stomach cancer | 6 | 134 996 | 1.01 (0.90, 1.15) |
| Lung cancer | 5 | 111 635 | 1.02 (0.88, 1.19) |
| Colorectal cancer | 4 | 91 099 | 0.95 (0.81, 1.12) |
| Prostate cancer | 4 | 71 759 | 0.85 (0.74, 0.96) |
| Breast cancer | 3 | 62 158 | 0.99 (0.90, 1.10) |
| Esophageal cancer | 3 | 45 643 | 1.00 (0.88, 1.14) |
| Hematological cancer | 2 | 33 277 | 0.98 (0.71, 1.33) |
| Urinary tract cancer | 2 | 27 314 | 1.25 (0.84, 1.84) |
| Vitamin E alone vs. control | |||
| Total mortality | 6 | 36 465 | 1.00 (0.94, 1.05) |
| Colorectal cancer | 2 | 24 114 | 1.05 (0.79, 1.39) |
| Prostate cancer | 2 | 24 114 | 0.86 (0.70, 1.06) |
| Total cancer | 3 | 19 694 | 1.00 (0.90, 1.12) |
| Cancer mortality | 3 | 15 395 | 1.02 (0.83, 1.26) |
| Vitamin E with other supplements vs. control | |||
| Total mortality | 6 | 99 877 | 0.99 (0.96, 1.03) |
| Total cancer | 6 | 106 454 | 0.96 (0.92, 1.01) |
| Cancer mortality | 5 | 93 417 | 1.00 (0.93, 1.08) |
| Prostate cancer | 3 | 62 218 | 0.79 (0.67, 0.93) |
| Vitamin E ≥ 300 mg/d | |||
| Total mortality | 7 | 52 861 | 1.01 (0.97, 1.06) |
| Cancer mortality | 4 | 41 607 | 1.04 (0.92, 1.17) |
| Cancer incidence | 4 | 45 906 | 0.98 (0.92, 1.05) |
| Lung cancer | 2 | 30 077 | 0.97 (0.81, 1.16) |
| Prostate cancer | 2 | 30 077 | 0.94 (0.79, 1.11) |
| Vitamin E < 300 mg/d | |||
| Total mortality | 4 | 74 584 | 0.97 (0.92, 1.02) |
| Cancer mortality | 3 | 61 547 | 0.98 (0.90, 1.06) |
| Cancer incidence | 4 | 74 584 | 0.96 (0.91, 1.01) |
| Stomach cancer | 4 | 74 584 | 0.96 (0.84, 1.10) |
| Lung cancer | 2 | 41 682 | 0.97 (0.85, 1.10) |
| Prostate cancer | 2 | 41 682 | 0.69 (0.55, 0.87) |
Figure 1.
Relative risk (fixed) and 95% confidence intervals for prostate cancer vs. controls associated with vitamin E alone or with other supplements. Test for heterogeneity: χ2=5.70; df=3 (P=0.13), F=47.4%. Test for overall effect: Z=2.50 (P=0.01).
Table 3.
Data from four randomized, controlled trials that evaluated the role of vitamin E in the prevention of prostate cancer, and used in the pooled analysis (Figure 1).
| Symbols from Figure 1 | Study | Treatment group (n/N) | Control (n/N) | Weight (%) |
|---|---|---|---|---|

