Abstract
BACKGROUND
Saudi Arabia is facing an epidemic of type 2 diabetes that is complicated by a high rate of chronic complications such as kidney disease, which have a major impact on the healthcare system and economy. The Saudi diabetic kidney disease (SAUDI-DKD) study was launched to understand the implications of chronic diabetic kidney disease.
OBJECTIVES
Examine the hematological, biochemical and metabolic parameters of the selected cohorts to look for biomarkers of diabetic nephropathy.
DESIGN
Cross-sectional, hospital-based.
SETTING
Four general hospitals and two dialysis centers in Riyadh.
PATIENTS AND METHODS
We recruited adult type 2 diabetic patients aged between 35 and 70 years, with a duration of diabetes >10 years, including subjects with microalbuminuria, macroalbuminuria and end stage renal disease (ESRD). They were compared with subjects with normal albumin excretion classified according to American Diabetes Association (ADA) criteria.
MAIN OUTCOME MEASURES
The effect of different stages of diabetic nephropathy on hematological and biochemical parameters.
RESULTS
Of 427 subjects with nephropathy, 184 (43%) had microalbuminuria, 83 (19%) had macroalbuminuria and 160 (37%) had end stage renal disease (ESRD). The remaining 213 (50%) subjects did not have nephropathy. Patients with nephropathy were older with a mean age (SD) of 55.62 (6.00) years and had a longer duration of diabetes (mean [SD], 19.04 [6.33]) years), and had a lower monthly income and body mass index (BMI) than patients without nephropathy. Insulin resistance, elevated uric acid level, low red blood cells (RBCs) count and low hemoglobin level were associated with significantly increased risk of macroalbuminuria and ESRD. Elevated uric acid and LDH were associated with significantly increased risk of microalbuminuria and ESRD, while elevated red blood cell distribution width was significantly associated with an increased risk of ESRD.
CONCLUSION
Diabetic nephropathy is associated with insulin resistance, changes in liver enzymes and uric acid in addition to abnormalities in the red blood cell count and red blood cell shape that warrant frequent monitoring among patients with diabetic kidney disease.
LIMITATIONS
Cross-sectional study design and exclusion of patients with some risk factors.
Diabetic nephropathy is one of the major causes of increased morbidity and mortality among diabetic patients worldwide, where it remains the single largest cause of chronic kidney disease.1 This complication has put a major burden on health care systems, particularly in developing countries.2 Saudi Arabia is one of the leading countries in terms of diabetes prevalence and associated complications,3,4 where almost one-third of type 2 Saudi diabetic population has diabetic nephropathy.5 In 2011, it was estimated that 42.5% of end stage renal disease (ESRD) cases in Saudi Arabia were related to diabetes,6 and the cost for dialysis in this country amounts to SR 173 784 (US$ 46 332) per patient per year.7
Diabetic nephropathy needs to be extensively studied to explore risk factors that might be related to its gradually increasing incidence in Saudi Arabia,5 especially when it is known that diabetic patients in this country have higher rate of complications,3 which can be attributed to poor diabetes control in addition to genetic factors that may result from the high rate of consanguineous marriage.8 For that reason the Strategic Center for Diabetes Research initiated the Saudi Diabetes Kidney Disease (SAUDI-DKD) study, selecting a cohort of type 2 diabetic patients. This study will evaluate genetics, proteomics, and biomarkers related to diabetic kidney disease. Such a holistic approach will be useful in early detection and prevention of this devastating and costly complication. This article describes the SAUDI-DKD cohort from a clinical point of view to evaluate hematology, liver and biochemical markers related to type 2 diabetic patients with various degrees of albuminuria and chronic kidney disease (CKD).
PATIENTS AND METHODS
The SAUDI-DKD study was designed to be a hospital- based cohort study of four groups of Saudi type 2 diabetic patients defined according to the American Diabetes Association (ADA) criteria 2005 with or without diabetic nephropathy.9 Normoalbuminuria, microalbuminuria, and macroalbuminuria patients were recruited from two university hospitals: King Abdulaziz Hospital, King Khalid Hospital and other two Ministry of Health general hospitals: Al-Iman Hospital and King Salman bin Abdulaziz Hospital. Patients with ESRD were recruited either from the dialysis units of those hospitals or from the hemodialysis care project of The Custodian of the Two Holy Mosques King Abdullah bin Abdulaziz Al-Saud Foundation for Humanitarian Affairs. Any diabetic patients who developed ESRD unrelated to diabetes were excluded.
Subject sampled by convenience between 1 April 2014 and 18 June 2015 were type 2 diabetic patients of either gender aged between 35 and 70 years and with a diabetes duration that exceeded 10 years. Pregnant women, patients with history of smoking and patients suffering from cancer or any renal disease were excluded. Patients exposed to radiocontrast agents or drugs that might affect their kidney functions like aminoglycoside, amphotericin, beta-lactam antibiotics, methotrexate, cisplatin, cyclosporine, or tacrolimus were excluded.
All subjects signed the consent form and their clinical data were obtained during the interview. On a mutually agreed day, patients were asked to attend the clinic after overnight fasting for blood and urine sampling. Patients were then classified according to their estimated glomerular filtration rate (eGFR) value into two groups with patients having eGRF <15 mL/min/1.73 m2 who had ESRD and were on hemodialysis and 480 subjects had eGFR of ≥30 mL/min/1.73 m2. Subjects in the second group were reclassified according to their urinary albumin excretion into three groups using ADA diabetic nephropathy stages cutoff values for urinary albumin excretion. Personal and social data were collected from all subjects using a pre-designed data collection sheet. Diabetes-related data including duration, family history, and presence of other chronic diabetes complications namely: vasculopathy, retinopathy and neuropathy and associated diseases, such as hypertension and hyperlipidemia, were collected from medical records. Anthropometric measurements and blood pressure were measured during the clinical visit.
Each subject was asked to fast for more than 10 hours and 20 cc of venous blood sample was collected using an EDTA tube for hematology including hemoglobin A1c (HbA1c) and a plain tube for serum to assess all other biochemistry markers. Each subject was asked to provide a fresh 10 cc urine sample in plain tube for ACR assessment. All the samples were transferred using a portable refrigerator with temperature adjusted between 4 and 8 C to the Strategic Center for Diabetes Research Central Laboratory.
