Abstract
BACKGROUND
Food intolerance mediated by food specific IgG antibodies has been implicated in a variety of disorders.
OBJECTIVES
To assess the prevalence of food specific IgG antibodies among patients clinically presenting with allergic symptoms lacking laboratory evidence of allergy.
DESIGN
Descriptive retrospective cross-sectional study.
SETTING
King Khalid University Hospital, Riyadh between 2010–2015.
PATIENTS AND METHODS
Patients were screened for food specific IgG antibodies. All symptomatic patients lacking laboratory evidence of allergy who underwent food specific IgG testing during the study duration were included.
MAIN OUTCOME MEASURE(S)
Levels of IgG antibodies in patients with unidentified allergic symptoms.
RESULTS
We selected 71 patients with allergic symptoms lacking laboratory evidence of allergy. There were 49 female and 22 male patients mean age 38.8 (16.0) years. The majority (85.7%) had urticaria. The most frequently occurring food specific IgG antibodies were against cola nut in 80.3% of patients followed by yeast in 78.9%, wheat in 77.5%, red kidney bean in 71.8%, pea in 63.4%, corn in 62% and egg white in 62% of the patients. Compared with male patients, females harbored significantly higher food specific IgG antibodies for frequently occurring food materials, particularly against wheat (74% vs 25.5%; P<.0001), corn (77.3% vs 22.7%; P<.0001) and cola nut (71.9% vs 28.1%; P<.001). Patients aged less than 40 years had higher levels of food specific IgG against gliadin (P<.003), egg white (P<.03) and barley (P<.05) compared with older patients.
CONCLUSION
The detection of a variety of food specific IgG antibodies among patients with allergic symptoms indicates a possible link to food intolerance allergy. Females are prone to develop food intolerance more than males.
LIMITATIONS
Difficulty of comparison of results with previous studies because of lack of data. Follow-up studies could not be performed to assess the effects of elimination from the diet due to limited time allocated for this study.
Adverse food reactions are generally classified as food allergy and food intolerance.1 Whereas food allergy is typically mediated by IgE antibodies, food intolerance is mediated by IgG class of antibodies.2–4 The prevalence of food intolerance is believed to be 5–20% of the general population; however, the true prevalence of food intolerance remains unknown due to insufficient data.5 IgG-mediated food intolerance is believed to be caused by increased gut permeability, which permits food substances to gain access to the circulation and trigger food specific IgG production.3 Increased production of food specific IgG antibodies coupled with decreased production of anti-inflammatory cytokines such as IL-10 and TGFβ1 have been implicated in irritable bowel syndrome.6
Despite conflicting reports, IgG-mediated food intolerance has been associated with a wide range of specific and non-specific symptomatology. It has been implicated in symptoms associated with allergy such as rashes, urticaria and asthma.2,7,8 Gastrointestinal symptoms suggestive of irritable bowel syndrome including abdominal cramps, diarrhea and constipation are frequently observed in patients with IgG-mediated food intolerance.2,9 Similarly, a strong association between neurological manifestations such as migraine and IgG-mediated food intolerance has also been reported.5,10 Recently, in follow-up studies, food intolerance has been linked with disorders such as atherosclerosis and asthma. Prohibiting ingestion of incriminating food that patients were intolerant to resulted in significant improvement in symptom scores.8,9,11,12 Most common non-specific symptoms such as chronic fatigue and hair loss have also been associated with IgG-mediated food intolerance.3,5,7
Patients clinically presenting with allergic symptoms are usually investigated for the presence of specific IgE antibodies that may not be present among a subgroup of patients presenting with allergic symptoms due to food intolerance. These symptoms, however, take time to manifest since the formation of IgG takes days to months.9 As a result, this group of patients may not only remain undiagnosed, but also continue to suffer from significant preventable morbidity associated with a considerable financial burden on both patients and healthcare resources.9,13,14 This study was performed to investigate the presence of food specific IgG antibodies among patients clinically presenting with allergic symptoms with undetectable food specific IgE antibodies.