|
Hercberg et al, 200417 | 49/6364 | 54/6377 | 11.34 |

|
HOPE, 200019 | 116/4761 | 119/4780 | 24.96 |

|
ATBC, 199412; Heinonen et al, 199815 | 99/14 472 | 151/14 469 | 31.74 |

|
MRC/BHF, 200216 | 138/10 269 | 152/10 267 | 31.95 |

|
Total (pooled analysis) | 402/35 866 | 476/35 893 | 100.00 |
Test for heterogeneity: χ2=5.70; df=3 (P=0.13), F=47.4% Test for overall effect: Z=2.50 (P=0.01). 0.2 Favors treatment 1 Favors control 2
In studies with vitamin E alone versus control (6 studies) there was no statistically significant reduction in any of the outcomes (Table 2). Two studies (including 24 114 individuals) evaluated the role of vitamin E alone in the prevention of prostate cancer (RR 0.86; 95% CI 0.70, 1.06).15–19
In studies of vitamin E and other supplements vs. control, vitamin E was associated with a significant reduction in the incidence of prostate cancer, but did not reduce the incidence of any other types of cancer (Table 2). Three studies (including 62 218 individuals) evaluated the role of vitamin E with other supplements in the prevention of prostate cancer (RR 0.79; 95% CI 0.67, 0.93, NNT=450, test for heterogeneity: P=0.16, I2=46%).15–17
With a high dose of vitamin E (≥300 mg/d) there was no statistically significant reduction in any of the outcomes in studies that used a high dose of vitamin E (Table 2). Two studies (including 30 077 individuals) evaluated the role of a high dose of vitamin E in the prevention of prostate cancer (RR 0.94; 95% CI 0.79, 1.11).16,19
With a low dose of vitamin E (<300 mg/d), vitamin E was associated with a significant reduction in the incidence of prostate cancer, but did not reduce the incidence of any other types of cancer that was available for analysis (Table 2). Two studies (including 41 682 individuals) evaluated the role of low dose of vitamin E in the prevention of prostate cancer (RR 0.69; 95% CI 0.55, 0.87, NNT=380, test for heterogeneity: P=0.31, I2=1.6%).15,17
DISCUSSION
The methodological quality of the included trials was high. Vitamin E had no effect on total mortality, cancer incidence and cancer mortality among the different groups of patients included in this meta-analysis. The only positive effect of vitamin E was in the reduction of the incidence of prostate cancer. This effect disappeared when we analyzed studies that evaluated the role of vitamin E alone; however, the sample size was insufficient (21 634 male patients) to have enough power to detect a difference in the incidence of prostate cancer. A minimum of 32 000 participants is needed to detect a difference in the effect of vitamin E on the incidence of prostate cancer.25,26
The effect of vitamin E in the reduction of prostate cancer was statistically significant in studies that evaluated the role of vitamin E combined with other supplements. In this analysis we had a large sample size of 62 000 participants, which allowed enough power to detect a difference in the incidence of prostate cancer.
There was no statistically significant reduction of prostate cancer in studies that used high dose of vitamin E (>300 mg); however, in this analysis the sample size was insufficient (22 515 male patients) to have enough power to detect a difference in the incidence of prostate cancer. There was a statistically significant reduction of prostate cancer in studies that used a low dose of vitamin E (<300 mg) and there was enough power to detect a difference in the incidence of prostate cancer.
Vitamin E supplementation results in a minimum of 0.2% absolute reduction in the incidence of prostate cancer, which can be translated into a a number needed to treat (NNT) of 500. This effect of vitamin E is important as prostate cancer is the leading form of cancer diagnosed in men, affecting 0.7% of the male population1. Vitamin E was well tolerated in all of the included trials. Previous meta-analysis showed that vitamin E did not affect the rate of coronary and cerebrovascular events.27,28
In conclusion, vitamin E supplementation can be used for the prevention of prostate cancer among high risk groups, including individuals >55 years old, patients with elevated prostate specific antigen (PSA), African Americans, and patients with family history of prostate cancer. The results of the SELECT trial and other ongoing trials (Table 4) will provide more precise answers on the role of vitamin E in the prevention of prostate cancer.
Table 4.
Ongoing studies to evaluate the effect of Vitamin E and other interventions on disease prevention.
| Study | Participants | Interventions | Outcomes |
|---|---|---|---|
| Physicians Health Study II (PHS II) (Christen et al 200029) | 15 000 US male physicians aged 55 years and older with no history of cancer, or cardiovascular disease | Vitamin E or beta-carotene or Vitamin C or a daily multivitamins or placebo in a factorial design. | Total and prostate cancer, CVD, and eye disease |
| Women’s Atherosclerosis Cardiovascular Study (WACS) (Manson et al 199530) | 8000 female nurses with history of cardiovascular disease | Vitamin E 400IU/d or vitamin C 1g/d, or B-carotene 20 mg/d, in a factorial design | Cardiovascular disease morbidity and mortality, cancer incidence as a secondary outcome |
| SELECT Trial (Lieberman 200126; Lippman 200525) | 32 400 Healthy men at least 50 years of age | Placebo or selenium (200 mcg and/or vitamin E 400 IU/day) | Prostate cancer incidence and mortality |
Footnotes
This paper was accepted and presented at The American Society of Clinical Oncology Meeting (ASCO) June 2–6, 2006. The authors declare no conflict of interest.
REFERENCES
- 1.Canadian cancer statistics 2000. Toronto (ON): National Cancer Institute of Canada; 2005. pp. 58–60. [Google Scholar]