The hematological parameters were determined using Mindray BC-3200 autohematology analyzer (Mindray Medical International Limited, Shenzhen, China), while liver function, renal function and lipid profile as well as urine parameters were performed in a RX Daytona clinical chemistry analyzer by Randox (UK). The HbA1c was assessed using a latex agglutination inhibition assay using the same chemistry analyzer. Insulin and c-peptide measurement were conducted using the biochip assay methodology in a Randox Evidence Biochip analyzer by Randox (UK) which is based on the standard immunoassay technique. Blood glucose assessment was done using glucose oxidase-peroxidase methodology, and serum cholesterol assessment was done using cholesterol oxidase-peroxidase methodology. The HDL, LDL and triglyceride were measured using direct and glycerol kinase oxidase-peroxidase methodology. The eGFR and albumin creatinine ratio (ACR) were calculated by specific calculators.10,11 This study was reviewed and approved by the institutional review (IRB), at College of Medicine, King Saud University.
Data were analyzed using IBM SPSS statistical package version 21. Continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as percentages. The t test was used for continuous variables and the chi square test was used for categorical variables. Homeostatic model assessment (HOMA) insulin resistance (IR) and β-cell function (B) were calculated using the software HOMA 2 calculator.12 The association between different parameters and stages of diabetic nephropathy was expressed as odds ratio (OR) and its 95% confidence intervals (CI) using a multivariate regression analysis model. A P value of less than .05 was used as a level of significance.
RESULTS
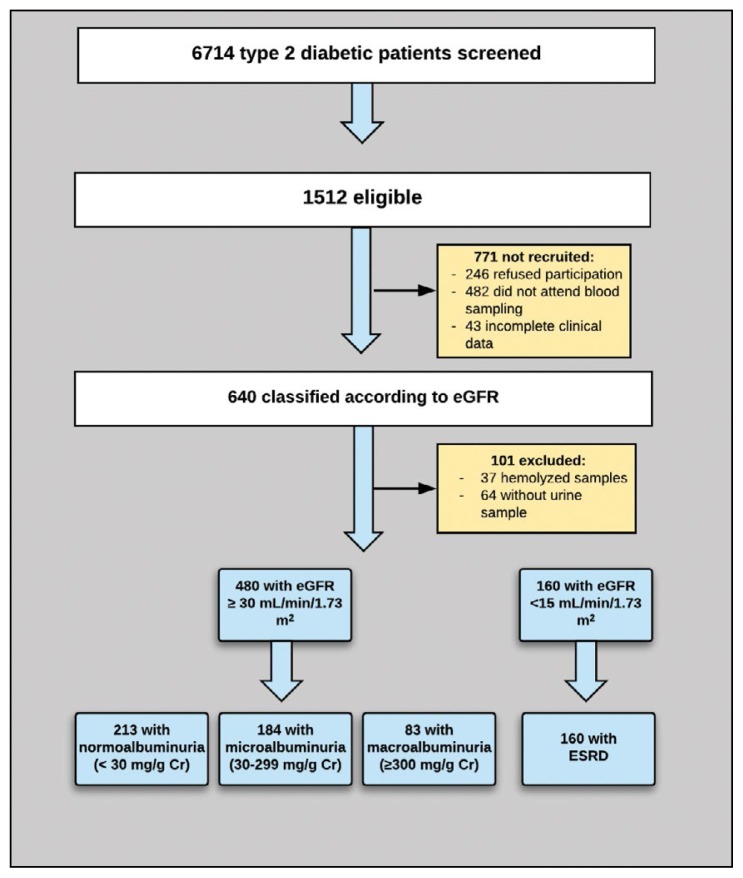
Of 6714 Saudi type 2 diabetic patients screened, 1512 were eligible and 741 were recruited after exclusions. One hundred one had to be excluded (unavailability of urine sample for 64 subjects and having a hemolyzed blood sample in 37 subjects) (Figure 1). Patients with nephropathy were older, had a longer diabetes duration, and had lower monthly income than patients without nephropathy (Table 1). The mean body mass index (BMI) was significantly lower among patients with nephropathy and was lowest among ESRD patients (P=.008). Despite control measures, systolic blood pressure (SBP) was significantly higher among patients with nephropathy (P<.001), which was not the case for diastolic blood pressure (DBP). The mean eGFR for nephropathic patients was 47.98 (45.65) mL/min/1.73 m2 compared to 79.01 (46.17) mL/min/1.73 m2 for patients with microalbuminuria and 50.14 (21.91) mL/min/1.73 m2 for patients with macroalbuminuria. The mean eGFR for cases with ESRD was 7.79 (4.32) mL/min/1.73 m2 (Table 1).
Figure 1.
Study flow chart for subject recruitment and classification.
Table 1.
Baseline characteristics of the study subjects according to nephropathy stage.
| Characteristics | Total Sample n=640 | Without nephropathy n=213 | With nephropathy n=427 | P value | Microal-buminuria n=184 | Macroal-buminuria n=83 | ESRD n=160 | P value |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age (years) | 56.64 (6.62) | 55.62 (6.00) | 57.52 (7.00) | <.0001 | 56.46 (6.05) | 55.93 (7.77) | 59.14 (7.27) | <.001 |
| Diabetes duration (years) | 18.21 (5.93) | 17.25 (5.26) | 19.04 (6.33) | <.0001 | 18.86 (5.77) | 19.52 (6.62) | 19.03 (6.77) | .751 |
| Monthly income (SR) | 10097 (7594) | 12017 (8171) | 8368 (6582) | <.0001 | 9877 (6947) | 10746 (7067) | 5538 (4678) | <.001 |
| Height (cm) | 160.50 (9.31) | 159.90 (8.80) | 161.02 (9.71) | .082 | 161.73 (9.72) | 161.42 (9.55) | 160.14 (9.75) | .264 |
| Weight (kg) | 82.15 (16.48) | 83.21(15.34) | 81.22 (17.38) | .076 | 84.91 (16.62) | 83.47 (17.55) | 76.66 (17.11) | <.001 |
| BMI (kg/m2) | 32.05 (6.20) | 32.65 (5.95) | 31.52 (6.38) | .008 | 32.57 (5.88) | 31.95 (6.27) | 30.27 (6.72) | .002 |
| Hip (cm) | 109.73 (13.00) | 110.06 (12.00) | 109.43 (13.84) | .480 | 110.63 (11.72) | 108.36 (14.01) | 108.58 (15.71) | .277 |
| Waist (cm) | 106.37 (12.94) | 105.70 (12.05) | 106.97 (13.68) | .151 | 107.95 (11.70) | 106.94 (13.10) | 105.94 (15.70) | .366 |
| W/H ratio | 0.97 (0.08) | 0.96 (0.08) | 0.98 (0.08) | .002 | 0.98 (0.07) | 0.98 (0.08) | 0.98 (0.09) | .941 |
| SBP (mm Hg) | 137.88 (20.05) | 131.56 (17.55) | 143.84 (20.46) | <.001 | 140.69 (19.35) | 147.09 (19.21) | 146.26 (21.79) | .014 |
| DBP (mm Hg) | 72.55 (11.44) | 72.29 (9.95) | 72.79 (12.68) | .532 | 74.61 (11.20) | 76.03 (13.10) | 69.26 (13.37) | <.001 |
| eGFR (mL/min/1.73m2) | 58.13 (41.69) | 79.43 (18.44) | 47.98 (45.65) | <.001 | 79.01 (46.17) | 50.14 (21.91) | 7.79 (4.32) | <.001 |
Data are mean (standard deviation). BMI: body mass index; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; SBP: systolic blood pressure; W/H: waist to hip; SR: Saudi Riyals.