METHODS
This descriptive retrospective cross-sectional study was performed in the Allergy Clinic at King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia between 2014 and 2015. Data were extracted from the records of all patients who presented with clinical signs and symptoms of allergy with no laboratory evidence of allergy. These patients were offered microarray testing because of the lack of evidence of IgE-mediated allergic symptoms. Patients who tested positive for the allergy skin prick test or had elevated levels of specific IgE antibodies in blood detected by the radioallergosorbent test (RAST) were excluded from the study. In addition patients suffering from non-allergic disorders such as celiac disease, food intolerance due to enzyme deficiencies or known cases of inflammatory bowel disease were also excluded. Along with demographic details, results of microarray test were extracted from the patient’s medical records. This study was approved by Institutional Review Board of the College of Medicine, King Saud University, Riyadh.
Microarray for food specific IgG antibodies
Food-specific IgG antibodies were detected using a microarray test (Genarrayt, Omega Diagnostics Group, Scotland, United Kingdom) that measures IgG levels against 223 food substances using a single blood sample. This microarray testing procedure involved incubation of the patient serum sample for 30 minutes at room temperature on a glass microscope slide containing a microarray of 223 food extracts. After this primary incubation, the slide was washed to remove unbound proteins. Following this anti-human IgG conjugated to horseradish peroxidase was added to the slide and incubated for another 30 minutes at room temperature. The slide was washed again to remove the unbound conjugate. After washing 3,3′,5′,5′-tetramethybenzidine (TMB) substrate was added to detect specific antibody binding. A third incubation was performed for 10 minutes and then the slide was washed with distilled water. Finally, the slides were centrifuged and scanned by a high-resolution flatbed scanner associated with computer software that interprets the optical densities of the samples. IgG levels less than 30 U/mL were considered negative, whereas levels either equal to or more than 30 U/mL were considered positive. Values of food specific IgG antibodies either equal to or more than 30 U/mL were included in the study.
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) software version 21.0 (IBM, New York, United States). Descriptive statistics were performed for determination of proportions and Pearson’s chi-square test was used for comparison of data for age and gender differences. A P<.05 was considered statistically significant.
RESULTS
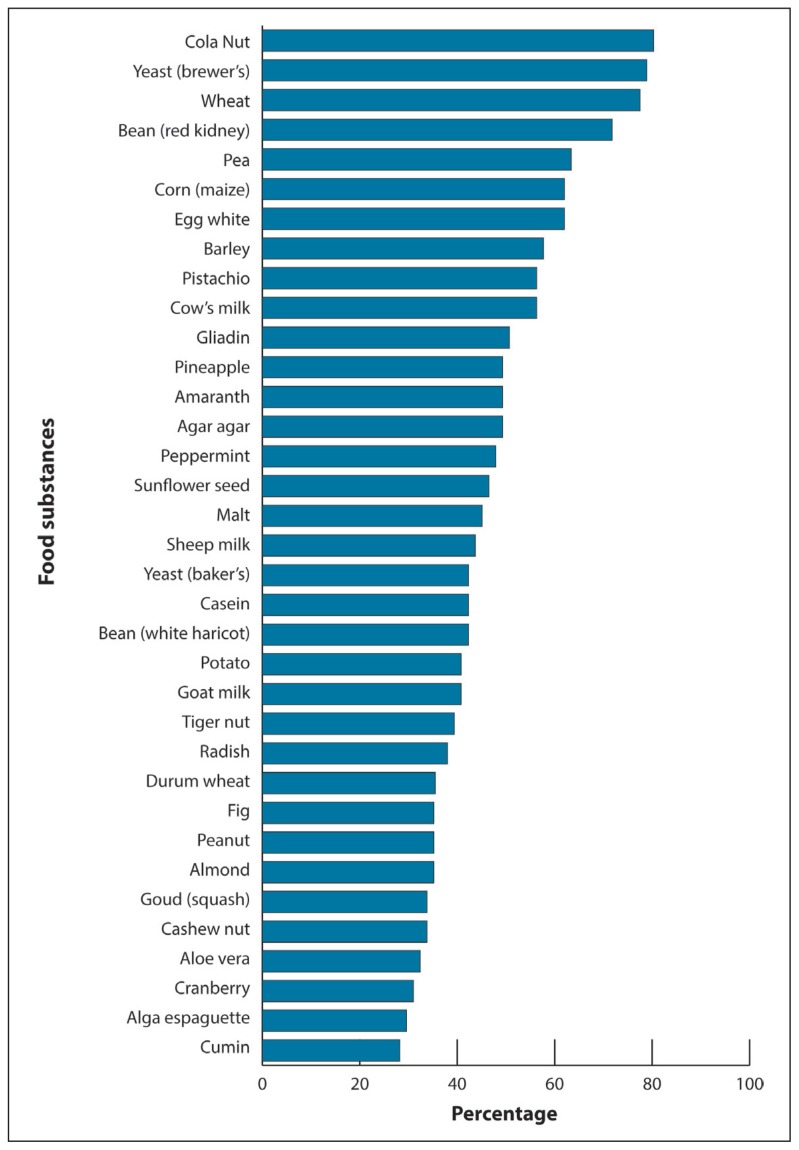