- 2.Prasad KN, Edwards-Prasad J. Expressions of some molecular cancer risk factors and their modification by vitamins. J Am Coll Nutr. 1990;9(1):28–34. doi: 10.1080/07315724.1990.10720346. [DOI] [PubMed] [Google Scholar]
- 3.Wattenberg LW. Inhibition of carcinogenesis by minor dietary constituents. Cancer Res. 1992;52(7 Suppl):2085s–91s. [PubMed] [Google Scholar]
- 4.Birt DF, Bresnick E. Chemoprevention by non-nutrient components of vegetables and fruits. In: Alfin-Slater RB, Kritchevsky D, editors. Cancer and nutrition. New York (NY): Plenum Press; 1991. pp. 221–61. [Google Scholar]
- 5.Prasad KN, Edwards-Prasad J. Vitamin E and cancer prevention: recent advances and future potentials. J Am Coll Nutr. 1992;11(5):487–500. doi: 10.1080/07315724.1992.10718253. [DOI] [PubMed] [Google Scholar]
- 6.Shah CP, Shah SS, Shah RR. Public Health and Preventive Medicine in Canada. 3rd ed. Toronto (ON): C.P. Shah; 1994. [Google Scholar]
- 7.Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Institute of Medicine; 2000. pp. 507–509. [PubMed] [Google Scholar]
- 8.Jadad AR, Moore A, Carroll D, Jenkinson C, Reyolds DJM, Gavaghan D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Lee I-M, Cook NR, Gazianno JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the Primary Prevention of Cardiovascular Disease and Cancer The Women’s Health Study: A Randomized Controlled Trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Blot WJ, Li JY, Taylor PR, Li B, Wang GQ, Dawsey S, et al. Nutrition Intervention Trial in Linaxin, China: Supplementation with specific vitamin/mineral combination, cancer incidence, and disease specific mortality. J Natl Cancer Inst. 1993;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 12.The ATBC cancer prevention study group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 13.Albanes D, Maila N, Taylor PR, Huttunen JK, Virtamo J, Edwards BK, et al. Effects of supplemental alphatocopherol and B-carotene on colorectal cancer:results from controlled trial (Finland) Cancer Causes and Control. 2000;11:197–205. doi: 10.1023/a:1008936214087. [DOI] [PubMed] [Google Scholar]
- 14.Virtamo J, Edwards BK, Virtanen M, Taylor PR, Malila N, Albanes D, et al. Effects of supplemental alphatocopherol and beta-carotene on urinary tract cancer: incidence and mortality in a controlled trial (Finland) Cancer Causes Control. 2000;11(10):933–9. doi: 10.1023/a:1026546803917. [DOI] [PubMed] [Google Scholar]
- 15.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, et al. Prostate cancer and supplementation with alpha-tocopherol and B- carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–6. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 16.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-ris individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 17.Herberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study. Arandomized, Placebo-Controlled Trial of the Health Effects of Antioxidant Vitamins and Minerals. Arch Intern Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 18.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 19.The Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high risk patients. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative Group of the Primary Prevention Project (PPP) Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research Group. A Randomized Placebo-Controlled, Clinical Trial of High Dose Supplementation with Vitamins C and E and Beta Carotene for age related cataract and vision loss. Arch Ophthal. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JY, Taylor PR, LI B, Dawsey S, Wang GQ, Ershow AG, et al. Nutrition intervention trials in Linaxin, China: Multiple vitamin/mineral supplementation, cancer incidence, and disease specific mortality among adults with eosophageal dysplasia. J Natl Cancer Inst. 1993;85:1492–8. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 23.Davey PJ, Schulz M, Gliksman M, Dobson M, Aristides M, Stephens NG. Cost-effectiveness of vitamin E therapy in the treatment of patients with angiographically proven coronary narrowing (CHAOS trial). Cambridge Heart Antioxidant Study. Am J Cardiol. 1998;82:414–7. doi: 10.1016/s0002-9149(98)00354-3. [DOI] [PubMed] [Google Scholar]
- 24.Boaz M, Smetana S, Weinstien T, Matas Z, Gafter U, Laina A, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage disease (SPACE): randomized placebo-controlled trial. Lancet. 2000;356:1213–8. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 25.Lippman S, Goodman, Klein E, Parnes H, Thompson I, Kristal A, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman R. Prostate Cancer Chemoprevention: Strategies for Designing Efficient Clinical Trials. UROLOGY. 2001;57(Suppl 4A):224–229. doi: 10.1016/s0090-4295(00)00981-x. [DOI] [PubMed] [Google Scholar]
- 27.Alkhenizan A, Al-Omran M. The Role of Vitamin E in the prevention of Cardiovascular disease; Meta-analysis of Randomized Controlled Trials. Saudi Med J. 2004;25(12):447–453. [PubMed] [Google Scholar]
- 28.Eidelman R, Hollar D, Hebert P, Lamas G, Hennekens C. Randomized Trials of Vitamin E in the Treatment and Prevention of Cardiovascular Disease. Arch Intern Med. 2004;164:1552–1556. doi: 10.1001/archinte.164.14.1552. [DOI] [PubMed] [Google Scholar]
- 29.Christen WG, Gaziano JM, Hennekens CH. Design of physicians’ health study II; a randomized trial of beta-carotene, vitamin E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 30.Manson J, Gaziano J, Spelberg A, Ridker P, Cook N, Buring J, et al. A secondary prevention trial of antioxidant vitamins and cardiovascular disease in women; Rational, design, and methods. Ann Epidemiol. 1995;5:261–269. doi: 10.1016/1047-2797(94)00091-7. [DOI] [PubMed] [Google Scholar]