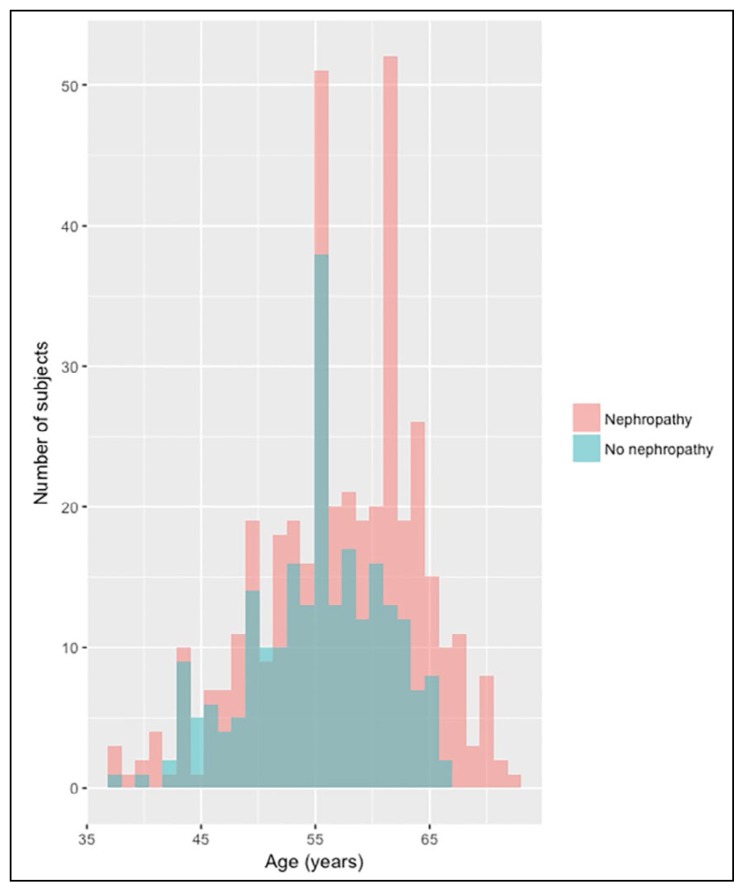
Subjects with diabetic nephropathy were older than those without nephropathy , but age distribution was normal in both groups (Figure 2) while ESRD cases had a mean age of more than 60 years. The longer the duration of diabetes, the more frequent diabetic nephropathy increased with longer diabetes duration, being the highest for a diabetes duration between 15 and 30 years. Positive family history of diabetes was more frequent among patients without nephropathy when compared with patients with nephropathy which is the same finding when looking at family history of renal disease. On the other hand, out of patients with positive family history of renal disease, 64.55% were having nephropathy, while 35.45% were among patients without nephropathy (Table 1).
Figure 2.
Age distribution of subjects with and without nephropathy.
Diabetic nephropathy in the studied cohort was significantly more frequent among lower social class. Subjects with diabetic nephropathy, had significantly higher rate of retinopathy and vasculopathy with P <.001, but not neuropathy when compared with patients without diabetic nephropathy. Both hypertension and hyperlipidemia were significantly more frequent among subjects with diabetic nephropathy (P<.001) as shown in Table 2.
Table 2.
Clinical and social characteristics by diabetic kidney disease stage.
| Characteristics | All Subjects n (%) | Without nephropathy n(%) | With nephropathy n(%) | P value | Diabetic kidney disease | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Micro-albuminuria | Macro-albuminuria | ESRD | |||||||
|
| |||||||||
| Number | 640 | 213 | 427 | 184 | 83 | 160 | |||
| Age | 30-<45 years | 31 (4.84) | 9 (4.23) | 22 (5.15) | <.001 | 8 (4.35) | 8 (9.64) | 6 (3.75) | .001 |
| 45–60 years | 408 (63.75) | 164 (77.00) | 244 (57.14) | 122 (66.30) | 48 (57.83) | 74 (46.25) | |||
| > 60 years | 201 (31.41) | 40 (18.77) | 161 (37.71) | 54 (29.35) | 27 (32.53) | 80 (50.00) | |||
| Gender | Male | 285 (44.53) | 79 (37.09) | 206 (48.24) | .062 | 84 (45.65) | 48 (57.83) | 74 (46.25) | .189 |
| Female | 355 (55.47) | 134 (62.91) | 221 (51.76) | 100 (54.35) | 35 (42.17) | 86 (53.75) | |||
| Diabetes duration | <15 years | 182 (28.44) | 73 (34.27) | 109 (25.53) | .017 | 46 (25.00) | 19 (22.89) | 44 (27.50) | .411 |
| 15–30 years | 444 (69.38) | 138 (64.79) | 306 (71.66) | 135 (73.37) | 61 (73.49) | 110 (68.75) | |||
| >30 years | 14 (2.18) | 2 (0.94) | 12 (2.81) | 3 (1.63) | 3 (3.62) | 6 (3.75) | |||
| Family history | Diabetes | 551 (86.09) | 192 (90.14) | 359 (84.07) | .036 | 159 (86.41) | 74 (89.16) | 126 (83.13) | .793 |
| Renal disease | 110 (17.19) | 39 (18.31) | 71 (16.63) | .052 | 37 (20.11) | 7 (8.43) | 27 (16.87) | .005 | |
| Marital status | Single | 22 (3.44) | 5 (2.35) | 17 (3.98) | <.001 | 7 (3.81) | 2 (2.42) | 8 (5.00) | .289 |
| Married | 514 (80.31) | 188 (88.26) | 326 (76.35) | 141 (76.63) | 71 (85.53) | 114 (71.25) | |||
| Divorced | 23 (3.59) | 5 (2.35) | 18 (4.22) | 9 (4.89) | 2 (2.42) | 7 (4.38) | |||
| Widow | 81 (12.66) | 15 (7.04) | 66 (15.46) | 27 (14.67) | 8 (9.63) | 31 (19.37) | |||
| Social class | I | 201 (31.41) | 43 (20.19) | 158 (37.00) | <.001 | 51 (27.72) | 21 (25.30) | 86 (53.75) | <.001 |
| II | 218 (34.06) | 73 (34.27) | 145 (33.96) | 64 (34.78) | 23 (27.71) | 58 (36.25) | |||
| III | 169 (26.41) | 75 (35.21) | 94 (22.01) | 52 (28.26) | 28 (33.73) | 14 (8.75) | |||
| IV | 52 (8.12) | 22 (10.33) | 30 (7.03) | 17 (9.24) | 11 (13.26) | 2 (1.25) | |||
| DM complications | Neuropathy | 323 (50.47) | 106 (49.77) | 217 (50.82) | .38 | 96 (52.17) | 42 (50.60) | 79 (49.38) | .863 |
| Retinopathy | 341 (53.28) | 63 (29.58) | 278 (65.11) | <.001 | 97 (52.27) | 59 (71.08) | 122 (76.25) | <.001 | |
| Vasculopathy | 138 (21.56) | 15 (7.04) | 123 (28.81) | <.001 | 40 (21.74) | 25 (30.12) | 58 (36.25) | <.001 | |
| Associated diseases | Hypertension | 477 (74.53) | 119 (55.87) | 358 (83.84) | <.001 | 144 (78.26) | 69 (83.13) | 145 (90.63) | .003 |
| Hyperlipidemia | 32 (5.00) | 9 (4.23) | 23 (5.39) | <.001 | 9 (4.89) | 8 (9.64) | 6 (3.75) | .062 | |
Data are mean (standard deviation) or number (percentage). ESRD: end-stage renal disease.