The study population of 71 patients presenting with symptoms of allergy included 49 females and 22 males with the mean (SD) age of 38.8 (16.0) years (range 6 to 80 years). The most frequent manifestation was urticaria (85.7%) followed by asthma (11.4%) and rhinitis (2.9%). Appendices 1, 2 and 3 describe the list of allergens tested by skin prick test and RAST test, respectively (see at www.annsaudimed.net). Online appendix contains the list of all the food substances tested by microarray assay. Figure 1 shows data for the distribution of food specific IgG antibodies among the patients clinically presenting with allergic symptoms. The most frequent food specific IgG antibodies detected among the patients were against cola nut in 80.3% of patients followed by yeast (brewer’s) in 78.9%, wheat in 77.5%, red kidney bean in 71.8%, pea in 63.4%, corn in 62.0%, egg white in 62.0%, barley in 57.7%, pistachio in 56.3%, cow’s milk in 56.3% and gliadin in 50.7% of the patients.
Figure 1.
Distribution of food-specific IgG antibodies in patients presenting with symptoms of allergy.
To investigate the gender differences food-specific IgG antibodies found in 50% or more number of patients were selected for analysis. Table 1 compares data of male and female patients harboring food specific IgG antibodies against 11 food substances found in 50% or higher proportion of patients. Females consistently and significantly had higher levels of food-specific IgGs for all the food substances when compared with the male patients. However, the most significant differences were noted for wheat (male vs. female: 25.5% vs. 74.5%; P≤.0001), corn (male vs. female: 22.7% vs. 77.3%; P≤.0001) and cola nut (male vs. female: 28.1% vs. 71.9%; P≤.001). Table 2 compares food-specific IgGs between two groups: aged 20–39 and 40–80 years old patients to assess the effect of age on IgG-mediated food intolerance. Among the 11 most prevalent food-specific IgGs tested the 20–39 years old group had significantly higher food-specific IgGs against gliadin (76.7% vs. 23.3%; P<.003) followed by egg white (68.6% vs. 31.4%; P<.03) and barley (65.8% vs. 34.2%; P<.05) when compared with the 40–80 year old group of patients presenting with symptoms of allergy.
Table 1.
Genders differences for food specific IgG among patients clinically presenting with allergy-like symptoms.
| Food substance | Males | Females | P value |
|---|---|---|---|
|
| |||
| Cola Nut | 16 (28.1) | 41 (71.9) | .001 |
| Yeast (Brewer’s) | 17 (30.4) | 39 (69.6) | .003 |
| Wheat | 14 (25.5) | 41 (74.5) | .0001 |
| Bean (Red kidney) | 15 (29.4) | 36 (70.6) | .003 |
| Pea | 14 (31.1) | 31 (68.9) | .01 |
| Corn (Maize) | 10 (22.7) | 34 (77.3) | .0001 |
| Egg white | 14 (31.8) | 30 (68.2) | .02 |
| Barley | 13 (31.7) | 28 (68.3) | .02 |
| Pistachio | 12 (30) | 28 (70) | .01 |
| Cow’s milk | 12 (30) | 28 (70) | .01 |
| Gliadin | 11 (30.6) | 25 (69.4) | .02 |
Values are n (%).