In Table 3, subjects with microalbuminuria demonstrated significantly high mean values of white blood cells (WBC) and mean corpuscular hemoglobin (MCH) when compared to patients with without diabetic nephropathy. The subjects with microalbuminuria had demonstrated decreased mean values for RBCs count, hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), Red blood cell distribution width (RDW-SD) and Red blood cell distribution width (RDW-CV), while significantly increased mean values of WBC and mean corpuscular hemoglobin concentration (MCHC) compared with those without nephropathy (P value=. 015 and 0.37 respectively). The subjects with ESRD showed a significant decrease in the mean values of Hb, RBC, Hct, platelets and plateletcrit (PCT) but a significant increase in MCV, MCH, MCHC, RDW-SD, and RDW-CV.
Table 3.
Hematology, biochemistry and lipid parameters by diabetic kidney disease group.
| Parameters (measurement, unit) | No kidney disease | Diabetic kidney disease | Total | Micro-albuminuria | P value | Macro-albuminuria | P value | ESRD | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Complete blood count | WBC (109/L) | 6.81 (2.47) | 6.43 (2.37) | 6.99 (2.50) | 6.92 (2.74) | .048 | 7.30 (2.79) | .015 | 6.92 (2.39) | .057 |
| RBC (1012/L) | 4.63 (0.95) | 4.89 (0.68) | 4.51 (1.03) | 4.85 (0.87) | .647 | 4.65 (0.97) | .053 | 4.04 (1.06) | <.001 | |
| Hb (%) | 12.79 (2.63) | 13.41 (2.49) | 12.50 (2.66) | 13.36 (2.18) | .809 | 12.59 (2.38) | .013 | 11.47 (2.93) | <.001 | |
| Platelets (109/L) | 268.26 (79.71) | 272.42 (76.88) | 266.16 (81.20) | 275.96 (75.15) | .650 | 276.32 (88.94) | .717 | 249.71 (81.82) | .008 | |
| Hct (%) | 39.47 (8.61) | 41.74 (7.37) | 38.41 (8.96) | 41.08 (8.29) | .410 | 38.13 (7.17) | <.001 | 35.40 (9.60) | <.001 | |
| MCV (fL) | 85.61(8.18) | 85.61 (9.94) | 85.62 (7.20) | 84.83 (7.58) | .386 | 83.38 (7.07) | .036 | 87.71 (6.24) | .015 | |
| MCH (pg/cell) | 28.04 (2.85) | 27.58 (3.35) | 28.27 (2.56) | 28.22 (2.64) | .035 | 27.43 (2.41) | .674 | 28.75 (2.43) | <.001 | |
| MCHC (g/dL) | 32.71 (2.27) | 32.37 (2.74) | 32.86 (1.99) | 32.78 (2.28) | .110 | 33.02 (2.12) | .037 | 32.86 (1.50) | .032 | |
| RDW-SD (fL) | 44.67 (5.09) | 44.22 (5.23) | 44.87 (5.04) | 43.58 (4.78) | .241 | 42.40 (4.61) | .011 | 47.35 (4.41) | <.001 | |
| RDW-CV (%) | 14.35 (1.19) | 14.33 (1.27) | 14.36 (1.15) | 14.11 (1.05) | .089 | 13.91 (0.93) | .004 | 14.83 (1.18) | <.001 | |
| MPV (fL) | 11.78 (1.49) | 11.85 (1.58) | 11.75 (1.45) | 11.81 (1.59) | .800 | 11.64 (1.53) | .297 | 11.75 (1.23) | .468 | |
| PCT (%) | 0.33 (0.10) | 0.33 (0.10) | 0.32 (0.10) | 0.34 (0.10) | .652 | 0.33 (0.10) | .879 | 0.30 (0.09) | <.001 | |
| Renal function | Na (mmol/L) | 146.41 (12.62) | 144.59 (7.73) | 147.199 (14.18) | 148.50 (7.73) | <.001 | 144.057 (8.99) | .660 | 147.03 (18.69) | .125 |
| K (mmol/L) | 4.98 (0.82) | 4.54 (0.48) | 5.18 (0.86) | 4.74 (0.43) | <.001 | 4.95 (0.57) | <.001 | 5.81 (0.96) | <.001 | |
| Ca (mg/dL) | 10.63 (2.15) | 10.27 (1.83) | 10.80 (2.27) | 11.37 (1.35) | <.001 | 10.70 (2.65) | .201 | 10.25 (2.68) | .944 | |
| Urea (mg/dL) | 61.54 (50.85) | 29.49 (15.13) | 76.53 (54.72) | 38.49 (17.51) | <.001 | 57.95 (35.19) | <.001 | 133.50 (45.02) | <.001 | |
| Creatinine (mg/dL) | 2.63 (2.87) | 0.96 (0.28) | 3.42 (3.19) | 1.15 (0.85) | .004 | 1.71 (1.00) | <.001 | 7.02 (2.36) | <.001 | |
| Uric acid (mg/dL) | 5.69 (1.70) | 4.79 (1.33) | 6.11 (1.69) | 5.62 (1.63) | <.001 | 6.42 (1.84) | <.001 | 6.55 (1.53) | <.001 | |
| ACR (mg/g) | 98.00 (123.18) | 10.67 (10.18) | 165.45 (128.16) | 111.31 (73.43) | <.001 | 357.89 (95.47) | <.001 | 188.13 (112.28) | .012 | |
| Liver function tests | D bilirubin (mg/dL) | 0.07 (0.09) | 0.07 (0.09) | 0.07 (0.09) | 0.07 (0.11) | .811 | 0.04 (0.06) | .001 | 0.08 (0.07) | .270 |
| T bilirubin (mg/dL) | 0.47 (0.21) | 0.45 (0.24) | 0.47 (0.20) | 0.48 (0.23) | .213 | 0.43 (0.21) | .398 | 0.48 (0.16) | .140 | |
| T protein (g/L) | 71.87 (9.88) | 71.19 (10.33) | 72.25 (9.67) | 73.95 (10.42) | .009 | 72.03 (8.22) | .518 | 70.29 (9.07) | .384 | |
| Albumin (g/L) | 43.33 (6.39) | 43.78 (4.28) | 43.15 (7.17) | 45.37 (7.12) | .008 | 43.50 (6.21) | .703 | 40.24 (6.70) | <.001 | |
| ALT (U/L) | 12.65 (7.94) | 13.91 (7.79) | 12.09 (7.97) | 13.12 (8.27) | .328 | 12.96 (6.32) | .290 | 10.39 (8.10) | <.001 | |
| AST (U/L) | 22.73 (9.52) | 22.48 (8.69) | 22.87 (9.92) | 23.69 (9.73) | .194 | 23.06 (6.93) | .593 | 21.74 (11.31) | .500 | |
| LDH (U/L) | 163.57 (60.03) | 140.40 (51.16) | 174.70 (60.78) | 153.49 (56.34) | .016 | 174.40 (48.70) | <.001 | 201.33 (61.72) | <.001 | |
| GGT (U/L) | 42.28 (30.23) | 36.45 (25.61) | 45.02 (31.88) | 36.87 (22.83) | .864 | 47.35 (29.08) | .002 | 53.52 (39.37) | <.001 | |
| Lipid profile | Cholesterol (mg/dL) | 181.25 (45.94) | 174.34 (36.51) | 184.77 (49.43) | 190.10 (52.47) | .001 | 195.11 (46.34) | .001 | 173.12 (44.91) | .776 |
| HdL (mg/dL) | 45.27 (13.03) | 45.89 (11.67) | 45.06 (13.64) | 47.45 (13.93) | .228 | 44.95 (11.93) | .546 | 42.12 (13.61) | .006 | |
| LdL (mg/dL) | 124.78 (41.88) | 131.79 (41.20) | 121.65 (41.92) | 130.44 (42.58) | .751 | 130.46 (38.92) | .806 | 106.97 (38.54) | <.001 | |
| Triglyceride (mg/dL) | 183.13 (89.43) | 155.91 (62.29) | 195.93 (97.16) | 188.00 (90.83) | <.001 | 218.40 (100.83) | <.001 | 194.32 (101.60) | <.001 | |
| Metabolic markers | FBS (mg/dL) | 216.32 (93.53) | 188.63 (74.85) | 230.07 (98.44) | 230.64 (92.58) | <.001 | 243.96 (103.63) | <.001 | 222.35 (102.36) | .001 |
| HbA1c (%) | 10.40 (2.06) | 10.13 (1.51) | 10.52 (2.27) | 10.86 (1.95) | <.001 | 11.44 (2.45) | <.001 | 9.65 (2.26) | .023 | |
| Insulin (uIU/mL) | 35.07 (32.72) | 33.50 (32.66) | 35.70 (32.53) | 29.53 (28.36) | .195 | 46.82 (4.023) | .010 | 37.22 (30.72) | .313 | |
| C-peptide (ng/mL) | 1.68 (2.55) | 0.63 (0.68) | 2.16 (2.92) | 0.68 (0.98) | .622 | 0.69 (0.67) | .509 | 4.64 (3.44) | <.001 | |
| HOMA-IR | 4.40 (10.20) | 2.95 (2.20) | 5.23 (12.65) | 4.16 (3.50) | <.001 | 8.03 (24.87) | <.001 | 5.35 (11.76) | .057 | |
| HOMA-B | 63.88 (63.42) | 65.85 (53.35) | 62.79 (68.44) | 59.69 (72.33) | .408 | 51.49 (53.22) | .111 | 73.67 (68.11) | .307 | |
Mean (standard deviation). ACR: albumin creatinine ratio; ALT: alanine aminotransferase; AST: aspartate aminotransferase; D bilirubin: direct bilirubin; FBS: fasting blood glucose; GGT: gamma-glutamyl transpeptidase; Hb: hemoglobin; HbA1c: hemoglobin A1c; Hct: hematocrit; HdL high-density lipoprotein; HOMA B: homeostatic model assessment β-cell function (B); HOMA IR: homeostatic model assessment insulin resistance; LDH: lactate dehydrogenase; LdL: low-density lipoprotein; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; MPV: mean platelet volume; PCT: plateletcrit; RBC: red blood cells; RDW: RDW-CV: Red blood cell distribution width coefficient of variation; RDW-SD: Red blood cell distribution width standard discrepancy; SD: standard deviation; T bilirubin: total bilirubin; T protein: white blood cells. P value was calculated between patients with and without diabetic kidney disease.
The mean values of potassium, urea, creatinine and uric acid were significantly increasing with the progression of diabetic nephropathy. Total protein and albumin were significantly higher for subjects with microalbuminuria, while subjects with microalbuminuria did not show any significant difference in the mean values of both proteins. The LDH and gamma-glutamyl trans-peptidase (GGT) have shown significant higher values among microalbuminuria subjects and ESRD patients.
In this study, the mean values of total cholesterol and triglycerides were significantly higher among microalbuminuria and macroalbuminuria subjects which was not the case for high-density lipoprotein (HDL) and low-density lipoprotein (LDL), except for those who have ESRD, where they demonstrated significantly lower values.
Glycemic control represented by the values of fasting blood glucose and HbA1c was the worst among subjects with microalbuminuria followed by subjects with microalbuminuria and ESRD. Insulin level was significantly higher among subjects with microalbuminuria, while C-peptide was significantly higher among patients with ESRD. HOMA-IR as indication for insulin resistance was significantly higher in all-stages of diabetic nephropathy (P<.001), while beta cell function expressed by HOMA-B did not show any significant difference.