Table 2.
Comparison of food specific IgG between different age groups of patients clinically presenting with allergic symptoms.
| Food substance | Age 20–39 Years Number (%) | Age 40–80 Years Number (%) | P value |
|---|---|---|---|
|
| |||
| Cola Nut | 31 (62) | 19 (38) | .09 |
| Yeast (Brewer’s) | 31 (63.3) | 18 (36.7) | .06 |
| Wheat | 30 (61.2) | 19 (38.8) | .11 |
| Bean (Red kidney) | 25 (58.1) | 18 (41.9) | .29 |
| Pea | 24 (63.2) | 14 (36.8) | .11 |
| Corn (Maize) | 23 (60.5) | 15 (39.5) | .19 |
| Egg white | 24 (68.6) | 11 (31.4) | .03 |
| Barley | 25 (65.8) | 13 (34.2) | .05 |
| Pistachio | 22 (64.7) | 12 (35.3) | .09 |
| Cow’s milk | 22 (64.7) | 12 (35.3) | .09 |
| Gliadin | 23 (76.7) | 7 (23.3) | .003 |
Values are n (%).
DISCUSSION
Of 223 food substances tested, food specific IgG against cola nut (80.3%), brewer’s yeast (78.9%) and wheat (77.5%) were the most prevalent among the patients clinically presenting with allergic symptoms lacking laboratory evidence of allergy. Data on the prevalence of food specific IgG antibodies among allergic patients are scarce. A study investigating the presence of food specific IgG antibodies among patients suffering from urticaria, asthma, rhinitis and atopic eczema has reported higher prevalence of specific IgGs against egg (77.8%), milk (62%) and casein (57.8%).15 Similarly among patients suffering from asthma specific IgG antibodies against cow’s milk (56%), tiger nut (48%) and casein (48%) were found to be the most frequently occurring antibodies.8 Collectively these data suggest that food specific IgG may have some association with allergic symptoms, but the pattern of most common food specific IgG frequently observed may vary in different populations.
The relationship between harboring high levels of food specific IgG and the development of asthma does not appear to be casual. A study investigating development of asthma in children under five years of age has shown that a high level of egg-specific IgG antibodies is a better predictor of having asthma compared with IgE levels with a sensitivity of 64%.16 Although 62% of the patients in the present study had egg-specific IgG antibodies it is difficult to compare the results of the present study with the previous study as the majority of patients in the current study were suffering from urticaria and not asthma.
Similarly, cola nut specific IgG antibodies were present in the majority of the patients suffering from allergic disorder in the present study. The low prevalence of specific IgG antibodies against cola nut (4.9%) reported among patients suffering from migraine suggests that prevalence of frequently occurring food specific IgG antibodies may vary in different disorders.17 Furthermore modified food materials tend to support production of higher amounts of food specific IgG, IgM, IgA and IgE antibodies compared to raw food materials.18 Cola nut, extensively used as a flavoring agent in beverages after having been processed, could have possibly triggered higher amounts of cola nut specific IgG antibody production observed in the present study. It is highly likely that differences in cultural and dietary habits in different parts of the world contribute to variations in the global prevalence rates of food specific IgG antibodies.
The results of this study revealed that food intolerance is significantly more common in females than in males. These observations are similar to a study from China documenting a higher concentration of food specific IgG in females compared to males for 12 out of 14 food substances.1 Similarly, higher levels of food-specific IgG levels among females compared to males for 10 out of 11 food materials have also been reported.19 In addition female gender has also been linked with an increased likelihood of suffering from hypersensitivity reactions.20 Although it is difficult to explain female preponderance for food specific IgG antibodies, female sex steroids are known for exerting a strong pro-inflammatory effect for induction of allergies.21 Despite a number of studies supporting female predilection for developing food intolerance, there is evidence suggesting that there are no gender differences in development of IgG-mediated food intolerance.22 Findings of the present study are however consistent with majority of previously published reports indicating a higher predisposition of the female gender for food intolerance.