When looking at the association of different hematological and biochemical parameters with different stages of diabetic nephropathy, the presence of uric acid >6.8 mg/dL for males and >6 mg/dL for females was associated with increased risk for microalbuminuria and macroalbuminuria (P<.001). Higher level of LDH was associated with high risk of microalbuminuria (P=.009). Insulin resistance presented by HOMA-IR>4.32 was associated with increased risk of microalbuminuria and ESRD only (P=.004). Increased RDW was only associated with ESRD. Increased Hct and WBCs were not associated with any risk for diabetic nephropathy. The low RBCs count and hemoglobin level in both males and females were associated with more than two times increased risk for microalbuminuria (P=.013). All the tested parameters were significantly associated with increased risk of ESRD (P<.001), except for higher levels of WBCs count and Hct as seen in Table 4.
Table 4.
Multinomial logistic regression analysis of factors associated with different types of nephropathy.
| Risk factors | Microalbuminuria | Macroalbuminuria | ESRD | |||
|---|---|---|---|---|---|---|
| Odds Ratio | P value | Odds Ratio | P value | Odds Ratio | P value | |
|
| ||||||
| Uric acid for males >6.8 mg/dL | 6.30 (2.25–17.54) | <.001 | 3.97 (1.58–9.99) | <.001 | 13.42 (5.04–35.70) | <.001 |
| Uric acid for females >6.0 mg/ dL | 4.70 (2.04–10.83) | <.001 | 1.67 (0.82–3.41) | .161 | 11.30 (4.90–26.09) | <.001 |
| LDH >190 U/L | 2.33 (1.23–4.39) | .009 | 1.37 (0.81–2.32) | .246 | 7.34 (4.19–12.85) | <.001 |
| HOMA-IR ≥4.32 | 1.85 (0.86–3.96) | .116 | 2.11 (1.23–3.62) | .007 | 2.57 (1.34–4.90) | .004 |
| WBCs count >11 109/L | 1.82 (0.57–5.86) | .313 | 1.41 (0.52–3.78) | .499 | 1.10 (0.32–3.75) | .877 |
| RBCs count <4.7 1012/L | 1.190 (0.72–1.99) | .496 | 2.40 (1.20–4.78) | .013 | 13.26 (5.83–30.15) | <.001 |
| Hemoglobin for females <12% | 1.14 (0.56–2.32) | .718 | 2.82 (1.07–7.38) | .035 | 7.77 (3.33–18.12) | <.001 |
| RDW ≥14.5% | 0.72 (0.38–1.38) | .329 | 0.87 (0.54–1.39) | .562 | 2.84 (1.70–4.74) | <.001 |
| Hemoglobin for males <13% | 0.71 (0.26–1.96) | .508 | 3.62 (1.20–4.78) | .019 | 19.66 (5.89–65.60) | <.001 |
| Hct ≥52% | 0.57 (0.11–2.87) | .497 | 1.45 (0.57–3.69) | .437 | 1.01 (0.33–3.15) | .981 |
Hb: hemoglobin; Hct: hematocrit; HOMA B: homeostatic model assessment b-cell function (B); HOMA IR: homeostatic model assessment insulin resistance LDH: lactate dehydrogenase; RBC: red blood cells; RDW: Red blood cell distribution width; WBC: white blood cells.
Adjust for age, diabetes duration, BMI, SBP and DBP.
Model-fitting information: −2 Log Likelihood:Uric Acid for males >6.8 μmol/L: 772.2; Uric Acid for females ≥6.8 μmol/L: 772.2; 1670.0; LDH >190 U/L: 1670.0; HOMA-IR ≥4.32: 1057.5; WBCs count >11×109/L:1635.8; RBCs<4.7 1012 /L: 1622.6; Hemoglobin for females <12%: 857.8; RDW ≥14.5%: 1498.6; Hemoglobin for Male <13%: 758.0; HCT ≥ 53%: 622.2
DISCUSSION
Since SAUDI-DKD study is aiming to assess clinical and biochemical parameters related to diabetic kidney disease, subjects were recruited using ADA case definition for different stages of diabetic nephropathy namely: microalbuminuria, marcoalbuminuria and ESRD. We started screening of more than 5000 Saudi type 2 diabetic patients to come up with 427 subjects with diabetic kidney disease. The real challenge for the patients’ recruitment was to come up with enough number cases with marcoalbuminuria since in this society similar to other societies patients with marcoalbuminuria swiftly go to ESRD.13
In consistence with other studies, subjects with diabetic nephropathy were older with longer diabetes duration, and had higher blood pressure which are well-established risk factors for diabetic nephropathy.14,15 This observation has been previously reported among a large cohort of Saudi type 2 diabetic patients at a country level.16 The significantly lower mean BMI among subjects with nephropathy observed in this study could be as a consequence of renal failure.17 The weight loss observed among patients with diabetic nephropathy, especially among patients with ESRD could result from poor appetite and metabolic acidosis that would reduce both muscle and fat mass.18
In consistence with other studies that have shown family history of renal disease as a strong predictor for kidney disease,19 the current study has shown that the vast majority of patients with positive family history of renal disease were suffering from diabetic nephropathy.