Comparison of patients aged between 20–39 and 40–60 years of age revealed that the younger age group had higher levels of egg white and gliadin specific IgG among the 11 most frequently occurring food specific IgG in the present study. A similar comparison between younger and older age groups has been described previously where the younger age group was found to harbor higher levels of egg-specific IgG antibodies compared with the older age group.1 Patients suffering from food intolerance may have increased gut permeability that allows food macromolecules to gain access to the circulation resulting in the formation food specific IgG antibodies.23 Maturation of intestinal mucosa with increasing age may influence food intolerance resulting in differential immune responses to egg and gliadin specific IgG antibodies. Additionally, food specific IgG antibodies tend to have a negative correlation with increasing age that could also contribute to the observed differences between the younger and the older patients.24 Anti-gliadin antibodies are frequently associated with celiac disease and are found in a vast majority of patients suffering from celiac disease. Exposure to gliadin among the younger population may predispose to the development of celiac disease in this subgroup of patients.3
In conclusion, cola nut, yeast (brewer’s) and wheat were the most common food substances implicated in food intolerances among patients presenting with allergy symptoms in the current study. The results also revealed that females are more likely to have “leaky guts” predisposing them to the development of food intolerance than males. In addition, younger age was associated with a higher predisposition for IgG-mediated food intolerance particularly to egg white and gliadin. The results of this study do not reflect the status of food intolerance among the general population of Saudi Arabia as it was conducted only in one center in Riyadh. Large-scale follow up studies are recommended to assess the effects of food elimination diet on symptom scores. In addition, it would be of interest to assess the prevalence of food specific IgG antibodies in an otherwise asymptomatic normal healthy population to establish local reference ranges for various food materials.
Footnotes
Conflict of Interest
None declared.
REFERENCES
- 1.Zeng Q, Dong S-Y, Wu L-X, Li H, Sun Z-J, Li J-B, et al. Variable food-specific IgG antibody levels in healthy and symptomatic Chinese adults. PloS One. 2013;8(1):e53612. doi: 10.1371/journal.pone.0053612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaur S, Kumar R. Food allergy or food intolerance.? Indian J Allergy Asthma Immunol. 2013;27(2):93. [Google Scholar]
- 3.Beyer K, Teuber SS. Food allergy diagnostics: scientific and unproven procedures. Curr Opin Allergy Clin Immunol. 2005;5(3):261–6. doi: 10.1097/01.all.0000168792.27948.f9. [DOI] [PubMed] [Google Scholar]
- 4.Turner PJ, Kemp AS. Intolerance to Food Additivies - Does It Exist? J Pediatr Child Health. 2012;48(2):E10–4. doi: 10.1111/j.1440-1754.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- 5.Priedite V, Nikiforenko J, Kurjanev N, Kroica J. Antigen Specific IgG4 in Patients with Gastrointestinal Complaints. Brit J Med & Med Res. 2014;4(1):194–201. [Google Scholar]
- 6.Sentsova TB, Vorozhko IV, Isakov VA, Morozov SV, Shakhovskaia AK. [Immune status estimation algorithm in irritable bowel syndrome patients with food intolerance]. Eksp Klin Gastroenterol. 2014;(7):13–7. [Article in Russian] [PubMed] [Google Scholar]
- 7.Collard J. Food Allergy and Intolerance. Pract Nurse. 2010;39(1):17–21. [Google Scholar]
- 8.Kumar R, Kumar M, Singh M, Bisht I, Gaur S, Gupta N. Prevalence of food intolerance in bronchial asthma in India. Indian J Allergy Asthma Immunol. 2013;27(2):121. [Google Scholar]