The majority of the studied cohort were married subjects, however with the disease progression and owing to advanced age and longer diabetes duration, the percentage of widows was higher in subjects with ESRD compared with subjects with microalbuminuria and marcoalbuminuria. More than 70% of patients with diabetic nephropathy were in the low and middle social class with monthly income of <10 000 SR and this observation is even worse among patients with ESRD. This is in consistence with the finding that low socioeconomic status is associated with both the development and progression of chronic kidney disease.20
Diabetic retinopathy had the highest frequency among subjects with nephropathy especially in marcoalbuminuria and ESRD which is a well-established association, where microalbumiuria is considered as a state of generalized vascular dysfunction.21 The presence of vasculopathy was more frequent among patients with diabetic nephropathy, which could be explained on one hand by the fact that both conditions are co-existing traditional risk factors. On the other hand, microvascular diseases promote atherosclerosis through processes such as hypoxia and changes in vasa vasorum. 22 Hypertension affected more than 80% of subjects with nephropathy compared with 50% among patients with normal albumin excretion, which could be due to several mechanisms including the activation of sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS), endothelial cell dysfunction (ECD), and excess sodium retention as well as oxidative stress.23 The hyperlipidemia observed in diabetic patients, which could be secondary to diabetes, may exaggerate the effect of hyperglycemia on the kidney tissue, since the triglyceride-rich lipoprotein may induce glomerular damage through the activation of the transforming growth factor-beta (TGF-β) pathway.24 It can also activate monocytes and disrupt cellular glycocalyx which exaggerate permeability of the glomerulus.25 However, the frequency of hyperlipidemia did not show an increasing pattern with the progression of diabetic nephropathy which could reflect using lipid lowering agents among such patients.26
Anemia was found to be an independent predictor for progression of diabetic nephropathy in many studies, especially among patients with ESRD.27 This supports our observation of the significant decrease in the mean concentration of Hb and RBCs count in subjects with microalbuminuria and ESRD which could be due to impaired erythropoietin production and factors that may suppress bone marrow erythropoiesis and shortened red cell survival.28 This could also explain the significant association that was observed in the current study between the two parameters and different stages of diabetic nephropathy when calculating the OR. Therefore, this hematological changes can be considered an indicative for renal damage in diabetic patients. The picture of anemia observed among the study cohort was more likely to be anemia of chronic diseases for patients with microalbuminuria and marcoalbuminuria, where the mean values of both MCV and MCH were not affected significantly.29 However, it was more in favor of megaloblastic anemia with high mean values of MCV and MCH among ESRD subjects. Such finding could be due to vitamin B12 deficiency that is associated with poor nutritional intake among dialysis patients, in addition to addition to the fact that patients on dialysis are limited to low B12 foods, since foods rich in B12 are known to contain high concentrations of electrolytes harmful to dialysis patients.30 Hematocrit, on the other hand decreases with the progression of nephropathy, especially among microalbuminuria and ESRD which could result from fluid retention associated with renal impairment.31
Similar to what has been observed among Caucasians, our population also showed a progressive impairment in red blood cell deformability in the current study, especially in microalbuminuria and marcoalbuminuria stages. The impairment of red blood cell deformability could be related to hyperglycemia, accumulation of advanced glycation end products (AGEs), and impaired renal functions.32 On the other hand, patients with ESRD had shown elevated RDW, which is similar to the finding of Tekce et al, and could be due to adverse effects of inflammation, malnutrition, and excess intradialytic weight gain (IDWG) in the patients being on hemodialysis.33
The leukocytosis observed in this study among patients with diabetic nephropathy is similar to what has been reported among Taiwanese type 2 diabetic that could be due to the inflammatory process associated with microangiopathy.34 The mechanisms for such change could be related to the increased plasma cortisol and insulin levels in renal disease since both are known to increase WBC counts by increasing neutrophil influx from marrow storage and decreasing efflux from the blood stream.35,36 Although the literature support the increase in the platelets count with progression of diabetic nephropathy,37 our study did not demonstrate this finding which could be explained on the basis that impaired erythropoietin secretion decreases the platelet count due to the extensive homology between the erythropoietin and thrombopoietin thus it acts as a humoral regulator of platelet mass.28 Additionally, the significant drop in platelets counts among patients with ESRD could be a consequence of hemodialysis procedure.38
This study did not demonstrate any correlation between DKD and total bilirubin, although direct bilirubin was significantly lower among patients with marcoalbuminuria which is consistent with the observation that hyperbilirubinemia is associated with reduced risk of diabetic nephropathy.39 Total protein and albumin were significantly higher among patients with microalbuminuria, while they were lower among patients with marcoalbuminuria most likely due to protein loss and may reflect as well nutritional status.
The progression of diabetic kidney disease is associated with increased levels of LDH in this study indicating kidney damage, while increasing GGT reflects endothelial dysfunction that is strongly associated with advanced chronic kidney disease. Therefore, it could be speculated that elevated serum GGT level might be a biomarker rather than a reflection of oxidative stress or inflammatory process.40,41
Insulin resistance has been reported to increase glomerular hydrostatic pressure and renal vascular permeability.42 This will aggravate glomerular hyper-filtration and enhance renal sodium reabsorption43 which goes with the current study findings presented by higher insulin and C-peptide level along with high values of HOMA-IR in subjects suffering from marcoalbuminuria and ESRD. In this study the significantly high uric acid level observed among subjects with kidney disease could be secondary to decreased eGFR and increased uric acid reabsorption.44
The current study is limited by being cross-sectional study design which only provides the basis for associations rather than causality. Another limitation of this study was excluding patients with some risk factors such as smoking which might affect the generalizability of the study results. On the other hand, this study draws its strength from the large sample size and clearly defined cases. All laboratory assessment were performed in an internationally accredited central laboratory, where all performed test had low intra and inter assay variation coefficient.
In conclusion, the presence of diabetic kidney disease on the top of hyperglycemia would exaggerate its effect on hematological, biochemical and metabolic parameters. This warrants proper monitoring of such patients, especially with their increased risk of anemia with nephropathy progression. Early initiation of lipid lowering agent may be beneficial in preserving renal functions among such patients. Improving insulin sensitivity could be one of the strategies to delay the progression of diabetic nephropathy and it is highly recommended that patients should be advised to take all measures that improve insulin sensitivity. Liver enzymes as well as uric acid level should be monitored frequently among patients with diabetic kidney disease.
Further investigations with longitudinal prospective studies to evaluate the values in these laboratory findings through the course of the disease is recommended.
Footnotes
Conflict of interest
The authors have no conflict of interest.
Funding
Study funded by King Abdulaziz City for Science and Technology (KACST), grant for project no A-T-34-194.
REFERENCES
- 1.Lee SY, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol. 2015;30(7):1063–75. doi: 10.1007/s00467-014-2888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–70. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 3.Al-Rubeaan K, Al-Manaa H, Khoja T, Ahmad N, Al-Sharqawi A, Siddiqui K, et al. The Saudi Abnormal Glucose Metabolism and Diabetes Impact Study (SAUDI-DM) Ann Saudi Med. 2014;34(6):465–75. doi: 10.5144/0256-4947.2014.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbar DH, Mira SA, Zawawi TH, Malibary HM. Subclinical diabetic neuropathy: a common complication in Saudi diabetics. Saudi Med J. 2000;21(5):433–7. [PubMed] [Google Scholar]
- 5.Alwakeel JS, Al-Suwaida A, Isnani AC, Al-Harbi A, Alam A. Concomitant macro and microvascular complications in diabetic nephropathy. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2009;20(3):402–9. [PubMed] [Google Scholar]
- 6.Al-Sayyari AA, Shaheen FA. End stage chronic kidney disease in Saudi Arabia. A rapidly changing scene. Saudi Med J. 2011;32(4):339–46. [PubMed] [Google Scholar]
- 7.Al Saran K, Sabry A. The cost of hemodialysis in a large hemodialysis center. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2012;23(1):78–82. [PubMed] [Google Scholar]
- 8.el-Hazmi MA, al-Swailem AR, Warsy AS, al-Swailem AM, Sulaimani R, al-Meshari AA. Consanguinity among the Saudi Arabian population. J Med Genet. 1995;32(8):623–6. doi: 10.1136/jmg.32.8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4–36. [PubMed] [Google Scholar]
- 10.GFR Calculator - Glomerular Filtration Rate - DaVita [Internet] [cited 2017 Nov 15]. Available from: https://www.davita.com/gfrcalculator/
- 11.Albumin: Creatinine Ratio: MediCalc® [Internet] [cited 2017 Nov 15]. Available from: http://www.scymed.com/en/smnxps/psdjb222.htm.
- 12.The Oxford Centre for Diabetes. HOMA Calculator [Internet] Software from the DTU; Endocrinology & Metabolism. Diabetes Trial Unit. [cited 2016 Nov 13]. Available from: https://www.dtu.ox.ac.uk/ToolsSoftware/ [Google Scholar]
- 13.Alwakeel JS, Isnani AC, Alsuwaida A, Alharbi A, Shaffi SA, Almohaya S, et al. Factors affecting the progression of diabetic nephropathy and its complications: a single- center experience in Saudi Arabia. Ann Saudi Med. 2011;31(3):236–42. doi: 10.4103/0256-4947.81528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45) Diabetes Care. 2007;30(8):2019–24. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 15.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, et al. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30(10):2523–8. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 16.Al-Rubeaan K, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Al-Mutlaq HM, et al. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PloS One. 2014;9(2):e88956. doi: 10.1371/journal.pone.0088956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen KL, Lee C. Body composition in chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24(3):268–75. doi: 10.1097/MNH.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2(1):9–25. doi: 10.1007/s13539-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int. 2006;70(10):1694–705. doi: 10.1038/sj.ki.5001794. [DOI] [PubMed] [Google Scholar]
- 20.Bello AK, Peters J, Rigby J, Rahman AA, El Nahas M. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol CJASN. 2008;3(5):1316–23. doi: 10.2215/CJN.00680208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rani PK, Raman R, Gupta A, Pal SS, Kulothungan V, Sharma T. Albuminuria and Diabetic Retinopathy in Type 2 Diabetes Mellitus Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular Genetic Study (SN-DREAMS, report 12) Diabetol Metab Syndr. 2011;3(1):9. doi: 10.1186/1758-5996-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–51. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawanami D, Matoba K, Utsunomiya K. Dyslipidemia in diabetic nephropathy. Ren Replace Ther. 2016;2:16. [Google Scholar]
- 24.Chen S, Tseng C-H. Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud RDS. 2013;10(2–3):88–100. doi: 10.1900/RDS.2013.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saja MF, Baudino L, Jackson WD, Cook HT, Malik TH, Fossati-Jimack L, et al. Triglyceride- Rich Lipoproteins Modulate the Distribution and Extravasation of Ly6C/Gr1(low) Monocytes. Cell Rep. 2015;12(11):1802–15. doi: 10.1016/j.celrep.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campese VM. Dyslipidemia and progression of kidney disease: role of lipid-lowering drugs. Clin Exp Nephrol. 2014;18(2):291–5. doi: 10.1007/s10157-014-0934-9. [DOI] [PubMed] [Google Scholar]
- 27.Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66(3):1131–8. doi: 10.1111/j.1523-1755.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 28.Suresh M, Mallikarjuna N, Sharan B, Singh M, Hari Krishna B, Shravya KG, et al. Hematological Changes in Chronic Renal Failure. IJSRP September 2012 Publication. Int J Sci Res Publ. 2012;2(9):1–4. [Google Scholar]
- 29.Wians FH, Urban JE, Keffer JH, Kroft SH. Discriminating between iron deficiency anemia and anemia of chronic disease using traditional indices of iron status vs transferrin receptor concentration. Am J Clin Pathol. 2001;115(1):112–8. doi: 10.1309/6L34-V3AR-DW39-DH30. [DOI] [PubMed] [Google Scholar]
- 30.Saifan C, Samarneh M, Shtaynberg N, Nasr R, El-Charabaty E, El-Sayegh S. Treatment of confirmed B12 deficiency in hemodialysis patients improves Epogen® requirements. Int J Nephrol Renov Dis. 2013;6:89–93. doi: 10.2147/IJNRD.S44660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda H, Ishimura E, Shoji T, Emoto M, Morioka T, Matsumoto N, et al. Factors affecting progression of renal failure in patients with type 2 diabetes. Diabetes Care. 2003;26(5):1530–4. doi: 10.2337/diacare.26.5.1530. [DOI] [PubMed] [Google Scholar]
- 32.Brown CD, Ghali HS, Zhao Z, Thomas LL, Friedman EA. Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int. 2005;67(1):295–300. doi: 10.1111/j.1523-1755.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 33.Tekce H, Kin Tekce B, Aktas G, Tanrisev M, Sit M. The evaluation of red cell distribution width in chronic hemodialysis patients. Int J Nephrol. 2014;2014:754370. doi: 10.1155/2014/754370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung F-M, Tsai JC-R, Chang D-M, Shin S-J, Lee Y-J. Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes Care. 2005;28(7):1710–7. doi: 10.2337/diacare.28.7.1710. [DOI] [PubMed] [Google Scholar]
- 35.Collier A, Patrick AW, Hepburn DA, Bell D, Jackson M, Dawes J, et al. Leucocyte mobilization and release of neutrophil elastase following acute insulin-induced hypoglycaemia in normal humans. Diabet Med J Br Diabet Assoc. 1990;7(6):506–9. doi: 10.1111/j.1464-5491.1990.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 36.Bjornson BH, Harvey JM, Rose L. Differential effect of hydrocortisone on eosinophil and neutrophil proliferation. J Clin Invest. 1985;76(3):924–9. doi: 10.1172/JCI112091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterner G, Carlson J, Ekberg G. Raised platelet levels in diabetes mellitus complicated with nephropathy. J Intern Med. 1998;244(6):437–41. [PubMed] [Google Scholar]
- 38.Gafter U, Bessler H, Malachi T, Zevin D, Djaldetti M, Levi J. Platelet count and thrombopoietic activity in patients with chronic renal failure. Nephron. 1987;45(3):207–10. doi: 10.1159/000184118. [DOI] [PubMed] [Google Scholar]
- 39.Toya K, Babazono T, Hanai K, Uchigata Y. Association of serum bilirubin levels with development and progression of albuminuria, and decline in estimated glomerular filtration rate in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(2):228–35. doi: 10.1111/jdi.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee D-H, Blomhoff R, Jacobs DR. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38(6):535–9. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 41.Yamada J, Tomiyama H, Yambe M, Koji Y, Motobe K, Shiina K, et al. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis. 2006;189(1):198–205. doi: 10.1016/j.atherosclerosis.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 42.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, et al. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care. 2014;37(11):3033–9. doi: 10.2337/dc14-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu C-C, Chang H-Y, Huang M-C, Hwang S-J, Yang Y-C, Tai T-Y, et al. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes Care. 2011;34(4):982–7. doi: 10.2337/dc10-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson RJ, Kang D-H, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertens Dallas Tex 1979. 2003;41(6):1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]