- 9.Guo H, Jiang T, Wang J, Chang Y, Guo H, Zhang W. The Value of Eliminating Foods According to Food-Specific Immunoglobulin G Antibodies in Irritable Bowel Syndrome with Diarrhoea. J Int Med Res. 2012;40(1):204–10. doi: 10.1177/147323001204000121. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell N, Hewitt CE, Jayakody S, Islam M, Adamson J, Watt I, et al. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J. 2011;10:85. doi: 10.1186/1475-2891-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arroyave Hernández CM, Echavarría Pinto M, Echevarría Pinto M, Hernández Montiel HL. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex Tecamachalco Puebla Mex 1993. 2007;54(5):162–8. [PubMed] [Google Scholar]
- 12.Wilders-Truschnig M, Mangge H, Lieners C, Gruber H-J, Mayer C, März W. IgG antibodies against food antigens are correlated with inflammation and intima media thickness in obese juveniles. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. 2008;116(4):241–5. doi: 10.1055/s-2007-993165. [DOI] [PubMed] [Google Scholar]
- 13.Halbrich M, Ben-Shoshan M, Rex G. Friend or foe? Figuring out the difference between FPIES, IgE-mediated allergy and food intolerance. BMJ Case Rep. 20142014 doi: 10.1136/bcr-2013-200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aydinlar EI, Dikmen PY, Tiftikci A, Saruc M, Aksu M, Gunsoy HG, et al. IgG-Based Elimination Diet in Migraine Plus Irritable Bowel Syndrome. Headache J Head Face Pain. 2013;53(3):514–25. doi: 10.1111/j.1526-4610.2012.02296.x. [DOI] [PubMed] [Google Scholar]
- 15.Antico A, Pagani M, Vescovi PP, Bonadonna P, Senna G. Food-Specific IgG4 Lack Diagnostic Value in Adult Patients with Chronic Urticaria and Other Suspected Allergy Skin Symptoms. Int Arch Allergy Immunol. 2011;155(1):52–6. doi: 10.1159/000318736. [DOI] [PubMed] [Google Scholar]
- 16.Vance GHS, Thornton CA, Bryant TN, Warner JA, Warner JO. Ovalbumin-specific immunoglobulin G and subclass responses through the first 5 years of life in relation to duration of egg sensitization and the development of asthma. Clin Exp Allergy. 2004;34(10):1542–9. doi: 10.1111/j.1365-2222.2004.02058.x. [DOI] [PubMed] [Google Scholar]
- 17.Rees T. A Prospective Audit of Food Intolerance Among Migraine Patients in Primary Care Clinical Practice. Headache Care. 2005;1:11–4. [Google Scholar]
- 18.Vojdani A1. Detection of IgE, IgG, IgA and IgM antibodies against raw and processed food antigens. Nutr Metab (Lond) 2009;6:22. doi: 10.1186/1743-7075-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizza V. Food Intolerance in Migraine. 2013;1 [Google Scholar]
- 20.Schäfer T, Böhler E, Ruhdorfer S, Weigl L, Wessner D, Filipiak B, et al. Epidemiology of contact allergy in adults. Allergy. 2001;56(12):1192–6. doi: 10.1034/j.1398-9995.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- 21.Harish Babu BN, Mahesh PA, Venkatesh YP. A cross-sectional study on the prevalence of food allergy to eggplant (Solanum melongena L.) reveals female predominance. Clinical and experimental allergy: J Brit Soci Allergy & Clin Immunol. 2008;38:1795–1802. doi: 10.1111/j.1365-2222.2008.03076.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang RL, Li FY, Li WS, Jiang YM. [An epidemiological study of food intolerance in 2434 children]. Zhongguo Dang Dai Er Ke Za Zhi. 2013;(7):550–4. [Article in Chinese] [PubMed] [Google Scholar]
- 23.Dannaeus A, Inganäs M. A follow-up study of children with food allergy. Clinical course in relation to serum IgE- and IgG-antibody levels to milk, egg and fish. Clin Allergy. 1981;11(6):533–9. doi: 10.1111/j.1365-2222.1981.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 24.Jansen A, Mandić AD, Bennek E, Frehn L, Verdier J, Tebrügge I, et al. Anti-food and anti-microbial IgG subclass antibodies in inflammatory bowel disease. Scand J Gastroenterol. 2016:1–9. doi: 10.1080/00365521.2016.1205130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]